Abstract
Weight gain is associated with an increase in intrahepatic triglycerides (IHTGs), and is the primary cause of nonalcoholic fatty liver disease in obese people. We combined imaging and stable isotope tracer techniques to evaluate the physiologic mechanisms of weight gain-induced steatosis in 27 obese people. Weight gain appeared to increase IHTG content by generating an imbalance between hepatic fatty acid availability and disposal, and resulted in increased hepatic de novo lipogenesis, decreased intrahepatic fatty acid oxidation, and inadequate increases in IHTG export via very low-density lipoprotein secretion.
ClinicalTrials.gov number, NCT01184170.
Keywords: VLDL, overweight, apoB100, fatty acid oxidation
Obesity is an important risk factor for the development of nonalcoholic fatty liver disease (NAFLD); the prevalence of NAFLD is ~15% and ~65% in lean and obese adults, respectively.1 The mechanisms responsible for excessive accumulation of intrahepatic triglyceride (IHTG) induced by weight gain are not known,2,3 but likely involve an imbalance between free fatty acid (FFA) delivery to the liver and de novo fatty acid synthesis and the rate of fatty acid oxidation and export (as triglyceride [TG] within very low-density lipoprotein [VLDL]).4
The purpose of this study was to evaluate the physiological mechanisms responsible for the accumulation of IHTG after moderate weight gain in obese people. Twenty-seven obese subjects (7 men, 20 women; age 48±10 years old) (Table 1) were studied before and after they gained a target of ~6% body weight by consuming an additional ~1000 kcal/d of foods containing the same macronutrient distribution (percent calories from carbohydrate, fat and protein) as their usual diet. Magnetic resonance spectroscopy and imaging were used to evaluate body composition and fat distribution. Stable isotopically-labeled glycerol, palmitate, leucine, acetate and β-hydroxybutyrate tracer infusions were used to evaluate: i) the rate of release of FFA from adipose tissue into the bloodstream, which is an important source of fatty acids delivered to the liver for IHTG synthesis; ii) hepatic de novo lipogenesis (DNL), which provides fatty acids synthesized from carbohydrate precursors for esterification into IHTG; iii) hepatic β-hydroxybutyrate secretion rate, which is a marker of intrahepatic fatty acid oxidation; iv) hepatic VLDL-TG secretion rate, which exports TG out of the liver, and are comprised of fatty acids derived from both systemic (plasma FFA) and non-systemic (lipolysis of intrahepatic, visceral adipose tissue, and circulating TGs, and/or DNL) sources;5 and v) VLDL-apolipoprotein B100 (VLDL-apoB100) secretion rate, which is a marker of the number of VLDL particles secreted by the liver because each particle contains one molecule of apoB100 (see Supplemental Material for details of experimental protocol, sample analyses, calculations and statistical analyses).
Table 1.
Body composition and metabolic characteristics before and after weight gain
| Before | After | |
|---|---|---|
| Body mass index (kg/m2) | 34.6±3.5 | 36.6±3.7* |
| Body weight (kg) | 96.8±13.8 | 102.4±14.6* |
| Fat-free mass (kg) | 52.5±11.0 | 54.1±11.3* |
| Fat mass (kg) | 43.8±6.8 | 47.7±7.1* |
| Body fat mass (%) | 45.5±5.8 | 46.8±5.3* |
| Visceral adipose tissue (g) | 1057 (902, 1239) | 1183 (1012, 1381)* |
| Intrahepatic triglyceride content (%) | 4.8 (3.3, 7.0) | 7.1 (4.7, 10.7)* |
| Glucose (mg/dL) | 97 (94, 100) | 99 (96, 103) |
| Insulin (mU/L) | 13 (10, 16) | 15 (11, 19)* |
| HOMA-IR | 3.1 (2.4, 4.0) | 3.7 (2.8, 4.8)* |
| Free fatty acid (μmol/L) | 350±80 | 360±90 |
| Free fatty acid Ra (μmol/min) | 300±69 | 305±72 |
| Triglyceride (mg/dL) | 110±48 | 126±51* |
| Apolipoprotein B100 (mg/dL) | 82±22 | 89±25§ |
| β-hydroxybutyrate (μmol/L) | 79 (52, 121) | 46 (37, 58)* |
| Respiratory quotient | 0.73±0.03 | 0.76±0.05** |
| Fat oxidation (g/kg/d) | 1.5±0.4 | 1.3±0.4** |
| Carbohydrate oxidation (g/kg/d) | 0.6±0.6 | 0.9±1.0** |
Values are mean ± SD or 95% CIs.
HOMA-IR, homeostasis model assessment of insulin resistance; Ra, rate of appearance.
Value significantly different than corresponding value before weight gain:
P<0.001,
P<0.05,
P=0.07.
Studies were performed before and after subjects consumed the high-calorie diet for up to 12 weeks in an effort to gain ~6% body weight. The average duration of high-calorie diet consumption was 8±3 weeks, which caused a 5.8±1.1% (range 3% to 9 %) weight gain that was mostly due to an increase in fat mass (Table 1). Genotyping for the PNPLA3 SNP rs738409 (I148M) was performed because of the known association between this SNP and steatosis.6 We identified 16 non-carriers (genotype C/C) and 11 carriers (G/C and G/G); 2 of the 11 carriers were homozygous for the risk allele (G/G). Although there was a trend for carriers to have higher baseline IHTG content (8±6% carriers vs. 6±7% non-carriers) and to increase IHTG content more after weight gain than non-carriers (13±11% carriers vs. 9±9% non-carriers), these differences were not statistically significant. Weight gain caused a 4.0±4.1% absolute increase (55±49% relative increase) in IHTG content (Table 1), which corresponds to an accrual of ~60 g of IHTG, assuming a liver weight of 1,500 g. Basal plasma glucose did not significantly change with weight gain, whereas plasma insulin and HOMA-IR values increased by 15% to 20%, demonstrating a deterioration in insulin sensitivity (Table 1).
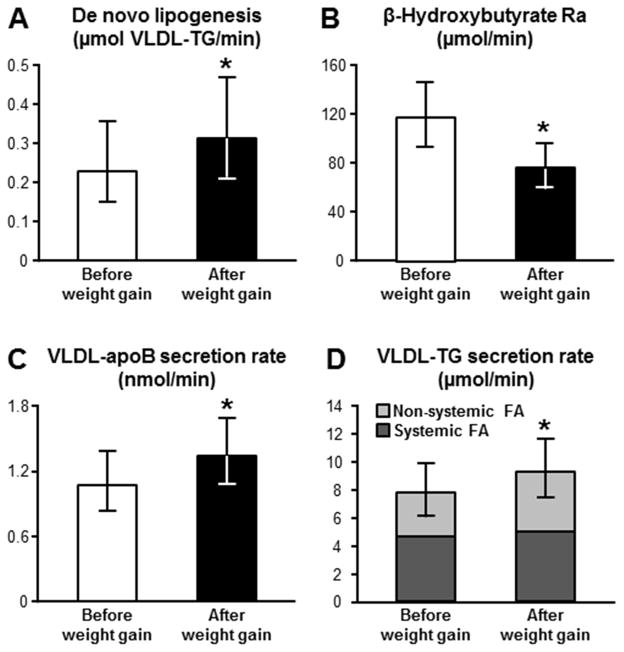
We then evaluated the effect of weight gain on the metabolic pathways that influence IHTG production rate, namely hepatic fatty acid availability from plasma and DNL from carbohydrate precursors. Plasma FFA concentration and the rate of appearance (Ra) of FFA into plasma, a measure of adipose tissue lipolytic rate and fatty acid availability to the liver, did not change with weight gain (Table 1). In contrast, the rate of intrahepatic de novo synthesis of fatty acids increased by ~20% with weight gain (Figure 1A).
Figure 1.
Effect of moderate weight gain on: A) hepatic de novo lipogenesis, assessed as fatty acid produced from carbohydrate precursors and incorporated into very low-density lipoprotein-triglyceride (VLDL-TG); B) β-hydroxybutyrate rate of appearance (Ra); C) hepatic secretion of VLDL-apolipoprotein B100 (VLDL-apoB100); and D) hepatic secretion of VLDL- TG, and contribution of systemic (dark grey) and non-systemic (light grey) fatty acid sources to triglyceride secreted within VLDL. *Value different from before weight gain value, P<0.05. Data are back-transformed from the log and are presented as means and 95% CIs.
We also evaluated the effect of weight gain on the metabolic pathways that are involved in the removal of IHTG, namely intrahepatic fatty acid oxidation and export of TG within VLDL particles. Fatty acids are used by the liver to produce energy through a multi-step process that involves β-oxidation and production of β-hydroxybutyrate (the most predominant ketone body). Accordingly, the rate of release of β-hydroxybutyrate into the systemic circulation reflects the rate of hepatic fatty acid oxidation.7,8 We found that weight gain caused a reduction in hepatic fatty acid oxidation rate, as demonstrated by a decrease in both β-hydroxybutyrate concentration (Table 1) and β-hydroxybutyrate Ra (Figure 1B). Weight gain also caused a decrease in whole-body fatty acid oxidation rate and an increase in carbohydrate oxidation rate (Table 1). VLDL particles produced by the liver are composed primarily of TG, a single molecule of apoB100 and some cholesterol, phospholipids and small, exchangeable lipoproteins.9 The secretion of VLDL provides a mechanism for exporting water-insoluble lipids from the liver as a water soluble particle into the bloodstream to peripheral tissues. The rate of VLDL-apoB100 secretion represents the number of VLDL particles secreted by the liver, and the rate of secretion of VLDL-TG is a measure of the amount of triglyceride exported from the liver. Weight gain caused an increase in both VLDL-apoB100 (Figure 1C) and VLDL-TG (Figure 1D) secretion rates, and an increase in plasma total triglyceride and total apolipoprotein B100 (Table 1). Moreover, the increase in VLDL-TG secretion rate was almost entirely attributable to a marked increase in the contribution of fatty acids originating from non-systemic sources (lipolysis of intrahepatic, visceral adipose tissue, and circulating TGs, and/or DNL) (Figure 1D).
Our study has several limitations. First, although we tried to keep dietary macronutrient content stable throughout the study by providing careful diet instructions, diet plans, and weekly reviews of food intake, it is possible that changes in macronutrient composition occurred when subjects consumed the high-calorie diet, which could have had independent effects on our outcome measures. Second, our study evaluated the effect of weight gain after ~8 weeks of a high-calorie diet, so we cannot exclude the possibility that a slower rate of weight gain over a much longer period of time would produce different results. Finally, all metabolic assessments were performed after subjects fasted for ~12 h overnight, so we cannot determine the effect of weight gain on total 24-h metabolic activity, which includes both fasted and fed conditions. Nonetheless, it is likely that the effect of weight gain on our basal measurements would have the same qualitative effect on measurements made throughout the day.
The results from the present study elucidate the physiological mechanisms responsible for IHTG accumulation caused by moderate weight gain in obese people. Our data demonstrate that weight gain induced by a macronutrient-balanced, high-calorie diet causes alterations in specific metabolic pathways that contribute to an increase in steatosis. Weight gain affected both sides of intrahepatic triglyceride balance by increasing the de novo synthesis of fatty acids from carbohydrate, in conjunction with a decrease in the elimination of fatty acids by intrahepatic fatty acid oxidation. In fact, the decrease in fatty acid oxidation in the liver was associated with a shift in whole-body substrate oxidation from lipid to carbohydrate. Weight gain also caused an increase in the export of TG out of the liver by secreting a greater number of TG-rich VLDL particles, manifested by the increase in VLDL-apoB100 and VLDL-TG secretion rates. The increase in VLDL-TG secretion was due entirely to a contribution from non-systemic fatty acid sources, presumably derived from lipolysis of visceral adipose tissue, intrahepatic and circulating TGs, and/or DNL. However, the increase in TG export was not adequate to fully compensate for the increased rate of IHTG production, because IHTG content increased. The alteration in VLDL kinetics was likely responsible for the observed increase in plasma TG and apoB100 concentrations. In contrast, weight gain did not cause an increase in the basal delivery of fatty acids to the liver from lipolysis of subcutaneous adipose tissue triglycerides.
Supplementary Material
Acknowledgments
Grant support: This work was supported by NIH grants UL1 RR024992 (Clinical Translational Science Award), DK 56341 (Nutrition and Obesity Research Center), DK 20579 (Diabetes Research Center), DK 37948, a grant from the Longer Life Foundation, and support from the Kilo Foundation.
The authors thank Emily Lake for assistance in recruiting the study subjects, Freida Custodio, and Jennifer Shew for technical assistance, Melisa Moore, Kathryn Gratza and the staff of the Clinical Research Unit for help in performing the studies, and the study subjects for their participation. Dr. Samuel Klein is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
Abbreviations
- apoB100
apolipoprotein B100
- DNL
de novo lipogenesis
- NAFLD
nonalcoholic fatty liver disease
- Ra
rate of appearance
- VLDL
very low-density lipoprotein
Footnotes
Disclosures: The authors have no conflicts of interest to disclose
Author contribution: S.K. designed the study; E.F. M.Y. and G.F. performed the metabolic studies; C.T.L. provided the dietary intervention, A.L.O., L.L., and B.W.P. performed the sample analyses; E.F., L.L, and B.W.P collated and analyzed the data; E.F. performed the statistical analyses; E.F. and S.K. interpreted the data and wrote the manuscript; all authors critically revised the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Browning JD, et al. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Kimura T, et al. J Gastroenterol Hepatol. 2015;30:909–917. doi: 10.1111/jgh.12861. [DOI] [PubMed] [Google Scholar]
- 3.Fabbrini E, et al. J Clin Invest. 2015;125:787–795. doi: 10.1172/JCI78425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabbrini E, et al. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabbrini E, et al. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romeo S, et al. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havel RJ, et al. J Clin Invest. 1970;49:2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beylot M. Diabetes Metab. 1996;22:299–304. [PubMed] [Google Scholar]
- 9.Converse CA, et al. Lipoprotein Analysis: A Practical Approach. New York, NY: Oxford University Press; 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.