Abstract
Background
Animal models capable of differentiating the neurobiological intricacies between physical and emotional stress are scarce. Current models rely primarily on physical stressors (e.g. chronic unpredictable or mild stress, social defeat, learned helplessness), and neglect the impact of psychological stress alone. This is surprising given extensive evidence that a traumatic event needs not be directly experienced to produce enduring perturbations on an individual’s health and psychological well-being. Post-traumatic stress disorder (PTSD), a highly debilitating neuropsychiatric disorder characterized by intense fear of trauma-related stimuli, often occurs in individuals that have only witnessed a traumatic event.
New method
By modifying the chronic social defeat stress (CSDS) paradigm to include a witness component (witnessing the social defeat of another mouse), we demonstrate a novel behavioral paradigm capable of inducing a robust behavioral syndrome reminiscent of PTSD in emotionally stressed adult mice.
Results
We describe the vicarious social defeat stress (VSDS) model that is capable of inducing a host of behavioral deficits that include social avoidance and other depressive-and anxiety-like phenotypes in adult male mice. VSDS exposure induces weight loss and spike in serum corticosterone (CORT) levels. A month after stress, these mice retain the social avoidant phenotype and have an increased CORT response when exposed to subsequent stress.
Comparison with existing method(s)
The VSDS is a novel paradigm capable of inducing emotional stress by isolating physical stress/confrontation in mice.
Conclusions
The VSDS model can be used to study the short- and long-term neurobiological consequences of exposure to emotional stress in mice.
Keywords: emotional stress, PTSD, depression, social defeat, witness stress
Introduction
Post-traumatic stress disorder (PTSD) is a trauma-related disorder characterized by the persistent fear of trauma-related stimuli that may emerge after exposure to severe stress (American Psychiatric Association, 2013). This debilitating disorder affects approximately 10% of the population in the United States (Kessler, 2000, Kessler et al., 2005), and carries an economic burden now approximating 85 billion dollars per year (Greenberg et al., 2015). The neurobiology underlying PTSD and related disorders is not well understood (Newport and Nemeroff, 2000), and adding to its complexity is the fact that PTSD can also develop in individuals who simply witness a fearful/traumatic event (Perlman et al., 2011, van Wingen et al., 2011). Unfortunately, there is a considerable gap in our basic understanding of the neurobiological consequences of psychological/emotional stress alone and its influence on mental health of the individual.
Most animal models of stress rely on physical stressors to induce a pathological-like state, but neglect psychological aspects (i.e., involving indirect, non-physical conflict). A witness foot-shock model has been proposed to delineate the biological differences between physical and emotional stress (Van den Berg et al., 1998, Pijlman et al., 2003). In this paradigm, a rat is forced to witness another rat receive unpredictable foot shocks from the safety of an adjacent compartment. In this model, the witness rats showed locomotor hyperactivity and increased sensitivity to saccharin when compared to non-stressed controls, while the foot-shocked rats showed opposite effects (Van den Berg et al., 1998, Pijlman et al., 2003). Given that in this paradigm the witness and foot-shocked rats were housed together, it is possible that differences between experimental groups could be the result of a dominance hierarchy (Warren et al., 2013). Although this paradigm has proven useful in delineating biological effects of witnessing stress, it lacks ethological validity. This highlights the necessity for the continued development of refined animal models of emotional stress and demonstrates the viability of a witness component for the study of psychological stress effects.
The chronic social defeat stress (CSDS) paradigm is an effective, ethologically relevant, and reliable model for inducing a depressive-like phenotype, PTSD-, and mood- and anxiety-related symptomology in rodents (Berton et al., 2006, Golden et al., 2011, Huhman, 2006, Nestler and Hyman, 2010). This paradigm has strong construct, face, and predictive validity, since repeated, but not acute, antidepressant treatment reverses CSDS-induced deficits (Berton et al., 2006, Nestler and Hyman, 2010, Warren et al., 2013). In mice, CSDS involves an adult male C57BL/6J mouse being forced to intrude upon the home cage of an aggressive CD-1 male mouse. The intruder is quickly overpowered and adopts a submissive posture characterized by rearing, vocalization, and escape-like behaviors. Socially defeated mice display lasting deficits in the elevated plus-maze and forced swim test, behavioral measures of mood dysregulation (Berton et al., 2006, Krishnan et al., 2007). However, the CSDS paradigm alone is not capable of discriminating between the emotional and physical aspects of stress. This limitation can be overcome by the addition of a witness component (i.e., the emotionally-stressed mouse) to the CSDS paradigm. The result is a novel vicarious social defeat stress (VSDS) paradigm, which elicits a robust social avoidant phenotype and other mood-related behavioral deficits. This improved model offers ethological relevance, strong face validity, and easy testability (Warren et al., 2013). The VSDS paradigm also offers a model that removes the confounding influence of physical injury from studies assessing the role of stress on immune and inflammatory systems (Hodes et al., 2014). The VSDS paradigm can help bridge the gap in our understanding of psychological stress by simultaneously eliciting behavioral abnormalities in both physically and strictly emotionally stressed mice. VSDS not only provides an unparalleled method for studying the behavioral consequences of physical and emotional stress, but also potentially provide valuable insight into the cellular and molecular mechanisms underlying them.
1. Methods
All experimental procedures described here are in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 2003) and with approval of the Institutional Animal Care and Use Committee at Florida State University.
1.1. Animals
Eight week-old male C57BL/6J mice (Jackson Laboratory) and CD-1 retired breeders (Charles River) were used in this study. Animals were housed in a vivarium at 23–25°C on a 12 h light/dark cycle (lights on between 7:00 A.M. and 7:00 P.M.). C57BL/6J mice (four per cage) and CD-1 mice (one per cage) were housed in clear polypropylene boxes containing wood shavings.
1.2. Materials
1.2.1. Social Defeat
Clear polypropylene mice breeding cages (23.5cm × 45.5cm × 15cm)
Clear polypropylene mice cages (29.5cm × 18.5cm × 13cm)
Wood shaving bedding
Water bottles
Cage cards
Animal feed (Standard Mouse Chow)
Paired steel-wire tops
Clear perforated Plexiglas dividers (45.5cm × 0.5cm × 14cm)
1.2.2. Social Interaction Test
Video tracking hardware and software (EthovisionXT; Noldus).
Social interaction arena custom made from opaque white Plexiglas (40cm × 40cm × 40cm).
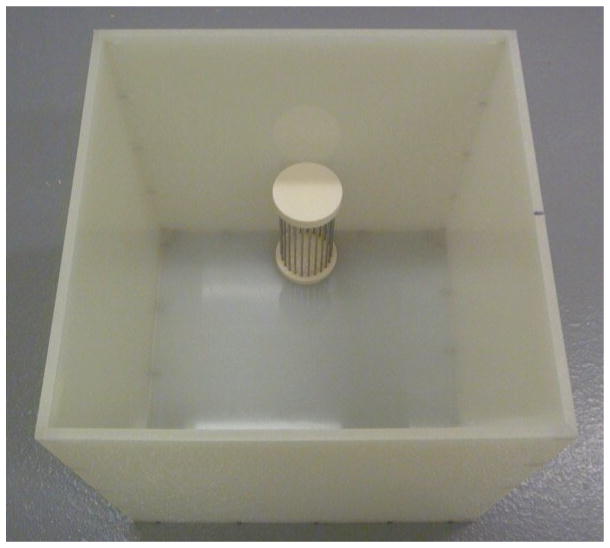
Wire mesh cage (one per social interaction arena) – large enough to hold a CD-1 and allow for their snout and paw to fit though the space between wires is needed. See Appendix A, Figure G.
50% Ethanol for cleaning.
2. Procedure
2.1. 10 days before defeat – Ordering Mice
Eight week-old male C57BL/6J mice (The Jackson Laboratory) arrive and are housed no more than five to a cage. In our experiments, group size per condition is usually 10. However, it is important to have 3–5 extra mice in the unlikely event of losing PS mice to attrition. CD-1 retired breeder mice (Charles River) should be between 4–6 months of age and single housed upon arrival. (Note: not all of the CD-1 mice will meet the aggressive behavior threshold required (see screening process below). At least 10 C57 screeners should be ordered per 50 aggressors at the same age as the experimental C57. All mice should be allowed to habituate to the living colony for one week.
2.2. < 3–5 days before defeat – Screening Process
Retired breeder CD-1 (aggressors) mice are screened using non-experimental C57 screeners. The screening process involves placing a screener mouse into the aggressor’s home cage once a day for three consecutive days. Different screeners should be used between consecutive screening sessions. Screeners should be close to the same age as the experimental C57 mice and may be used in future screenings.
Place the C57 screener in the home cage of the aggressor and record the amount of time it takes the CD-1 to attack the intruding C57 in seconds as latency to aggression. If it takes the aggressor one second, then it should be recorded as 1. If an aggressor does not fight, annotate with an “X.” Screenings should last 180 s and the C57 should be left in the aggressor’s cage for the entirety of the 180 s (unless there are open wounds or an overly submissive poster is observed, such as the C57 laying down on its back, or not moving). To assess aggression in CD-1 mice, look for several key behaviors. When the CD-1 attacks, they follow, try to bite and pull on the C57. This bout of aggression lasts approximately 5 seconds. When screening, it is important that the CD-1 show at least 3–5 of these bouts of aggression for inclusion. Furthermore, CD-1s must have a latency of less than 60 s in the last two consecutive sessions to be used for defeats.
2.3. 1 day before defeat – Cage Setup
Assemble defeat cages as seen in Figure A–E in Appendix A. To ensure that all C57BL/6J mice encounter novel CD-1 mice each day, a minimum of 10 CD-1 cages should be used at a time. Multiples of 10 are easiest to manage. The CD-1 should be put into their respective side (right side) of the divider so that they may territorialize their “home” overnight. Food and water should be provided on both sides of the divider ad libitum.
The C57BL/6J mice should be randomly assigned to be either physical stressed (PS), emotional stressed (ES) or control stress (CON) conditions. ES and PS mice are alternately housed adjacent to CD-1 mice, while the CON mice must be housed separately, away from the social defeat environment.
2.4. Chronic social defeat (10 days)
Place the ES mouse in the compartment adjacent to the CD-1 mouse, in alternating cages. Then place the intruding PS mouse into the compartment containing the CD-1 mouse. The defeats should last 10 minutes or less for 10 consecutive days. When the time is up, move the ES-exposed mouse to the cage to the left (into the empty compartment next to a novel CD-1). The PS-exposed mouse should be moved across the divider to remain overnight adjacent to the attacker. The next day, PS mice will be moved to the home of the aggressor in the cage to the right for the defeat. This ensures that everyday a novel aggressor is defeating the PS mice and that the ES animal observes a novel defeat each day.
Mice in the CON condition are housed in divided cages, one CON mouse on each side (see Appendix A, Figure C–D). Before defeats start, the divider is removed and the CON mice are allowed to interact with their cage mate for 10 minutes. No aggressive behavior is observed/expected between these mice. Though it is not necessary to house the CON mice in a different room than the ES/PS, it is important that to note we perform the defeats in a separate room to avoid influencing the CON mice.
2.5. Social Interaction Test (1d)
The social interaction (SIT) behavioral assay is a test of social avoidance. Briefly, this is a two-session test. In the first session, a mouse is allowed to explore an open field arena (40cm × 40 cm) for 2.5 min (see Figure G in Appendix A). Along one side of the arena is a wire mesh cage that remains empty during the first trial (no target). The mouse is then removed from the arena and a novel CD-1 male mouse is placed into the wire mesh cage. The test mouse is placed back into the arena and the amount of time it spends in the “interaction zone” (an 8 cm wide corridor surrounding the cage) is measured during the 2.5 min trial (target present). Socially defeated mice explore the interaction zone significantly less when another mouse is present. The CON mice have strong tendency to spend greater amount of time in the interaction zone in each session. Interestingly, chronic, but not acute, antidepressant treatment alleviates this avoidant behavioral phenotype (Van den Berg et al. 1998; Berton et al. 2006; Nestler and Hyman 2010). This makes the social defeat/interaction test a valid model of antidepressant efficacy given that repeated, not acute antidepressant treatment is efficacious in humans (Iribarren et al. 2005; Kessler et al. 2005).
2.5.1. Video Tracking apparatus and software
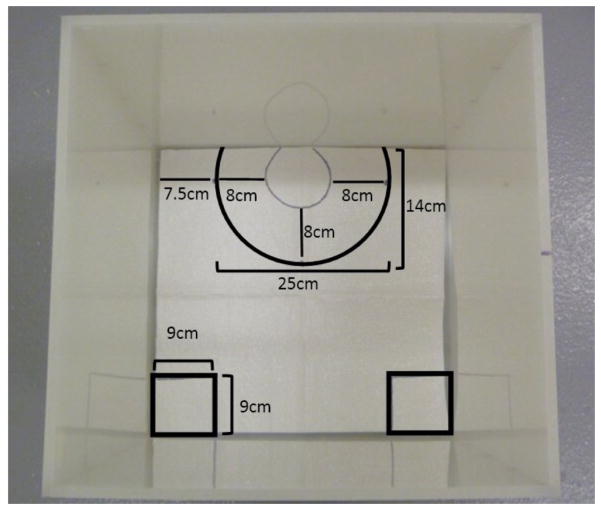
A video tracking system is used to record, track and analyze the animals’ behavior within multiple arenas. Other less sophisticated systems may be used (e.g., regular video recording system) at the risk of loss of efficiency, since this would mean recording interaction sessions and scoring behavior manually one animal at a time. The tracking software should be configured to recognize two distinct areas in the arena, the interaction zone as described above, and two 9 cm square boxes on the opposite wall corners (see of figure C in Appendix A). The tracking system should score the amount of time spent in both areas and total time spent moving. All social interaction testing should be conducted under red-light conditions in a sound attenuated room.
Choose novel CD-1 mice that have not been used during the defeat process. Screen the target CD-1 to ensure aggressive characteristics 2–3 days before starting the interaction task.
Assemble the social interaction arena and the video tracking system.
The first phase of this two-part test involves allowing the experimental mouse to explore the open field arena without a target CD-1 inside of the wire enclosure for 150 s.
Place the experimental mouse directly into the rear center of the arena opposite to the wire enclosure.
For the second phase of the task the experimental mouse is then removed and placed back in its home cage, a novel CD-1 aggressor added to the wire enclosure and then the experimental mouse is replaced for another 150 s.
The arenas should be wiped down with 50% ethanol between each set of trials.
2.6. Cleaning of Equipment
Cages do not need to be cleaned after each defeat session. However in the event that there is blood present on the walls, it should be wiped off with a wet towel after the end of the defeat. After all 10 defeat sessions, all cages and tops should be autoclaved and the Plexiglas dividers hand washed, as they are not autoclave safe.
2.7. Physical wounding of defeated mice
Wounding of the C57 mice is a concern posed by the social defeat model. Subsequently, it is crucial that the experimenter watch over the defeat sessions very carefully to ensure that no mouse is showing overt submissive postures or injuries. In the event that a mouse develops an open wound larger than 1 cm, it should be removed from the experiment and euthanized. If an aggressor is consistently causing wounding to different C57 mice, it should be removed from the study. Defeat protocols should be followed as approved of the necessary review boards and standards. Though death of an animal is not a common occurrence, it is possible that an animal may die in the hours following the last defeat session, thus it is critical to closely monitor the health of the mice throughout the study. Veterinary assistance is highly recommended when new experimenters are learning the protocol. It is crucial that the experimenter ensure each cage divider is properly sealing the compartments to prevent the mice from crawling over to the other side.
3.8 Elevated Plus Maze (EPM)
EPM is a classic test of an anxiety-like behavior in rodents. (Ramos et al., 2008). The apparatus contains two perpendicular runways, one with no walls (open arms) and the other with tall dark walls (closed arms). The runways are 6 cm wide, 33 cm long and the closed walls are 25 cm tall. The runways are 50 cm from floor. Mice are placed into the closed arm and allowed to explore for 5 min. Mice tend to prefer the closed arms but will begin to explore the arms. Anxiolytics such as diazepam will increase time spent in the open arms and decrease time spent in closed arms. The same mice used for the SIT can be used for EPM as well (Warren et al., 2013).
3.9 Corticosterone Enzyme Immuno Assay
One group of mice was sacrificed 40 min following a single session of control stress (CON), emotional stress (ES), or physical stress (PS) exposure. A second group was sacrificed 24 h after 10 sessions of CON, ES, or PS. The third group was exposed to CON, ES, or PS, and then sacrificed 40 min after the forced swim test (FST). Trunk blood from each animal was individually collected in EDTA lined tubes and kept on ice until use. Whole blood samples were centrifuged at 1500 X g for 30 min at 4°C. Serum supernatant was decanted for analysis with the corticosterone enzyme immuno assay per manufacturer’s instructions (Assay Designs). Briefly, serum was diluted to 10% using the provided buffer and added to the wells of an immuno-lined 96-well plate and allowed to incubate for 2 h with provided antibodies. The plate was washed with a provided wash buffer, developed, and optical density was read using a 96-well plate reader (Biotek). Serum corticosterone was calculated by comparing these values to optical density values obtained from corticosterone standards (Warren et al., 2013).
3.10 Fluoxetine Reversal
In order to demonstrate predictive validity, mice were exposed to ten days of ES, PS, or CON stress. Reference levels of social interaction were assessed 24 h after the last stress session. Mice were then divided into acute or chronic treatment groups. Mice in the chronic treatment group were given daily injections of fluoxetine (20 mg/kg), dissolved in sterile water (20 mg/mL) or saline for 30 days, whereas the mice assigned to acute treatment received a single fluoxetine injection 30 days after the last stress session. Twenty-four h after the last injection, the social interaction test was repeated.
3.11 Statistics
Statistical analysis of the data can be performed using analysis of variance (ANOVAs) followed by the appropriate post-hoc comparisons when the ANOVA reaches statistical significance (p<0.05). Results can be reported and analyzed as total time spent by the C57BL/6J mouse in the interaction zone during each SIT session (i.e., target absent or present), or as the ratio of these two scores. When analyzing interaction ratios, a one-way ANOVA is appropriate (comparing CON, ES, and PS). Mixed-designed, or Split-plot, ANOVA is used when analyzing total times spent in the interaction zone. Dependent measures are time spent in interaction and corner zones. Similar statistical analyses can be performed on data resulting from behavioral paradigms assessing anxiety- and reward-related functional outcomes. The Behavioral data presented here have been previously published (Warren et al., 2013) and were analyzed using mixed-design ANOVAs followed by Fisher least significant difference (LSD) post hoc tests. Student t-tests were used to determine statistical significance of pre-planned or focused comparisons (Rosenthal and Rosnow, 1985). Data are expressed as the mean e SEM. Statistical significance was set at p< 0.05.
It should be noted that behavioral assessments can be performed 24 h, to assess short-term effects, and/or weeks (e.g. up to a month as in the data presented here) after the last stress session, to assess long-term effects.
3. Results
3.1. Short-Term Effects of Emotional Stress
Exposure to physical stress (PS) produces a series of behavioral deficits (i.e. depression- and anxiety-like phenotype), and we have recently demonstrated that mice exposed to emotional stress (ES) using this paradigm have similar behavioral abnormalities. Mice exposed to ES and PS displayed reduced weight gain compared to controls (Figure 1A). They also showed an increase in serum corticosterone levels both 40 min after a single session of stress (acute), and 24 h after the end of 10 stress sessions (Figure 1B). Exposure to ES and PS altered mood-related behavioral measures. ES and PS exposure reduced the time spent interacting with a CD-1 mouse when compared to control (CON) mice 24 h following the last stress session (Figure 2B). ES- and PS-exposed mice spent significantly less time in the open arms of the elevated plus-maze (EPM) 48 h after the last stress session (Figure 2B).
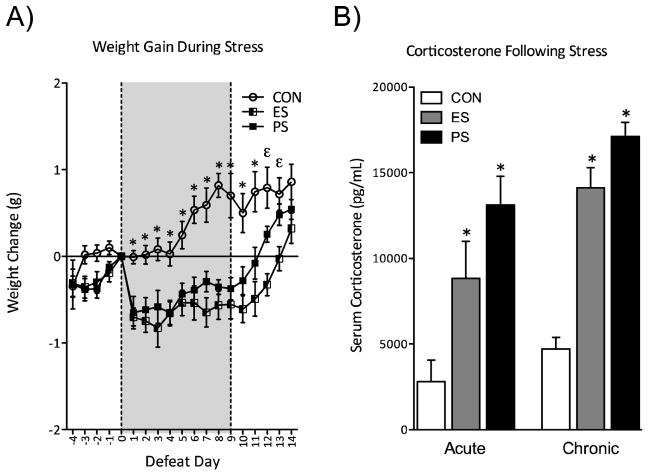
Figure 1.
Emotional (ES) and physical (PS) stress disrupts neuroendocrine reactivity (A) ES and PS caused a reduction in weight gain across all 10 days of stress exposure, returning to control (CON) levels after 5 days of last stress session (n=11–12). (B) ES and PS also influenced serum corticosterone levels (CORT) both 40 minutes after a single defeat session (acute, n=9–10) and 24 hours (chronic, n=10) after the 10th defeat session. Both ES- and PS-exposed mice have significantly increased CORT levels compared to CON-exposed mice 40 minutes after a single defeat (p<0.05). Elevated CORT levels were also observed in ES- and PS-exposed mice 24 hours after the last defeat session compared to CON (p<0.001) Data is presented as weight change in grams, and serum corticosterone as pg/mL (mean ± SEM). (Data from Warren et al., 2013, with permission)
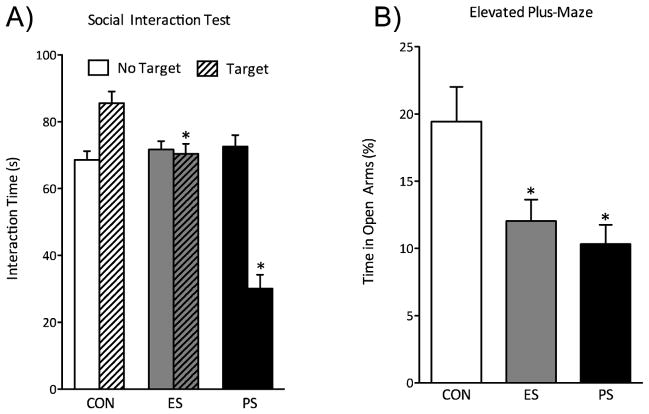
Figure 2.
Emotional (ES) and physical (PS) stress alter mood and anxiety-related behavioral measures 24-hr after the last stress exposure. (A) ES and PS exposure reduced the time spent interacting with the CD-1 mouse when compared to control (CON) mice (n=38; p< 0.05). (B) A group of mice was exposed to the elevated maze test (EPM) 48 hours after the last stress session (n=10). ES- and PS-exposed mice showed reduced time spent in the open arms of the EPM when compared to CON (p<0.05). (C) To determine the long-lasting effects of prior ES and PS exposure on subsequent neuroendocrine stress responses, these mice were used 40 min after the FST exposure and serum corticosterone (CORT) was assessed (n=8). ES and PS exposed mice had significantly increased (CORT) levels when compared to CON mice (p<0.05, respectively). (Data from Warren et al., 2013, with permission)
3.2. Long-Term Effects of Emotional Stress
Given that CSDS induces an enduring social avoidance one month after the last stress session, mice were re-exposed to the social interaction test. As expected, PS-exposed mice showed reduced time spent interacting with the social target. Exposure to ES also resulted in robust social avoidance when compared to the CON-exposed mice (Figure 3A), indicating that social avoidance induced by witnessing stress persists for up to 1 month. To determine the long-lasting effects of VSDS on anxiety-like behaviors, separate groups of mice were tested in the EPM 1 month after the last stress session. ES and PS exposure reduced time spent in the open arms of the EPM as compared to control mice (Figure 3B). To characterize the lasting effects of VSDS on neuroendocrine measures, CORT levels were assessed in these mice 40 min after exposure to forced swimming (Figure 3C). Corticosterone concentrations varied as a function of stress exposure. Both ES and PS exposure significantly elevated CORT levels when compared to control mice (p<0.05, respectively), suggesting that witnessing stressful events causes long-lasting sensitivity of the neuroendocrine system.
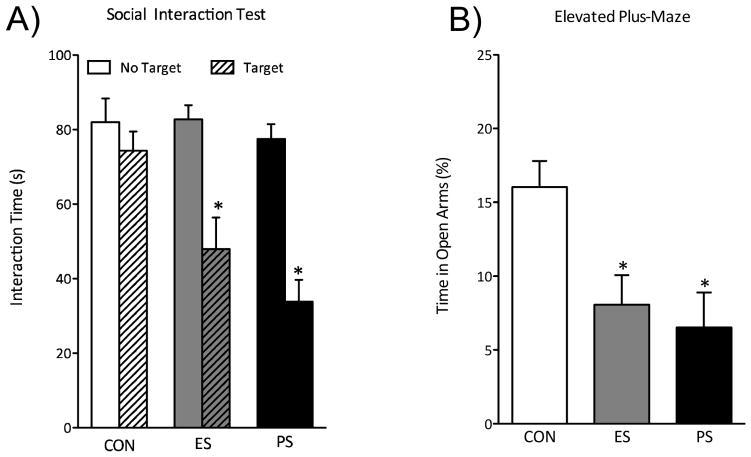
Figure 3.
(A) One month after the last exposure to various stress conditions a group of mice was re-exposed to the social interaction test (n=12). ES and PS exposure significantly reduced the time the mice spent interacting when compared to CON-exposed mice (p<0.05). (B) A different group of mice was exposed to CON, ES, or PS conditions, and anxiety-like behavior was assessed one month after the last defeat session using the EPM (n=8). (C) Exposure to ES and PS signigicantly reduced time spent in the open arms of the EPM when compared to the CON-exposed mice. (p<0.05). (Data from Warren et al., 2013, with permission)
3.3. Fluoxetine Reversal of VSDS Effects
Separate groups of mice were exposed to ES, PS, or CON conditions for 10 days. Twenty-four hours after the last stress exposure, social interaction was assessed. Mice were then divided into either acute or chronic fluoxetine treatment groups with equivalent mean social interaction scores, and were given either a single day or thirty days of fluoxetine injections (20 mg/kg/day). A single injection of fluoxetine was not capable of reversing stress-induced social avoidance in PS- or ES-exposed mice (Figure 4A). However, chronic treatment with fluoxetine reversed stress-induced social avoidance in both ES- and PS-exposed mice (Figure 4B), demonstrating that chronic, but not acute, fluoxetine is capable of reversing VSDS-induced social aversion, thus demonstrating predictive validity of the model.
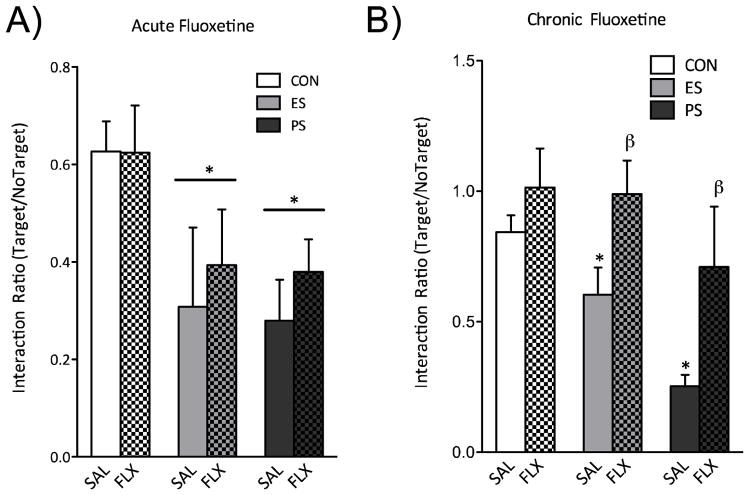
Figure 4.
(A) A single day of fluoxetine exposure was unable to reverse stress-induced social avoidance (p>0.05). (B) Chronic (30 d) treatment with fluoxetine reversed the social interaction deficits observed in ES- and PS-exposed mice (n=5–8). (Data from Warren et al., 2013, with permission)
4. Discussion
The lack of animal models that can approximate human neuropsychiatric disorders contributes to the considerable gap in our understanding of the neurobiological consequences of psychological/emotional stress and its influence on mental health (Yehuda and LeDoux, 2007, Nestler and Hyman, 2010, Daskalakis et al., 2013). The vicarious social defeat stress (VSDS) model is a new informative behavioral assay of psychological stress that possesses ethological relevance, strong face and predictive validity, and is easily testable. This unique paradigm offers an opportunity to assess the functional, cellular and molecular consequences of psychological/emotional stress without a physical component (Russo and Nestler, 2013, Warren et al., 2013, Daskalakis and Yehuda, 2014).
The witnessing of social defeat serves as a potent stressor in adult male mice capable of inducing long-lasting dysregulation in several functional outputs. Exposure to ES induces deficits in weight gain, serum CORT levels, anxiety-like behavior, and social interaction (Warren et al., 2013). Previous studies have shown that exposure to CSDS reduces weight gain in mice (Berton et al., 2006, Krishnan et al., 2007), and we have demonstrated that exposure to ES induces similar reductions in weight gain (Warren et al., 2013). Here, data from Warren et al. indicate that the PS-exposed mice show a trend towards returning to CON levels faster than the ES mice. Factor(s) accounting for this trend are not known, but given that the PS mice undergo physical trauma, it is plausible that this may be a compensatory mechanism by which these mice increase their food intake due increased basal metabolic immune response. This reduction in weight gain is accompanied by significant increases in CORT levels shortly after exposure to a single, or 10 days of ES or PS stress sessions. Interestingly, even a month after the last stress session the mice show enhanced responsivity to subsequent stress (Warren et al. 2013), therefore signifying enduring dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis after exposure to ES. Together, similar deficits in weight gain and elevations in CORT levels between the ES- and PS-exposed mice further indicate that exposure to VSDS is a potent stressor.
As previously demonstrated with the CSDS paradigm, exposure to VSDS induces social avoidance. The social avoidance phenotype induced by VSDS is important because avoidance of trauma-related cues is a hallmark of PTSD and subset of depression, and PTSD can develop simply by witnessing a traumatic event (Teicher et al., 2006, Teicher et al., 2010, Perlman et al., 2011, van Wingen et al., 2011). Under normal conditions, naïve mice will interact more with a novel target CD-1, however, after exposure to CSDS mice will avoid this interaction. Exposure to VSDS induces social avoidance that is significantly different from controls, but this avoidance is not as pronounced when compared to the PS-exposed mice 24 h after the last stress exposure (see Fig. 2A). A more robust social avoidance phenotype emerges 1 month after cessation of stress (see Fig. 3A). This is remarkable as it indicates that the biological adaptations resulting in social avoidance must incubate before they fully emerge following ES but not PS. Exposure to ES also elicits an anxiety-like phenotype. Mice in the ES and PS conditions spent significantly less time in the open arms of the EPM as compared to CON mice 24 h after stress exposure, and this phenotype still is preserved 1 month after stress exposure. This indicates that ES induces a persistent increase in anxiety-like behavior. Therefore, our representative data further illustrates that exposure to ES is a potent stressor capable of inducing long-lasting sensitivity to aversive situations.
We also demonstrate that the social avoidance phenotype elicited by ES exposure is reversed with chronic (1 month) but not acute fluoxetine treatment (Warren et al., 2013). This makes the VSDS an attractive model, in contrast to other models that respond to acute antidepressants, that is comparable to human condition and lends the VSDS paradigm with strong predictive validity. Importantly, it is unlikely that the deficits in social interaction are due to changes in locomotor activity, since we have shown that total distance traveled does not differ when target is not present in SIT (Warren et al., 2013).
There are several caveats to consider as potential limitations of the VSDS paradigm. These experiments necessitate a relatively large space in the animal vivarium (e.g. single housing of CD-1 mice), moderate financial investment, and moderate time investment per animal and inherent variability due to external factors (e.g. aggression of CD-1 mice). This is particularly pertinent when using younger mice, such adolescents, in this paradigm. When studying the effects of VSDS in adolescence, it is crucial that the aggressors be more stringently screened for aggression, because most aggressors are less likely to attack small adolescent mice.
Although this model also presents a tantalizing opportunity to assess the effects of witnessing social defeat in female mice, an understudied population, we have not been successful. In our hands, this paradigm has not been successful with female mice, as the CD1 does not readily confront and defeat the male PS mouse when a female ES mouse is present. Instead the CD-1 engages in sniffing and social behavior with the female mouse. Nevertheless, we are actively working toward adapting this model to explore the effects of witnessing social defeat in female mice.
The data presented here only show social avoidance induced by ES exposure toward CD-1 strains of mice. In PS-exposed mice, CSDS readily induces avoidance of both aggressive CD-1 and non-aggressive C57 strains. Given that both ES- and PS-exposed mice show deficits in anxiety- and depression-like behavioral measures, we believe that the model does induce a maladaptive phenotype, but subsequent experiments will be needed to extend this interpretation to social avoidance.
Despite these limitations, the versatility of the VSDS paradigm makes it a powerful model. The ability to incorporate viral-mediated gene transfer, genetically modified mice, or optogenetic manipulation, makes the VSDS paradigm an incredibly useful tool for understanding of the neurobiological sequalae of emotional stress.
5. Conclusion
In summary, the VSDS paradigm can be used to study the neurobiological consequences of exposure to emotional stress without the physical component often present in other stress paradigms. Exposure to ES produces physiological and psychological effects such as dysregulated weight gain and increased sensitivity of the HPA axis, and a negative emotional state characterized by increased anxiety and depressive-like behavior. These exciting results indicate that emotional stress alone serves as a potent stressor in male mice, and that VSDS paradigm could serve as a relevant model of stress-induced neuropsychiatric disorders such as MDD and PTSD. Combining the CSDS model with a witness component provides a promising avenue to uncovering the neurobiological consequences of emotional stress and may revolutionize the way in which the field approaches studying the effects of different types of stress.
Highlights.
A novel animal model for vicarious defeat stress (VSDS) is described.
VSDS can model posttraumatic stress, depression, and stress-related disorders.
This model has high face, construct, and predictive validity.
VSDS is a tool for studying the neurobiological consequences of emotional stress.
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse R01DA026854 to CABG. EMP was supported by an NRSA F31MH103939 from the National Institute of Mental Health (NIMH). LFA was supported by a McKnight Fellowship from the Florida State Educational fund. We are grateful to Rachael Elliott and John Chalcraft for the artwork.
Appendix A
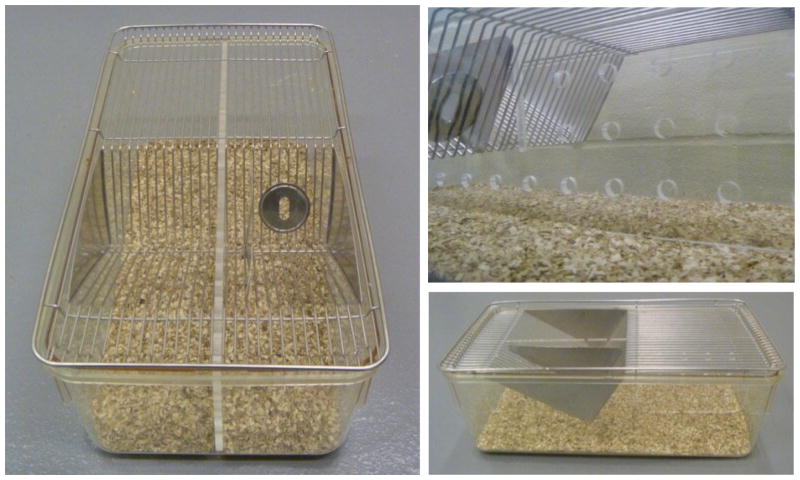
Figure A.
Picture of empty mice breeding cage without divider used in repeated social defeat stress.
Figure B.
Picture of empty mice breeding cage with divider, and close up of perforated Plexiglas dividers. Care must be taken to ensure that the divider is firmly secure to prevent mice from escaping their overnight compartments.
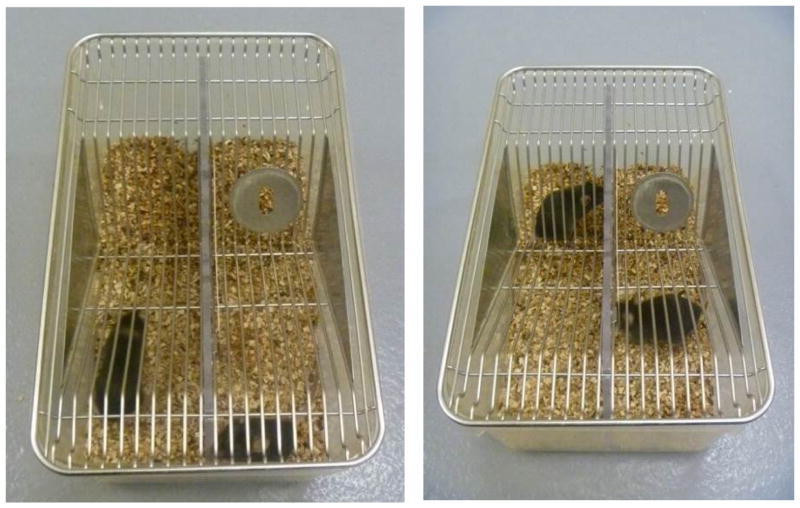
Figure C.
Standard mice cage without divider use to house control mice.
Figure D.
Standard mice cage with divider and control mice.
Figure E.
Defeat cage with CD-(right side) and C57 (left side).
Figure F.
Social Interaction box with template layout
Figure G.
Social Interaction box with wire mesh enclosure
Figure H.
Procedure to move ES- and PS-exposed mice during defeat sessions.
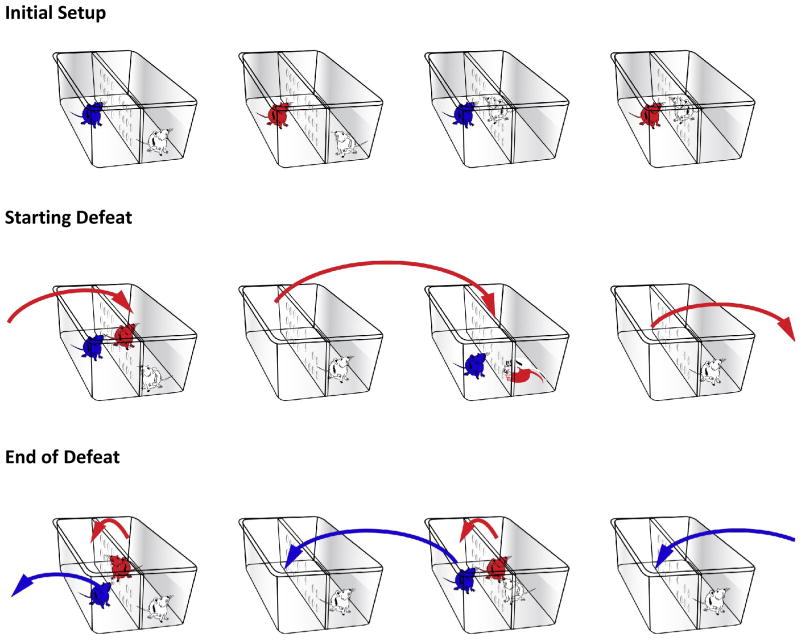
Emotional Stress (ES) mice are shown in blue, Physical Stress (PS) mice are shown in red, and CD1 aggressors are shown in white. Notice aggressors are on the right side of the divider in all cages. ES and PS mice are alternated (as shown in Initial Setup) in every other cage. To begin the defeat, PS mice are moved to the next cage to the right into the aggressor’s territorialized home cage (there should be an ES mouse on the other (left) side of the divider). After the defeat session, ES mice are moved to another cage to the left (still on the left side of the divider). The PS mice are moved over the divider (to the left) in the same cage (where the ES mice was previously). This ensures that the PS-exposed mice are defeated by different aggressors and sleep in the aggressor’s home cage overnight.
Footnotes
Disclosures: The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Association AP. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolaños CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Yehuda R. Principles for developing animal models of military PTSD. Eur J Psychotraumatol. 2014;5 doi: 10.3402/ejpt.v5.23825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Yehuda R, Diamond DM. Animal models in translational studies of PTSD. Psychoneuroendocrinology. 2013;38:1895–1911. doi: 10.1016/j.psyneuen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76:155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nature Protocols. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonté B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Krishnan V, Bolaños-Guzmán CA, Murrough JW, Merad M, Russo SJ. Individual differences in the peripheral immune system promote resilience versus suseptibility to social stress. Proc Natl Acad Sci USA. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl 5):4–12. discussion 13–14. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Nemeroff CB. Neurobiology of posttraumatic stress disorder. Current opinion in neurobiology. 2000;10:211–218. doi: 10.1016/s0959-4388(00)00080-5. [DOI] [PubMed] [Google Scholar]
- Perlman SE, Friedman S, Galea S, Nair HP, Eros-Sarnyai M, Stellman SD, Hon J, Greene CM. Short-term and medium-term health effects of 9/11. Lancet. 2011;378:925–934. doi: 10.1016/S0140-6736(11)60967-7. [DOI] [PubMed] [Google Scholar]
- Pijlman FT, Wolterink G, Van Ree JM. Physical and emotional stress have differential effects on preference for saccharine and open field behaviour in rats. Behav Brain Res. 2003;139:131–138. doi: 10.1016/s0166-4328(02)00124-9. [DOI] [PubMed] [Google Scholar]
- Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;10:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL. Contrast Analysis: Focused Comparisons in the Analysis of Variance. Cambridge Univ. Press; Cambridge, London, UK: 1985. p. 692. [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews Neuroscience. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am J Psychiatry. 2006;163:993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Sheu YS, Polcari A, McGreenery CE. Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. Am J Psychiatry. 2010;167:1464–1471. doi: 10.1176/appi.ajp.2010.10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg CL, Lamberts RR, Wolterink G, Wiegant VM, Van Ree JM. Emotional and footshock stimuli induce differential long-lasting behavioural effects in rats; involvement of opioids. Brain Res. 1998;799:6–15. doi: 10.1016/s0006-8993(98)00397-7. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolaños-Guzmán CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]