Abstract
PURPOSE
To assess the clinical utility of routine electroencephalograms (EEGs) in the prediction of epilepsy onset in asymptomatic infants with Tuberous Sclerosis Complex (TSC)
PROCEDURES
This multicenter prospective observational study recruited infants less than 7 months of age, seizure-free on no antiepileptic drugs at enrollment, who all underwent serial physical examination and video-EEGs throughout the study. Parental education on seizure recognition was completed at initial enrollment. Once seizure onset occurred, standard of care was applied, and subjects were followed until 24 months.
FINDINGS
40 patients were enrolled, 28 over age 12 months with completed EEG evaluation at the time of this interim analysis. Of those, 19 (67.8%) developed seizures. Epileptic spasms occurred in 10 (52.6%), focal seizures occurred in 5 (26.3%), generalized tonic-clonic seizure in 1 (5.3%), and a combination of epileptic spasms and focal seizures in 3 (15.7%). Fourteen infants (73.6%) had the first emergence of epileptiform abnormalities on EEG at an average age of 4.2 months, preceding seizure onset by a median of 1.9 months. Hypsarrhythmia or modified hypsarrhythmia was not found in any infant prior to onset of epileptic spasms. All children with epileptiform discharges subsequently developed epilepsy (100% positive predictive value), and the negative predictive value for not developing epilepsy after a normal EEG was 64%.
CONCLUSIONS
Serial routine EEGs in TSC infants is a feasible strategy to identify those at high risk for epilepsy. The most frequent clinical presentation was epileptic spasms followed by focal seizures, and then a combination of both seizure types.
Keywords: Epileptic Spasms, EEG, Video EEG Use in Epilepsy, Partial Seizures
Introduction
Tuberous Sclerosis Complex (TSC) is an autosomal dominant disease that affects ~1 in 6000 people, represents one of the most common genetic causes of epilepsy1-3, and is caused by TSC1 or TSC2 mutation. The neurological manifestations in TSC are common and in children represent the most disabling problems of the disease, including epilepsy, intellectual disabilities, psychiatric problems and autism. Epilepsy is particularly prevalent, affecting about 80% of individuals with TSC4-6 with over 60% having seizures that are severe and refractory4,7,8. Almost half of TSC infants develop epileptic spasms, which is associated with poor neurological prognosis4.
Increasingly TSC is diagnosed at a young age before the onset of epilepsy from non-neurological findings, such as cardiac rhabdomyomas9. The earlier diagnosis of TSC provides a unique opportunity to identify and validate a biomarker for epilepsy. A predictive biomarker would allow earlier intervention that may alter or curtail epileptogenesis and its adverse effects. A recent open-label study suggests treating TSC patients with an abnormal electroencephalogram (EEG) prior to onset of epileptic spasms with vigabatrin may improve neurological outcome10. An earlier retrospective study reported similar benefit with early treatment11. Nonetheless, the use of clinical EEG as a reliable biomarker of epilepsy has not been rigorously validated and has been limited to retrospective analyses subject to referral, recording, and recall biases4,12. Our prospective study provides a unique opportunity to document the evolution of epileptogenesis, development of clinical seizures, and the utility of EEG as an early biomarker for epilepsy in TSC.
Methods
Subject Recruitment
Infants with TSC in this multicenter prospective observation study were enrolled from the neonatal nursery, pediatric cardiology, general pediatrics, genetics, pediatric neurology, and obstetrics/perinatology/maternal-fetal medicine clinics. TSC diagnosis was based on clinical features (i.e., cardiac rhabdomyomas, intracranial tubers/subependymal nodules/giant cell astrocytomas, characteristic skin findings, and/or other evidence on prenatal/perinatal cardiac echocardiography, neuroimaging, and skin exams) or genetic diagnosis13.
Each TSC infant enrolled met all the following inclusion criteria: 1) age < 7 months, 2) seizure-free at enrollment, and 3) the genetic or clinical diagnosis for TSC13. Infants were excluded if any one of the following criteria were present: 1) ≥ 7 months of age, 2) history of seizure(s) of any type, or 3) current or past treatment with vigabatrin or inhibitors of the mammalian target of rapamycin (mTOR), prior to study enrollment. Prematurely born TSC infants as young as 32 weeks gestation could participate only if there were no medical complications from prematurity, involving the brain or other major organs, such as hypoxic-ischemic encephalopathy, any intracranial hemorrhage, necrotizing enterocolitis, any respiratory diagnoses requiring ventilator support, or cardiovascular compromise. The earliest time of enrollment for these premature infants was when they reached full-term (37 weeks gestation).
Infants with TSC were recruited from the TSC centers at each of the 5 sites (University of Alabama at Birmingham, University of California at Los Angeles, Boston Children’s Hospital, Cincinnati Children’s Hospital Medical Center, and University of Texas Medical School at Houston).
Study Design
This study was approved by the institutional review boards of all five institutions. Parental consent was obtained for all subjects. At designated time points following enrollment (1.5, 3, 4.5, 6, 9, 12, 18, and 24 months chronological age), physical/neurological exam and a 1-hour research video-EEG (to include both sleep and wakefulness) were performed. The 1 hour duration of the video-EEG was chosen to maximize capturing both wakefulness and sleep during the same study, yet sufficiently brief to utilize in and extend to the clinical setting, as well as taking into consideration families’ time commitment and staying within funding constraints.
Subjects referred for initial screening and enrollment were seen within 2 weeks. Initial evaluation included physical/neurologic exam, baseline video-EEG (1 hour wakefulness and sleep). As part of our research protocol, a seizure recognition educational video was shown to the parents/caregivers at the time of enrollment. Enrolled subjects were followed until the age of 2 years.
If the infant/child at any point in the study developed seizures, history and additional clinical video-EEG(s) of varying duration were completed to confirm epilepsy onset, and antiepileptic drug (AED) treatment was initiated at the managing neurologist’s discretion as dictated by individual clinical scenarios, but this clinical information was recorded, as were all medical therapies throughout the duration of the study. The research video-EEGs continued at the designated time points stated above, even after clinical seizure onset.
In addition to the scheduled serial research 1-hour video-EEG studies to monitor for the development and evolution of EEG abnormalities, the parent/caregiver maintained a seizure log throughout the study. Once a diagnosis of seizures was made, the subject continued in the study to monitor developmental progress, seizure control, and response to AEDs.
Video-EEG Acquisition and Interpretation
Video-EEGs were uniformly acquired across all five TSC centers, with standard 23 electrodes placed according to the 10-20 international placement system. All video-EEG studies were recorded for one hour, incorporating both sleep and wakefulness, at a high sampling rate of 2000 Hz, with a high frequency/low pass filter of 500 Hz. All video-EEGs from all 5 sites were anonymized, then uploaded to a secure central server, located and maintained at the University of California at Los Angeles. To view the video-EEGs from all 5 sites, which collectively utilized 3 different video-EEG vendors, video-EEG analysis was viewed digitally with Persyst software (San Diego, CA), in the standard time scale of 30 mm/sec and standard filter settings of 1 Hz low frequency filter (high pass) and 70 Hz high frequency (low pass), along with a 60 Hz notched filter.
To render a more balanced interpretation, each video-EEG study was reviewed by two independent central EEG reviewers (JMP, MG), who are both board-certified pediatric electroencephalographers and blinded to all clinical information except the age of the subject necessary for the EEG interpretation. Differences in the EEG interpretation, when present, were adjudicated by a third, blinded board-certified pediatric electroencephalographer (JYW).
The EEG results were classified based on age-appropriate norms, as either normal or abnormal. EEG abnormalities were evaluated in terms of the presence or absence of background abnormalities, such as generalized or focal slowing, epileptiform discharges (focal, regional, bilateral, or generalized spike or spike and wave discharges), (modified) hypsarrhythmia, voltage attenuation, as well as clinical and/or electrographic seizures, in accordance with the National Institutes of Neurologic Disorders and Stroke Common Data Element Tools for Epilepsy.
Statistical Analysis
The study design made the following assumptions for statistical sample size. In a population of TSC patients the study assumed the incidence of epilepsy to be 85% and that the overall frequency of epileptiform discharges is 50%. Based on the statistical analysis of the preliminary data from the University of Alabama Birmingham and the University of California Los Angeles, enrolling 30 patients results in >80% power at a non-directional alpha of 0.05 assuming that the ratio of patients with normal EEG vs. EEG with epileptiform discharges will vary from 0.2 to 0.5. The association between epileptiform discharges and epilepsy was analyzed by multiple methods, performed by a statistician at the University of Alabama Birmingham (GC): 1) time-to-event survival analysis to determine the temporal relationship between epileptiform discharges and seizure onset, 2) multivariable Cox proportional hazard analysis, to assess the contribution of multiple variables to development of epilepsy, and 3) logistical regression analysis to determine strength of association between epileptiform discharges and epilepsy at the end of the 24 month follow-up period.
Results
Cohort and Clinical Characteristics
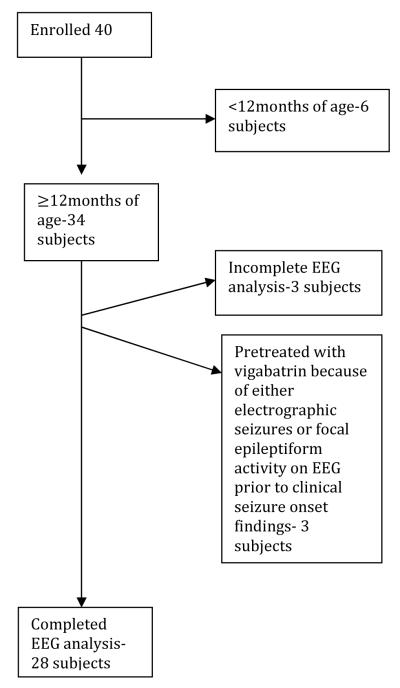
A total of 40 subjects were prospectively enrolled into the study (Table 1). At the time of 2/1/15 data cut-off for this interim analysis, 28 infants were older than 1 year of age and were included in the current analysis (Figure 1). Three additional subjects older than 12 months of age were excluded since most recent EEG analysis by the blinded reviewers was not completed at the time of cut-off. Of the remaining subjects, 3 subjects had either electrographic seizures or epileptiform discharges on EEG and were excluded because they were treated with AEDs prior to the onset of clinical seizures. These subjects could not be included in the statistical calculation for positive and negative predictive values because they had received a therapeutic intervention. The remaining 6 subjects were excluded from this interim analysis because they were still younger than 12 months of age and therefore needed longer follow-up time.
Table 1.
Summary of TSC Infants over 12 months of age
| Subject | Race | Gender | TSC1/TSC2 | Age enrolled (months) |
Age EEG Abnormal (months) |
EEG abnormality |
Age clinical seizure onset (months) |
Clinical Seizure Type (s) |
Age at data cut-off (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Caucasian | Male | NMI | 5.4 | 16 | ||||
| 2 | Caucasian | Male | Not tested | 3.8 | 15 | ||||
| 3 | Caucasian | Female | Not tested | 2.1 | 22 | ||||
| 4 | Caucasian | Female | TSC2 | 1.4 | 18 | ||||
| 5 | Caucasian | Female | TSC1 | 7.2 | 20 | ||||
| 6 | Caucasian | Male | TSC2 | 2.1 | 25 | ||||
| 7 | Caucasian | Male | TSC2 | 1.5 | 24 | ||||
| 8 | Caucasian | Female | TSC1 | 7 | 27 | ||||
| 9 | Caucasian | Male | TSC1 | 4.4 | 26 | ||||
| 10 | Caucasian | Female | TSC2 | 2.3 | 6 | ES | 13 | ||
| 11 | Caucasian | Female | TSC1 | 2.6 | 5.5 | GTC | 15 | ||
| 12 | Caucasian | Female | TSC2 | 1.5 | 2 | Focal | 14 | ||
| 13 | African American |
Female | TSC2 | 0.4 | 11 | Focal | 14 | ||
| 14 | Caucasian | Female | TSC2 | 1.6 | 3.5 | ES | 20 | ||
| 15 | Caucasian | Male | TSC2 | 0.7 | 4.2 | Focal spikes | 6 | Focal + ES | 15 |
| 16 | Caucasian | Female | TSC1 | 3.3 | 3.4 | Regional Spikes | 4 | ES | 12 |
| 17 | Caucasian | Female | TSC2 | 6.5 | 6.5 | Regional Spikes | 8 | ES | 17 |
| 18 | Caucasian | Male | TSC2 | 6 | 9 | Regional spikes | 12.5 | ES | 16 |
| 19 | Caucasian | Male | TSC2 | 1.1 | 4 | Regional spikes | 7 | ES | 17 |
| 20 | Caucasian | Female | TSC2 | 4 | 4 | Bilateral spikes | 5.5 | ES | 15 |
| 21 | Caucasian | Male | TSC2 | 3.9 | 4 | Regional spikes | 5 | ES | 18 |
| 22 | Caucasian | Male | TSC2 | 1.1 | 6 | Bilateral spikes | 6.2 | ES | 22 |
| 23 | Asian | Male | TSC2 | 1.4 | 1.5 | Focal spikes | 3.5 | Focal | 21 |
| 24 | Caucasian | Female | TSC2 | 2.5 | 4 | Bilateral spikes | 6 | Focal + ES | 20 |
| 25 | American Indian | Male | TSC2 | 1.5 | 1.6 | Bilateral spikes | 3.5 | Focal | 20 |
| 26 | Hispanic | Female | TSC2 | 6 | 6 | Focal spikes | 20 | Focal | 29 |
| 27 | Caucasian | Male | TSC2 | 4.2 | 4.2 | Bilateral spikes | 6 | ES | 28 |
| 28 | Caucasian | Male | TSC2 | 1.1 | 1.2 | Bilateral spikes | 6 | ES + Focal | 25 |
Figure 1.
Cohort of TSC study participants
Gender was distributed evenly amongst the 28 infants included for the interim analysis, and the average age at time of enrollment was 2.7 ± 2.0 months of age. Of the 28 subjects enrolled, 26 underwent TSC genetic testing, 20 (76.9%) had a pathologic mutation in TSC2. Mutations in TSC1 were identified in 5 (19.2%). In 1 (3.8%), no mutation in either TSC1 or TSC2 was found. In this cohort of 28 infants over the age of 12 months, 19 (67.9%) developed clinical seizures during the observation period. The average age at time of seizure onset was 6.7 ± 4.1 months, the youngest within 2.0 months of age and oldest at 20 months of age. Epileptic spasms were the most common seizure type and occurred in 10 infants (52.6%). Focal seizures occurred either as the sole seizure type (5 subjects, 26.3%) or with epileptic spasms (3 subjects, 15.8%). In contrast, seizures with generalized onset were rare, with generalized tonic-clonic seizures occurring only in 1 (5.3%) subject, and no other clinical seizure types were reported (Table 2).
Table 2. Clinical seizure semiology and EEG Characteristics.
Seizure characteristics in 19 subjects who have had a clinical seizure since enrollment, by group:
| Seizure Type | Epileptiform Discharges (n=14) |
Normal EEG (n=5) |
|---|---|---|
| Focal seizures (FS) | 3 | 2 |
| Epileptic Spasms (ES) | 8 | 2 |
| Focal seizures + Epileptic Spasms | 3 | 0 |
| Generalized seizures (GS) | 0 | 1 |
|
EEG characteristics 19 subjects who have had a clinical seizure since enrollment: 14/19 (73.6%) had epileptiform activity detected on EEG before the onset of clinical seizures | ||
|---|---|---|
| Average | Median | |
| Age at time of 1st epileptiform discharges | 4.2 (sd=2.1months) | 4.0 months |
| Age at time of 1st clinical seizure | 6.7 (sd= 4.1 months) | 6.0 months |
| Time Interval between epileptiform discharges and Seizure | 2.8 (sd=3.4 months) | 1.9 months |
| 5/19 (26.3%) had no epileptiform activity detected on EEG before the onset of clinical seizures | ||
|---|---|---|
| Subject ID | Last Normal EEG | Clinical Seizure Onset |
| 10 | 4 months | 6 months |
| 11 | 4 months | 5.5 months |
| 12 | 1.5 months | 2 months |
| 13 | 9 months | 11 months |
| 14 | 1.5 months | 3.5 months |
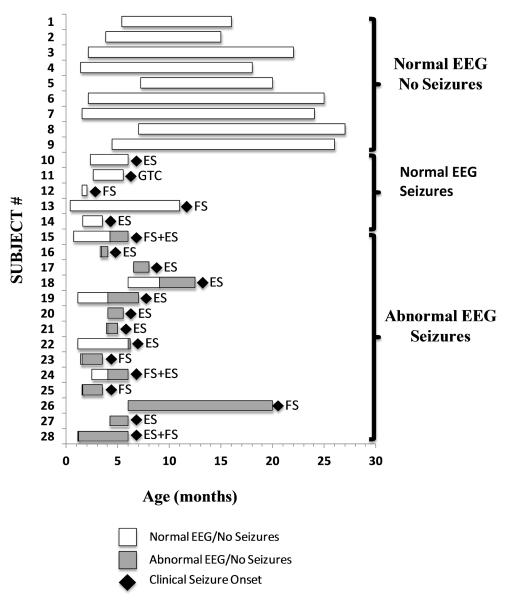
Of these EEG interictal and ictal findings, the presence of epileptiform discharges preceded the onset of first clinical seizure in 14 of 19 infants (73.7%), which occurred between 1.2-9.0 months of age (Table 2). The interval between the 1st EEG with epileptiform discharges and the 1st clinical seizure was an average of 2.8 ± 3.4 months, median of 1.9 months (interquartile range 1.5, 3.0 months). No epileptiform discharges were detected with any of the video-EEGs in 5 subjects (26.3%) prior to the onset of clinical seizures. Seizure type in this latter group included focal seizures, epileptic spasms, and generalized tonic-clonic seizure (Figure 2).
Figure 2.
Summary of EEG in Relation to Clinical Seizure Onset
The remaining 9 infants of the cohort of 28 have remained seizure-free, on no AED, at the time of this interval analysis, and all 9 infants have had normal video-EEGs, without the presence of epileptiform discharges on any of the video-EEGs (Table 3).
Table 3.
Statistical Analysis Summary
| Clinical seizure | No Clinical seizure |
|
|---|---|---|
|
Epileptiform
discharges |
14 | 0 |
|
No epileptiform
discharges/NL EEG |
5 | 9 |
| Sensitivity | Specificity | Positive Predictive Value |
Negative Predictive Value |
|---|---|---|---|
| 73.7% | 100% | 100% | 64% |
The positive predictive value (PPV), or how often the presence of a biomarker can correctly predict the disease in a population, can be determined from the ratio of true positives (those subjects with both abnormal EEG and subsequent seizures) to the sum of true positives and false positives (those with abnormal EEG and no subsequent seizures). The corresponding PPV (Table 3) for the presence of epileptiform activity on an EEG preceding the development of clinical seizure onset in infants with TSC is then = 14/(14 + 0), or 100% CI (76.8%, 100.0%).
Similarly, the negative predictive value (NPV), or how often the absence of a biomarker can correctly predict the non-disease state in a population, is determined from the ratio of true negatives (subjects with both normal EEG and no seizures) to the sum of true negatives and false negatives (subjects with normal EEG but subsequently developing seizures). The corresponding NPV (Table 3) of the absence of epileptiform activity on the EEG and no subsequent epilepsy in infants with TSC is then = 9/(9 + 5), or 64.3% CI (35.1%, 87.2%).
The other EEG findings of focal or generalized slowing, attenuation, hypsarrhythmia or modified hypsarrhythmia, and ictal events did not consistently precede the onset of clinical seizures and none reached statistical significance.
Discussion
This prospective multicenter observational study provides unique insight into the development of epilepsy in TSC, and the clinical utility of serial EEGs in the identification of patients at high-risk for impending seizure onset. This preliminary report is driven by compelling strength of interim analysis and provides early supportive data for risk-stratification in planned prospective research on effects of pre-emptive anti-epileptic treatment in TSC.
The risk for epilepsy in TSC has been previously estimated to be 80%, based on retrospective studies in patients of all ages, including older children and adults4,14,15. Determining prevalence in infants is more difficult as not all the studies separated their analyses into younger cohorts. Jozwiak et al. reported seizures present by 2 years of age in 83%14, whereas Chu-Shore et al. reported 63% by age 1 and 82% by age 3 years4. Using an observational study design in which infants were enrolled prior to seizure onset, and followed prospectively, we calculated in this study the incidence of TSC infants developing clinical seizures before the age of two years as 67.8 %. While our approach overcomes recall and reporting bias inherent to retrospective studies, the estimate in this prospective study may yet underestimate the true incidence, as not all subjects have been observed past 24 months of age. Continuing to follow our present cohort prospectively will allow more definitive calculation of annual and cumulative incidence of epilepsy for TSC infants, by age, throughout infancy and childhood.
Only one prior published study has evaluated EEG findings in TSC infants prior to the onset of seizures, consisting of 5 subjects16. Patients were enrolled from 9 days to 9 weeks of age and had serial EEGs at 4 week intervals. EEG abnormalities were detected in 4 subjects between 0.5-5.0 months of age, all of whom (100%) subsequently developed seizures within 1-8 days of the first abnormal EEG. The remaining subject with a normal EEG never developed clinical seizures. In this study, we found a similar high correlation between epileptiform discharges and subsequent seizures, with the average age when epileptiform discharges were first detected as 4.2 months. However, sensitivity was notably lower (73.7%) and with a much longer time interval between epileptiform discharges and clinical seizure onset that averaged 2.8 months time and median of 1.9 months. Our study involved multiple centers, larger cohort than previous studies, and multiple blinded EEG readers, adding additional patient diversity, power, and scientific rigor to the calculated lower limit of sensitivity. An unresolved variable that could contribute to the differences observed is the frequency of surveillance EEGs in the asymptomatic cohort. The every 6 weeks EEG at earlier time points in our study was chosen to balance study sensitivity with clinical feasibility for participating families. This longer time interval between scheduled EEGs, especially at later time points when expanded to 3-6 month intervals, may also explain the relatively high false negative rate observed (and corresponding calculated NPV) in our study. The longer interval between scheduled EEGs increases the likelihood that newly emerging epileptiform abnormalities before the onset of clinical seizures may have gone undetected in the interim. However, the calculated time between epileptiform activity and seizures was an average 2.8 months, median 1.9 months. We suspect that with more frequent sampling, the interval time, here measured only in monthly increments, would likely be shorter with the possibility of showing higher sensitivity and lower NPV. A sizeable window between epileptiform discharges and clinical seizure onset is key, as such a window provides a unique and feasible opportunity to design and implement antiepileptogenic treatment strategies that may delay or prevent clinical seizure onset.
In our study infants with TSC are as likely to present with focal seizures, epileptic spasms, or focal seizures mixed with epileptic spasms (either concurrently or subsequently to onset of focal seizures). Furthermore, similar to results of the Domańska-Pakieła et al study16, classic or modified hypsarrhythmia was not found in any infant prior to the onset of focal seizures or epileptic spasms. This would suggest that classic or modified hypsarrhythmia, reported to occur in up to 71% of TSC patients with TSC and clinical epileptic spasms17, occurs after seizure onset and corresponds to later events in the epileptogenesis process. These observations on the evolution of epilepsy onset in infants with TSC have important impact on clinical management, as treatment delay may adversely affect long-term epilepsy and developmental outcome 11,17.
First, parents and clinicians should know that either focal onset seizures or epileptic spasms may be an initial seizure manifestation in infants with TSC. Secondly, because hypsarrhythmia may follow epileptic spasms, clinicians should not wait for hypsarrhythmia, either in classic or modified form, to appear on EEG before initiating appropriate treatment for epileptic spasms. Finally, the earliest signs of seizures, whether focal seizures, epileptic spasms, or a mix thereof, may be very subtle and could go unrecognized or misdiagnosed without a high index of clinical suspicion on the part of parents and clinicians. We found it very useful to show videos of multiple clinical seizure types, both classic and subtle forms, to parents in order to increase their likelihood of recognizing and reporting to clinicians the earliest clinical events of concern. We also encouraged parents and caregivers to send video files obtained with mobile phones for clinician review and confirmation.
While an EEG is currently recommended at the time of initial TSC diagnosis11,13,19, the results of this study not only support the importance of that initial EEG, but also the importance of subsequent EEGs in monitoring the development of seizures and epileptiform discharges. This recommendation is consistent with the European recommendation, which suggested close EEG monitoring in the first few months of life and consideration of preventative treatment in the presence of EEG ictal discharges18.
In conclusion, this study is the first multicenter prospective study to evaluate serial EEGs as a biomarker for subsequent epilepsy in the infant TSC population. Our study demonstrates the feasibility and importance of close EEG surveillance in infants with TSC, with high positive predictive value of epileptiform discharges for predicting those who subsequently develop epilepsy. This interim analysis highlights the value of early diagnosis of infants with TSC and the value of serial EEG beginning at the time of diagnosis. Importantly, our study suggests there is a critical window of time between emergence of epileptiform discharges and clinical seizure onset which provides a unique opportunity to investigate potentially disease-modifying antiepileptogenic treatment strategies in this population.
Acknowledgments
Study Funding: Supported by the National Institute of Neurological Diseases and Stroke of the National Institutes of Health (U01-NS082320, P20-NS080199) and the Tuberous Sclerosis Alliance. JYW also supported by the NIH (R01-NS082649), the Department of Defense Congressionally Directed Medical Research Program, and the Today’s and Tomorrow’s Children Fund from Mattel Children’s Hospital at the University of California Los Angeles. MS supported by Senior Investigator award from Boston Children’s Translational Research Program. This study also utilized clinical research facilities and resources supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health Grant (8UL1TR000077 and UL1RR033176).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sparagana SP, Roach ES. Tuberous sclerosis complex. Curr Opin Neurol. 2000;13:115–9. doi: 10.1097/00019052-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kwiatkowski DJ. Cancer Biol Ther. 2003;2:471–6. doi: 10.4161/cbt.2.5.446. [DOI] [PubMed] [Google Scholar]
- 3.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 4.Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–41. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb DW, Fryer AE, Osborne JP. On the incidence of fits and mental retardation in tuberous sclerosis. J Med Genet. 1991;28:395–7. doi: 10.1136/jmg.28.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparagana SP, Delgado MR, Batchelor LL, Roach ES. Seizure remission and antiepileptic drug discontinuation in children with tuberous sclerosis complex. Arch Neurol. 2003;60:1286–9. doi: 10.1001/archneur.60.9.1286. [DOI] [PubMed] [Google Scholar]
- 7.Curatolo P. Mechanistic target of rapamycin (mTOR) in tuberous sclerosis complex-associated epilepsy. Pediatr Neurol. 2015;52:281–289. doi: 10.1016/j.pediatrneurol.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Aboian MS, Wong-Kisiel LC, Rank M, Wetjen NM, Wirrell EC, Witte RJ. SISCOM in children with tuberous sclerosis complex-related epilepsy. Pediatr Neurol. 2011;45:83–8. doi: 10.1016/j.pediatrneurol.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Datta AN, Hahn CD, Sahin M. Clinical presentation and diagnosis of tuberous sclerosis complex in infancy. J Child Neurol. 2008;23:268–73. doi: 10.1177/0883073807309250. [DOI] [PubMed] [Google Scholar]
- 10.Jozwiak S, Kotulska K, Domanska-Pakiela D, et al. Antiepielptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. Eur J Peadiatr Neurol. 2011;15:424–31. doi: 10.1016/j.ejpn.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol. 2010;14:146–9. doi: 10.1016/j.ejpn.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Jozwiak S, Goodman M, Lamm SH. Poor mental development in patients with tuberous sclerosis complex: clinical risk factors. Arch Neurol. 1998;55:379–84. doi: 10.1001/archneur.55.3.379. [DOI] [PubMed] [Google Scholar]
- 13.Northrup H, Krueger D, et al. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatric Neurology. 2013;49:243–254. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jozwiak S, Schwartz RA, Janniger CK, Bielicka-Cymerman J. Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. 2000;15:652–9. doi: 10.1177/088307380001501003. [DOI] [PubMed] [Google Scholar]
- 15.Devlin LA, Shepherd CH, Crawford H, Morrison PJ. Tuberous sclerosis complex: clinical features, diagnosis, and prevalence within Northern Ireland. Dev Med Child Neurol. 2006;48:495–9. doi: 10.1017/S0012162206001058. [DOI] [PubMed] [Google Scholar]
- 16.Domańska-Pakieła D, Kaczorowska M, Jurkiewicz E, Kotilska K, Dunin-Wasowicz Jozwiak S. EEG abnormalities preceding the epilepsy onset in tuberous sclerosis complex patients- a prospective study in 5 patients. Eur J Paediatr Neurol. 2014;18:456–68. doi: 10.1016/j.ejpn.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Muzykewicz DA, Costello DJ, Halpern EF, Thiele EA. Infantile spasms in tuberous sclerosis complex: prognostic utility of EEG. Epilepsia. 2009;50:290–6. doi: 10.1111/j.1528-1167.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 18.Curatolo P, Jóźwiak S, Nabbout R, TSC Consensus Meeting for SEGA and Epilepsy Management Management of epilepsy associated with tuberous sclerosis complex (TSC): clinical recommendations. Eur J Paediatr Neurol. 2012 Nov;16(6):582–6. [Google Scholar]
- 19.Krueger D, Northrup H, et al. Tuberous Sclerosis Complex Surveillance and Management: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatric Neurology. 2013;49:255–265. doi: 10.1016/j.pediatrneurol.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]