Abstract
Objective
To characterize the course of neuropsychiatric symptoms (NPS) in adults with mild cognitive impairment (MCI), and to examine baseline individual-level predictors and associated cognitive and functional outcomes.
Design
A two-year prospective cohort study.
Setting
Multi-center clinical settings.
Participants
Five hundred and sixty individuals with MCI at baseline.
Measurements
NPS severity (measured using Neuropsychiatric Inventory Questionnaire) and cognitive and functional outcomes were assessed at baseline and every six months thereafter. Potential individual-level predictors were collected at baseline.
Results
Three latent classes of NPS courses were identified using growth mixture modeling: a stable class in which a low NPS burden remained relatively unchanged over time (n = 503, 89.8%); a worsened class in which an initially moderate NPS burden increased (n = 39, 7.0%); and an improved class in which an initially high NPS burden decreased (n = 18, 3.2%). There were no associations between class membership and baseline individual characteristics. Members of the worsened class were 1.74 times more likely to be diagnosed with incident Alzheimer’s disease (AD) than members of the stable class (95% CI = 1.07–2.84). The worsened class also showed significantly more rapid declines in cognitive and functional outcomes than the stable class. Class membership did not predict rate of brain atrophy.
Conclusions
Patients with MCI may experience different trajectories of NPS over time. Patients with worsening NPS may be at greater risk of developing AD and severe cognitive and functional impairment.
Search Terms: Neuropsychiatric symptoms, Mild cognitive impairment, Latent class analysis
Objective
Alzheimer’s disease (AD) is a progressive neurodegenerative disease marked by deficits in cognition and function. It affects roughly 4.7 million in the United States, and it is expected to affect 13.8 million by 2050.1 Mild cognitive impairment (MCI), a condition marked by cognitive deficits without functional impairment, is thought to be an early manifestation of AD pathophysiology.2 Patients with MCI develop dementia at a rate of 10% – 15% per year, compared to 1% – 2% in the general population.3,4
Neuropsychiatric symptoms (NPS) are mental and behavioral disturbances often found in dementia and MCI.5 They are thought to be caused by damage in brain regions that are compromised in dementia.6,7 Estimates of NPS prevalence range from 61% – 75% in older adults with dementia,5,8 and from 31% – 51% in those with MCI.4,9 NPS have been associated with increased caregiver burden,10,11 and more pronounced cognitive decline.3,4,7
Recent literature has sought to address the relationship between NPS and the risk of progression from MCI to AD. Rosenberg et al. found that MCI patients with baseline NPS are at greater risk of conversion to dementia,12 and Edwards et al. reported that the number of baseline NPS is correlated with both the type of MCI patients exhibit (amnestic or non-amnestic MCI) and their risk of developing dementia.13 Such studies, however, do not account for the fact that, while often persistent, NPS may wax and wane.14–17 Evidence from Steenland et al. suggests that cognitively normal adults who were persistently or intermittently depressed throughout follow-up were more likely to progress to MCI than adults who were depressed at baseline but whose symptoms improved.18
Studies that have evaluated NPS at one time point may be insufficient to characterize a patient’s NPS burden because they give a static view of an essentially dynamic set of symptoms. This is further complicated when using datasets that have entry criteria restricting the use of psychotropics or the type and severity of NPS at screening, essentially injecting a bias in the sample selection when it comes to the examination of NPS. The heterogeneous patterns of NPS that patients experience may provide a means of predicting which patients with MCI are most at risk for developing AD. To our knowledge, no studies have examined the heterogeneity in NPS trajectories over time in MCI and their relationship to cognitive and functional outcomes.
In the present study, our primary aim was to identify different courses of NPS over two years in patients with a baseline diagnosis of MCI. We determined whether patient characteristics were associated with particular courses of NPS. Finally, we examined whether different NPS courses predicted cognitive and functional outcomes over time.
Methods
Study sample
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials.
The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and University of California – San Francisco. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the U.S. and Canada. The initial goal of ADNI was to recruit 800 subjects but ADNI has been followed by ADNI-GO and ADNI-2. To date these three protocols have recruited over 1500 adults, ages 55 to 90, to participate in the research, consisting of cognitively normal older individuals, people with early or late MCI, and people with early AD. The follow up duration of each group is specified in the protocols for ADNI-1, ADNI-2 and ADNI-GO. Subjects originally recruited for ADNI-1 and ADNI-GO had the option to be followed in ADNI-2. For up-to-date information, see www.adni-info.org.
Participants
The present study used data obtained July 2014 from ADNI. Our sample included 560 older adults who began ADNI-1 with a diagnosis of MCI, or who were newly enrolled in ADNI-GO or ADNI-2 with a diagnosis of late MCI (lMCI), which had the same diagnostic criteria as a diagnosis of MCI in ADNI-1. The MCI group included participants with amnestic single- and multiple-domain MCI. Each participant was enrolled with a study partner who could provide functional evaluation.
The diagnosis of MCI or lMCI was made by a psychiatrist or neurologist at each study site and reviewed by a Central Review Committee. Diagnoses were based on a subjective memory complaint and performance on neurocognitive testing, including the Logical Memory II subscale of the Wechsler Memory Scale-Revised (score ≤ 8, cut-off adjusted for education level), the Mini-Mental State Exam (MMSE; score 24 – 30), and the Clinical Dementia Rating (CDR; global score = 0.5). These subjects did not meet the NINCDS-ADRDA criteria for AD.19
ADNI entry criteria exclude use of antidepressants with significant anticholinergic side effects (other antidepressants were allowed in stable doses), neuroleptics, chronic anxiolytics or sedative hypnotics for at least 4 weeks prior to screening. Furthermore, active major depression or bipolar disorder within the past 12 months as well as psychotic features, agitation, or behavioral problems within the last three months prior to screening that the investigators believed could lead to difficulty complying with the protocol were exclusionary. This limits the severity of NPS at study entry.
Assessment of NPS
NPS were evaluated using the Neuropsychiatric Inventory Questionnaire (NPI-Q).20 The NPI-Q asked the study partner whether a patient had exhibited NPS in the past 30 days in each of twelve symptom domains. If the informant answered “no” to a screening question, that domain was scored as 0; if the informant answered “yes”, he or she was asked to rate the severity of the symptom from 1 (mild) to 3 (severe). Total scores ranged from 0 to 36, higher scores indicating worse symptoms.20 Individual items were also analyzed as dichotomous variables based on the absence or presence of the particular symptom. The NPI-Q was administered at baseline and every six months thereafter for two years, either in person or over the phone.
Baseline demographic and health information
Basic demographic information, including age, sex, and years of formal education were obtained during screening. Additional data included a blood draw, which was analyzed for APOE4 (having ≥ 1 allele defined carrier status), and a structural MRI.
Outcome measures
All outcomes were assessed at baseline and every six months for two years thereafter.
Diagnostic outcome
Diagnoses of conversion to AD dementia or reversion to normal cognition were made by site clinicians and confirmed by a Central Review Committee. After each follow-up visit, a site psychiatrist or neurologist reviewed the participant’s medical history, laboratory tests, and neuropsychological test results (including results from the MMSE and the CDR) to determine an appropriate diagnosis. Diagnoses did not take NPS into account.
Criteria for diagnosing AD were similar to the criteria for diagnosing MCI except that MMSE scores had to be between 20 and 26, CDR had to be 0.5 or 1.0, and the patient had to meet NINCDS-ADRDA criteria for probable AD. Normal cognition was defined by absence of memory complaints, a score on the Logical Memory II subscale of the Wechsler Memory Scale-Revised of 9 or greater (cut-off adjusted for education level), an MMSE score between 24 and 30, and a CDR of 0.
Cognitive outcomes
Cognition was evaluated using the MMSE21 and two composite indices for memory and executive function. The composite memory index (ADNI-Mem) was based on the memory domains of the MMSE, AD Assessment Scale-Cognition subscale (ADAS-Cog), Rey Auditory Verbal Learning Test (RAVLT), and Logical Memory test.22 The composite executive function index was based on the Wechsler Memory Scale- Revised Digit Span Test, Digit Span Backwards, Category Fluency, Trails A and B, and the Clock Drawing Test.23
Functional outcome
The CDR sum of boxes (CDR-SB) has psychometric properties that make it a sensitive measure of both cognitive and functional disability.24 It was completed by a site clinician who interviewed each subject and his or her study partner. The CDR-SB evaluates five degrees of impairment in each of six categories of cognitive functioning—which include memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care—to generate a sum score from 0 to 18. In this analysis, CDR-SB was added to 1 and then log transformed to ascertain a normal distribution.
Neuroimaging and MRI data collection
MRI acquisition and preprocessing have been described previously.25 Briefly, high-resolution T1 structural images were obtained from all participants on either a 1.5T GE scanner (Waukesha, WI, USA) or a 1.5T Siemens Medical Solutions MRI (Erlangen, Germany) using standardized MRI protocols and acquisition parameters, system-specific corrections for gradient nonlinearity and intensity nonuniformity, and phantom-based monitoring of imaging instruments to control for cross-site variation. Additional preprocessing and quality control procedures included gradient warping, scaling, B1 correction, and N3 inhomogeneity correction on all T1-weighted images as performed by the Mayo Clinic. Cross-sectional image processing was performed using FreeSurfer version 5.1. Each scan is segmented according to an atlas defined by FreeSurfer. This allows for group comparisons at a single time point.26 Images that did not pass a thorough visual quality control were excluded.
Data analysis
Growth Mixture Modeling (GMM) from Mplus version 7.0 was used to find the smallest number of classes of participants with similar courses of NPS over time. GMM is a method of identifying unique classes within a set of heterogeneous individual growth patterns by examining both the intercept (baseline) and slope (change over time) of individual patterns. It provides insight into the dynamics of developmental processes. More details on GMM can be found elsewhere. 27
A series of models were evaluated beginning with a 1-class solution and ending with a 5-class solution. The optimal number of classes was decided based on Bayesian, Akaike, and Adjusted Bayesian Information Criteria in which lower values indicate a more parsimonious model; and based on the Lo-Mendell-Rubin (LMR) adjusted likelihood ratio test, which compares the current class solution with the class - 1 solution to determine whether the two solutions are similar (p > .05) or different (p ≤ 0.05).27 Each class was described by the shape of trajectory (i.e. intercept and slope) and the number of participants belonging to the class.
After deciding the number of latent classes (n = 3 in the present study), remaining analyses were performed in IBM SPSS 19.0. Analysis of variance (ANOVA) was used to compare continuous baseline demographic and health variables, and Chi-square tests were applied to compare categorical baseline variables.
The association between courses of NPS and conversion/reversion of diagnosis was analyzed using Cox Proportional Hazard Regressions. Generalized Estimating Equation (GEE) modeling with AR(1) working correlated matrix was applied to assess the longitudinal relationships of the latent class of NPS with individual NPS items, as well as other cognitive and functional outcomes adjusted for covariates. Individual NPS items were dichotomous outcomes, while memory, executive function, MMSE, and CDR-SB were continuous outcomes. The equation was: y = β0 + β1Cov1 + … + βnCovn + βn+1Time + βn+2 NPS class + β3 Time × NPS class + ε. Time referred to baseline, and 6-, 12-, 18-, and 24-month follow-ups; ε referred to error term; y referred to each health outcome. The binary logistic function was used for the dichotomous outcome, and the identify link function was used for the continuous outcomes (equal to linear regression). In Cox proportional hazards regression, we used time as the underlying timescale to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for incident AD cases by NPS class. Two methods (i.e., visual inspection of log minus log survival curves and test of Schoenfeld residuals) were used to verify the proportional hazard assumption. In Cox regression and GEE analyses for cognitive and functional outcomes, age, sex, education, and APOE4 carrier status were included as covariates. All tests were two-tailed and values of p < 0.05 were considered significant in all analyses.
Results
Latent class of change in NPS over two years
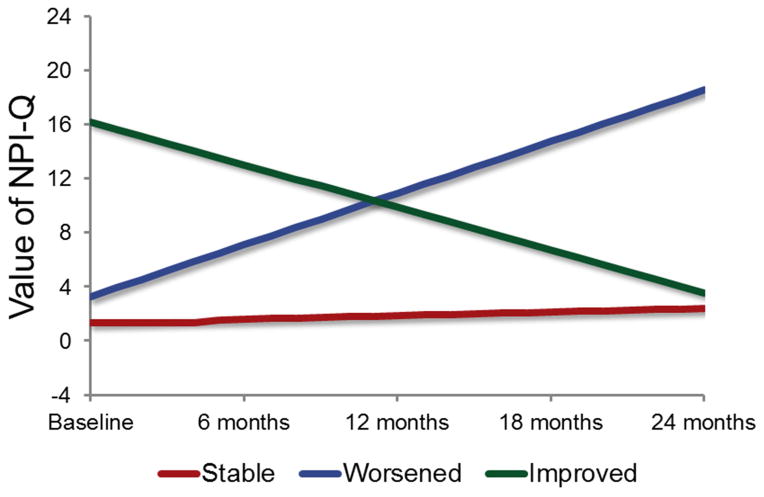
Table 1 summarizes the series of model fit statistics of GMM. Synthesizing the model fit indices and the number of participants in each class, the three-class model was considered the best solution. Figure 1 displays the three classes. Class I (n = 503, 89.8%) was characterized by a low initial NPI-Q score (intercept: B = 1.35, SE = 0.12, Wald χ2 = 123.23, df = 1, p < 0.001) that had significant but mild increase over time (slope: B = 0.27, SE = 0.06, Wald χ2 = 21.48, df = 1, p < 0.001); we labeled this class stable. Class II (n = 39, 7.0%) was characterized by moderate initial NPI-Q scores (intercept: B = 3.27, SE = 0.53, Wald χ2 = 38.81, df = 1, p < .001) that increased significantly over time (slope: B = 3.82, SE = 0.26, Wald χ2 = 210.10, df = 1, p < 0.001); we labeled this class worsened. Class III (n = 18, 3.2%) was characterized by an initially high NPI-Q score (intercept: B = 16.21, SE = 1.34, Wald χ2 = 145.68, df = 1, p < 0.001) that decreased significantly over time (slope: B = −3.17, SE = 0.76, Wald χ2 = 17.28, df = 1, p < 0.001); we labeled this class improved. Of note, the three classes were significantly different from each other in their baseline level of NPI-Q (F = 172.74, df1 = 2, df2 = 399. p < .001. LSD post-hoc t test all p < .005).
Table 1.
Growth Mixture Model Fit Statistics for Different Class Solutions of NPI.
| Model | Latent Class | N | AIC | BIC | Adjusted BIC | Lo-Mendell_Rubin adjusted Likelihood ratio test, χ2 test (p) |
|---|---|---|---|---|---|---|
| One-class | 1 | 560 | - | - | - | |
| Two-class | 1 | 536 (95.7%) | 9619.74 | 9676.00 | 9634.73 | 190.65 (.009) |
| 2 | 24 (4.3%) | |||||
| Three-class | 1 | 503 (89.8%) | 9520.82 | 9590.07 | 9539.28 | 99.66 (.05) |
| 2 | 39 (7.0%) | |||||
| 3 | 18 (3.2%) | |||||
| Four-class | 1 | 12 (2.1%) | 9465.38 | 9547.61 | 9487.30 | 58.37 (.06) |
| 2 | 498 (88.9%) | |||||
| 3 | 41 (7.3%) | |||||
| 4 | 9 (1.6%) | |||||
| Five-class | 1 | 12 (2.1%) | 9421.05 | 9516.27 | 9446.43 | 47.81 (.43) |
| 2 | 441 (78.8%) | |||||
| 3 | 37 (6.6%) | |||||
| 4 | 58 (10.4%) | |||||
| 5 | 12 (2.1%) |
Figure 1.
Graphical Representation of NPI-Q Scores over Time by the Latent Class.
Note: Higher scores of NPI-Q indicated worse NPS.
We also examined the individual items of NPS by latent class using GEE (see Table 2). The results were consistent with the overall NPS trajectories. The improved class had significantly higher prevalence of individual NPS, except delusion and hallucination, than the stable class at baseline, but their individual NPS (i.e., agitation, depression, apathy, disinhibition, irritability) improved over time. The worsened class had significantly higher prevalence of individual NPS (i.e., delusions, elation, disinhibition, motor disturbance) than the stable class at baseline, and all individual NPS except delusion, hallucination, disinhibition, and appetite worsened significantly faster than the stable class over time.
Table 2.
Presence of Individual NPS by Latent Class using GEE Analysis (B(SE)).
| Class# | Time | Class# × Time | |||
|---|---|---|---|---|---|
| Worsened | Improved | Worsened | Improved | ||
| a. Delusions | −1.95 (0.74)** | −1.51 (1.11) | −0.17 (0.11) | −0.04 (0.19) | 0.20 (0.33) |
| b. Hallucinations | −0.51 (1.55) | - | −0.33 (0.16)* | −0.26 (0.40) | - |
| c. Agitation/aggression | −0.32 (0.41) | −4.30 (0.64)*** | −0.02 (0.04) | −0.60 (0.16)*** | 0.44 (0.18)* |
| d. Depression/dysphoria | −0.23 (0.41) | −4.53 (0.74)*** | −0.11 (0.04)** | −0.40 (0.13)** | 0.94 (0.25)*** |
| e. Anxiety | −0.41 (0.40) | −2.45 (0.47)*** | −0.09 (0.04)* | −0.43 (0.15)** | 0.08 (0.14) |
| f. Elation/euphoria | 3.08 (1.39)* | −1.24 (0.93) | 0.14 (0.09) | −0.93 (0.31)** | −0.21 (0.22) |
| g. Apathy/indifference | −0.80 (0.43) | −3.47 (0.74)*** | −0.18 (0.04)*** | −0.43 (0.13)** | 0.56 (0.19)** |
| h. Disinhibition | −1.35 (0.43)** | −4.03 (0.72)*** | −0.12 (0.05)* | −0.20 (0.11) | 0.49 (0.22)* |
| i. Irritability/lability | −0.76 (0.42) | −3.77 (0.78)*** | −0.06 (0.04) | −0.37 (015)* | 0.60 (0.18)** |
| j. Motor disturbance | −1.14 (0.57)* | −2.11 (0.58)*** | −0.09 (0.08) | −0.33 (0.16)* | 0.07 (0.11) |
| k. Nighttime behaviors | −0.46 (0.48) | −1.65 (0.72)* | −0.07 (0.04) | −0.33 (0.14)* | −0.08 (0.22) |
| l. Appetite and eating | −0.70 (0.46) | −2.03 (0.53)*** | −0.10 (0.05)* | −0.24 (0.12) | 0.12 (0.16) |
Note.
Taking the stable class as the reference group. Wald χ2 test was conducted for hypothesis test. All df = 1.
p < .05;
p < .01;
p < .001.
Baseline demographic and health variables by latent class of NPS trajectory
Table 3 displays the demographic and health variables at baseline by latent class using ANOVA or Chi-square test. Baseline differences in age, sex, race, years of education, APOE4 allele carrier status were not significant. Similarly, there were no differences in brain volume or cognitive measures at baseline except in the CDR-SB score. The stable class had the best global functional ability at baseline, while the improved class had the worst.
Table 3.
Baseline Demographic and Health Characteristics by Latent Class
| Characteristic | Classes | Values | F or χ2 test (df) |
|---|---|---|---|
| Age, M (SD) | Stable | 74.05 (7.65) | 0.11 (2, 557) |
| Worsened | 73.78 (6.45) | ||
| Improved | 73.29 (8.12) | ||
| Male, n (%) | Stable | 304 (60.6%) | 3.72 (2) |
| Worsened | 27 (71.1%) | ||
| Improved | 12 (66.7%) | ||
| White, n (%) | Stable | 460 (91.6%) | 1.86 (2) |
| Worsened | 35 (92.1%) | ||
| Improved | 16 (88.9%) | ||
| Years of education, M (SD) | Stable | 15.85 (3.02) | 0.18 (2, 557) |
| Worsened | 15.54 (4.82) | ||
| Improved | 15.78 (2.46) | ||
| APOE4 allele carrier, n (%) | Stable | 268 (53.3%) | 3.72 (2) |
| Worsened | 27 (69.2%) | ||
| Improved | 10 (55.6%) | ||
| Hippocampal volume (mm3), M (SD) | Stable | 6448.49 (1116.09) | 1.37 (2, 548) |
| Worsened | 6416.79 (932.56) | ||
| Improved | 6514.61 (1464.47) | ||
| Amygdala volume (mm3), M (SD) | Stable | 2509.43 (468.13) | 2.01 (2, 548) |
| Worsened | 2353.79 (372.17) | ||
| Improved | 2523.00 (658.09) | ||
| Frontal lobe volume (mm3), M (SD) | Stable | 104,399.37 (12793.85) | 0.54 (2, 548) |
| Worsened | 104,103.28 (13442.30) | ||
| Improved | 101,195.11 (14353.14) | ||
| Memory, M (SD) | Stable | −0.04 (0.57) | 0.50 (2, 559) |
| Worsened | −0.14 (0.58) | ||
| Improved | −0.03 (0.76) | ||
| Executive function, M (SD) | Stable | 0.02 (0.79) | 1.18 (2, 558) |
| Worsened | −0.15 (0.79) | ||
| Improved | −0.17 (0.89) | ||
| MMSE, M (SD) | Stable | 27.18 (1.83) | 0.15 (2, 559) |
| Worsened | 27.03 (1.76) | ||
| Improved | 27.11 (1.64) | ||
| CDR-SB, M (SD) | Stable | 1.59 (0.89) a | 15.83 (2, 559)*** |
| Worsened | 1.92 (0.92) b | ||
| Improved | 2.72 (1.25) c |
Note.
p < .001;
different letter indicates the value was significantly different between classes with LSD post-hoc t test.
Latent class of neuropsychiatric symptom trajectory and health outcomes over time
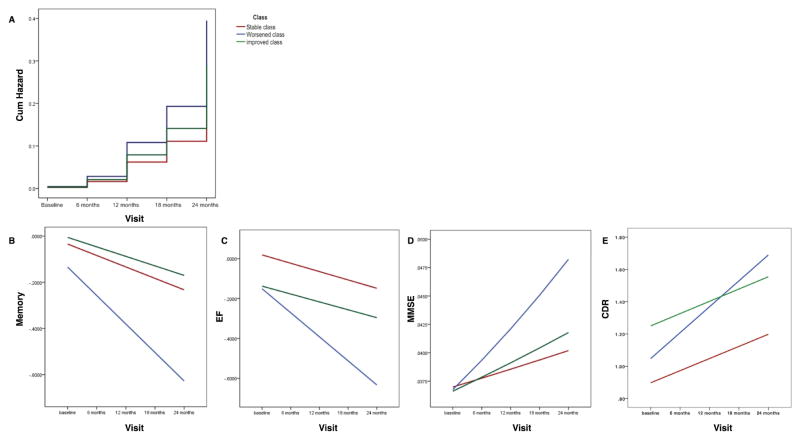
Cox proportional hazard regression used the stable class as the referent and controlled for age, sex, education, and APOE4 carrier status. Members of the worsened class were significantly more likely to be diagnosed with incident AD (HR = 1.74, 95% CI = 1.07 – 2.84, Wald = 4.89, df = 1, p = 0.027). There was no difference in incidence rate between the improved and stable classes (HR = 1.27, 95% CI = 0.56 – 2.91, Wald =0.33, df = 1, p = 0.57) (see Figure 2A). Reversion from MCI to normal cognition was rare, and rates did not differ significantly between classes (data not shown).
Figure 2.
Longitudinal changes of cognitive and functional outcomes by latent class of Neuropsychiatric Inventory Questionnaire (NPI-Q) scores
Note: MMSE was reversed coded and log transformed; CDR-SB was added 1 and then log transformed; higher scores of MMSE and CDR-SB indicated worse function. Memory: the composite score was based on the memory domains of the MMSE, AD Assessment Scale-Cognition subscale (ADAS-Cog), Rey Auditory Verbal Learning Test (RAVLT), and Logical Memory test; EF: the composite executive function index was based on the Wechsler Memory Scale- Revised Digit Span Test, Digit Span Backwards, Category Fluency, Trails A and B, and the Clock Drawing Test.
Table 4 summarizes the continuous health outcomes by class, taking the stable class as the referent and controlling for age, sex, education, and APOE4 carrier status. Hippocampal and amygdala volumes declined significantly; the worsened class had significantly smaller amygdala volumes at baseline than the stable class; there were no differences in the rate of decline in regional brain volumes by class. All cognitive domains (memory, executive function, and MMSE) declined significantly; the worsened class had significantly better MMSE scores at baseline than the stable class; the declines in all cognitive domains were significantly faster in the worsened class than the stable class; there were no differences in the rate of change in any cognitive domain between the improved and stable classes (see Figures 2B to 2D). The CDR-SB showed significant decline over time; the improved class had significantly worse CDR-SB scores at baseline than the stable class; CDR-SB scores deteriorated significantly faster in the worsened class than in the stable class (see figure 2E).
Table 4.
Parameter Estimate (B(SE) of Health Outcomes over Time by NPI Latent Class using GEE Analysis.
| Variable | Time | Class | Time × Class | |
|---|---|---|---|---|
| Hippocampal volume | −183.94 (9.49)*** | Stable | 1 | 1 |
| Worsened | −269.96 (176.87) | −27.96 (29.67) | ||
| Improved | −44.90 (256.19) | 37.57 (43.27) | ||
| Amygdala volume | −65.01 (5.59)*** | Stable | 1 | 1 |
| Worsened | −165.47 (69.16)* | 41.13 (33.26) | ||
| Improved | −65.68 (118.70) | −1.73 (15.82) | ||
| Frontal lobe volume | −1659.55 (145.02) | Stable | 1 | 1 |
| Worsened | 234.23 (2059.90) | −1095.06 (597.14) | ||
| Improved | −5315.02 (3403.45) | 1081.36 (882.06) | ||
| Memory | −0.10 (.01)*** | Stable | 1 | 1 |
| Worsened | 0.11 (0.11) | −0.15 (0.06)* | ||
| Improved | 0.02 (0.13) | 0.02 (0.08) | ||
| Executive function | −0.08 (0.02)*** | Stable | 1 | |
| Worsened | 0.01 (0.13) | −0.16 (0.07)* | ||
| Improved | −0.16 (0.25) | 0.01 (0.10) | ||
| MMSE† | 0.042 (0.004)*** | Stable | 1 | 1 |
| Worsened | −0.10 (0.05)* | 0.09 (0.04)* | ||
| Improved | −0.03 (0.03) | 0.02 (0.02) | ||
| CDR-SB‡ | 1.50 (0.13)*** | Stable | 1 | 1 |
| Worsened | −0.04 (0.08) | 0.17 (0.04)*** | ||
| Improved | 0.35 (0.14)* | 0.002 (0.08) |
Note. Controlled for age, sex, education, and APOE4 carrier;
MMSE was reversed coded and log transformed;
CDR-SB scores were added 1 and log transformed; for MMSE and CDR-SB, higher score indicating worse cognition; Wald χ2 test was conducted for hypothesis test. All df = 1.
p < .05;
p < .001.
Conclusions
Applying GMM, we found that the longitudinal development of NPS in older adults with a baseline diagnosis of MCI can be classified into three patterns. The majority of participants experienced a consistently low NPS burden, while a small proportion (7.0%) had an NPS burden that worsened over time, and another small proportion (3.2%) had initially high NPS burdens that improved over time. Baseline characteristics such as age, sex, years of education, and APOE4 carrier status did not predict class membership. However, class membership did predict rates of conversion from MCI to AD, as well as cognitive and functional outcomes. In particular, members of the worsened class had higher rates of conversion from MCI to AD, and faster rates of cognitive and functional decline. The rates of change in regional brain volumes did not differ between classes.
The novelty of the present study is that it takes into account the variability of NPS trajectories in individual patients and thereby seeks to refine our understanding of the relationship between NPS and cognitive and functional prognosis. It is premised on the observation that NPS are chronic conditions that develop heterogeneously.15–17 Tsoi et al. found that community-dwelling older adults with early signs of executive dysfunction were more likely to develop worsening NPS,28 while Wetzels et al. found that NPS among nursing home residents tended to improve over a two-year period.29 Others have found that clinically significant NPS generally remain substantial at follow up.15,17 Despite sample differences in these studies, the discrepancy in their findings suggests that NPS trajectories in older adults may be heterogeneous, which is what we found in our sample of patients with MCI.
The diagnostic role of NPS as a sentinel feature of neurocognitive decline is well-established.6,7,10,12 There has been significant effort to characterize baseline NPS profiles that predispose older adults to develop AD. Peters et al. found that the presence of mild NPS increases a patient’s risk of progression from MCI to dementia,4 while Edwards et al. reported that patients with a greater number of NPS at baseline are more likely to convert from MCI to dementia.13 Three separate studies have found that baseline symptoms of apathy,30 anxiety,31 or depression32 can be associated with conversion from MCI to AD. Work by Steenland et al., however, suggests that it is not merely the presence of depression at baseline but also the pattern of depressive symptoms over time that best predicts a patient’s risk of cognitive decline.18 The present study expands Steenland et al’s insight by determining the heterogeneous trajectories of NPS over time and demonstrating that these patterns are associated with different cognitive and functional outcomes.
Examining longitudinal trajectories exposes heterogeneities at the level of individual NPS as well. For example, the overall the level of depression worsened over time; but depression in the improved class got better while depression in the worsened class got worse despite higher levels of depression in the improved class at baseline. These findings appear to validate our model and suggest that individual NPS tend to track with the trajectories we found for overall NPS burden. Further, assessing NPS at a single time point may misinterpret the development of NPS across individuals and its relation to cognitive and functional outcomes.
The literature has not focused on individual characteristics as predictors of NPS.5,8,9 We did not find any baseline characteristics that predicted an individual’s trajectory of NPS burden. But different individual characteristics may predict the development of particular NPS. Geda et al. found gender differences in the types of NPS exhibited by cognitively normal older adults who go on to develop MCI.3
We did not find that the course of NPS predicted brain region volumes. There are two potential explanations. First, our analysis considered brain volume, and NPS may be more related to regional function or connectivity.6 Second, some clusters of NPS are attributed to specific areas of the brain (e.g. the frontal symptoms of apathy, disinhibition, and irritability).33,34 Grouping these different clusters together may have obscured relationships between regional brain volumes and NPS.
Several limitations to the present study bear mentioning. First, we did not control for medication use. Psychotropic drugs and cholinesterase inhibitors are often used to help cognitive function and alleviate NPS in older adults.35,36 Second, the NPI-Q may be biased by caregiver burden.37,38 The newer Neuropsychiatric Inventory-Clinician rating scale (NPI-C) may provide a more objective evaluation of NPS.37 Third, although we are supported by consistently finding the most pronounced declines in the “worsened” class across multiple clinical domains, we cannot exclude the possibility that generation of the two classes with extreme cases (“improved” and “worsened” classes) may be affected by the regression to mean in addition to the true NPS changes over time. Future comparison of rates and amounts of change to the estimates reported here may help shed light on this issue. Fourth, we did not examine more functional metrics of brain activity, such as cerebral blood flow and functional connectivity. This data is being collected as part of ADNI-2 and may provide insight into the neurobiological underpinnings of NPS. Finally, the ADNI database represents a highly selective sample of participants with low vascular load, relatively high education, and low baseline NPS burden, which may affect the generalizability of the current findings and inject a bias in the sample selection when it comes to the examination of NPS.
Nevertheless, the present study has important implications for clinicians caring for patients with MCI. Regular follow-up may be indispensable to identify early risk factors for progression to dementia. NPS trajectories may have predictive value that is beyond mere presence of NPS at a single time point and independent of well-known risk factors such as age, sex, education level, and APOE4 carrier status. Administering caregivers a brief questionnaire (e.g., NPI-Q) to monitor a patient’s NPS may be a convenient and clinically meaningful way to predict longitudinal cognitive and functional changes.
In conclusion, we suggest that patients with MCI experience different NPS trajectories that are associated with different cognitive and functional outcomes over time. Pharmacological and non-pharmacological interventions that target NPS can improve quality of life for both patients and caregivers,10 and early intervention in AD can delay institutionalization and neurological decline.39,40 Further investigation of the longitudinal relationship between NPS trajectories and cognitive and functional outcomes may enhance efforts to diagnose and prevent the risk of developing AD. Early identification and disruption of NPS trajectories associated with poor outcomes may have implications not only for the quality of life of older adults and their caregivers, but also for their cognitive and functional prognosis.
Acknowledgments
The project was partially supported by the National Institutes of Health, Grant R25 MH071544/MH/NIMH (PI: Dilip V. Jeste, M.D.) and the University of California, San Diego, Stein Institute for Research on Aging. The manuscript preparation was supported by the University of Rochester CTSA award No. KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health to F.L. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Source of Funding:
Mr. David is funded by NIH grant # R25 MH071544/MH/NIMH.
Dr. Lin is funded by the University of Rochester CTSA award number KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health, and Alzheimer’s Association New Investigator Grant (NIRG-14-317353). Dr Porsteinsson reports receipt of a grant to his institution from AstraZeneca, Avanir, Baxter, Biogen, BMS, Eisai, Elan, EnVivo, Genentech/Roche, Janssen Alzheimer Initiative, Medivation, Merck, Pfizer, Toyama, Transition Therapeutics, the National Institutes of Health (NIH), the National Institute of Mental Health (NIMH), the National Institute on Aging (NIA), and the Department of Defense; paid consultancy for Elan, Janssen Alzheimer Initiative, Lundbeck, Pfizer, and TransTech Pharma; membership on data safety and monitoring boards for Quintiles, Functional Neuromodulation, and the New York State Psychiatric Institute; participation on a speaker’s bureau for Forest; and development of educational presentations for CME Inc and PRI.
Footnotes
This paper was previously presented at the Medical Student Training and Research Experience in Aging and Mental Health (M-STREAM) conference held at the University of California San Diego from August 1st – August 2nd, 2014 and funded by the National Institute of Mental Health
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population based study. Am J Psychiatry. 2014;171(5):572–581. doi: 10.1176/appi.ajp.2014.13060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters ME, Rosenberg PB, Steinberg M, et al. Neuropsychiatric symptoms as risk factors for progression from CIND to dementia: the Cache County Study. Am J Geriatr Psychiatry. 2013;21(11):1116–1124. doi: 10.1016/j.jagp.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. JAMA. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 6.Geda YE, Schneider LS, Gitlin LN, et al. Neuropsychiatric symptoms in Alzheimer’s disease: past progress and anticipation of the future. Alzheimers Dement. 2013;9(5):602–608. doi: 10.1016/j.jalz.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stella F, Radanovic M, Balthazar MLF, et al. Neuropsychiatric symptoms in the prodromal stages of dementia. Curr Opin Psychiatry. 2014;27(3):230–235. doi: 10.1097/YCO.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 8.Lyketsos CG, Steinberg M, Tschanz JT, et al. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory and Aging. Am J Psychiatry. 2000;157(5):708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 9.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65(10):1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaugler JE, Wall MM, Kane RL, et al. The effects of incident and persistent behavioral problems on change in caregiver burden and nursing home admission of persons with dementia. Med Care. 2010;48(10):875–883. doi: 10.1097/MLR.0b013e3181ec557b. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg PB, Mielke MM, Appleby BS, et al. The association of neuropsychiatric symtpoms in MCI with incident dementia and Alzheimer’s disease. Am J Geriatr Psychiatry. 2013;21(7):685–695. doi: 10.1016/j.jagp.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edward ER, Spira AP, Barnes DE, et al. Neuropsychiatric symptoms in mild cognitive impairment: differences by subtype and progression to dementia. Int J Geriatr Psychiatry. 2009;24(7):716–722. doi: 10.1002/gps.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geiatr Psychiatry. 2008;23(2):170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu SH, Ha JH, Park DH, et al. Persistence of neuropsychiatric symptoms over six months in mild cognitive impairment in community-dwelling Korean elderly. Int Psychogeriatr. 2011;23(2):214–220. doi: 10.1017/S1041610210001766. [DOI] [PubMed] [Google Scholar]
- 16.Aalten P, de Vugt ME, Jaspers N, et al. The course of neuropsychiatric symptoms in dementia. Part I: findings from the two-year longitudinal Maasbed study. Int J Geriatr Psychiatry. 2005;20(6):523–530. doi: 10.1002/gps.1316. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg M, Tschanz JT, Corcoran C, et al. The persistence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2004;19(1):19–26. doi: 10.1002/gps.1025. [DOI] [PubMed] [Google Scholar]
- 18.Steenland K, Karnes C, Seals R, et al. Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. J Alzheimers Dis. 2012;31(2):265–275. doi: 10.3233/JAD-2012-111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. Journal of Neuropsychiatry and Clinical Neuroscience. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 21.Fosltein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Crane PK, Carle A, Gibbons LE, et al. Development of assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6(4):502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbons LE, Carle AC, Mackin RS, et al. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6(4):517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24(4):637–639. [PubMed] [Google Scholar]
- 25.Jack CR, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natle Acad Sci USA. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ram N, Grimm KJ. Growth mixture modeling: a method for identifying differences in longitudinal change among unobserved groups. International Journal of Behavioral Development. 2009;33(6):565–576. doi: 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsoi T, Baillon S, Lindesay J. Early frontal executive impairment as a predictor of subsequent behavior disturbance in dementia. Am J Geriatr Psychiatry. 2008;16(2):102–108. doi: 10.1097/JGP.0b013e318151fb42. [DOI] [PubMed] [Google Scholar]
- 29.Wetzels RB, Zuidema SU, de Jonghe JFM, et al. Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. Am J Geriatr Psychiatry. 2010;18(12):1054–1065. doi: 10.1097/jgp.0b013e3181f60fa1. [DOI] [PubMed] [Google Scholar]
- 30.Palmer K, Di lulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis. 2010;20(1):175–183. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 31.Palmer K, Berger AK, Monastero R, et al. Predictors of progression from mild cognitive impairment to Alzheimer’s disease. Neurology. 2007;68(19):1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Kang HS, Kim HJ, et al. Neuropsychiatric predictors of conversion to dementia both in patients with amnestic mild cognitive impairment and those with subcortical vascular MCI. Clin Neurol Neurosurg. 2013;115(8):1264–1270. doi: 10.1016/j.clineuro.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 33.Trzepacz PT, Saykin A, Yu P, et al. Subscale validation of the Neuropsychiatric Inventory Questionnaire: comparison of Alzheimer’s Disease Neuroimaging Initiative and National Alzheimer’s Coordinating Center cohorts. Am J Geriatr Psychiatry. 2013;21(7):607–622. doi: 10.1016/j.jagp.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters KR, Rockwood K, Black SE, et al. Neuropsychiatric symptom clusters and functional disability in cognitively-impaired-not-demented individuals. Am J Geriatr Psychiatry. 2008;16(2):136–144. doi: 10.1097/JGP.0b013e3181462288. [DOI] [PubMed] [Google Scholar]
- 35.Smeets CHW, Smalbrugge M, Gerritsen DL, et al. Improving psychotropic drug prescription in nursing home patients with dementia: design of a cluster randomized controlled trial. BMC Psychiatry. 2013;13:280. doi: 10.1186/1471-244X-13-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy ML, Cummings JL, Kahn-Rose R. Neuropsychiatric symptoms and cholinergic therapy for Alzheimer’s disease. Gerontology. 1999;45(Suppl 1):15–22. doi: 10.1159/000052760. [DOI] [PubMed] [Google Scholar]
- 37.De Medeiros K, Robert P, Gauthier S, et al. The Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22(6):984–994. doi: 10.1017/S1041610210000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conde-Sala JL, Reñé-Ramirez R, Turró-Garriga O, et al. Factors associated with the variability in caregiver assessments of the capacities of patients with Alzheimer disease. J Geriatr Psychiatry Neurol. 2013;26(2):86–94. doi: 10.1177/0891988713481266. [DOI] [PubMed] [Google Scholar]
- 39.Barnett JH, Lewis L, Blackwell AD, et al. Early intervention in Alzheimer’s disease: a health economic study of the effects of diagnostic timing. BMC Neurol. 2014;14:101–109. doi: 10.1186/1471-2377-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosseini SM, Kramer JH, Kesler SR. Neural correlates of cognitive intervention in persons at risk of developing Alzheimer’s disease. Front Aging Neurosci. 2014;6 doi: 10.3389/fnagi.2014.00231. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]