Abstract
Amyloid-β (A(β) peptide plays an essential role in the pathogenesis of Alzheimer's disease (AD) and is generated from amyloid-β precursor protein (APP) through sequential proteolytic cleavages by β-site APP cleaving enzyme 1 (BACE1) and γ-secretase. Trafficking dysregulation of APP, BACE1 and γ-secretase may affect Aβ generation and disease pathogenesis. Sorting nexin 15 (SNX15) is known to regulate protein trafficking. Here we report that SNX15 is abundantly expressed in mouse neurons and astrocytes. In addition, we show that although not affecting the protein levels of APP, BACE1 and γ-secretase components and the activity of BACE1 and γ-secretase, overexpression and downregulation of SNX15 reduces and promotes Aβ production, respectively. Furthermore, we find that overexpression of SNX15 increases APP protein levels in cell surface through accelerating APP recycling, whereas downregulation of SNX15 has an opposite effect. Finally, we show that exogenous expression of human SNX15 in the hippocampal dentate gyrus by adeno-associated virus (AAV) infection can significantly reduce Aβ pathology in the hippocampus and improve short-term working memory in the APPswe/PSEN1dE9 double transgenic AD model mice. Together, our results suggest that SNX15 regulates the recycling of APP to cell surface and thus its processing for Aβ generation.
Keywords: Alzheimer's disease, amyloid-β, amyloid-β precursor protein, SNX15, trafficking
Introduction
Alzheimer's disease (AD) is the most common irreversible, progressive and degenerative brain disease that causes dementia[1]. Pathological features of AD are extracellular senile plaques composed of fibrillar amyloid-β (Aβ) peptides and intracellular neurofibrillary tangles containing hyperphosphorylated tau, accompanied by synaptic dysfunction and neuronal death[1–3]. Aβ peptides are derived from the transmembrane glycoprotein amyloid-β precursor protein (APP) through the amyloidogenic proteolysis, during which process APP is first cleaved by the β-secretase (β-site APP cleaving enzyme 1, BACE1) to generate an extracellular soluble sAPPβ and a membrane-associated APP β-cartoxyl terminal fragment (CTF)[4,5]. APP β-CTF is then cleaved by the γ-secretase complex that is composed of presenilin (PS, including PS1 and PS2), Nicastrin, APH-1 and PEN-2, to release Aβ[6–9]. Alternatively, APP can be processed through a non-amyloidogenic pathway by α-secretase (mainly ADAM10), which cleaves APP within the Aβ sequence, precluding Aβ generation and releasing the neuroprotective soluble sAPPα instead[10–12]. Since APP is cleaved by various secretases during its trafficking within the cell, identifying proteins that can regulate APP transport and thus affect its processing for Aβ generation may shed light on elucidating the pathogenesis of AD. Recent studies have identified multiple APP-interacting proteins, such as Mints[13], LRP[14,15], RanBP9[16], SorLA/LR11[17,18], AP-4[19], FBL2[20], APLP1[21], SNX17[22], ApoER2[23], HAP1[24] and flotillin-2[25], that can regulate APP trafficking and Aβ generation.
Sorting nexin (SNX) family proteins contain a phox homology (PX) domain that mediates their binding to specific phosphatidylinositol phosphates. To date, 33 mammalian and 10 yeast SNXs have been identified, and many SNXs have been reported to regulate intracellular protein trafficking[26–28]. Among these SNXs, SNX17 has been found to interact with APP and affect APP processing/Aβ generation [22]; and SNX33 can regulate APP endocytosis dependent on its interaction with dynamin[29]. In addition, several other SNX family members such as SNX6[30], SNX8[31], SNX12[32], and SNX27[33] have been found to regulate the trafficking of other proteins involved in AD[34], and thus also affect Aβ generation. However, whether and how the rest SNX family members affect Aβ production has yet to be determined.
The SNX family member SNX15 contains a PX domain which binds to phosphatidylinositol 3-phosphate (PtdIns(3)P)[35] and an microtubule interacting and trafficking (MIT) domain which binds to PtdIns(3)P more weakly than phosphatidylinositol 4-phosphate in a Ca2+-dependent manner[36]. Overexpressed GFP-SNX15 was localized to early endosome and early to late transition endosome, but not to late endosome/lysosome and TGN[35,37]. SNX15 overexpression also altered endosome morphology and affected the endocytosis of transferrin and platelet-derived growth factor receptors, as well as the recycling of TGN38 and furin, resulting in a mislocalization of furin and a following inhibition of post-translational processing of insulin receptor and hepatocyte growth factor receptor precursors[37,38]. Another recent study also revealed that SNX15 could regulate the endocytosis and degradation of epidermal growth factor receptor[35]. However, the role of SNX15 in AD is unknown.
In the present study, we found that SNX15 was abundantly expressed in adult mouse brain, especially in neurons and astrocytes. We demonstrated that SNX15 could affect APP processing and Aβ generation through regulating the recycling of endocytotic APP to cell surface. Moreover, we found that exogenous expression of human SNX15 significantly reduced Aβ plaques and improved short-term working memory in an AD mouse model. Together, these findings suggest that SNX15 regulates APP trafficking/Aβ generation and could represent a potential therapeutic target for AD.
Materials and Methods
1. Antibodies
SNX15, EEA1 and Flag antibodies were purchased from Sigma Aldrich. Aβ (6E10) and sAPPβ antibodies were purchased from Covance. GAPDH, β-actin, MAP2, PSD95, NICD, CHC, and GFP antibodies were purchased from Cell Signaling Technology. Myc (9E10) antibody and mouse IgG were purchased from Santa Cruz Biotechnology. GFAP antibody was purchased from Millipore. ADAM10 antibody was purchased from Abcam. GluR1 antibody was purchased from Chemicon. Iba1 antibody was purchased from Wako Pure Chemical Industries. Alexa Fluor 594 F(ab)2 Fragment of Goat anti-rabbit IgG, Fluor 488 F(ab)2 Fragment of Goat anti-mouse IgG and Alexa Fluor 350 F(ab)2 Fragment of Goat anti-mouse IgG were purchased from Invitrogen. Antibodies against APP (369), PS1-NTF (Ab14) and Nicastrin (719) were developed in our laboratory [39]. The 3D5 antibody against BACE1 was kindly provided by Dr. Robert Vassar.
2. DNA Constructs
The pCI-neo-SNX15-myc vector was a generous gift from Dr. Wanjin Hong. For constructing the SNX15-EGFP vector, human SNX15 protein encoding sequence was amplified by polymerase chain reaction (PCR) using sense primer: 5′-CCC AAG CTT ATG CCT ACA ACA CAG CAG-3′ and antisense primer: 5′-CCC GGA TCC AGA AGG ATG AGA CCT TCA TA-3′, and sublconed into the pEGFP-C3 (Clontech) vector at HindIII and BamHI sites. The SNX15-2M–EGFP vector containing D216A/F217A double mutations was generated by using mutagenic PCR primers: 5′-CTT CAG GGA GTC CCC tCc GAC CCG TTG CCT GCC-3′ and 5′-GGC AGG CAA CGG GTC gGa GGG GAC TCC CTG AAG-3′. The pcDNA3.1-Notch-NδE and APP β-CTF expression plasmids were described previously[32].
3. Cell Culture, Transfection and Adeno-Associated Virus Infection
Human embryonic kidney (HEK 293T), human neuroblastoma SH-SY5Y and HT22 cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) (Thermo Scientific) supplemented with 10% fetal bovine serum (FBS) (Gibco), L-glutamine (2 mM), penicillin (100 units/ml) and streptomycin (100 µg/ml) (Invitrogen). Mouse neuroblastoma N2a cells were cultured in DMEM and Opti-MEM I (Invitrogen) (V/V=1:1) supplemented with 10% FBS L-glutamine (2 mM), penicillin (100 units/ml) and streptomycin (100 µg/ml). N2a cells stably expressing human wild-type APP695 (N2a695) were cultured in N2a media supplemented with additional 500 µg/ml G418. Primary cortical neurons were isolated from wild-type C57BL/6 or APPswe/PSEN1dE9 mice at embryonic day 17–18 (E17-18) and cultured in Neurobasal (Gibco) medium supplemented with B27 (Gibco), penicillin (100 units/ml) and streptomycin (100 µg/ml). All the cells were cultured in a humidified 37°C incubator with 5% CO2. Upon cell confluency, mammalian expression vecotrs were transiently transfected with Lipofectamine 2000 Reagent (Invitrogen) or TurboFect Transfection Reagent (Thermo Scientific) for 36 h, following the manufacturers’ instructions. Adeno-associated viral vector (serotype 8) containing human SNX15 cDNA was used to assemble AAV8-EGFP-SNX15. Purifed viral particles (titer: 2×1012) were used to infect mouse primary neurons after 9 days in culture. Neurons were harvested for western blot analysis at 4–5 days post-infection.
4. RNA Interference
For gene silencing, short hairpin RNA (shRNA) targeting mouse SNX15 was designed and cloned into lentiviral vector pLL3.7 at HpaI and XhoI sites. The sense sequences were as follows: GTT TGA AGC CTC TGT GAT C (shRNA-1) and GCC ATA TCT GTA TTA ACTG (shRNA-2). The shRNA was transfected into N2a cells using Lipofectamine 2000 Reagent (Invitrogen) for 72 h, following the manufacturer's protocol. The human SNX15 siRNA used was: siSnx15-1: 5′-ATG ACT TCC TGC GGC ACT ACA CA-3′ and siSnx15-2: 5′-ATG ACT TCC TGC GGC ACT ACA CA-3′. The negative control siRNA was purchased from Ribobio. The siRNA was transfected into HEK 293T or SH-SY5Y cells using Lipofectamine RNAi MAX Transfection Reagent (Invitrogen) for 72 h, following the manufacturer's protocol.
5. Animals
Animals used in this study include C57BL/6 wild type mice and APPswe/PSEN1dE9 AD model mice co-expressing the Swedish mutant APP and the exon-9 deletion mutant PS1 provided by Nanjing Biomedical Research Institute of Nanjing University, China. All animal procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Xiamen University.
6. Western Blotting
Cells and mouse tissues were lysed in RIPA buffer (150 mM sodium chloride, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris-HCl, pH 8.0, supplemented with protease inhibitors and phosphatase inhibitors). Protein lysates were separated on SDS-polyacrylamide gels, electrophoretically transferred onto PVDF membrane, and immunoblotted with antibodies.
7. Immunofluorescence
Cells grown on coverslips were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100 in PBS, incubated with primary antibody and then with fluorescence-conjugated secondary antibody, and observed under laser scanning fluorescence confocal microscope (Olympus, Japan) with 60× oil immersion objective. Quantitation of APP and BACE1 colocalization, indicated by Pearson’s coefficient and overlap coefficient, was carried out by using Olympus imaging analysis software, following the manufacturer’s instructions.
8. Reverse Transcription and Quantitative Real-Time PCR
Total RNAs were extracted from cells or mouse tissues using Trizol reagent (Invitrogen), and equal amounts of total RNAs were used to synthesize the first strand cDNA using ReverTra Ace qPCR RT Kit (TOYOBO) following the manufacturer’s instructions. Quantitative real-time PCR was carried out using ABI 7500 Fast Real-Time PCR System (Life technologies) with FastStart Universal SYBR Green Master (ROX) (Roche). Primers used in real-time PCR amplification were shown in Table S1.
9. Aβ ELISA Assay
The levels of Aβ40 and Aβ42 in conditioned media of treated cells were analyzed by sandwich ELISA using Human Abeta1-40/1-42 ELISA Kits (Cloud-Clone), following the manufacturer’s instructions. In addition, hippocampal tissues of treated APPswe/PSEN1dE9 mice were homogenized with 1×TBSX (Tris-buffered saline buffer containing 1% Triton X-100) and centrifuged at 20,000×g for 1 h at 4°C. The supernatant, TBSX-soluble fraction, was allocated. The TBSX-insoluble pellets were resuspended in 5 M GuHCl, mixed by rotation at room temperature for 6 h, and centrifuged at 16,000×g for 30 min. Both TBSX-soluble and GuHCl-soluble fractions were assayed for Aβ40 and Aβ42 levels by ELISA.
10. BACE1 Activity Assay
BACE1 activity was determined by using a commercial kit from Sigma-Aldrich, following the manufacturer’s protocol.
11. Cell Surface Biotinylation and Endocytic Protein Recycling Experiments
Assays for cell surface biotinylation have been described previously[40,41]. Protein recycling experiments were performed as described previously[42,43]. Briefly, cells were labeled with EZ-Link™ Sulfo-NHS-SS-Biotin (Thermo Scientific) at 4°C. After washing, cells were incubated at 37°C for 30 min to drive protein endocytosis. At the end of this incubation, cells were treated with glutathione at 4°C to cleave biotin from biotinylated proteins remaining at the cell surface. Cells were then incubated with serum-free media containing 50 mM glutathione at 37°C for various times to allow recycling to occur. Finally, cells were incubated with glutathione at 4°C to ensure complete cleavage of biotin from surface proteins. Residual biotinylated (internalized) proteins in cell lysates were affinity precipiated by Streptavidin Agarose Resin (Thermo Scientific) and detected by western blot. The reduction of biotinylated target proteins indicates their increased recycling to cell surface.
12. Stereotactic Injection of AAV and Mouse Studies
Stereotactic bilateral injections of AAV8 containing EGFP-2A-SNX15-3Flag or EGFP (1.5 µl, titer 2×1012) into hippocampal dentate gyrus of APPswe/PSEN1dE9 mice were carried out at the following coordinates: −2.0 anterior/posterior, ±1.6 medial/lateral, and 2 dorsal ventral relative to bregma (in millimeters)[44]. Six weeks after injection, mice were subjected to behaviroal tests including open field test[45] and Y maze test[46], following previously described protocols. Eight weeks after virus injection, mice were sacrificed and brain samples were dissected for immunohistochemistry and biochemical analyses.
13. Immunohistochemistry
Brain sections of treated APPswe/PSEN1dE9 mice were first incubated with anti-Aβ antibody (6E10) and then with biotinylated secondary antibody. Sections were stained with diaminobenzidine and subsequently with hematoxylin. Images were captured and the percentages of Aβ-immunolabeled area (positive pixels) were calculated by quantitative image analysis (Image Pro Plus).
14. Statistical Analyses
All statistical analyses were performed with GraphPad Prism 5. Results were expressed as means ± standard error of the mean (SEM). Statistical significance was assessed by paired or unpaired t test.
Results
1. Expression Pattern of Mouse SNX15
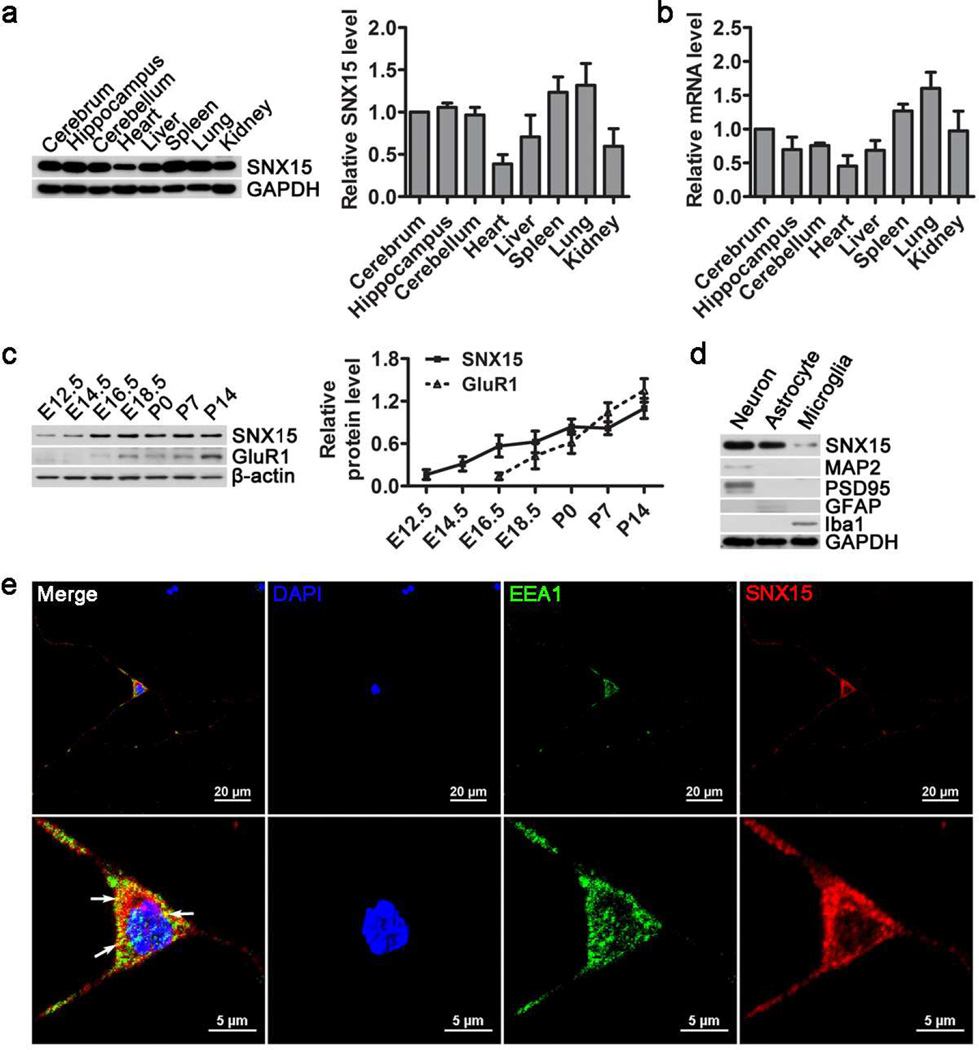
Sequence analysis revealed that human SNX15 protein is highly homologous to those of mouse and rat (Fig. S1). To study the expression pattern of SNX15, we compared SNX15 protein (Fig. 1a) and mRNA (Fig. 1b) levels in various adult C57BL/6 mouse tissues. The results showed that SNX15 was ubiquitously expressed in all tissues examined including brain, heart, liver, spleen, lung and kidney, with a relatively low expression in heart. Within the brain, SNX15 was comparably expressed in cerebrum, cerebellum and hippocampus. In addition, we found that during development, SNX15 expression was detected in the brain early at embryonic day 12.5 (E12.5) and kept increasing until reaching a plateau at postnatal day 0 (P0) (Fig. 1c), whereas the expression of the glutamate receptor subunit, GluR1 appeared later at E16.5. Furthermore, in different neural cell types, we found that SNX15 expression was much higher in neurons and astrocytes than in microglia (Fig. 1d). Moreover, immunofluorescence study showed that endogenous SNX15 was distributed in the form of punctate spots in the cytosol of mouse primary cortical neurons and partially colocalized with the early endosome marker EEA1 (Fig. 1e), consistent with a previous study showing partial localization of SNX15 in early endosomes [35].
Fig. 1.
SNX15 is abundantly expressed in adult mouse brain. a Equal protein amounts of lysates from various C57BL/6 mouse (two months old) tissues were analyzed by western blot for SNX15 and GAPDH. SNX15 protein levels were quantified by densitometry, normalized to those of GAPDH, and compared to that of cerebrum (set as one arbitrary unit), n=4. b SNX15 mRNA levels in various mouse tissues were determined by quantitative real time PCR, normalized to those of β-actin, and compared to that of cerebrum (set as one arbitrary unit), n=4. c Equal protein amounts of brain lysates from C57BL/6 mice at different embryonic and postnatal developmental days were analyzed by western blot for SNX15, GluR1 and β-actin. SNX15 and GluR1 levels were quantified by densitometry, normalized to those of β-actin for comparison, n=3. d Equal amounts of protein lysates from primary cultured neurons, astrocytes and microglia were analyzed by western blot for SNX15, MAP2 (neuron marker), PSD95 (neuron marker), GFAP (astrocyte marker), Iba1 (microglia marker) and GAPDH (loading control). e Mouse primary cortical neurons were subjected to immunofluorescent staining of SNX15 (in red) and EEA1 (in green). The nuclei were stained with DAPI (in blue). Lower panels were enlargements of neuron soma in upper panels. Scale bars: 20 µm in upper panels and 5 µm in lower panels.
2. SNX15 Regulates APP Processing and Aβ Generation
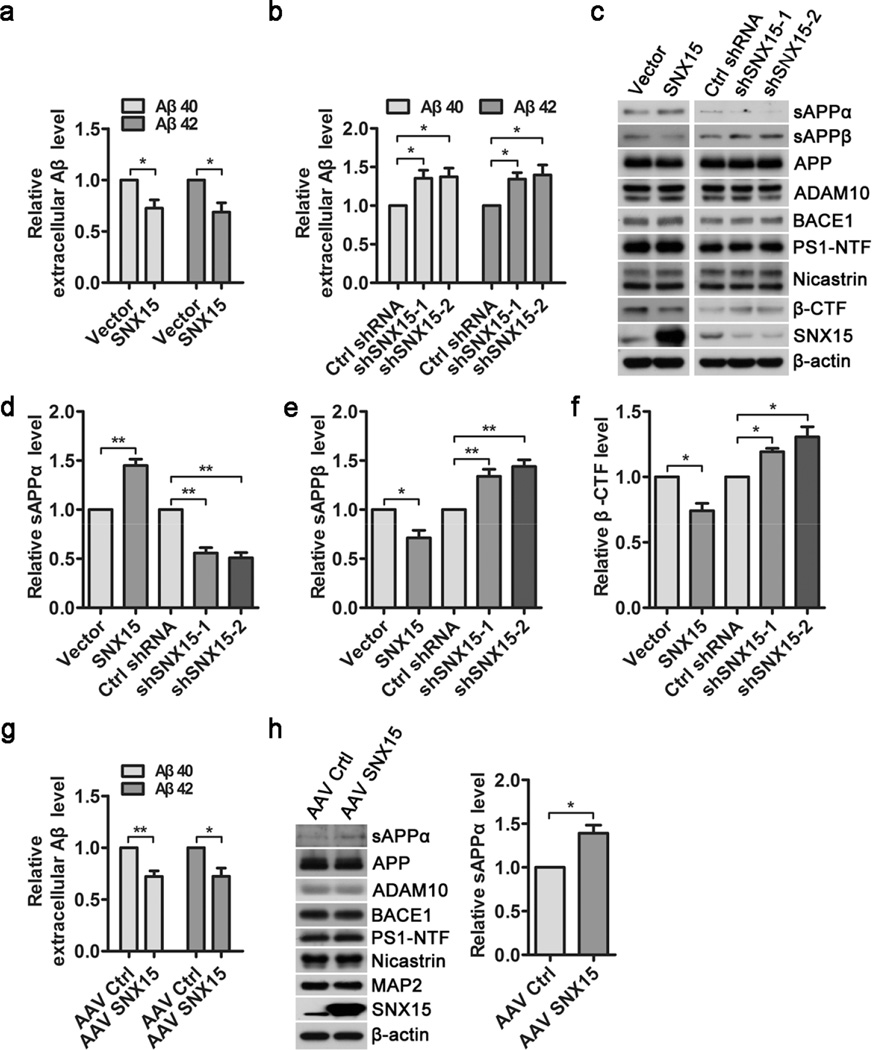
When SNX15 was transiently overexpressed in N2a cells stably expressing APP695 (N2a695), we found that levels of secreted Aβ40 and Aβ42 in conditioned media were significantly decreased (Fig. 2a). On the other hand, short hairpin RNA (shRNA)-mediated downregulation of SNX15 in N2a695 cells resulted in a dramatic increase of secreted Aβ40 and Aβ42 levels (Fig. 2b). In addition, although modulation of SNX15 levels (overexpression to about 8–10 folds of endogenous levels or downregulation to about one quarter of control levels) did not affect protein levels of full-length APP, ADAM10, BACE1, PS1-amino-terminal fragment (PS1-NTF) and Nicastrin (Fig. 2c), overexpression of SNX15 markedly increased sAPPα levels (Fig. 2c, d), and decreased levels of sAPPβ (Fig. 2c, e) and APP β-CTF (Fig. 2c, f), whereas downregulation of SNX15 had opposite effects. In cultured primary neurons derived from E17.5 embryos of the APPswe/PSEN1dE9 AD model mice, adeno-associated virus (AAV)-mediated exogenous expression of human SNX15 also decreased Aβ40 and Aβ42 secretion (Fig. 2g) and increased sAPPα levels (Fig. 2h), without affecting protein levels of full-length APP, ADAM10, BACE1, PS1-NTF, Nicastrin, and MAP2 (Fig. 2h).
Fig. 2.
SNX15 regulates APP processing and Aβ generation. a N2a695 cells were transiently transfected with SNX15 and control vectors for 36 h, and secreted Aβ40 and Aβ42 in conditioned media were quantified by ELISA, n =5, *p<0.05. b N2a695 cells were transiently transfected with mouse SNX15 shRNA (shSNX15-1 and −2) and Control shRNA (Ctrl shRNA) for 72 h, and secreted Aβ40 and Aβ42 in conditioned media were quantified by ELISA, n =4, *p<0.05. c Equal amounts of conditioned media in (a and b) were subjected to western blot analysis for sAPPα and sAPPβ, and equal protein amounts of treated cell lysates were analyzed by western blot analysis for indicated proteins. d-f Levels of sAPPα (d), sAPPβ (e) and APP β-CTF (f) in (c) were quantified by densitometry, normalized to those of β-actin, and compared to those of controls (set as one arbitrary units), n=3, *p<0.05, **p<0.01. g, h Primary cultured APPswe/PSEN1dE9 mouse neurons were infected with AAV8 expressing SNX15 (AAV SNX15) or EGFP (as control, AAV Ctrl) for 72 h. g Secreted Aβ40 and Aβ42 in conditioned media were quantified by ELISA, n=3, *p<0.05, **p<0.01. h sAPPα in conditioned media and indicated proteins in cell lysates were subjected to western blot. sAPPα levels were quantified by densitometry for comparison, n=3, *p<0.05.
3. SNX15 Does Not Affect β- and γ-Secretase Activity
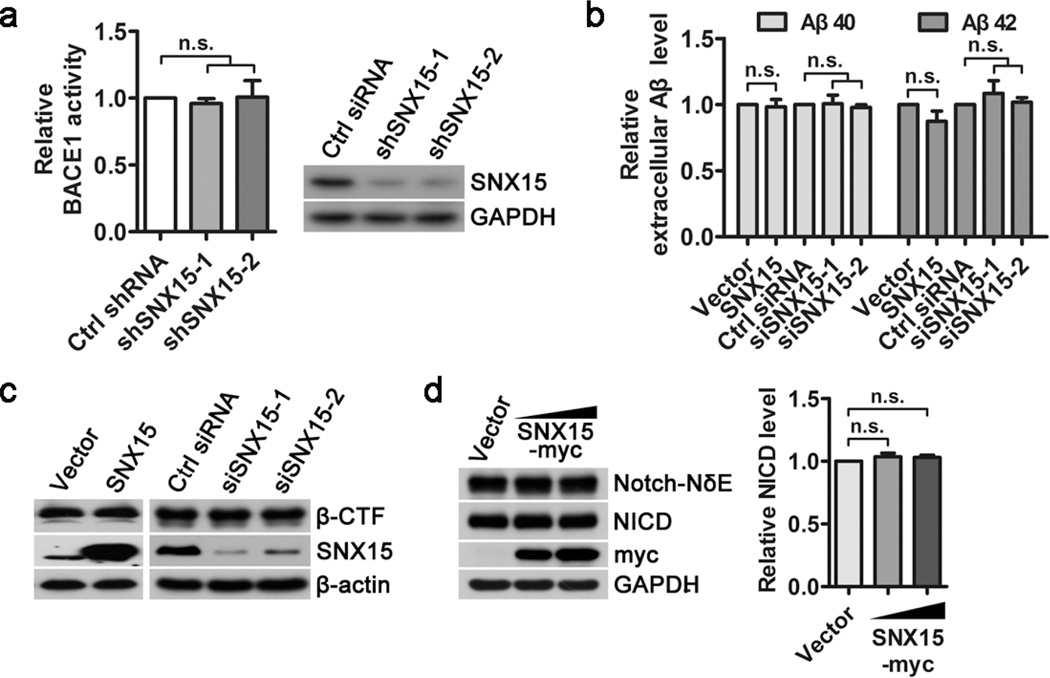
Consistent with the results in N2a695 (Fig. 2c) and APPswe/PSEN1dE9 mouse primary neurons (Fig. 2h), we found that neither overexpression nor downregulation of SNX15 affected protein levels of endogenous APP, ADAM10, BACE1, and γ-secretase components including PS1-NTF and Nicastrin in SH-SY5Y cells (Fig. S2a), in N2a cells (Fig. S2b) and in primary neurons from C57BL/6 mice (Fig. S2c). In addition, downregulation of SNX15 had no effect on mRNA levels of APP, ADAM10, BACE1, PS1 and Nicastrin (Fig. S2d).
Since Aβ is generated from APP through sequential cleavages by β-secretase (BACE1) and γ-secretase, we checked whether SNX15 affects the two enzymes’ activity. As shown in Fig. 3a, downregulation of SNX15 by shRNAs in N2a cells did not affect BACE1 activity. APP β-CTF is the direct substrate of γ-secretase for Aβ production[1,47]. In HEK 293T cells transiently expressing APP β-CTF, we found that overexpression or downregulation of SNX15 did not affect secreted Aβ40 and Aβ42 levels in conditioned media (Fig. 3b), as well as APP β-CTF levels in cell lysates (Fig. 3c). Notch is another important substrate of γ-secretase and can be cleaved to release Notch intracellular domain (NICD)[48]. When Notch-NδE, a truncated Notch fragment that can be directly cleaved by γ-secretase, was transiently expressed in HEK 293T cells, we found additional overexpression of SNX15 did not affect the generation of NICD (Fig. 3d). Together, these results suggest that SNX15 does not affect BACE1 and γ-secretase activity.
Fig. 3.
SNX15 does not affect β- and γ-secretase activity. a N2a cells were transfected with mouse SNX15 shRNA (shSNX15-1 and −2) or Control shRNA (Ctrl shRNA) for 72 h. Cell lysates were assayed for BACE1 activity using a commercial kit or analyzed for SNX15 and GAPDH by western blot, n=3, n.s.: not significant. b, c HEK 293T cells were pre-transfected with APP β-CTF. After splitting equally, cells were transfected with SNX15 or control vectors for 36 h, and human SNX15 siRNA (siSNX15-1 and −2) or Control siRNA (Ctrl siRNA) for 72 h. b Secreted Aβ40 and Aβ42 in conditioned media were quantified by ELISA, n=3, n.s.: not significant. c Cell lysates were analyzed for β-CTF, SNX15 and β-actin by western blot. d HEK 293T cells were pre-transfected with Notch-NδE. After splitting equally, cells were transfected with different amounts of SNX15 or control vectors for 36 h. Cell lysates were analyzed by western blot for Notch-NδE, NICD, SNX15 and GAPDH. NICD levels were quantified by densitometry, normalized to those of Notch-NδE, and compared to those of controls (set as one arbitrary units), n=3, n.s.: not significant.
4. SNX15 Affects Cell Surface Levels of APP through Regulating APP Recycling
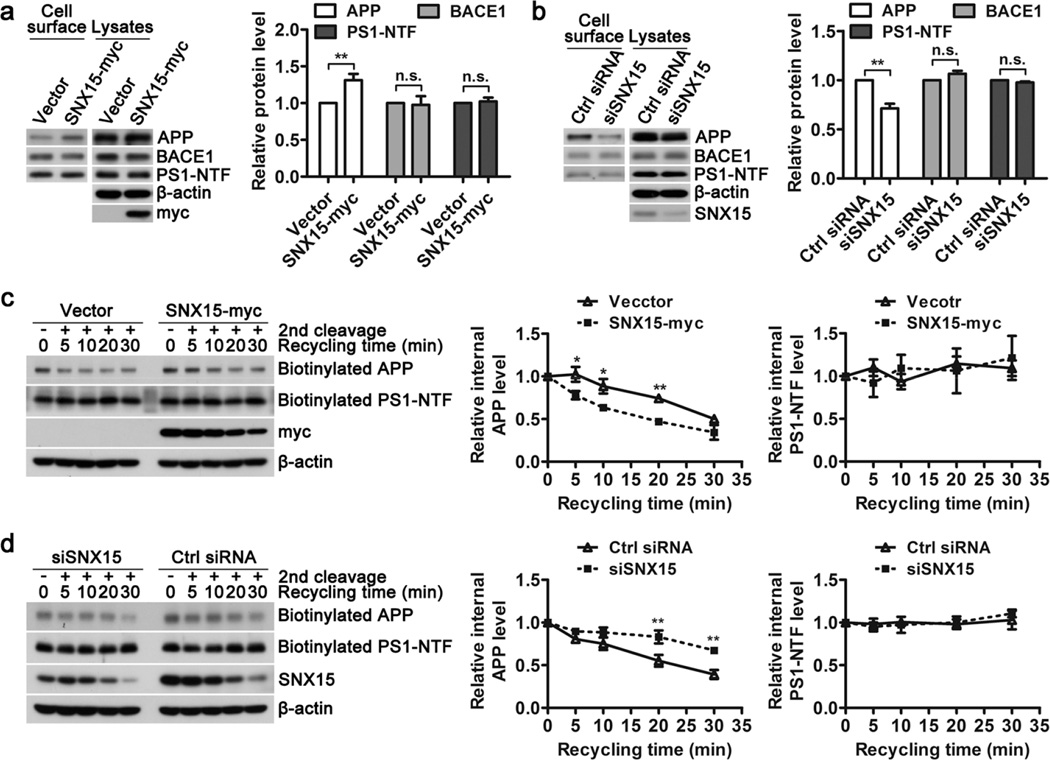
Altered APP trafficking may affect Aβ generation and SNX15 has been known to regulate protein trafficking[37,38]. Therefore we asked whether SNX15 affects APP trafficking. Cell surface biotinylation experiments showed that overexpression (Fig. 4a) and downregulation (Fig. 4b) of SNX15 resulted in increased and decreased cell surface levels of APP, respectively, without affecting levels of BACE1 and PS1-NTF, indicating that SNX15 indeed affects APP trafficking.
Fig. 4.
SNX15 regulates the recycling of APP to the cell surface. a, c HEK 293T cells were transfected with SNX15 and control vectors for 36 h, or (b, d) with SNX15 siRNA and control (Ctrl) siRNA for 72 h. a, b Cells were subjected to surface biotinylation. After lysing and affinity precipitation, biotinylated (cell surface) and lysate proteins were analyzed by western blot for APP, BACE1, and PS1-NTF. Their levels in cell surface were quantified by densitometry, normalized to those in lysates, and compared to those of controls (set as one arbitrary units), n=3, **p<0.01; n.s.: not significant. c, d Alternatively, cells were subjected to cleavable biotinylation at 4°C, incubated at 37°C for 30 min to induce endocytosis, treated with glutathione at 4°C to cleave biotin of remaining surface proteins, incubated at 37°C again for indicated time periods, and then treated with glutathione at 4°C again to cleave biotin of recycling proteins. After lysing and affinity precipitation, biotinylated (internal) APP and PS1-NTF were analyzed by western blot, quantified by densitometry, and normalized to those at time zero (set as one arbitrary units) for comparison, n=3, *p<0.05, **p<0.01.
Since SNX family members including SNX15 mainly regulates the endocytic pathways of protein trafficking, we first studied whether SNX15 affects APP endocytosis. Our results showed that when SNX15 was downregulated, the endocytic rate of APP was not affected (Fig. S3a). Endocytic proteins can be either recycled back to the cell surface or delivered to lysosome for degradation. However, neither overexpression nor downregulation of SNX15 altered APP degradation rate when protein synthesis was inhibited by cycloheximide (Fig. S3b, c). Instead, we found that overexpression of SNX15 promoted APP recycling back to cell surface (Fig. 4c), whereas downregulation of SNX15 reduced APP recycling (Fig. 4d). In contrast, recycling of PS1-NTF to cell surface was not affected upon overexpression or downregulation of SNX15 levels (Fig. 4c, d). Therefore, these results suggest that SNX15 regulates recycling of endocytic APP to cell surface.
5. Downregulation of SNX15 Promotes Colocalization of APP and BACE1 within the Cell
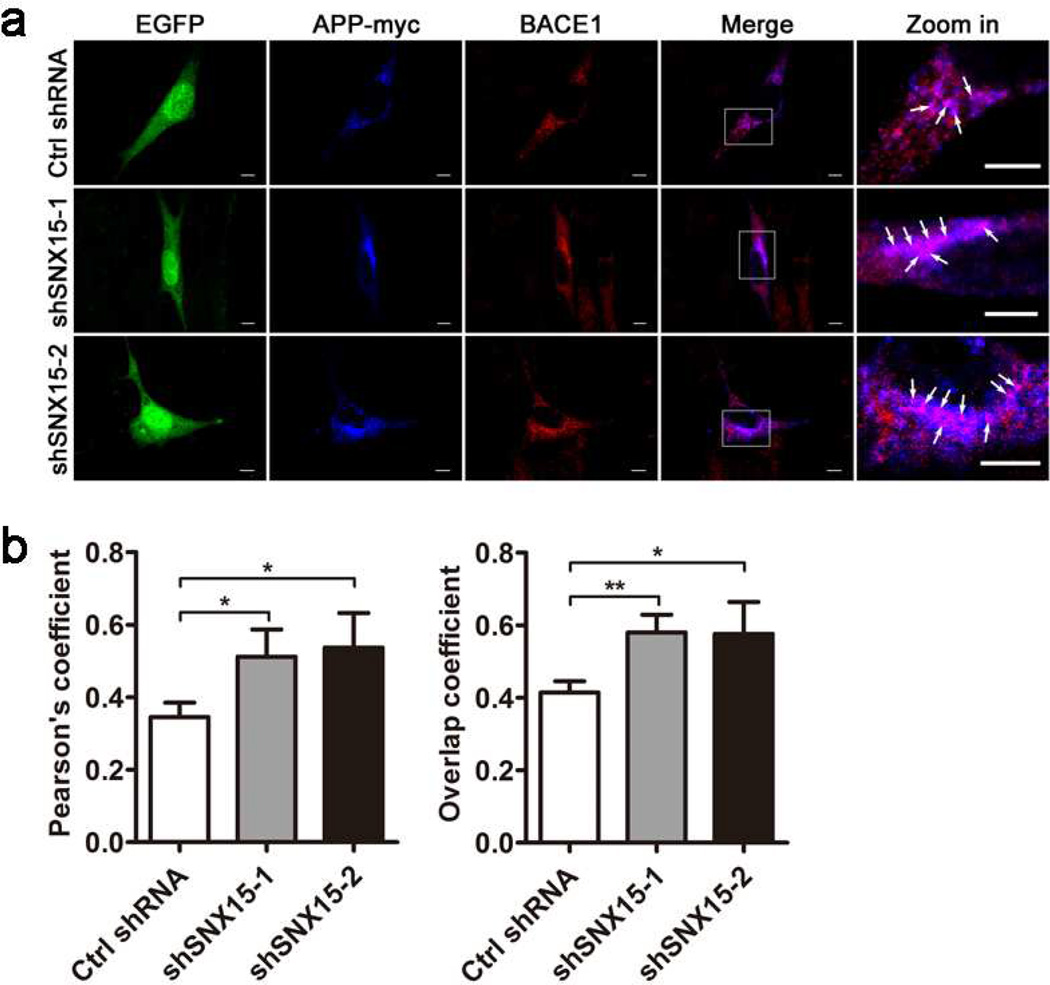
Downregulation of SNX15 reduces cell surface levels of APP without affecting total levels of APP, implying that APP is accumulated within the cell. Since recycling of endocytic APP is reduced upon downregulating SNX15 and BACE1 is mainly localized in acidic organelle such as endosomes for its optimal activity, we speculate that APP may have increased interaction with BACE1 possibly in endosomes upon SNX15 downregulation. Indeed, co-immunostaining of APP and BACE1 revealed that downregulation of SNX15 resulted in enhanced colocalization of APP and BACE1 within the cell (Fig. 5a, b), implying that APP is subjected to increased BACE1 cleavage for Aβ production upon SNX15 downregulation.
Fig. 5.
Downregulation of SNX15 increases the colocalization of APP and BACE1. a HT22 cells co-transfected with APP-myc and Ctrl shRNA, shSNX15-1 or shSNX15-2 (EGFP, shown in green), were immunostained with anti-myc primary antibody followed by fluorescence-conjugated secondary antibody (in blue) and with anti-BACE1 primary antibody followed by fluorescence-conjugated secondary antibody (in red), and then observed under the Olympus FV1000 confocal microscope. Enlarged areas in the merged images on the right show colocalization of APP and BACE1 in purple (white arrows). Scale bars: 5 µm. b Both Pearson's coefficient and overlap coefficient were measured by the Olympus imaging analysis software to indicate colocalization of APP and BACE1. At least ten randomly selected neurons from each treatment were measured for comparison. *p<0.05, **p<0.01.
6. Exogenous Expression of Human SNX15 Reduces Aβ Deposition and Improves Short-Term Working Memory in APPswe/PSEN1dE9 Mice
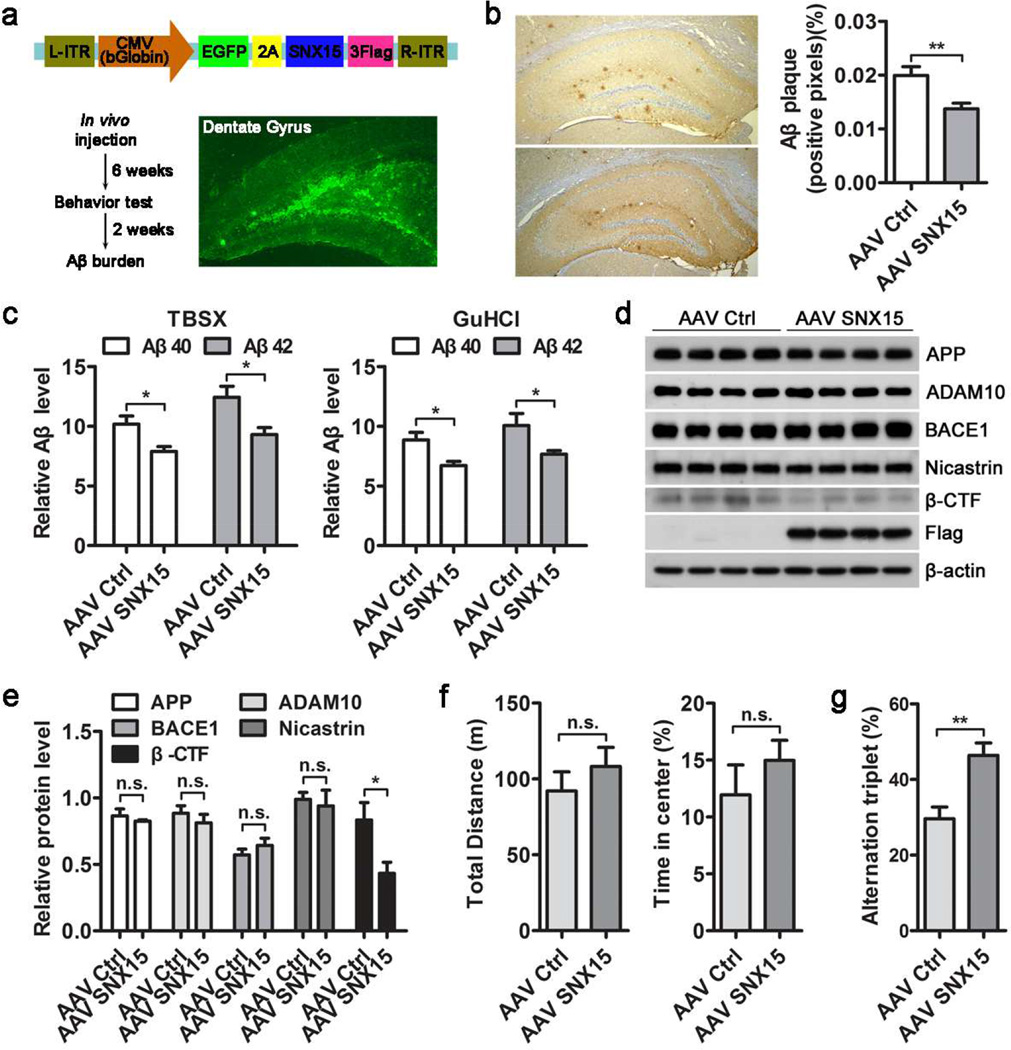
To confirm the role of SNX15 in regulating Aβ generation in vivo, we generated an adeno-associated virus serotype 8 (AAV8) containing human SNX15 cDNA (Fig. 6a). AAV8-EGFP-SNX15 (AAV SNX15) or AAV8-EGFP (as control, AAV Ctrl) were bilaterally injected into the hippocampal dentate gyrus area of 7.5–8 month-old APPswe/PSEN1dE9 transgenic AD model mice. Stereotactic injection resulted in local expression of EGFP (Fig. 6a). The protein expression of exogenous Flag-tagged SNX15 in the hippocampus was also confirmed by western blot (Fig. 6d). Eight weeks after AAV8 injection, Aβ plaques in hippocampal sections were detected by immunohistochemistry. We found that Aβ plaques in the hippocampus were dramatically reduced in mice expressing exogenous SNX15 when compared to control group (Fig. 6b). However, there was no significant change of Aβ plaques in cortical regions and this is possibly because the AAV spread was limited to the hippocampus (data not shown). In addition, we extracted proteins from treated mouse hippocampal tissues and measured Aβ40 and Aβ42 levels by ELISA analysis. The results showed that both TBSX (Tris-buffered saline with 1% Triton X-100)-soluble and TBSX-insoluble (redissolved in 5M GuHCl) Aβ40 and Aβ42 levels were significantly reduced in APPswe/PSEN1dE9 mice expressing exogenous SNX15 (Fig. 6c). Moreover, western blot results showed that although the levels of APP, ADAM10, BACE1, and Nicastrin were not altered, the levels of APP β-CTF were markedly reduced in the hippocampus of APPswe/PSEN1dE9 mice expressing exogenous SNX15 (Fig. 6d, e).
Fig. 6.
Expression of human SNX15 decreases Aβ deposition and promotes spontaneous alternation in APPswe/PSEN1dE9 mice. a Schematic illustration of the AAV8 construct for expression of human SNX15 and EGFP (upper) and the workflow of stereotactic AAV injection experiments (lower left). A representative EGFP fluorescence image of a dentate gyrus section at 8 weeks after AAV injection (lower right). b Hippocampal sections of treated mice were subjected to immunohistochemical detection of Aβ plaques using the 6E10 antibody. Percentages of Aβ-immunolabeled area captured (positive pixels) were calculated by quantitative image analysis, n=3, **p<0.01. c Hippocampal tissues of treated mice were lysed in TBSX and TBSX-insoluble remnants were redissolved in 5M GuHCl. Aβ40 and Aβ42 in TBSX and GuHCl fractions were measured by ELISA assays, n=6, *p<0.05. d, e Indicated proteins from hippocampal lysates of treated mice were (d) analyzed by western blot and (e) compared after densitometry quantification, n=4, *p<0.05; n.s.: not significant. f, g Treated mice were subjected to various behavioral test analyses, including: (f) total distance moved and the percentage of time in the center during open field test; (g) spontaneous alternations during Y maze test, AAV Ctrl, n=8 mice; AAV SNX15, n=12 mice, **p<0.01; n.s.: not significant.
Behavioral tests were carried out 6 weeks after AAV8 injection. In open field test, there were no significant differences bewteen AAV8-EGFP-SNX15 and AAV8-EGFP infected APPswe/PSEN1dE9 mice in terms of total distance traveled and time spent in the center (Fig. 6f), indicating that exogenous expression of SNX15 did not alter the locomotor activity and anxiety responses of APPswe/PSEN1dE9 mice. In Y maze test, the percentage of alternations triplet was tested to assess spontaneous alternation behavior and we found a significant increase in AAV8-EGFP-SNX15 injected APPswe/PSEN1dE9 mice when compared to control mice (Fig. 6g), indicating that exogenous expression of SNX15 can improve short-term working memory in APPswe/PSEN1dE9 mice.
Discussion
Subcellular localication of APP greatly affects its amyloidogenic processing to generate Aβ, whose progressive aggregation and deposition in the brain plays an important role in the pathogenesis of AD[34,49]. Although APP trafficking has been found to be regulated by various factors, the detailed underlying mechanisms are only partly understood. In this study, we demonstrate that SNX15, a protein abundantly expressed in neurons, can also regulate APP processing and Aβ generation through modulating the recycling of endocytic APP back to cell surface. Since another SNX family member, SNX17, is found to interact with APP intracellular domain and affect APP stability and Aβ production[22], we also explored whether SNX15 interacts with APP. However, our results showed that there was no direct interaction between the two (Fig. S4).
It has been reported that clathrin mediates endocytosis of APP[50]. One recent study showed that SNX15 can interact with clathrin heavy chain 1 (CHC) through its L214FDPF217 motif and deletion of this motif or individual mutation of amino acids within this motif abolishes their interaction [35] Therefore, we studied whether SNX15 regulates clathrin-mediated APP trafficking and thus processing through its interaction with CHC. Although we confirmed that SNX15 interacted with CHC but SNX15 double mutation (D216P217 to A216A217) did not (Fig. S5a), we found that overexpression or downregulation of SNX15 did not affect CHC levels (Fig. S5b). In addition, overexpression of both SNX15 and SNX15 double mutation (D216P217 to A216A217) similarly reduced secreted Aβ42 levels (Fig. S5c) and increased sAPPα levels (Fig. S5d). These results indicate that the effect of SNX15 on APP trafficking does not depend on the interaction between SNX15 and CHC.
Since there are many proteins interacting with and affecting APP trafficking, one possibility is that SNX15 regulates APP trafficking through modulating such proteins. For example, another SNX family member, SNX33, is found to regulate APP endocytosis through interacting with dynamin[29]. Therefore, it is possible that SNX15 may also regulate APP trafficking through interacting with dynamin, which deserves further scrutiny.
Consistent with in vitro results, we found that exogenous expression of human SNX15 in the hippocampal dentate gyrus also significantly reduced Aβ pathology in APPswe/PSEN1dE9 AD model mice (Fig. 6b, c). Notably, exogenous expression of SNX15 in APPswe/PSEN1dE9 mice promoted their spontaneous alternation during Y maze test (Fig. 6g), which is used to evaluate short-term working memory capacity[51–53]. Hence, our results suggest that exogenous expression of SNX15 is beneficial for short-term memory, implying that SNX15 might be a potential target for AD intervention.
Supplementary Material
Acknowledgements
We thank Dr. Robert Vassar for providing the BACE1 antibody and Dr. Wanjin Hong for providing the SNX15 plasmid. This study was supported by grants from National Institutes of Health (R01AG021173, R01AG038710, R01AG044420, R01NS046673, and R21AG049247) and from National Natural Science Foundation of China (Nos. 81225008, 81161120496, 91332112, and 91332114), and Fundamental Research Funds for the Central Universities of China.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Tuancheng Feng, Fujian Provincial Key Laboratory of Neurodegenerative Disease and Aging Research, Institute of Neuroscience, School of Pharmaceutical Sciences, College of Medicine, Xiamen University, Xiamen, 361102, China.
Mengmeng Niu, Fujian Provincial Key Laboratory of Neurodegenerative Disease and Aging Research, Institute of Neuroscience, School of Pharmaceutical Sciences, College of Medicine, Xiamen University, Xiamen, 361102, China.
Chengxiang Ji, Fujian Provincial Key Laboratory of Neurodegenerative Disease and Aging Research, Institute of Neuroscience, School of Pharmaceutical Sciences, College of Medicine, Xiamen University, Xiamen, 361102, China.
Yuehong Gao, Fujian Provincial Key Laboratory of Neurodegenerative Disease and Aging Research, Institute of Neuroscience, School of Pharmaceutical Sciences, College of Medicine, Xiamen University, Xiamen, 361102, China.
Jing Wen, Fujian Provincial Key Laboratory of Neurodegenerative Disease and Aging Research, Institute of Neuroscience, School of Pharmaceutical Sciences, College of Medicine, Xiamen University, Xiamen, 361102, China.
Guojun Bu, Fujian Provincial Key Laboratory of Neurodegenerative Disease and Aging Research, Institute of Neuroscience, School of Pharmaceutical Sciences, College of Medicine, Xiamen University, Xiamen, 361102, China.
Huaxi Xu, Fujian Provincial Key Laboratory of Neurodegenerative Disease and Aging Research, Institute of Neuroscience, School of Pharmaceutical Sciences, College of Medicine, Xiamen University, Xiamen, 361102, China; Degenerative Disease Research Program, Sanford-Burnham Medical Research Institute, La Jolla, CA 92037, USA.
Yun-wu Zhang, Fujian Provincial Key Laboratory of Neurodegenerative Disease and Aging Research, Institute of Neuroscience, School of Pharmaceutical Sciences, College of Medicine, Xiamen University, Xiamen, 361102, China; Degenerative Disease Research Program, Sanford-Burnham Medical Research Institute, La Jolla, CA 92037, USA.
References
- 1.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien C. Auguste D. and Alzheimer's disease. Science. 1996;273(5271):28. doi: 10.1126/science.273.5271.28. [DOI] [PubMed] [Google Scholar]
- 3.Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer's disease. Lancet. 1997;349(9064):1546–1549. doi: 10.1016/S0140-6736(96)10203-8. [DOI] [PubMed] [Google Scholar]
- 4.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 5.Vassar R, Kandalepas PC. The beta-secretase enzyme BACE1 as a therapeutic target for Alzheimer's disease. Alzheimers Res Ther. 2011;3(3):20. doi: 10.1186/alzrt82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391(6665):387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 7.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5(5):486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 8.Kaether C, Haass C, Steiner H. Assembly, trafficking and function of gamma-secretase. Neurodegener Dis. 2006;3(4–5):275–283. doi: 10.1159/000095267. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Bohm C, Dodd R, Chen F, Qamar S, Schmitt-Ulms G, Fraser PE, St George-Hyslop PH. Structural biology of presenilin 1 complexes. Mol Neurodegener. 2014;9(1):59. doi: 10.1186/1750-1326-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Constitutive and regulated alpha-secretase cleavage of Alzheimer's amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci U S A. 1999;96(7):3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtenthaler SF, Haass C. Amyloid at the cutting edge: activation of alpha-secretase prevents amyloidogenesis in an Alzheimer disease mouse model. J Clin Invest. 2004;113(10):1384–1387. doi: 10.1172/JCI21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, Flamez P, Dequenne A, Godaux E, van Leuven F, Fahrenholz F. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113(10):1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan SE, Dillon GM, Sullivan JM, Ho A. Mint proteins are required for synaptic activity-dependent amyloid precursor protein (APP) trafficking and amyloid beta generation. J Biol Chem. 2014;289(22):15374–15383. doi: 10.1074/jbc.M113.541003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knauer MF, Fouts DA, Knauer DJ. Identification of a domain in the amyloid precursor protein (APP) required for the LRP-mediated endocytosis of APP: Protease complexes. Neurobiol Aging. 2004;25:S442–S442. [Google Scholar]
- 15.Cam JA, Zerbinatti CV, Li YH, Bu GJ. Rapid endocytosis of the low density lipoprotein receptor-related protein modulates cell surface distribution and processing of the beta-amyloid precursor protein. J Biol Chem. 2005;280(15):15464–15470. doi: 10.1074/jbc.M500613200. [DOI] [PubMed] [Google Scholar]
- 16.Lakshmana MK, Yoon IS, Chen E, Bianchi E, Koo EH, Kang DE. Novel role of RanBP9 in BACE1 processing of amyloid precursor protein and amyloid beta peptide generation. J Biol Chem. 2009;284(18):11863–11872. doi: 10.1074/jbc.M807345200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CAF, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. P Natl Acad Sci USA. 2005;102(38):13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt V, Sporbert A, Rohe M, Reimer T, Rehm A, Andersen OM, Willnow TE. SorLA/LR11 regulates processing of amyloid precursor protein via interaction with adaptors GGA and PACS-1. J Biol Chem. 2007;282(45):32956–32964. doi: 10.1074/jbc.M705073200. [DOI] [PubMed] [Google Scholar]
- 19.Burgos PV, Mardones GA, Rojas AL, daSilva LL, Prabhu Y, Hurley JH, Bonifacino JS. Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev Cell. 2010;18(3):425–436. doi: 10.1016/j.devcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe T, Hikichi Y, Willuweit A, Shintani Y, Horiguchi T. FBL2 Regulates Amyloid Precursor Protein (APP) Metabolism by Promoting Ubiquitination-Dependent APP Degradation and Inhibition of APP Endocytosis. J Neurosci. 2012;32(10):3352–3365. doi: 10.1523/JNEUROSCI.5659-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann S, Schobel S, Jager S, Trautwein A, Haass C, Pietrzik CU, Lichtenthaler SF. Amyloid precursor-like protein 1 influences endocytosis and proteolytic processing of the amyloid precursor protein. J Biol Chem. 2006;281(11):7583–7594. doi: 10.1074/jbc.M508340200. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Retamal C, Cuitino L, Caruano-Yzermans A, Shin JE, van Kerkhof P, Marzolo MP, Bu GJ. Adaptor protein sorting nexin 17 regulates amyloid precursor protein trafficking and processing in the early endosomes. J Biol Chem. 2008;283(17):11501–11508. doi: 10.1074/jbc.M800642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuentealba RA, Barria MI, Lee J, Cam J, Araya C, Escudero CA, Inestrosa NC, Bronfman FC, Bu G, Marzolo MP. ApoER2 expression increases Abeta production while decreasing Amyloid Precursor Protein (APP) endocytosis: Possible role in the partitioning of APP into lipid rafts and in the regulation of gamma-secretase activity. Mol Neurodegener. 2007;2:14. doi: 10.1186/1750-1326-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang GZ, Yang M, Lim Y, Lu JJ, Wang TH, Qi JG, Zhong JH, Zhou XF. Huntingtin associated protein 1 regulates trafficking of the amyloid precursor protein and modulates amyloid beta levels in neurons. J Neurochem. 2012;122(5):1010–1022. doi: 10.1111/j.1471-4159.2012.07845.x. [DOI] [PubMed] [Google Scholar]
- 25.Schneider A, Rajendran L, Honsho M, Gralle M, Donnert G, Wouters F, Hell SW, Simons M. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J Neurosci. 2008;28(11):2874–2882. doi: 10.1523/JNEUROSCI.5345-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nature reviews Molecular cell biology. 2002;3(12):919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- 27.Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nature reviews Molecular cell biology. 2008;9(7):574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- 28.Teasdale RD, Collins BM. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease. Biochem J. 2012;441(1):39–59. doi: 10.1042/BJ20111226. [DOI] [PubMed] [Google Scholar]
- 29.Schobel S, Neumann S, Hertweck M, Dislich B, Kuhn PH, Kremmer E, Seed B, Baumeister R, Haass C, Lichtenthaler SF. A novel sorting nexin modulates endocytic trafficking and alpha-secretase cleavage of the amyloid precursor protein. J Biol Chem. 2008;283(21):14257–14268. doi: 10.1074/jbc.M801531200. [DOI] [PubMed] [Google Scholar]
- 30.Okada H, Zhang W, Peterhoff C, Hwang JC, Nixon RA, Ryu SH, Kim TW. Proteomic identification of sorting nexin 6 as a negative regulator of BACE1-mediated APP processing. Faseb J. 2010;24(8):2783–2794. doi: 10.1096/fj.09-146357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muirhead G, Dev KK. The expression of neuronal sorting nexin 8 (SNX8) exacerbates abnormal cholesterol levels. J Mol Neurosci. 2014;53(1):125–134. doi: 10.1007/s12031-013-0209-z. [DOI] [PubMed] [Google Scholar]
- 32.Zhao YH, Wang YS, Yang JY, Wang X, Zhao YJ, Zhang X, Zhang YW. Sorting nexin 12 interacts with BACE1 and regulates BACE1-mediated APP processing. Mol Neurodegener. 2012;7:30. doi: 10.1186/1750-1326-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Huang T, Zhao Y, Zheng Q, Thompson Robert C, Bu G, Zhang Y-w, Hong W, Xu H. Sorting Nexin 27 Regulates Aβ Production through Modulating γ-Secretase Activity. Cell Rep. 2014;9(3):1023–1033. doi: 10.1016/j.celrep.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Huang T, Bu G, Xu H. Dysregulation of protein trafficking in neurodegeneration. Mol Neurodegener. 2014;9:31. doi: 10.1186/1750-1326-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danson C, Brown E, Hemmings OJ, McGough IJ, Yarwood S, Heesom KJ, Carlton JG, Martin-Serrano J, May MT, Verkade P, Cullen PJ. SNX15 links clathrin endocytosis to the PtdIns3P early endosome independently of the APPL1 endosome. J Cell Sci. 2013;126(Pt 21):4885–4899. doi: 10.1242/jcs.125732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwaya N, Takasu H, Goda N, Shirakawa M, Tanaka T, Hamada D, Hiroaki H. MIT domain of Vps4 is a Ca2+-dependent phosphoinositide-binding domain. J Biochem. 2013;153(5):473–481. doi: 10.1093/jb/mvt012. [DOI] [PubMed] [Google Scholar]
- 37.Phillips SA, Barr VA, Haft DH, Taylor SI, Haft CR. Identification and characterization of SNX15, a novel sorting nexin involved in protein trafficking. J Biol Chem. 2001;276(7):5074–5084. doi: 10.1074/jbc.M004671200. [DOI] [PubMed] [Google Scholar]
- 38.Barr VA, Phillips SA, Taylor SI, Haft CR. Overexpression of a novel sorting nexin, SNX15, affects endosome morphology and protein trafficking. Traffic. 2000;1(11):904–916. doi: 10.1034/j.1600-0854.2000.011109.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YW, Wang R, Liu Q, Zhang H, Liao FF, Xu H. Presenilin/gamma-secretase-dependent processing of beta-amyloid precursor protein regulates EGF receptor expression. Proc Natl Acad Sci U S A. 2007;104(25):10613–10618. doi: 10.1073/pnas.0703903104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mammen AL, Huganir RL, O'Brien RJ. Redistribution and stabilization of cell surface glutamate receptors during synapse formation. J Neurosci. 1997;17(19):7351–7358. doi: 10.1523/JNEUROSCI.17-19-07351.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang YW, Luo WJ, Wang H, Lin P, Vetrivel KS, Liao F, Li F, Wong PC, Farquhar MG, Thinakaran G, Xu H. Nicastrin is critical for stability and trafficking but not association of other presenilin/gamma-secretase components. J Biol Chem. 2005;280(17):17020–17026. doi: 10.1074/jbc.M409467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28(2):511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Zhao YJ, Zhang XF, Badie H, Zhou Y, Mu YL, Loo LS, Cai L, Thompson RC, Yang B, Chen YM, Johnson PF, Wu CB, Bu GJ, Mobley WC, Zhang DX, Gage FH, Ranscht B, Zhang YW, Lipton SA, Hong WJ, Xu HX. Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down's syndrome. Nat Med. 2013;19(4):473–480. doi: 10.1038/nm.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckervordersandforth R, Deshpande A, Schaffner I, Huttner HB, Lepier A, Lie DC, Gotz M. In vivo targeting of adult neural stem cells in the dentate gyrus by a split-cre approach. Stem cell reports. 2014;2(2):153–162. doi: 10.1016/j.stemcr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng L, Person RE, Huang W, Zhu PJ, Costa-Mattioli M, Beaud AL, et al. Truncation of Ube3a–ATS unsilences paternal Ube3a and ameliorates behavioral defects in the Angelman syndrome mouse model. PLoS genetics. 2013;9(12):e1004039. doi: 10.1371/journal.pgen.1004039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuroda K, Yamada S, Tanaka M, Iizuka M, Yano H, Mori D, Tsuboi D, Nishioka T, Namba T, Iizuka Y, Kubota S, Nagai T, Ibi D, Wang R, Enomoto A, Isotani-Sakakibara M, Asai N, Kimura K, Kiyonari H, Abe T, Mizoguchi A, Sokabe M, Takahashi M, Yamada K, Kaibuchi K. Behavioral alterations associated with targeted disruption of exons 2 and 3 of the Disc1 gene in the mouse. Hum Mol Genet. 2011;20(23):4666–4683. doi: 10.1093/hmg/ddr400. [DOI] [PubMed] [Google Scholar]
- 47.Funamoto S, Sasaki T, Ishihara S, Nobuhara M, Nakano M, Watanabe-Takahashi M, Saito T, Kakuda N, Miyasaka T, Nishikawa K, Saido TC, Ihara Y. Substrate ectodomain is critical for substrate preference and inhibition of gamma-secretase. Nat Commun. 2013;4:2529. doi: 10.1038/ncomms3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398(6727):518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 49.Jiang S, Li Y, Zhang X, Bu G, Xu H, Zhang YW. Trafficking regulation of proteins in Alzheimer's disease. Mol Neurodegener. 2014;9:6. doi: 10.1186/1750-1326-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cossec JC, Simon A, Marquer C, Moldrich RX, Leterrier C, Rossier J, Duyckaerts C, Lenkei Z, Potier MC. Clathrin-dependent APP endocytosis and Abeta secretion are highly sensitive to the level of plasma membrane cholesterol. Biochim Biophys Acta. 2010;1801(8):846–852. doi: 10.1016/j.bbalip.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Ghosal K, Vogt DL, Liang M, Shen Y, Lamb BT, Pimplikar SW. Alzheimer's disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc Natl Acad Sci U S A. 2009;106(43):18367–18372. doi: 10.1073/pnas.0907652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B, Arime Y, Hall FS, Uhl GR, Sora I. Impaired spatial working memory and decreased frontal cortex BDNF protein level in dopamine transporter knockout mice. Eur J Pharmacol. 2010;628(1–3):104–107. doi: 10.1016/j.ejphar.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prior M, Dargusch R, Ehren JL, Chiruta C, Schubert D. The neurotrophic compound J147 reverses cognitive impairment in aged Alzheimer's disease mice. Alzheimers Res Ther. 2013;5(3):25. doi: 10.1186/alzrt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.