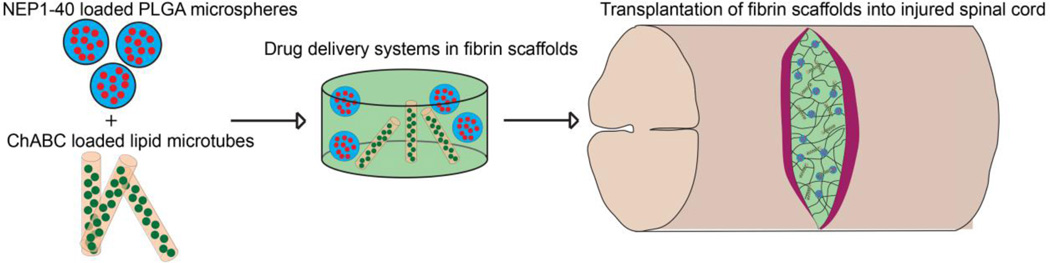
Abstract
Myelin-associated inhibitors (MAIs) and chondroitin sulfate proteoglycans (CSPGs) are major contributors to axon growth inhibition following spinal cord injury and limit functional recovery. The NEP1-40 peptide competitively binds the Nogo receptor and partially blocks inhibition from MAIs, while chondroitinase ABC (ChABC) enzymatically digests CSPGs, which are upregulated at the site of injury. In vitro studies showed that the combination of ChABC and NEP1-40 increased neurite extension compared to either treatment alone when dissociated embryonic dorsal root ganglia were seeded onto inhibitory substrates containing both MAIs and CSPGs. Furthermore, the ability to provide sustained delivery of biologically active ChABC and NEP1-40 from biomaterial scaffolds was achieved by loading ChABC into lipid microtubes and NEP1-40 into poly (lactic-co-glycolic acid) (PLGA) microspheres, obviating the need for invasive intrathecal pumps or catheters. Fibrin scaffolds embedded with the drug delivery systems (PLGA microspheres and lipid microtubes) were capable of releasing active ChABC for up to one week and active NEP1-40 for over two weeks in vitro. In addition, the loaded drug delivery systems in fibrin scaffolds decreased CSPG deposition and development of a glial scar, while also increasing axon growth after spinal cord injury in vivo. Therefore, the sustained, local delivery of ChABC and NEP1-40 within the injured spinal cord may block both myelin and CSPG-associated inhibition and allow for improved axon growth.
Keywords: PLGA microspheres, lipid microtubes, chondroitinase ABC, NEP1-40, controlled release
Graphical abstract
Introduction
Spinal cord injury (SCI) is a major medical problem affecting ~320,000 Americans, with 12,000 new cases occurring annually [1]. SCI typically results in partial or complete loss of function below the initial site of injury, leaving patients without the ability to perform basic daily activities. Loss of function occurs by disrupting signal transduction of ascending and descending neuronal tracts. After the initial contusion or compression injury, a secondary injury leading to the formation of a glial scar causes increased cell death at the site of injury, as well as the upregulation of inhibitory factors that limit recovery [2]. Current research focuses on reforming functional synapses between severed tracts by increasing the intrinsic growth capacity of neurons, removing the extrinsic barriers to regeneration from inhibitory cues, or replacing lost cells by transplantation [3–5].
Therapies for SCI are trending toward multifunctional systems that combine single therapies to overcome several inhibitory obstacles and further enhance functional recovery [3, 6, 7]. The use of biomaterial scaffolds allows for the development of multifunctional systems by providing a growth substrate for host and transplanted cells, delivery of growth factors that promote cell survival, and delivery of anti-inhibitory molecules to increase migration and axon extension following injury. The transplantation of neural progenitors, olfactory ensheathing cells, Schwann cells, and other cell types have shown improved benefits when transplanted within natural or synthetic biomaterial scaffolds [8–11]. Previous research in our lab has shown the benefits of transplanting embryonic stem cell-derived neural progenitor cells within a fibrin-based scaffold capable of sustained delivery of neurotrophic factors [12]. Transplantation of neural progenitor cells coupled with the delivery of neurotrophic factors promoted recovery by repopulating the cystic cavity in the lesion, increasing transplanted and host cell survival, and increasing the growth potential of neurons. However, further improvements to the scaffold include the addition of drug delivery systems that release anti-inhibitory molecules, which reduce the extrinsic barriers within the glial scar that block axonal extension.
The formation of a glial scar greatly limits axonal regeneration into and across the injury site. The glial scar is generated by reactive astrocytes that extend processes to form a physical and chemical barrier to axonal extension [5]. Key chemical inhibitors to axonal extension are chondroitin sulfate proteoglycans (CSPGs) and myelin-associated inhibitors (MAIs). CSPGs and MAIs have been studied extensively due to their ability to destabilize the axonal growth cone by initiating the Rho/ROCK signaling cascade [13, 14]. The deposition of CSPGs is significantly upregulated after SCI for up to 8 weeks, with most deposition occurring within 2 weeks after the primary injury [15]. Chondroitinase ABC (ChABC) has been extensively studied and shown to limit the effects of CSPGs by cleaving inhibitory sugar chains, leaving the core protein and stub carbohydrates [16–18].
Intrathecal delivery of ChABC into severely injured spinal cords for 10 days post-injury decreased the level of deposited CSPGs, increased axonal extension, and significantly improved functional recovery. Research has shown that the use of an implantable intrathecal pump to deliver ChABC every other day for 2 or 4 weeks in cat hemisection models led to differences in functional recovery and that longer treatment times increased the number of rubrospinal tract neurons below the lesion site [19]. The use of the implantable injection system verifies the need of prolonged delivery of ChABC but requires implantation of an invasive pump system for 4 weeks. The need for invasive pumps or microinjections has been removed by delivery of the highly labile ChABC enzyme through natural and synthetic drug delivery systems [20–24]. In addition, combination therapies with ChABC have also shown promise, but limited research has been performed with both ChABC drug delivery systems coupled with other promising therapies [6, 22, 25]. In this study, we developed a combination therapy of dual anti-inhibitory molecules to improve upon single treatment options that utilizes natural and synthetic drug delivery systems to remove the need for intrathecal pumps and microinjections.
In addition, several techniques have tried to limit the effects of MAIs following SCI by blocking the interaction of the myelin glycoprotein, Nogo-A with its receptor, NgR1, found on axons [26–30]. Specifically, the NEP1-40 peptide has been shown to limit the effect of MAIs through competitive inhibition of Nogo-A binding to NgR1. Intrathecal delivery of NEP1-40 for 4 weeks post-injury led to increased axonal extension and functional recovery after thoracic SCI in rats [26, 27, 31]. The NEP1-40 peptide has also been administered through intraperitoneal injection and subcutaneous osmotic pumps in mouse SCI with both showing improved axonal sprouting rostral to SCI [32]. Furthermore, transplantation of hybridoma cells expressing a monoclonal antibody, IN-1, that binds to Nogo and blocks myelin-associated inhibitor signaling also showed improved recovery over cells expressing a control antibody [33]. These demonstrate that the duration and therapeutic window for treatment with anti-myelin inhibitors is important to increase axon sprouting and functional recovery; extended treatment durations chronically suppress MAIs and delayed therapies of at least 1 week showed no difference to immediate treatments [32, 33].
While ChABC and NEP1-40 have been found effective individually, the combined delivery of ChABC and anti-myelin inhibitors using cannulas and intrathecal pumps, along with treadmill training, significantly increased functional recovery compared to either treatment alone [6]. Therefore we hypothesized that it would be beneficial to build upon previously published work by developing a drug delivery system that releases multiple anti-inhibitory molecules and can be combined with biomaterial-based cell transplantation therapies. Our drug delivery systems are introduced simultaneously but have varying release profiles. Thus the delivery of individual anti-inhibitory molecules can be tuned independently. In this study, we developed drug delivery vehicles capable of providing sustained delivery of both ChABC and NEP1-40 over one week in vitro. Also, proof of concept studies were performed by incorporating the drug delivery vehicles into fibrin scaffolds and implanting into injured rat spinal cords. The dual delivery of ChABC and NEP1-40 limited the development of the glial scar and increased the number of axons found around the injury site.
Materials and Methods
Inhibitory Spot Assays
Chick dorsal root ganglia (DRG) neurite outgrowth assays were used similar to previous publications [34]. Briefly, dissociated embryonic day 8 chick DRG cells were cultured on purified myelin, CSPG (EMD Millipore, Billerica, MA), or combination purified myelin and CSPG spots. Myelin was purified using previously published protocols [35]. Prior to plating, 24-well tissue culture plates were coated with poly-L-ornithine (Sigma, St. Louis, MO) for one hour at 37°C, then washed 3 times with water, and dried overnight. After drying, 1 µL spots of inhibitory substrate (87.5 µg/mL of myelin or 10 µg/mL of CSPG) in phosphate buffered saline (PBS, pH7.4) was placed onto the coated wells and dried overnight. Dissociated DRGs were plated at 100,000 cells/well, fixed with 4% paraformaldehyde (Sigma) after 6 hours, and stained for neurofilament (NF, Dev. Studies Iowa Hybridoma Bank, Iowa City, IA, 1:200). Stained neurites were imaged using a 20x objective on an Olympus IX70 (Olympus, Center Valley, PA) inverted microscope with an Optronics MICROfire camera (Optronics, Muskogee, OK) and neurite lengths were measured using ImageJ software with over 100 neurites measured per group. Culture medium was 1:1 DFK5:Neurobasal with B27 (Life Technologies, Carlsbad, CA) and varying concentrations of ChABC or NEP1-40. DFK5 media consisted of DMEM:F12 base media (Life Technologies) supplemented with 5% knockout serum replacement (Life Technologies), 50 µg/ml apo-transferrin (Sigma), 50 µM non-essential amino acids (Life Technologies), 5 µg/ml insulin (Sigma), 30 nM sodium selenite (Sigma), 100 µM β-mercaptoethanol (Life Technologies), 5 µM thymidine, and 15 µM of the following nucleosides: adenosine, cytosine, guanosine, and uridine (EMD Millipore).
Formation of Poly(lactic-co-glycolic acid) (PLGA) Microspheres
PLGA microspheres, with a 50:50 lactic acid to glycolic acid ratio, were fabricated using a water in oil in water double emulsion solvent evaporation technique [36]. PLGA (10% w/v, intrinsic vis. = 0.15-0.3 dL/g, Absorbable Polymers Inc., Pelham, NJ) was dissolved in 2 mL dichloromethane (DCM). 100 µL of 10 mg/mL NEP1-40 (Mw = 4627 Da, Sigma) was added to the PLGA/DCM solution and sonicated for 10 seconds (Microson, Misonix Inc.), added to 25 mL of water with 1% w/v polyvinyl alcohol) and 10% w/v NaCl and homogenized. The resulting emulsion was poured into 250 mL of water with 0.1% w/v poly(vinyl alcohol) and 10% w/v NaCl, then magnetically stirred for 3 hours, washed with water, frozen overnight at −80°C and lyophilized. PLGA microspheres were imaged using a scanning electron microscope (Nova NanoSEM 230, FEI) after gold sputter coating for 45 seconds. Diameters from individual microspheres were measured using ImageJ software and 100 microsphere diameters were averaged per batch. Loading efficiency of PLGA microspheres was measured using previously established protocols [37–39]. Specifically, microspheres were fully degraded with 0.5 M NaOH to release the loaded molecules. An equal volume of 10 mM phosphate buffered saline was added and the solution pH was adjusted to 7 using HCl. The amount of fluorescent loaded molecules was measured using a SpectraMax M2e fluorimeter (Molecular Devices, Sunnyvale, CA).
Formation of Lipid Microtubes
Lipid microtubes were formed similar to previous work [40]. 1,2-bis(tricosa-10,12-diynoyl)-sn-3-phosphocholine (DC8,9PC) lipids (Avanti Polar Lipids, Alabaster, AL) were dissolved at 55°C in 70% ethanol at 1 mg/mL and placed into a temperature controlled water bath (Haake A10, ThermoScientific, Asheville, NC). The total volume per batch was 5 mL. The temperature was decreased from 55°C to 25°C at a rate of 2.5°C/min. The lipid tubes were then stored at room temperature in the dark for one week. Trehalose (EMD Millipore) was added to a final concentration of 50 mM, the solution was centrifuged (1200 rcf, 5 minutes) to pellet the lipid microtubes, and 4 mL of solution was removed. The concentrated (5 mg/mL) microtube solution was lyophilized overnight to dry and stored at −20°C until further use.
Fluorescein isothiocyanate (FITC) conjugation to NEP1-40 and ChABC
FITC was covalently conjugated to NEP1-40 and ChABC (Sigma). To fluorescently label the molecules, a solution of 2 mg/mL ChABC or 2 mg/mL NEP1-40 was dissolved in 0.1 M sodium carbonate buffer, pH 9. FITC (Sigma) was freshly dissolved in dimethyl sulfoxide (DMSO) at 1 mg/mL, and 50 µL of the FITC solution was then slowly added to the ChABC/NEP1-40 solution in 5 µL aliquots while continually stirring. The solution was allowed to react for 8 hours at 4°C and protected from light. Ammonium chloride was added to a final concentration of 50 mM and incubated for 2 hours to stop the reaction. The finished reaction was then dialyzed against phosphate buffered saline, pH 7.4, to remove unconjugated FITC. The molecular weight cutoff for the ChABC dialysis cassette was 10 kD and the cutoff for the NEP1-40 dialysis cassette was 2 kD. Following dialysis, the conjugates were stored at 4°C. The ratio of fluorescein to ChABC/NEP1-40 was determined by measuring the absorbance at 495 nm and 280 nm. Electrophoresis with polyacrylamide gels was also used to verify the overlap of fluorescent ChABC protein bands with Coomassie Blue stained bands.
Release profiles of FITC conjugated NEP1-40 and ChABC
The release profiles of fluorescently labeled NEP1-40 (F-NEP) loaded in PLGA microspheres and fluorescently labeled ChABC (F-ChABC) loaded in lipid microtubes was measured by embedding the microparticles into fibrin scaffolds. Fibrin scaffolds with total volume of 200 µL were prepared by mixing 10 mg/ml fibrinogen, 2.5 mM CaCl2 (Sigma), 2 NIH units/mL thrombin (Sigma), and 30 mg of FITC-NEP1-40 loaded PLGA microspheres or 5 mg of FITC-ChABC loaded microtubes. Scaffolds were allowed to polymerize for one hour at 37°C in a 48 well plate, then 200 µL of 10 mM Tris buffered saline was placed on top. The saline solution was removed at 1, 3, 5, 7, 10, and 14 days after scaffold formation, and the fluorescence measured using a fluorimeter. Following the last time point, the fibrin scaffolds and PLGA microspheres were degraded using 1 M NaOH overnight at 37°C, then an equal volume of 10 mM Tris buffered saline was added and the pH was adjusted to 7 using HCl to measure the amount of fluorescent molecules remaining in the scaffold.
Active release of NEP1-40
PLGA microspheres loaded with 40 mg/mL NEP1-40 were incorporated into 10 mg/mL fibrin (EMD Millipore) scaffolds and allowed to release NEP1-40 into cell culture media. The media was removed and replaced on days 5, 12, and 20. The removed media, now containing released NEP1-40, was used as the culture media for inhibitory myelin spot assays. Cell culture media was 1:1 DFK5:Neurobasal with B27 supplement. Control experiments were performed with empty PLGA microspheres.
Active release of ChABC
Lipid microtubes loaded with 500 mU/mL ChABC (Sigma) dissolved in 1 M trehalose were encapsulated into 10 mg/mL fibrin scaffolds and allowed to release ChABC into 1 M trehalose solution. The trehalose solution was tested for enzymatic activity by measuring the formation of unsaturated disaccharides by degrading the chondroitin sulfate over time (Sigma enzymatic assay – EC 4.2.2.4 per manufacturer instructions). Control experiments were performed with lipid microtubes loaded with 1 M trehalose and ChABC directly added to the fibrin scaffold formation solution.
Spinal Cord Injury and Scaffold Implantation
All experimental procedures on animals complied with the Guide for the Care and Use of Laboratory Animals and were performed under the supervision of the Division of Comparative Medicine at Washington University. Long-Evans female rats (250–300 g) were anesthetized using 2–5% isoflurane gas and 5 mg/kg xylazine. A single incision was created through the skin to expose the back muscle. Parallel incisions were created through the back muscle on each side of the vertebral processes from T5-T11. A dorsal laminectomy was performed at T8 using fine tip rongeurs to expose the spinal cord. The dura mater was removed from the exposed cord at T8. Vitrectomy scissors mounted to a micromanipulator were lowered 1.5 mm into the spinal cord. A lateral incision was created across the spinal cord to from a dorsal hemisection.
Following the initial incision, fibrin scaffolds with total volume of 200 µL were prepared by mixing 10 mg/ml fibrinogen, 2.5 mM CaCl2 (Sigma), 2 NIH units/ml thrombin (Sigma), 30 mg of NEP1-40-loaded PLGA microspheres (1.5 mg per transplant) and 10 mg of ChABC-loaded microtubes (0.5 mg per transplant). For fibrin control group, empty drug PLGA microspheres and lipid microtubes were incorporated into the fibrin scaffold as controls. 4 animals were used per group and 12 rats total survived the injury and transplantation out of 15. A 10 µL fibrin scaffold was allowed to polymerize for 5 minutes prior to implantation into the injury site. Using fine tooth forceps, the fibrin scaffold was gently forced into the dorsal hemisection incision. Following implantation, a second 10 µL fibrin scaffold was allowed to polymerize in situ to hold the delivery system/scaffold within the injury site, covered with artificial dura, then the overlying back muscles closed using degradable sutures, and the skin stapled closed.
Immediately following surgery, animals were given cefazolin (25 mg/kg) and buprenorphine (0.04 mg/kg). Cefazolin was continued twice daily for 5 days with buprenorphine (0.04 mg/kg) given twice daily for 3 days and (0.01 mg/kg) for the next 3 days. Bladders were manually expressed twice a day until normal bladder function was resumed. Two weeks following treatment, animals were euthanized by an overdose of Euthasol. Spinal cords were harvested following transcardial perfusion with 4% paraformaldehyde and post-fixed in 4% paraformaldehyde overnight. Spinal cords were then cryoprotected in 30% sucrose in PBS. Prior to embedding, 3 cm sections of the spinal cords with the injury site in the center were cut and frozen on dry ice. Cords were embedded in Tissue-Tek OCT compound and cut into 20 µm sagittal sections with a cryostat (Leica CM1950).
Immunofluorescence
To determine expression of markers at the site of injury, immunohistochemistry was performed on 6 spinal cord sections per animal. OCT was washed from spinal cord sections with PBS. Sections were permeabilized with 0.1% Triton X-100 for 15 minutes and blocked with 10% bovine serum albumin and 2% normal goat serum (NGS). The following primary antibodies were applied overnight at 4°C in PBS with 2% NGS: β-tubulin III (β-tubIII, Covance, Dedham, MA, 1:400), glial fibrillary acidic protein (GFAP, Immunostar, Hudson, WI, 1:100), chondroitin sulfate (CS56, Sigma, 1:250), and CD68 (ED1, AbD Serotec, 1:200). Primary antibody staining was followed by 3 washes with PBS. Appropriate Alexa Fluor secondary antibodies (Life Technologies) in PBS with 2% NGS were applied for 1 hour at room temperature followed by an additional 3 washes in PBS. Sections were mounted using ProLong Gold anti-fade reagent with DAPI (Life Technologies).
Image Analysis of Immunohistochemistry
To quantify the staining of β-tubIII, GFAP, and CS56 markers at the injury site, a series of images spanning the lesion site were captured using a MICROfire camera attached to an Olympus IX70 inverted microscope using a 4x objective. As previously described, the images were merged using Adobe Photoshop, and the injury site traced and expanded 500 µm away from the injury [41]. The average pixel intensity within 500 µm of the traced injury was measured using a custom Matlab (Mathworks, Natick, MA) script that determined the intensity of each individual pixel and then averaged all the intensities of pixels within 500 µm. Previously published methods were used to quantify the staining of the ED1 marker; the images were merged using Adobe Photoshop and the area of inflammation was determined as the fraction of ED1+ tissue in 2 cm sections [42].
Statistical Analysis
Spot assay data was analyzed by ANOVA followed by Scheffe’s post-hoc test with a significance criterion of 95%. Statistical significance for in vivo studies was determined by the planned comparisons post-hoc test with a significance criterion of 95%.
Results
Combination ChABC and NEP1-40 Improve Neurite Extension on Inhibitory Spots
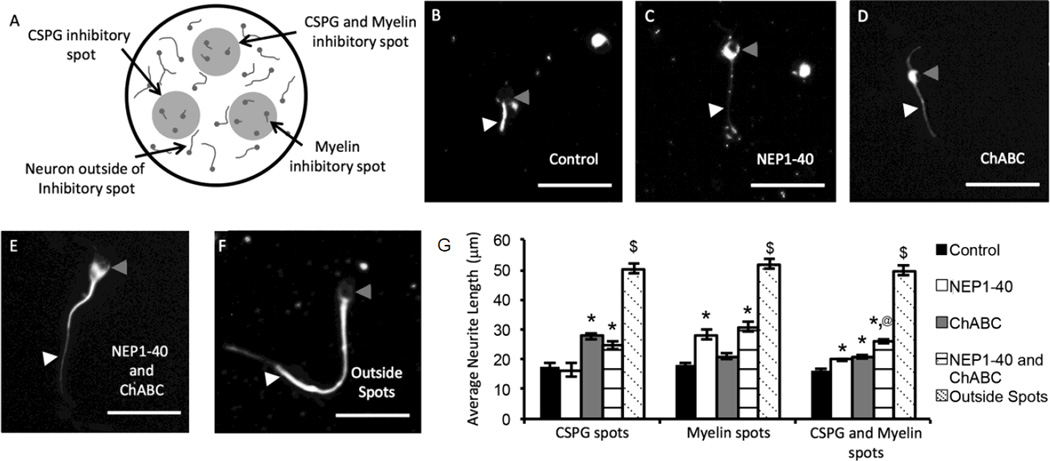
Spot assays were used to study the effect of culturing primary neurons on inhibitory substrates of myelin, CSPGs, or myelin and CSPGs combined. When E8 chick DRG explant cultures were plated onto inhibitory spots, neurite outgrowth was significantly inhibited compared to neurons plated outside of the inhibitory spots (Figure 1). To determine the percent of inhibition caused by plating on the inhibitory spots, the average neurite length of cells on the inhibitory substrates was divided by the average neurite length of cells plated outside the inhibitory substrates on poly-L-ornithine. Neurons plated outside of the inhibitory spots had an average neurite length over 50 µm compared to neurites inside inhibitory spots, which had average lengths of less than 20 µm. The ability of NEP1-40 to block myelin inhibition was studied by adding 500 nM of NEP1-40 to the cell culture media (Figure 1G). The presence of NEP1-40 attenuates neurite outgrowth inhibition on myelin spots while the addition of ChABC did not significantly affect extension. The presence of NEP1-40 led to an average neurite length of 28.3±1.9 µm, which corresponds to 54% outgrowth compared to the positive control group plated outside the inhibitory spot. The group with no NEP1-40 added to the cell culture media averaged 18.2±0.9 µm or 35% outgrowth compared to the positive control group. The combined addition of NEP1-40 and ChABC had similar effects compared to adding NEP1-40 alone for culture on myelin spots. Therefore, the addition of ChABC to the cell culture media did not affect neurite extension on myelin inhibitory spots but NEP1-40 significantly increased neurite extension.
Figure 1. Dissociated embryonic chick DRG cultured on myelin and CSPG inhibitory spots and treated with ChABC and NEP1-40.
(A) Schematic representation of dissociated dorsal root ganglia neurons cultured on inhibitory spots. Three 1 µL spots of CSPGs, myelin, or CSPGs and myelin were placed onto poly-L-ornithine coated 24 well plates. ChABC, NEP1-40, or ChABC and NEP1-40 were directly added to the cell media. Neurons that plate outside of the inhibitory spots were used to determine the average neurite extension of uninhibited neurons. (B–E) Representative images of dissociated DRG neurons cultured on combined CSPG and myelin inhibitory spots. White arrowheads indicate neurites and gray arrowheads indicate neuronal cell bodies. (B) No ChABC or NEP1-40 treatment (C) NEP1-40 (D) ChABC (E) ChABC and NEP1-40 and (F) Uninhibited neurons plated outside inhibitory spots. (G) The average neurite length was measured for all neurites. Error bars are standard error of the mean. * denotes significance from control (p<0.05). @ denotes significance versus NEP1-40 or ChABC treatment (p<0.05). $ denotes significance from all other groups (p<0.05). Scale bars are 50 µm.
Similar types of results were seen when culturing dissociated DRGs on CSPG spots. In this case, media containing 100 mU/mL ChABC significantly increased neurite extension compared to media containing no anti-inhibitory factors or containing NEP1-40 alone (Figure 1G). The presence of ChABC led to an average neurite length of 27.7±1.2 µm compared to the group without ChABC added which averaged of 17.5±1.1 µm. The presence of ChABC increased the percent outgrowth compared to the positive control to 53%, up from 34% outgrowth in the group without ChABC.
Spots containing both myelin and CSPGs were capable of providing robust inhibition to neurite outgrowth with an average neurite length of 16.2±0.5 µm when no anti-inhibitory drugs were added to the media (Figure 1G). The addition of NEP1-40 or ChABC caused a significant increase in average neurite length (19.9±0.4 µm and 20.9±0.6 µm, respectively). Interestingly, the combination of NEP1-40 and ChABC in the cell culture media led to a statistically significant increase in neurite length, of 26.1±0.8 µm, compared to either of the anti-inhibitory factors alone. The addition of both anti-inhibitory factors caused a 50% outgrowth compared to the positive control group, while a 38% outgrowth was measured for NEP1-40 addition and 40% outgrowth for ChABC addition. Therefore, the combined effects of NEP1-40 and ChABC treatment may lead to improved axonal extension after SCI.
Incorporation of NEP1-40 into PLGA microspheres enables the sustained release of active NEP1-40
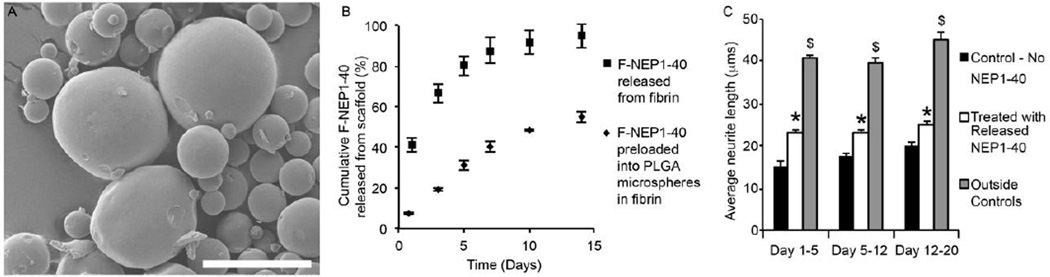
Delivery of NEP1-40 was achieved by encapsulation into PLGA microspheres. PLGA microspheres provide tunable release rates by controlling the ratio of lactic acid to glycolic acid, the intrinsic viscosity of the polymer, or by varying the formation conditions [43–45]. The microspheres were loaded with FITC conjugated NEP1-40 (F-NEP) and embedded into fibrin scaffolds to directly measure the release rate (Figure 2B). PLGA microspheres had an initial loading efficiency of 42.1±9.8% and an average diameter of 9.1±4.2 µm. When F-NEP1-40 was loaded directly into fibrin scaffolds over 80% of the fluorescent molecules were released in 5 days with a large burst release of 41% at day 1. In contrast, loading of F-NEP1-40 into PLGA microspheres lowered the initial burst release to 7%, and provided sustained release with 31% of loaded F-NEP1-40 released at day 5. The remaining F-NEP1-40 was recovered by degrading the fibrin scaffolds and PLGA microspheres using 0.5 M NaOH, adjusting the pH to 7, and then measuring the fluorescence. This gave us the total amount of NEP1-40 remaining within the fibrin scaffolds and PLGA microspheres. These amounts were within 10% of the initial loading dose. The release profile for loading into PLGA microspheres appears to have a first-order release up to 14 days.
Figure 2. Incorporation of NEP1-40 into PLGA microspheres and fibrin scaffolds.
(A) Scanning electron micrograph of NEP1-40-loaded PLGA microspheres. Average microsphere diameter measured 9.1±4.2 µm. Scale bar is 30 µm. (B) Release profile of F-NEP from PLGA microspheres encapsulated in fibrin scaffolds or from F-NEP in fibrin scaffolds alone. Loading efficiency of microspheres was 42.1±9.8%. (C) Dissociated DRGs plated onto inhibitory myelin spots suggest that media containing released NEP1-40 shows significantly less inhibition of neurite extension compared to control (media without NEP1-40) on the same day. Thus, the NEP1-40 loaded into PLGA microspheres within fibrin scaffolds maintains bioactivity after being released into the cell culture media. Error bars denote standard error of the mean. * denotes significance from control group on the same day (p < 0.05). $ denotes significance from all other groups (p < 0.05).
The ability to release NEP1-40 from PLGA microspheres is only useful if the peptide remains active after formation of the microspheres, incorporation into fibrin scaffolds, and upon release from the scaffolds. The activity of the released NEP1-40 was analyzed by loading NEP1-40 into PLGA microspheres, embedding the microspheres into fibrin scaffolds, and allowing the microspheres to release the loaded NEP1-40 into cell culture media. At days 5, 12, and 20 the cell culture media was collected and used for inhibitory spot assays with myelin spots. Dissociated DRGs that were plated onto the myelin spots showed a significant increase in average neurite length with media containing released NEP1-40 compared to control media which did not contain released NEP1-40 at every time point studied (Figure 2C). Treated media with released NEP1-40 from day 1 through day 5 increased the percent neurite outgrowth compared to outside positive controls to 56%, while media from day 5 through day 12 showed 57% outgrowth compared to positive controls, and media from day 12 through day 20 showed 60% neurite outgrowth. Thus, PLGA microspheres are capable of providing sustained release of NEP1-40, and the released peptide maintains its bioactivity for up to 20 days in vitro.
Incorporation of thermostabilized ChABC into lipid microtubes allows sustained active release of ChABC
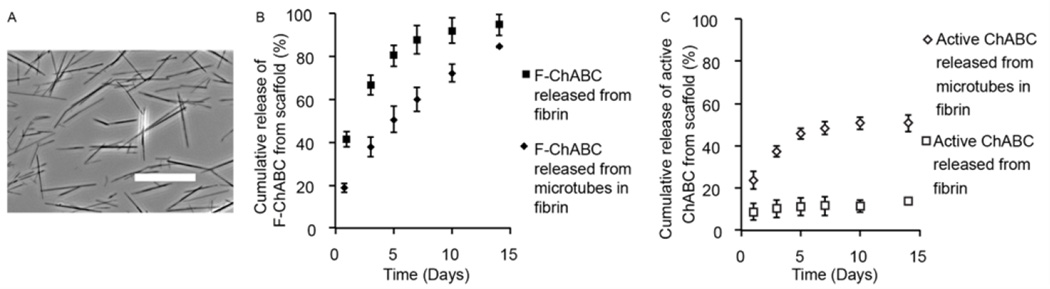
Delivery of thermostabilized ChABC enzyme was achieved by stabilization with trehalose and loading into lipid microtubes [46]. To study the release from lipid microtubes, either F-ChABC or unmodified ChABC was dissolved in 1 M trehalose at a concentration of 1 mg/mL or 500 mU/mL respectively, then loaded into lyophilized microtubes. The loaded lipid microtubes were encapsulated into 10 mg/mL fibrin scaffolds and allowed to release into a 1 M trehalose solution over 14 days. Release of F-ChABC was analyzed using a fluorimeter and the activity of ChABC was measured using an enzymatic activity assay to determine the amount of active units released from the fibrin scaffolds. The lipid microtubes allowed for sustained release of F-ChABC over 14 days compared to F-ChABC that was directly loaded into fibrin scaffolds, which released of over 85% of the F-ChABC in 7 days (Figure 3B).
Figure 3. Incorporation of ChABC into lipid microtubes and fibrin scaffolds.
(A) Phase contrast image of lipid microtubes. Average length measured 21±14 µm. Scale bar is 25 µm. (B) Fluorescent measurements of F-ChABC released from lipid microtubes within fibrin scaffolds or from F-ChABC in fibrin scaffolds alone (without microtubes). Loading efficiency of ChABC in microtubes was 89.5±4.7%. Loading F-ChABC into lipid microtubes allows for sustained release compared to adding F-ChABC directly into fibrin scaffolds. (C) Open data points are enzymatic activity measurements of ChABC and closed data points are fluorescence measurements of F-ChABC. Comparison between the enzymatic assay and fluorescence measurements suggests that the amount of released and active ChABC is similar to the release rate of F-ChABC loaded lipid microtubes for one week. In contrast, the amount of enzymatically active ChABC released when directly loaded into fibrin scaffolds shows a much lower release rate, suggesting that ChABC loses activity during the formation of the fibrin scaffolds when not protected within the lipid microtubes. ChABC activity was measured for up to 10 days after scaffold formation.
The ability to release active ChABC was measured from lipid microtubes encapsulated into fibrin scaffolds. ChABC loaded directly into fibrin scaffolds without lipid microtubes showed a total release of over 40% of F-ChABC on day 1 (Figure 3B), but only 8.7±3.9% of the enzyme remained active after release on day 1 (Figure 3C) and limited enzymatic activity was measured thereafter. In contrast, loading thermostabilized ChABC into lipid microtubes prior to encapsulation in fibrin scaffolds led to an initial release of 18.8±2.2% of F-ChABC on day 1 (Figure 3B) and 23.6±4.3% of the total loaded active enzyme was released on day 1 (Figure 3C). Enzymatic activity was detected up to day 10 with limited activity between 10 and 14 days. Overall, 50.7±4.0% of active ChABC was released from fibrin scaffolds with microtubes by day 14. Thus thermostabilized bioactive ChABC can be loaded and released from lipid microtubes for over one week in vitro.
Loaded PLGA microspheres and lipid microtubes limit formation of the glial scar and promote axonal extension in an acute dorsal hemisection injury
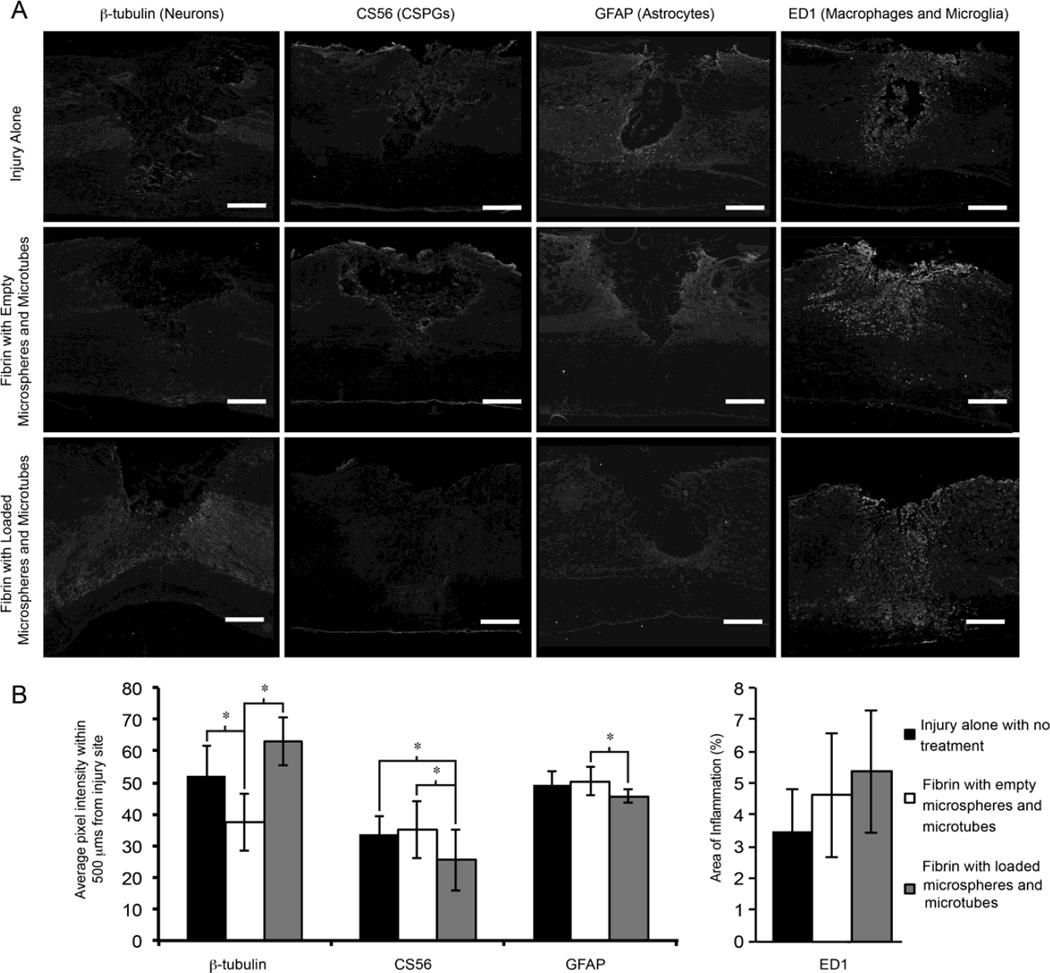
PLGA microspheres loaded with NEP1-40 and lipid microtubes loaded with thermostabilized ChABC were incorporated into fibrin scaffolds and implanted into a thoracic dorsal spinal cord hemisection immediately after injury. Two weeks after treatment, the spinal cords were harvested, sectioned, and stained for several markers to analyze the effect of the drug delivery systems at the site of injury (Figure 4A). The injury group had the thoracic hemisection with no treatment following the injury (n=4). The fibrin group underwent the thoracic hemisection and was acutely treated with a fibrin scaffold containing empty PLGA microspheres and lipid microtubes (n=4). The treatment group had a thoracic hemisection that was acutely treated with a fibrin scaffold containing NEP1-40-loaded PLGA microspheres and ChABC-loaded lipid microtubes (n=4). PLGA microspheres remained within the transplant site and appeared as spherical voids within the spinal cord sections, no evidence of lipid microtubes were seen within the sections. The loaded drug delivery systems promoted a significant decrease in the staining against CS56 (intact CSPGs, as assessed by average pixel intensity values) within 500 µm of the injury when compared to both injury alone and fibrin with empty microspheres and microtubes (Figure 4B). To measure neural fibers, antibodies against β-tubIII, a microtubule found in neurons, showed a significant increase in the average pixel intensity within 500 µm compared to the fibrin with empty delivery systems group although no difference was measured between the fibrin alone group and the fibrin with loaded microspheres and microtubes group.
Figure 4. Scaffold transplantation following a thoracic dorsal hemisection model of spinal cord injury.
(A) Immunohistochemistry of injured spinal cord sections two weeks after transplantation, 20 µm thick sections were stained with antibodies against CS56 (marker for CSPGs), ED1 (macrophages and microglia), GFAP (astrocytes and glial scar), or β-tubIII (neurons). (B) The average pixel intensity within 500 µm of the injury site was determined. Fibrin scaffolds with loaded delivery systems had increased β-tubIII intensities compared to fibrin with unloaded delivery systems. Deposition of CSPGs was decreased in the treated groups compared to the injury alone and fibrin with unloaded delivery systems. GFAP intensity was decreased in the fibrin with loaded delivery system groups. The percent area of inflammation was measured as the fraction of pixels that positively stained for ED1 in a 2 cm section. No significant changes were seen in the immune response between any groups. Error bars are standard deviation. * denotes significant difference between groups (p < 0.05). Scale bar is 250 µn.
Furthermore, the development of the glial scar was quantified by staining for GFAP, an intermediate filament found within astrocytes and upregulated in reactive astrocytes. Staining for GFAP showed that the fibrin with loaded delivery systems had significantly decreased GFAP staining compared to the fibrin with empty delivery systems group. The proof of concept animal study suggests that the sustained dual delivery of ChABC and NEP1-40 in an acute dorsal hemisection model may limit the presence of CSPGs, increase neuronal fiber extension, and affect the development of the glial scar after injury.
Discussion
After SCI, upregulation of extrinsic factors including CSPGs and MAIs affect functional recovery by limiting axonal outgrowth and preventing reformation of functional synapses [5]. Here we demonstrate that combination treatment with ChABC and NEP1-40 enhances neurite extension compared to either treatment alone when dissociated chick DRGs are cultured on inhibitory spot assays containing CSPGs and MAIs. Furthermore, active ChABC and NEP1-40 can be loaded and released from dual drug delivery systems incorporated into fibrin scaffolds.
CSPGs induce outgrowth inhibition through interactions of glycosaminoglycan chains with several receptor proteins found on neurons including PTPσ, LAR, NgR1, and NgR3 by initiating the Rho/ROCK signaling cascade [47–49]. CSPGs may also block growth promoting mechanisms by interfering with integrin signaling to further limit axonal growth potential [50]. In addition, CSPGs are known to contribute to formation of perineuronal nets that limit axonal outgrowth and control plasticity in the central nervous system. Deposition of CSPGs after injury is significantly upregulated by reactive astrocytes that form the glial scar. The time course of CSPG deposition is varied based on the type of CSPG, with expression levels typically peaking between 1 and 2 weeks and continued expression up to 4 weeks or more after injury [15]. Removal of inhibition via enzymatic cleavage of the glycosaminoglycan chains from the core protein with bacterially-expressed ChABC has consistently shown robust increases in neuronal outgrowth, although the core protein has some remaining inhibitory effect [16]. Our data further verifies that degradation of CSPGs using ChABC leads to increased neurite extension. Cells cultured on myelin spots alone did not have increased neurite extension with the addition of ChABC, while dual spots with myelin and CSPGs did have increased extension, but the outgrowth was not as robust as CSPGs alone. This distinction suggests that ChABC is capable of removing inhibition due to CSPGs but does not remove inhibition from MAIs.
The myelin-associated membrane proteins, such as Nogo-66, MAG, and OMgp, bind the Nogo receptor on neurons, which triggers the Rho/ROCK cascade through Lingo-1 and p75 transmembrane proteins [51–53]. Blocking the upstream activation of the ROCK signaling pathway can be achieved using Nogo receptor fragments, targeted Nogo receptor antibodies, or inactivating downstream effectors of the pathway [54, 55]. Similarly, limiting the activation of RhoA, RhoB, and RhoC proteins increased functional recovery in animal models [56–58]. Myelin inhibitory spots significantly decreased neurite extension of dissociated chick DRGs, and treatment with NEP1-40 allowed the neurites to overcome the inhibition and extend processes.
NEP1-40 is a small peptide sequence consisting of 40 amino acids, thus it diffuses away from the local injury site and can be taken up and degraded by infiltrating macrophages and microglia. Therefore, it is necessary to provide sustained delivery of the peptide to the injured spinal cord to continually block myelin-based inhibition. Another popular method to limit activation of the Nogo receptors is the use of anti-Nogo-A antibodies, which have been shown to improve functional recovery following SCI. Most treatments using anti-Nogo-A antibodies or NEP1-40 show improvements when treatment began immediately after injury and continued for 2 to 4 weeks post-injury [6, 26, 29, 30]. For example, intrathecal pump delivery of anti-Nogo-A antibodies for 2 weeks used in combination with ChABC treatment showed improved recovery over either treatment alone [6]. Therefore, sustained delivery of NEP1-40 over 2–4 weeks may be a target timeframe for SCI treatment. The 50:50 monomer ratio and low intrinsic viscosities were both chosen because of previous work showing these parameters allow for relatively rapid degradation of the microspheres and release of the loaded molecule, although smaller diameter microspheres may further increase the degradation rates [59–62]. Future research may benefit from adjusting the release profiles to explore the effect of the timing and duration of NEP1-40 treatment on regeneration in vivo.
Therapies combining ChABC and anti-myelin inhibitory molecules have shown varied results. Treatment of a thoracic contusion injury with ChABC, anti-Nogo-A antibodies, and treadmill training resulted in significant functional improvements over any single treatment, suggesting a synergistic effect with combination therapy [6]. However, another study reported combined treatment with ChABC and NEP1-40 did not increase axon growth in organotypic co-cultures taken from neonatal rats compared to either treatment alone, which suggests that the combined treatment may be limited because CSPGs and MAIs affect some of the same intracellular pathways [63]. Furthermore, several studies have shown that using ChABC or other anti-inhibitory molecules in severe SCI models lead to increased functional recovery, but moderate or mild injury models have limited or no significant recovery compared to untreated controls [64, 65]. Therefore, it may be necessary to use adult animals and severe injury models in order to demonstrate that combination treatment with ChABC and NEP1-40 improves recovery over either treatment alone. However, treatment with ChABC and NEP1-40 did not promote neurite extension to levels seen outside of the inhibitory spots. It may be beneficial to try various combinations of treatments that target both the intrinsic growth potential and extrinsic inhibitory environment to further improve extension and regeneration [66–69].
Delivery of active anti-inhibitory molecules to the injured spinal cord is typically achieved using invasive intrathecal pumps or cannulas and microinjections, which can cause increased scarring and compression of the spinal cord [70]. The ability to deliver active drugs to the injury using an implanted biomaterial scaffold may provide an improved alternative drug delivery method. PLGA microspheres were chosen to release the NEP1-40 peptide for their well-established formation methods, which allow for tunable control over the release rate and a longer release period [36, 44, 71]. The release rate is dependent upon the rate of hydrolytic degradation of the PLGA microspheres, which can be controlled by altering the ratio of lactic acid to glycolic acid, the intrinsic viscosity of the polymer (related to the molecular weight), or by changing formation conditions. PLGA with low intrinsic viscosity was chosen because decreasing the intrinsic viscosity increases the degradation and release rate of the microspheres. The PLGA microspheres formed had lower loading efficiencies but comparable release profiles to previously published research on delivery of alkaline phosphatase [36]. The ChABC enzyme was also tested with PLGA microspheres, but no enzymatic activity was measured after loading and release due to the highly labile and thermally unstable nature of the enzyme especially in organic solvents. The quantity of PLGA microspheres and lipid microtubes added to the scaffolds may be augmented to increase the amount of anti-inhibitory molecules delivered to the spinal cord. However, an upper limit will be reached when the amount of drug delivery systems begins to negatively affect the gelation of the fibrin scaffolds and potentially inhibit the ability of fibrin to form a stable hydrogel.
In order to maintain ChABC activity after release, the enzyme must be thermally stabilized and loaded into a delivery system under mild conditions. Stabilization of protein structure, using naturally occurring trehalose, is used to maintain activity [72–74]. In the absence of trehalose, bioactivity of ChABC drops dramatically in vitro [46]. Stabilized enzymes can be loaded into lyophilized lipid microtubes, embedded into biomaterial scaffolds, and upon release the enzyme is inactivated under normal deactivation kinetics [40, 46, 75]. For instance, the activity of TGF-β was shown to persist for up to 10 hours when released from microtubes, while the half-life of TGF-β in vitro is on the order of minutes [76]. Furthermore, the loading of ChABC led to maintained activity over 2 weeks while unstabilized ChABC lost activity after a few days [46]. ChABC added directly into fibrin scaffolds without prior loading into microtubes resulted in a loss of over 85% of ChABC activity in the first day, likely due to the rapid diffusion of trehalose away from ChABC causing normal deactivation kinetics. Interestingly, ChABC loaded directly into highly concentrated fibrin scaffolds was shown to maintain activity in vitro and in vivo, although, the fibrin used was 10 times more concentrated than the fibrin reported here [77].
As a proof of concept study, the drug delivery systems were incorporated into fibrin scaffolds and implanted into rats after SCI. A decrease in axonal extension was measured when unloaded microspheres and microtubes were transplanted within fibrin scaffolds by staining with antibodies against β-tubIII. This decrease may be caused by PLGA degradation byproducts which are acidic and lower the pH of the local environment [78]. Acidic environments have been shown to affect the immunoreactivity of astrocytes for GFAP, which corresponds to astrocytes in a more reactive state [79]. Therefore, the decreased pH caused by degradation of PLGA microspheres may drive astrocytes into a reactive state, as evidenced by an upregulation of GFAP. Astrocytes in a reactive state are detrimental to recovery because reactive astrocytes increase deposition of CSPGs and limit axonal extension; both an increase in GFAP and CSPG was measured with unloaded microspheres and microtubes. Therefore, the incorporation of PLGA microspheres into fibrin scaffolds may lead to increased CSPG deposition due to an enhanced conversion of astrocytes into reactive astrocytes and future research using the drug delivery systems should account for the potential negative effects on the local environment.
In contrast, the loaded drug delivery systems appeared to increase axonal extension and decrease the presence of CSPGs. Therefore, the sustained release of ChABC and NEP1-40 may increase axonal growth potential at the injury site by removing extrinsic barriers. The removal of extrinsic barriers including CSPGs and MAIs provide a more permissive environment leading to improved axonal extension. Sustained release of NEP1-40 and ChABC also had no effect on GFAP staining compared to the injury alone group, suggesting limited effect on astrocytes two weeks after injury. The inflammatory response after SCI is an important factor and acidic degradation byproducts PLGA may increase the inflammatory response [80]. No significant changes were measured in macrophage/microglia (ED-1) staining when PLGA microspheres and lipid microtubes were incorporated into fibrin scaffolds, although incorporation of microspheres and microtubes trended toward increased inflammation. This suggests that although the byproducts from the PLGA microspheres may increase macrophage infiltration and microglia activation, the increase was not significant when the microparticles were loaded with anti-inhibitory molecules and did not appear to limit neuronal growth at the site of injury. Future work using the microspheres and microtubes for drug delivery should monitor the local inflammatory response.
Conclusion
In conclusion, research has shown the promising effects of treating SCI with ChABC and anti-myelin associated inhibitors such as NEP1-40, but SCI is complex with a multitude of inhibitory cues that must be overcome to improve functional recovery. Therefore, it may be necessary to develop a treatment that is capable of providing mechanisms that eliminate or mitigate multiple obstacles. We have developed a dual drug delivery system capable of providing sustained release of ChABC from lipid microtubes and NEP1-40 from PLGA microspheres. The drug delivery system is capable of being embedded into fibrin scaffolds, which our lab has previously used to deliver growth factors and neural progenitor cells to the injury site, and removes the need for invasive intrathecal pumps or catheters. Future work will use the materials developed in combination with the delivery of specific neural progenitors and growth factors to synergistically aid in recovery after SCI.
Acknowledgments
Scanning electron images were taken at the Nano Research Facility, a member of the NSF National Nanotechnology Infrastructure Network. Animal surgical technique and care training was provided by OSU-SCITP, previously supported by the NIH/NINDS. Technical assistance was provided by Sara Oswald and Laura Marquardt. Funding supported by NIH grant R01 NS051454 and NSF DGE-1143954.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spinal cord injury facts and figures at a glance. Journal of Spinal Cord Medicine. 2012;35(6):480–481. doi: 10.1179/1079026812Z.000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiological Reviews. 1996;76(2):319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 3.Kadoya K, et al. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64(2):165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. 2012;122(11):3824–3834. doi: 10.1172/JCI64124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma K, Selzer ME, Li S. Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol. 2012;237(2):370–378. doi: 10.1016/j.expneurol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao RR, et al. Combination treatment with anti-Nogo-A and chondroitinase ABC is more effective than single treatments at enhancing functional recovery after spinal cord injury. Eur J Neurosci. 2013;38(6):2946–2961. doi: 10.1111/ejn.12276. [DOI] [PubMed] [Google Scholar]
- 7.van den Brand R, et al. Restoring Voluntary Control of Locomotion after Paralyzing Spinal Cord Injury. Science. 2012;336(6085):1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 8.Fortun J, Hill CE, Bunge MB. Combinatorial strategies with Schwann cell transplantation to improve repair of the injured spinal cord. Neuroscience Letters. 2009;456(3):124–132. doi: 10.1016/j.neulet.2008.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez J, Torres-Espin A, Navarro X. Adult stem cell transplants for spinal cord injury repair: current state in preclinical research. Curr Stem Cell Res Ther. 2011;6(3):273–287. doi: 10.2174/157488811796575323. [DOI] [PubMed] [Google Scholar]
- 10.Sahni V, Kessler JA. Stem cell therapies for spinal cord injury. Nat Rev Neurol. 2010;6(7):363–372. doi: 10.1038/nrneurol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tetzlaff W, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28(8):1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson PJ, et al. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter. 2010;6:5127–5137. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nature Reviews Neuroscience. 2010;11(12):799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- 14.Iaci JF, et al. Chondroitin sulfate proteoglycans in spinal cord contusion injury and the effects of chondroitinase treatment. Journal of Neurotrauma. 2007;24(11):1743–1759. doi: 10.1089/neu.2007.0366. [DOI] [PubMed] [Google Scholar]
- 15.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan brevican, phosphacan and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182(2):399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 16.Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416(6881):636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 17.Chau CH, et al. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 2004;18(1):194–196. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- 18.Lee YS, et al. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J Neurosci. 2013;33(26):10591–10606. doi: 10.1523/JNEUROSCI.1116-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondello SE, et al. Impact of treatment duration and lesion size on effectiveness of chondroitinase treatment post-SCI. Exp Neurol. 2015;267:64–77. doi: 10.1016/j.expneurol.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartus K, et al. Large-Scale Chondroitin Sulfate Proteoglycan Digestion with Chondroitinase Gene Therapy Leads to Reduced Pathology and Modulates Macrophage Phenotype following Spinal Cord Contusion Injury. Journal of Neuroscience. 2014;34(14):4822–4836. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YC, et al. Controlled release of chondroitinase ABC in chitosan-based scaffolds and PDLLA microspheres. Carbohydrate Polymers. 2011;84(2):788–793. [Google Scholar]
- 22.Liu T, et al. Sustained release of neurotrophin-3 and chondroitinase ABC from electrospun collagen nanofiber scaffold for spinal cord injury repair. Journal of Biomedical Materials Research Part A. 2012;100A(1):236–242. doi: 10.1002/jbm.a.33271. [DOI] [PubMed] [Google Scholar]
- 23.Pakulska MM, Vulic K, Shoichet MS. Affinity-based release of chondroitinase ABC from a modified methylcellulose hydrogel. Journal of Controlled Release. 2013;171(1):11–16. doi: 10.1016/j.jconrel.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Rossi F, et al. Sustained Delivery of Chondroitinase ABC from Hydrogel System. J Funct Biomater. 2012;3(1):199–208. doi: 10.3390/jfb3010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanno H, et al. Combination of Engineered Schwann Cell Grafts to Secrete Neurotrophin and Chondroitinase Promotes Axonal Regeneration and Locomotion after Spinal Cord Injury. Journal of Neuroscience. 2014;34(5):1838–1855. doi: 10.1523/JNEUROSCI.2661-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GrandPre T, Li SX, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417(6888):547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 27.GrandPre T, et al. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403(6768):439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 28.Huebner EA, et al. A Multi-domain Fragment of Nogo-A Protein Is a Potent Inhibitor of Cortical Axon Regeneration via Nogo Receptor 1. Journal of Biological Chemistry. 2011;286(20):18026–18036. doi: 10.1074/jbc.M110.208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebscher T, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Annals of Neurology. 2005;58(5):706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 30.Maier IC, et al. Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain. 2009;132(Pt 6):1426–1440. doi: 10.1093/brain/awp085. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, et al. Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat. Neurorehabilitation and Neural Repair. 2008;22(3):262–278. doi: 10.1177/1545968307308550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li SX, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. Journal of Neuroscience. 2003;23(10):4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merkler D, et al. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-a. Journal of Neuroscience. 2001;21(10):3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417(6888):547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 35.Norton WT, Poduslo SE. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973;21(4):749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Tator CH, Shoichet MS. Design of protein-releasing chitosan channels. Biotechnol Prog. 2008;24(4):932–937. doi: 10.1021/bp070352a. [DOI] [PubMed] [Google Scholar]
- 37.Kang F, Singh J. Effect of additives on the release of a model protein from PLGA microspheres. AAPS PharmSciTech. 2001;2(4):30. doi: 10.1007/BF02830570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutierro I, et al. Influence of dose and immunization route on the serum Ig G antibody response to BSA loaded PLGA microspheres. Vaccine. 2002;20(17–18):2181–2190. doi: 10.1016/s0264-410x(02)00146-9. [DOI] [PubMed] [Google Scholar]
- 39.Lam XM, et al. Sustained release of recombinant human insulin-like growth factor-I for treatment of diabetes. Journal of Controlled Release. 2000;67(2–3):281–292. doi: 10.1016/s0168-3659(00)00224-8. [DOI] [PubMed] [Google Scholar]
- 40.Meilander NJ, Yu X. Lipid-based microtubular drug delivery vehicles. Journal of Controlled Release. 2001;71:141–152. doi: 10.1016/s0168-3659(01)00214-0. [DOI] [PubMed] [Google Scholar]
- 41.McCreedy DA, et al. Survival, differentiation, and migration of high-purity mouse embryonic stem cell-derived progenitor motor neurons in fibrin scaffolds after sub-acute spinal cord injury. Biomaterials Science. 2014;2(11):1672–1682. doi: 10.1039/c4bm00106k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumann MD, et al. Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury. Biomaterials. 2010;31(30):7631–7639. doi: 10.1016/j.biomaterials.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Takai C, et al. Effect of Inorganic Salt on Formation of Porous PLGA Microspheres. Chemistry Letters. 2011;40(6):638–639. [Google Scholar]
- 44.Yang YY, Chia HH, Chung TS. Effect of preparation temperature on the characteristics and release profiles of PLGA microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. J Control Release. 2000;69(1):81–96. doi: 10.1016/s0168-3659(00)00291-1. [DOI] [PubMed] [Google Scholar]
- 45.Pean JM, et al. NGF release from poly(D,L-lactide-co-glycolide) microspheres. Effect of some formulation parameters on encapsulated NGF stability. Journal of Controlled Release. 1998;56(1–3):175–187. doi: 10.1016/s0168-3659(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 46.Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. PNAS. 2009;107(8):3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickendesher TL, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nature Neuroscience. 2012;15(5):703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher D, et al. Leukocyte Common Antigen-Related Phosphatase Is a Functional Receptor for Chondroitin Sulfate Proteoglycan Axon Growth Inhibitors. Journal of Neuroscience. 2011;31(40):14051–14066. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen YJ, et al. PTP sigma Is a Receptor for Chondroitin Sulfate Proteoglycan an Inhibitor of Neural Regeneration. Science. 2009;326(5952):592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou FQ, et al. Neurotrophins support regenerative axon assembly over CSPGs by an ECM-integrin-independent mechanism. Journal of Cell Science. 2006;119(13):2787–2796. doi: 10.1242/jcs.03016. [DOI] [PubMed] [Google Scholar]
- 51.Liu BP, et al. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297(5584):1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 52.McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends in Neurosciences. 2003;26(4):193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 53.Wang KC, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417(6892):941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 54.Liebscher T, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58(5):706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 55.Forgione N, Fehlings MG. Rho-ROCK Inhibition in the Treatment of Spinal Cord Injury. World Neurosurg. 2013;82:E535–E539. doi: 10.1016/j.wneu.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Dergham P, et al. Rho signaling pathway targeted to promote spinal cord repair. Journal of Neuroscience. 2002;22(15):6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. Journal of Neuroscience. 2003;23(4):1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007;27(15):4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced Drug Delivery Reviews. 2012;64:72–82. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 60.Kamei S, et al. New Method for Analysis of Biodegradable Polyesters by High-Performance Liquid-Chromatography after Alkali Hydrolysis. Biomaterials. 1992;13(13):953–958. doi: 10.1016/0142-9612(92)90120-d. [DOI] [PubMed] [Google Scholar]
- 61.Okada H, Toguchi H. Biodegradable Microspheres in Drug-Delivery. Critical Reviews in Therapeutic Drug Carrier Systems. 1995;12(1):1–99. doi: 10.1615/critrevtherdrugcarriersyst.v12.i1.10. [DOI] [PubMed] [Google Scholar]
- 62.Beck LR, Cowsar DR, Lewis DH. Systemic and local delivery of contraceptive steroids using biodegradable microspheres. In: Hafez ESE, Van Os WAA, editors. Biodegradables and Delivery of Systems for Contraception. Vol. 1. MTP Press Limited; 1980. [Google Scholar]
- 63.Nakamae T, et al. The effects of combining chondroitinase ABC and NEP1-40 on the corticospinal axon growth in organotypic co-cultures. Neuroscience Letters. 2010;476(1):14–17. doi: 10.1016/j.neulet.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 64.Caggiano AO, et al. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. Journal of Neurotrauma. 2005;22(2):226–239. doi: 10.1089/neu.2005.22.226. [DOI] [PubMed] [Google Scholar]
- 65.Mitsui T, et al. Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp Neurol. 2005;194(2):410–431. doi: 10.1016/j.expneurol.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 66.Blesch A, et al. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: Superiority to camp-mediated effects. Exp Neurol. 2012;235(1):162–173. doi: 10.1016/j.expneurol.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gopalakrishnan SM, et al. Role of rho kinase pathway in chondroitin sulfate proteoglycan-mediated inhibition of neurite outgrowth in PC12 cells. J Neurosci Res. 2008;86(10):2214–2226. doi: 10.1002/jnr.21671. [DOI] [PubMed] [Google Scholar]
- 68.Ma TC, et al. cAMP-responsive Element-binding Protein (CREB) and cAMP Co-regulate Activator Protein 1 (AP1)-dependent Regeneration-associated Gene Expression and Neurite Growth. Journal of Biological Chemistry. 2014;289(47) doi: 10.1074/jbc.M114.582460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walker CL, et al. Systemic Bisperoxovanadium Activates Akt/mTOR Reduces Autophagy and Enhances Recovery following Cervical Spinal Cord Injury. Plos One. 2012;7(1):315–326. doi: 10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones LL, Tuszynski MH. Chronic intrathecal infusions after spinal cord injury cause scarring and compression. Microscopy Research and Technique. 2001;54(5):317–324. doi: 10.1002/jemt.1144. [DOI] [PubMed] [Google Scholar]
- 71.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 72.Crowe JH, et al. Interactions of sugars with membranes. Biochim Biophys Acta. 1988;947(2):367–384. doi: 10.1016/0304-4157(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 73.Rhodes DG, et al. Structure of polymerizable lipid I--1 bilayers ,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine a tubule-forming phosphatidylcholine. Chem Phys Lipids. 1988;49(1–2):39–47. doi: 10.1016/0009-3084(88)90062-x. [DOI] [PubMed] [Google Scholar]
- 74.Yager P, Schoen PE. Formation of tubules by a polymerizable surfactant. Molecular Crystals and Liquid Crystals. 1984;106:371–381. [Google Scholar]
- 75.Johnson MR, et al. Sustained release of BMP-2 in a lipid-based microtube vehicle. Acta Biomater. 2009;5(1):23–28. doi: 10.1016/j.actbio.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spargo BJ, et al. Controlled-Release of Transforming Growth-Factor-Beta from Lipid-Based Microcylinders. J Microencapsul. 1995;12(3):247–254. doi: 10.3109/02652049509010293. [DOI] [PubMed] [Google Scholar]
- 77.Hyatt AJT, et al. Controlled release of chondroitinase ABC from fibrin gel reduces the level of inhibitory glycosaminoglycan chains in lesioned spinal cord. Journal of Controlled Release. 2010;147(1):24–29. doi: 10.1016/j.jconrel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 78.Sung HJ, et al. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004;25(26):5735–5742. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 79.Oh TH, et al. Acidic pH rapidly increases immunoreactivity of glial fibrillary acidic protein in cultured astrocytes. Glia. 1995;13(4):319–322. doi: 10.1002/glia.440130408. [DOI] [PubMed] [Google Scholar]
- 80.Kim MS, et al. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials. 2007;28(34):5137–5143. doi: 10.1016/j.biomaterials.2007.08.014. [DOI] [PubMed] [Google Scholar]