Preface
The prognostic study for the Japanese Breast Cancer Society (JBCS) registry in 2006 was finally published here (Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, Sup. Table 1–9). The JBCS registry has been started from 1975. To 2003, for 29 years, 188,265 cases have been registered. With the cooperation of the Non-Profit Organization Japan Clinical Research Support Unit (J-CRSU) and the Public Health Research Foundation, we have moved to the new system by the web registration from 2004.
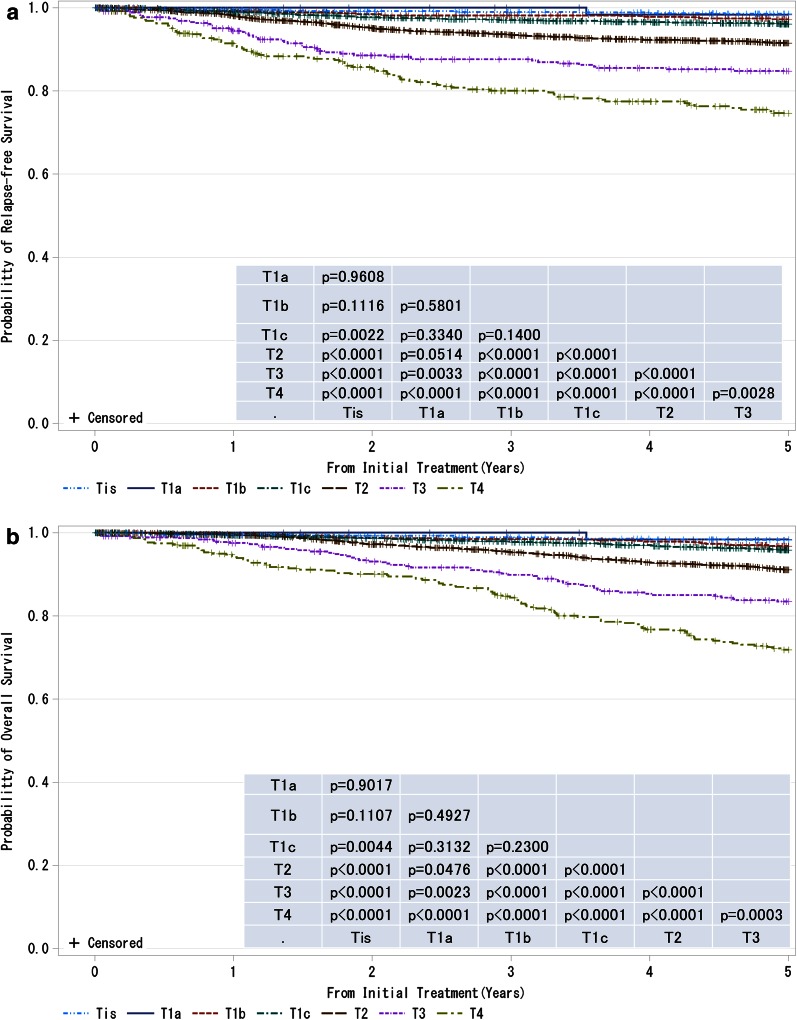
Fig. 1.
a, b Kaplan–Meier curves for relapse-free and overall survival of all cases by tumor classification (cT-category). P values were calculated using the log rank test. Tis non-invasive ductal carcinoma, lobular carcinoma in situ, or Paget disease, T1a ≤0.5 cm, T1b 0.5 < tumor ≤ 1.0 cm, T1c 1.0 < tumor ≤ 2.0 cm, T2 2.0 < tumor ≤ 5.0 cm, T3 >5.0 cm, T4 tumor of any size with direct extension to the chest wall and/or skin (ulceration or skin nodules) or inflammatory carcinoma
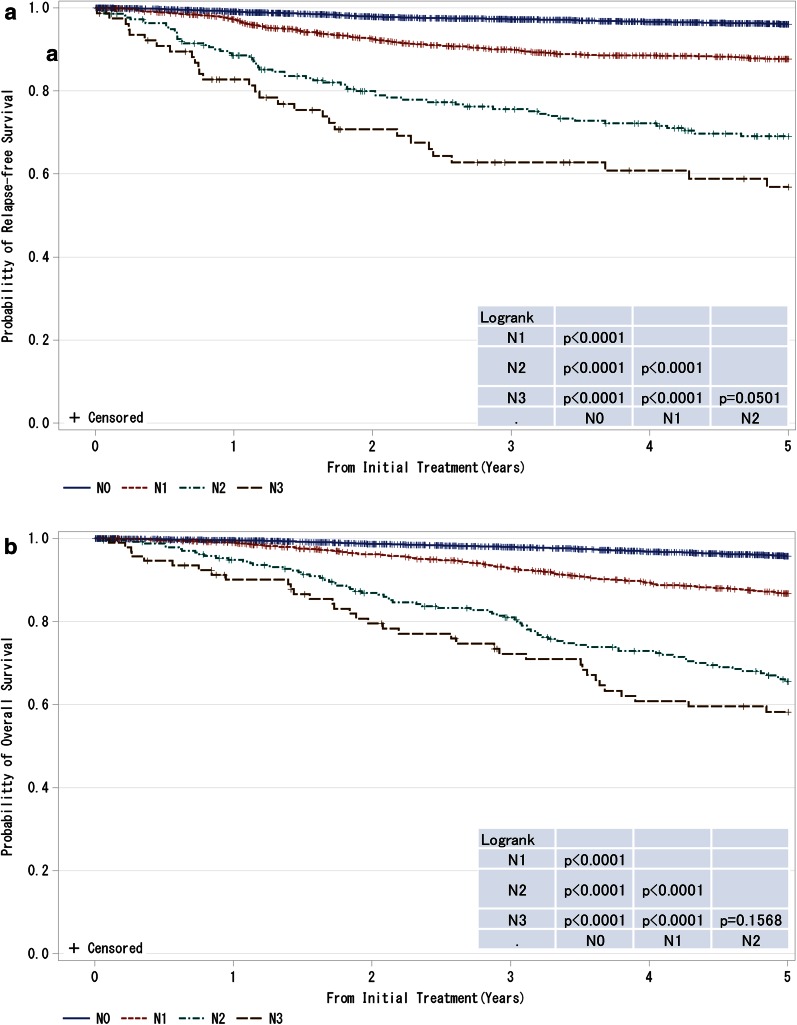
Fig. 2.
a, b Kaplan–Meier curves for relapse-free and overall survival of all cases by regional lymph nodes status (cN-category) N0 no regional lymph node metastases, N1 metastases in movable ipsilateral level I, II axillary lymph node(s), N2 metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted OR Metastases in clinically detected ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastases, N3 metastases in ipsilateral infraclavicular (level III axillary) lymph node(s) with or without level I, II axillary lymph node involvement OR Metastases in clinically detected ipsilateral internal mammary lymph node(s) with clinically evident level I, II axillary lymph node metastases OR Metastases in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement. P values were calculated using the log rank test
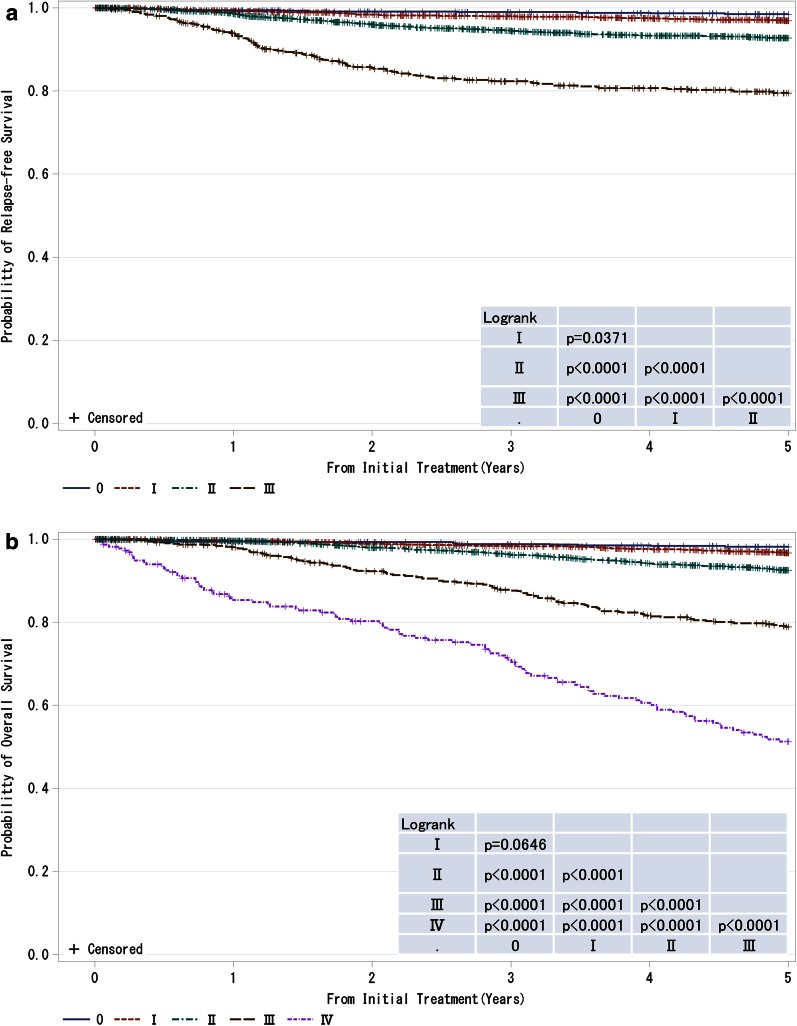
Fig. 3.
a, b Kaplan–Meier curves for relapse-free and overall survival of all cases by clinical stage (UICC). P values were calculated using the log rank test
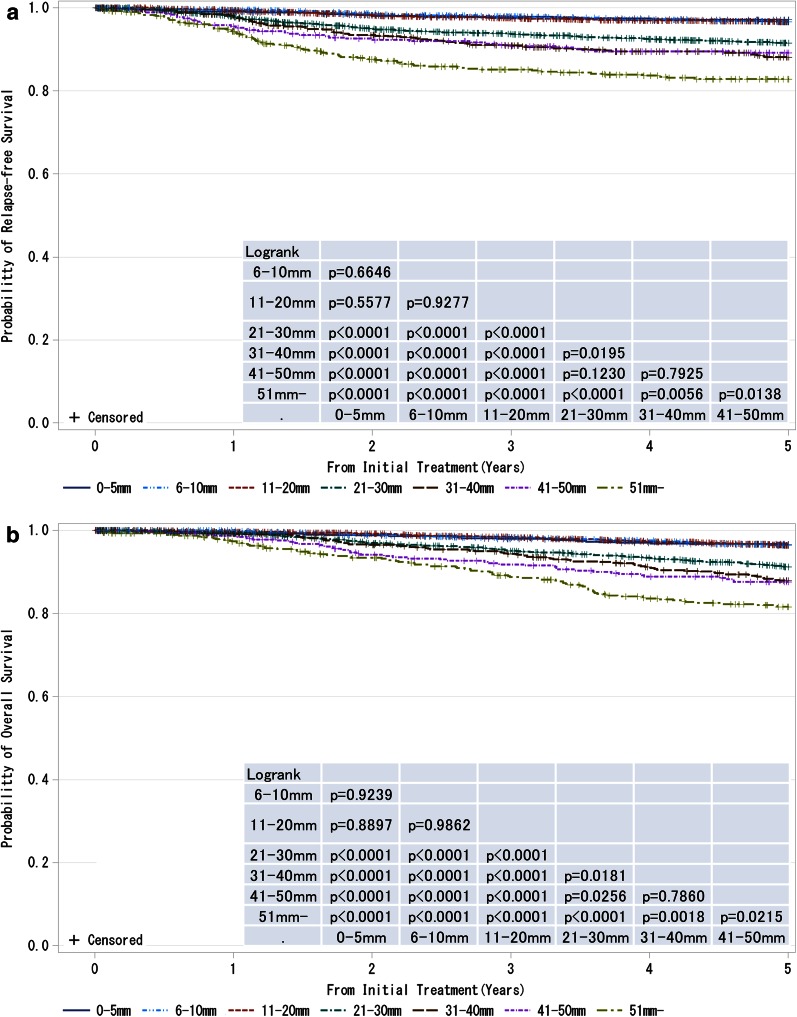
Fig. 4.
a, b Kaplan–Meier curves for relapse-free and overall survival of cases without neoadjuvant therapy by pathological tumor size (pT size). Tumor size is a marker of invasiveness. P values were calculated using the log rank test
Fig. 5.
a, b Kaplan–Meier curves for relapse-free and overall survival of cases without neoadjuvant therapy by the number of metastatic lymph nodes. P values were calculated using the log rank test
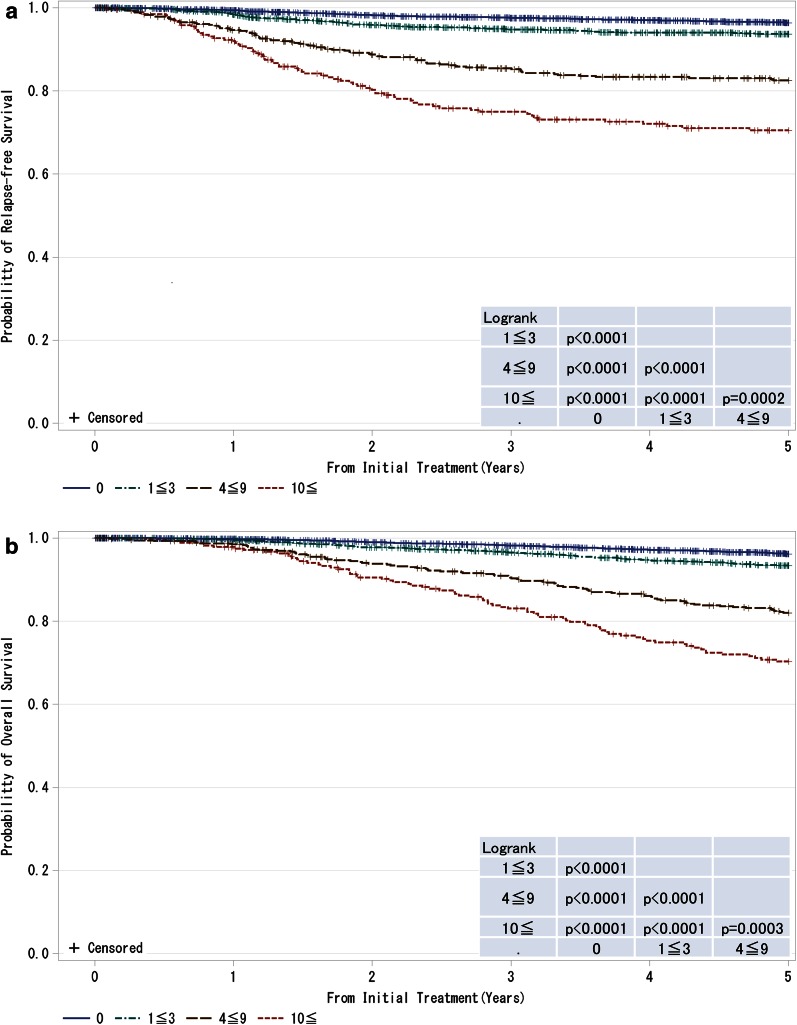
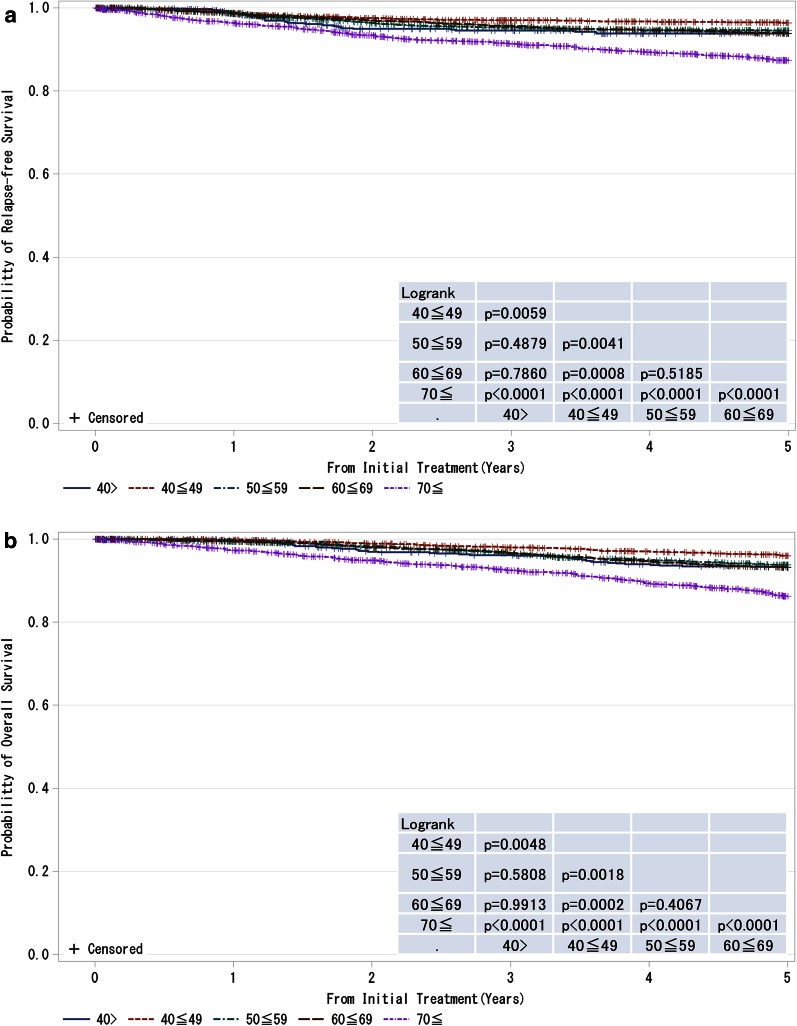
Fig. 6.
a, b Kaplan–Meier curves for relapse-free and overall survival of all cases by age. P values were calculated using the log rank test
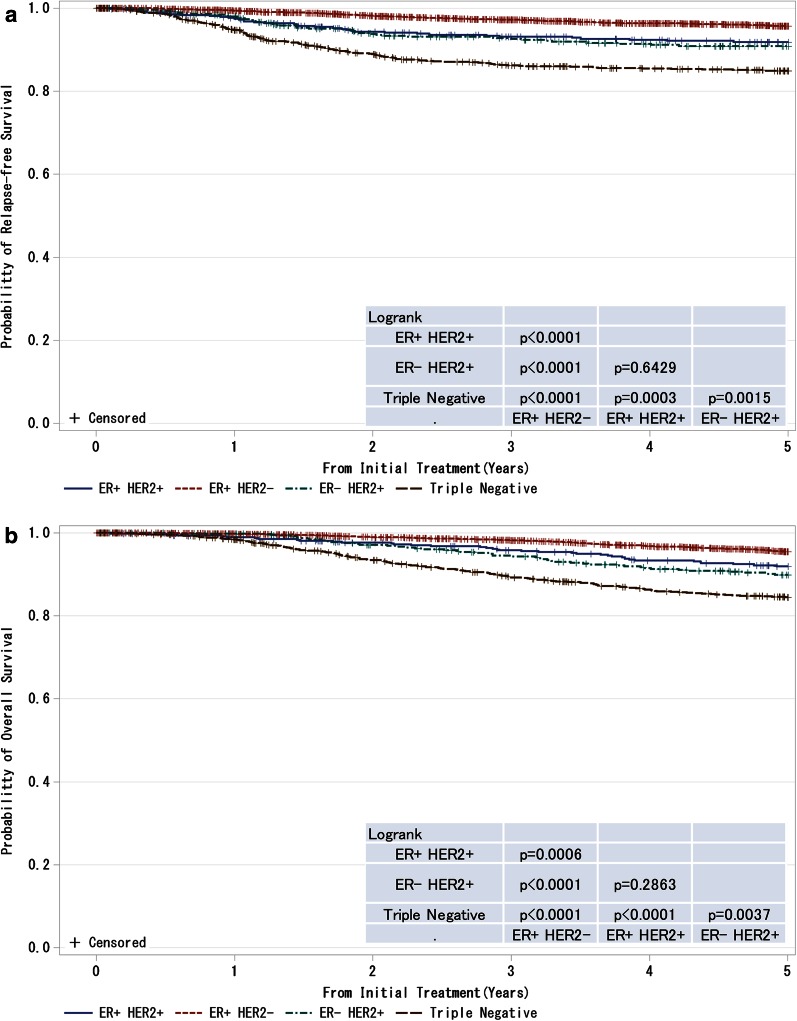
Fig. 7.
a, b Kaplan–Meier curves for relapse-free and overall survival of T1–T4, any N and M0 cases with respect to estrogen receptor (ER) status and HER2 (human epidermal growth factor receptor 2) amplification status. P values were calculated using the log rank test. Relapse-free survival and overall survival of patients with respect to combined ER and HER2 status
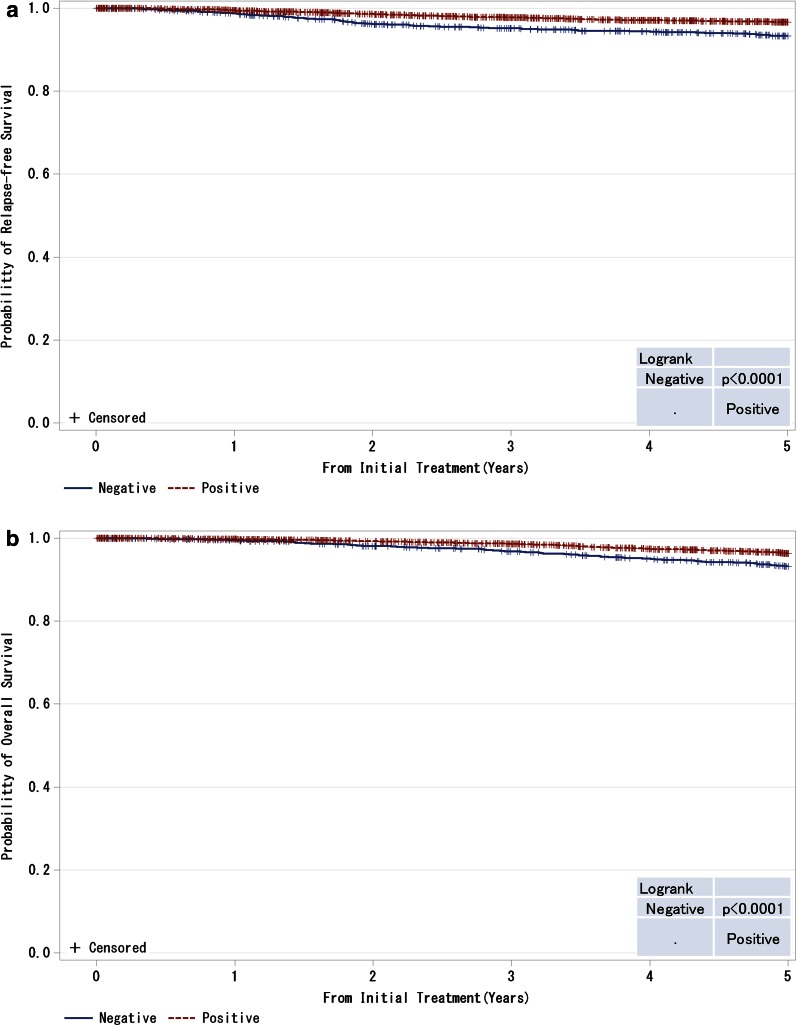
Fig. 8.
a, b Kaplan–Meier curves for relapse-free and overall survival of ER-positive and M0 cases by progesterone receptor (PgR) status. P values were calculated using the log rank test
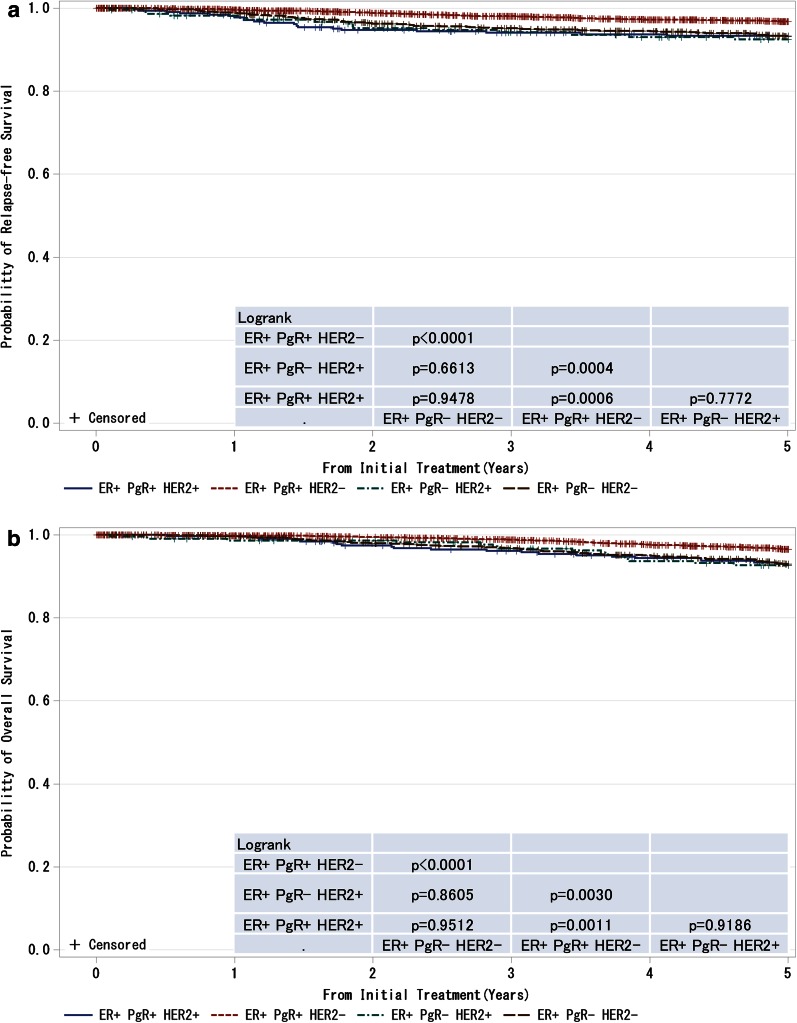
Fig. 9.
a, b Kaplan–Meier curves for relapse-free and overall survival of ER-positive and M0 cases with respect to PgR and HER2 amplifications. P values were calculated using the log rank test
In 2006, the number of the registry for institutions was 352 and cases were 22,005. The number of institutions in this prognostic study was 134 and cases were 8788, with 39.9 %. An assessment of 5-year prognosis for cases registered in 2006 has been carried out, and here we report the results thanks to a number of efforts and cooperation. We believe that it is necessary to further promote the registry for contributions to improving breast cancer care and prognosis.
Background characteristics of the patients are summarized in Table 1. The 5-year disease-free survival (DFS) was 93.5 %, and the 5-year overall survival (OS) was 92.7 % at a median follow-up of 60.0 months (range 0.0–60.0). The TNM classification and histological classification were registered according to the UICC staging [1] and WHO classification systems [2], respectively. The present report includes age- and subtype-based analyses in addition to the traditional TNM classification-based analyses.
Table 1.
Patient characteristics
| Age | ||
| Mean | S.D. | |
| 57.42 | 12.94 | |
| Tumor size(cm) | ||
| Mean | S.D. | |
| 2.60 | 2.05 | |
| Tumor size | Count | % |
| T0 | 95 | 1.08 |
| Tis | 729 | 8.3 |
| T1a | 68 | 0.77 |
| T1b | 742 | 8.44 |
| T1c | 2599 | 29.57 |
| T2 | 3043 | 34.63 |
| T3 | 369 | 4.2 |
| T4 | 390 | 4.44 |
| Unknown | 753 | 8.57 |
| N | ||
| N0 | 6758 | 76.90 |
| N1 | 1614 | 18.37 |
| N2 | 236 | 2.69 |
| N3 | 92 | 1.05 |
| Unknown | 88 | 1.00 |
| M | ||
| M0 | 8464 | 96.31 |
| M1 | 217 | 2.47 |
| Unknown | 107 | 1.22 |
| Stage | ||
| 0 | 700 | 7.97 |
| I | 3010 | 34.25 |
| II | 3336 | 37.96 |
| III | 622 | 7.08 |
| IV | 217 | 2.47 |
| Unknown | 903 | 10.28 |
| ER | ||
| Positive | 6514 | 74.12 |
| Negative | 2077 | 23.63 |
| Unknown | 197 | 2.24 |
| PgR | ||
| Positive | 5168 | 58.81 |
| Negative | 3404 | 38.73 |
| Unknown | 216 | 2.46 |
| HER2 | ||
| Positive | 1230 | 14.00 |
| Negative | 6759 | 76.91 |
| Unknown | 799 | 9.09 |
The TNM classification was identified by the UICC staging system
ER estrogen receptor, ER estrogen receptor, PgR progesterone receptor, HER2 human epidermal growth factor receptor 2
In addition to TNM classifications, estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) statuses, which are strong prognostic factors, have become frequently used to determine the therapeutic strategy in the clinical setting. Note that during the study period, not only the cutoff level of ER, PgR and HER2 positivity, but also test procedures for immunostaining and HER2 gene amplification have not yet been standardized, and trastuzumab had been gradually spread in daily clinic in Japan. For ER-negative/HER2-positive patients, DFS improved from 85.0 % in 2004 to 90.9 % in 2006, and OS from 85.02 to 89.88 %, respectively.
We appreciate the considerable support that we have received and would like to ask for continuing understanding and support of the registry.
Electronic supplementary material
Acknowledgments
This work was supported partly by JSPS KAKENHI Grant Numbers 15H04796. The authors thank all the affiliated institutes participating in the Breast Cancer Registry of the JBCS for their efforts to register the patients’ data. We extend our gratitude to staff members working at the three major institutions that contributed extensively to the present study: Sagara Hospital (Kagoshima), National Cancer Center Hospital (Tokyo), and Niigata Cancer Center Hospital (Niigata). We would like to ask for support from as many institutions as possible in the future to improve response rates in our survey on breast cancer prognosis. We also thank Dr. Muneaki Sano, a former director of Niigata Cancer Center Hospital, for his dedication to establishing the new registration system, and the staff members at the Japan Clinical Research Support Unit (J-CRSU) and the Public Health Research Foundation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6. New York: Springer; 2002. [Google Scholar]
- 2.World Health Organization . Tumours of the Breast and Female Genital Organs. Oxford: Oxford University Press; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.