Abstract
Objective: This pilot study evaluates efficacy of omega-3 fatty acid supplementation (Ω3), individual family psychoeducational psychotherapy (IF-PEP), and their combination in youth with subsyndromal bipolar disorders (bipolar disorder not otherwise specified [BP-NOS], cyclothymic disorder [CYC]).
Methods: This study was a 12 week, randomized trial of Ω3 versus placebo and IF-PEP versus active monitoring (AM) using a 2 × 2 design (Ω3 + PEP: n = 5; Ω3 + AM: n = 5; placebo + PEP: n = 7; placebo + AM: n = 6). Twenty-three youth ages 7–14 with BP-NOS or CYC were recruited via community advertisements and clinician referrals. Participants could be taking stable medication for attention-deficit/hyperactivity disorder and sleep aids, but no other psychotropics. Independent evaluators assessed participants at screen, baseline, and 2, 4, 6, 9, and 12 weeks. Primary outcome measures were the Kiddie Schedule for Affective Disorders (K-SADS) Depression (KDRS) and Mania (KMRS) Rating Scales, Children's Depression Rating Scale-Revised (CDRS-R), and Young Mania Rating Scale (YMRS). Ω3/placebo conditions were double-blind; independent evaluators were blind to psychotherapy condition.
Results: Most participants (83%) completed the 12 week trial. Side effects were uncommon and mild. Intent-to-treat analyses indicated significant improvement in depressive symptoms (KDRS) for combined treatment relative to placebo and AM (p = 0.01, d = 1.70). Across groups, manic symptoms improved over time without significant treatment effects. Effect of IF-PEP on child depression compared with AM was medium (d = 0.63, CDRS-R) to large (d = 1.24, KDRS). Effect of Ω3 on depression was medium (d = 0.48, KDRS).
Conclusion: IF-PEP and Ω3 are well tolerated and associated with improved mood symptoms among youth with BP-NOS and CYC.
Clinicaltrials.gov Identifier: NCT01507753
Introduction
Childhood-onset bipolar disorder (BP) can lead to greater impairment later in life than adult-onset BP (Perlis et al. 2009). It is important to develop/identify efficacious treatments for BP in children. Originally considered milder versions of BP, “subsyndromal” presentations of BP (bipolar disorder not otherwise specified [BP-NOS] and cyclothymic disorder [CYC]), which may not include a sufficient number of symptoms or duration to meet criteria for BP-I and BP-II, are now known to be highly impairing (Axelson et al. 2006; Van Meter et al. 2012, 2013;).
Two large phenomenologic studies, Course and Outcomes of Bipolar Youth (COBY) and Longitudinal Assessment of Manic Symptoms (LAMS), have used clear operational definitions to describe BP-NOS. COBY demonstrated that youth with BP-NOS (n = 153), BP-I (n = 255) and BP-II (n = 30) do not differ in age of onset, years having experienced manic or depressive symptoms, severity of worst week of manic and depressive symptoms, comorbidities (except anxiety disorders, which were highest in youth with BP-II), suicidal ideation, and family history of mental illness (Axelson et al. 2006). Youth with BP-I, however, had more functional impairment, suicide attempts, psychosis, and hospitalization than youth with BP-NOS. In longitudinal analyses, approximately one third of youth with BP-NOS met criteria for BP-I or BP-II within 2–4 years (Axelson et al. 2011). Additionally, BP-NOS manifested longer time to recovery, more frequent mood shifts, and more time in subsyndromal mood states than BP-I and BP-II (Axelson et al. 2006; Birmaher et al. 2006). Similarly, results from LAMS, a prospective study of youth with elevated symptoms of mania (Findling et al. 2010), indicated that youth with BP-NOS/CYC (n = 88) and those with BP-I (n = 71) do not differ in current symptom severity, functional impairment, parental psychiatric history, or rates of elated mood (Hafeman et al. 2013). Both youth with BP-NOS/CYC and those with BP-I in LAMS were more symptomatic and impaired and more likely to have a parent with a history of mania than youth with no bipolar spectrum disorder (BPSD) (n = 545) (Hafeman et al. 2013). A large CYC validation study including 894 youth demonstrated that youth with CYC have greater irritability, more comorbidity, more sleep problems, and were more likely to have a family history of BP than youth with non-BP disorders. Additionally, CYC was associated with earlier onset than depression or BP-II (Van Meter et al. 2013). Although research on BP-NOS and, even more so CYC, is limited, these findings indicate that BP-NOS and CYC are impairing disorders on a continuum with BP-I/II.
Despite mounting evidence of impairment and prevalence of BP-NOS/CYC, clinical trials have focused on BP-I. No clinical guidelines exist for the treatment of BP-NOS/CYC. Available evidence-based pharmacotherapy guidelines are for BP-I (Kowatch et al. 2009). Additionally, whereas efficacious antimanic agents have been identified, no study has demonstrated an effective antidepressant agent for youth with bipolar depression. Most pharmacotherapy studies have focused on adolescents with BP-I. Of the few that included BP-NOS, these youth were a small minority of the sample (DelBello et al. 2007; Geller et al. 2012; Joshi et al. 2012).
Further, efficacious medications are, unfortunately, associated with significant risk for adverse events (Fleischhaker et al. 2006; Kowatch et al. 2009). In a study monitoring side effects, atypical neuroleptics resulted in drowsiness and decreased motor activity; 30–60% of youth taking clozapine experienced constipation, increased salivation, orthostatic hypotension, and nasal congestion. Of patients taking olanzapine and risperidone, 5–15% experienced rigidity, tremor, and dystonia. All participants gained weight (Fleischhaker et al. 2006). A review of 24 trials of mood stabilizer and antipsychotic medication for pediatric BPSD found statistically and clinically significant weight increases in 18 (75%) (Correll 2007); and weight gain is often followed by increased cardiometabolic risk (Maayan and Correll 2011).
Previous research has demonstrated that omega-3 (Ω3) fatty acids have beneficial mood effects with little evidence of adverse side effects or deleterious drug interactions (Young and Martin 2003; Sarris et al. 2012). This suggests that Ω3 might function as either primary or adjunctive treatment with a more favorable risk–benefit ratio for children with BP-NOS/CYC than currently available drugs (Young and Martin 2003). Neuroimaging studies show that people with higher Ω3 intake have greater gray matter volume in the anterior cingulate cortex, right hippocampus, and right amygdala; these areas are involved in emotion arousal and regulation and are reduced in people with mood disorders (Conklin et al. 2007).
Ω3 may improve symptoms of many psychiatric disorders in youth including mood disorders (Wozniak et al. 2007; Clayton et al. 2009; McNamara et al. 2010; Sarris et al. 2012), autism (Amminger et al. 2007), attention-deficit/hyperactivity disorder (ADHD) (Sinn and Bryan 2007; Sorgi et al. 2007), and psychosis (Amminger et al. 2010). Ω3 has also been shown to significantly improve cardiovascular and metabolic health and decrease body fat, both independently and in combination with regular exercise (Hill et al. 2007). The latter is particularly important, as many current treatments for mood stabilization are associated with significant weight gain, obesity, and metabolic disorders.
A recent meta-analysis of RCTs examining Ω3 as an adjunctive treatment for BPSD in children and adults demonstrated a significant effect of Ω3 on depressive symptoms (effect size [ES] = 0.34, p = 0.029) and a trend on manic symptoms (ES = 0.20, p = 0.099) (Sarris et al. 2012). There are three Ω3 supplementation trials in youth with BPSD (Gracious 2006; Wozniak et al. 2007; Clayton et al. 2009; Gracious et al. 2010). An RCT of flax seed oil (alpha-linolenic acid [ALA]) versus olive oil as an adjunctive to lithium in youth ages 6–17 with BP-I/II found no significant difference on clinician-rated depression and mania. The authors hypothesized that individual variation in conversion from ALA to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) may explain the lack of findings by treatment group, emphasizing the importance of studying EPA and DHA directly (Gracious 2006, 2010). A 6 week open trial of Ω3 monotherapy (360 mg EPA, 1560 mg DHA) in youth with BP-I, BP-II,, or BP-NOS demonstrated significant improvements in clinician ratings of depression, mania, and global functioning and in parent-rated internalizing and externalizing behaviors (Clayton et al. 2009). Another open label trial examined 1.3–4.3 g of Ω3 monotherapy (7:1 EPA:DHA) over 8 weeks in 6–17-year-olds with BPSD (Wozniak et al. 2007). Both manic and depressive symptoms decreased significantly; youth who took ≥2.0 g of Ω3/day showed greater improvements than those who took <2.0 g/day (Wozniak et al. 2007). Side effects reported in these studies were primarily gastrointestinal and mild.
Previous research has indicated that medication and psychotherapy in combination are advantageous in treating youth with anxiety (Walkup et al. 2008), adolescent depression (March et al. 2004; The TADS Team 2007), autism (Aman et al. 2009), and ADHD (MTA Cooperative Group 1999a,b; 2004a,b). Whereas combination treatment is also recommended for youth with BPSD (McClellan et al. 2007; McNamara et al. 2010), it has not been examined in children with BP-NOS/CYC in a controlled trial. Because of adverse effects with mood stabilizers, antipsychotics, antidepressants, and psychostimulants in pediatric BPSD, McNamara and colleagues have suggested that safer and well-tolerated interventions with demonstrated efficacy be used to treat prodromal/subsyndromal BPSD (McNamara et al. 2010).
Ω3 and family-focused therapy provide this safety and efficacy. Previous research provides support for the efficacy of adjunctive family-focused psychoeducation and skills-based interventions for youth with BPSD, including psychoeducational psychotherapy (PEP), family-focused treatment, and child- and family-focused cognitive behavioral therapy (Fristad and MacPherson 2014; West et al. 2014). PEP has the strongest evidence base for treating children ≤12 years of age with BPSD (Fristad et al. 2002, 2003; Fristad 2006; Fristad et al. 2009; Mendenhall et al. 2009; Fristad and MacPherson 2014). Key goals of PEP are to provide psychoeducation about mood disorders and treatments, provide social support, and build skills in symptom management, emotion regulation, problem solving and communication. Fristad and colleagues have developed a PEP manual (Fristad et al. 2011c) and completed three previous randomized controlled trials (RCTs) (Fristad et al. 2002, 2003; Fristad 2006; Fristad et al. 2009; Mendenhall et al. 2009) and an effectiveness trial (MacPherson et al. 2014). These trials have demonstrated PEP's efficacy in improving mood symptoms, family interactions, parent understanding of mood disorders, access to quality mental healthcare, and high degrees of treatment satisfaction.
In summary, BP-NOS and CYC are highly impairing but lack evidence-based pharmacotherapy guidelines. First-line psychotropics pose significant risks, whereas Ω3 and psychotherapy are both safe and have potential benefits. Therefore, this trial investigated the feasibility and efficacy of Ω3 supplementation and psychotherapy (PEP) alone or in combination, relative to placebo (PBO) and active monitoring (AM), in treating youth with BP-NOS/CYC. We hypothesized that: 1) Youth receiving combined therapy would have faster rates of improvement in mood symptoms over 12 weeks than youth receiving no active treatment, or those receiving Ω3 or PEP monotherapy; 2) Ω3 would show greater improvement than PBO; and 3) PEP would demonstrate greater improvement than AM. We also expected that the active treatments would be well tolerated with >80% compliance for Ω3 and PEP sessions.
Methods
Participants
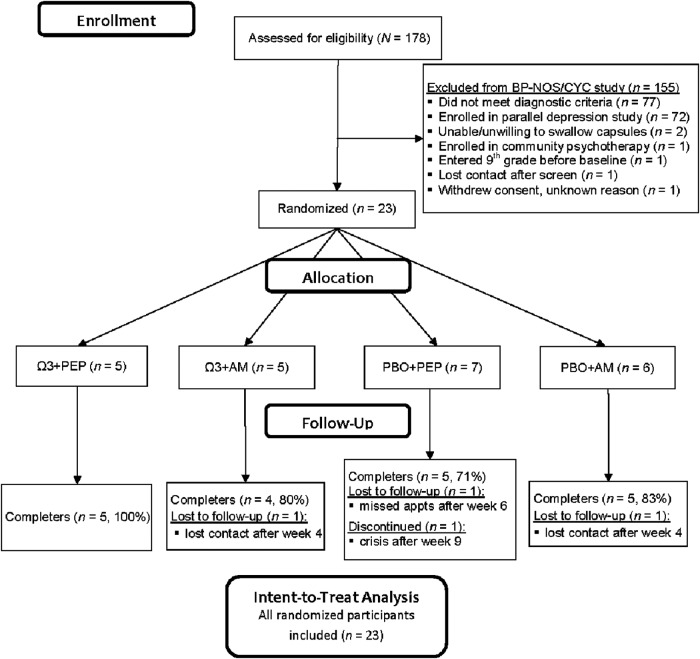
Participants were recruited for two parallel studies between July 2011 and May 2014, primarily from community advertisements and clinician referrals. Inclusion criteria for the current study were: 1) Diagnosis of BP-NOS or CYC, 2) age 7–14 years, 3) intelligence quotient (IQ) ≥70, 4) ability to swallow study capsules, and 5) one or more parent/caregiver (hereafter referred to as parent) and child completed the screening assessment and were willing and able to participate. Exclusion criteria were: 1) Major medical disorder, 2) autism, 3) psychotic states warranting antipsychotic medication, 4) active suicidal concern (passive suicidal ideation without plans/intent was permitted), 5) three or more “marked” or “severe” mood symptoms on the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS), 6) mental health intervention (pharmacotherapy and/or psychotherapy except for ADHD medication or sleep aids) in the month preceding randomization, and 7) enrollment in ninth grade or higher. Of 178 youth screened, 23 were enrolled and randomized (Fig. 1).
FIG. 1.
Consolidated Standards of Reporting Trials (CONSORT) chart of participation in a 12 week trial of IF-PEP and Ω3. BP-NOS/CYC, bipolar disorder not otherwise specified/cyclothymic disorder; Ω3, omega-3; AM, active monitoring; PBO, placebo; IF-PEP, individual-family psychoeducational psychotherapy. Total screens are for two parallel studies, this one for subsyndromal BP, and another for depression.
Procedures
Parents provided informed permission, and children provided assent prior to the screening assessment, as approved by the local institutional review board. Eligible youth participated in a baseline assessment (week 0), then were block-randomized into a 12 week clinical trial of Ω3 versus PBO and PEP versus active AM using a 2 × 2 design (Ω3 + PEP: n = 5; Ω3 + AM: n = 5; PBO + PEP: n = 7; PBO + AM: n = 6). Families participated in five additional assessments at the end of weeks 2, 4, 6, 9, and 12. Participants randomized into PEP participated in twice-weekly IF-PEP therapy sessions; AM groups attended assessments only. All participants willing to attend assessments were retained in the study for the intent-to-treat analyses.
Interviewers
Interviewers were graduate students pursuing a doctorate in clinical child psychology or postdoctoral clinicians. Interviewer training included didactics, mock interviews, and observing and rating videotaped and live interviews. Personnel interviewed independently after reliability was achieved in observed interviews. The Children's Interview for Psychiatric Syndromes (ChIPS) mean κ was 0.86, whereas mean intraclass correlations ranged from 0.81 on the KMRS to 0.89 on the KDRS. Following each assessment, interviewers prepared reports to review in a consensus conference with a co-principal investigator (Co-PI), during which the Co-PI reviewed and verified symptoms, diagnoses, and appropriateness of study admission.
Assessment
Youth and parent(s) were interviewed separately. The assessment battery included semistructured diagnostic interviews, rating scales, and questionnaires, described subsequently.
Demographics
At screen, parents provided information about the youth's sex, age, family structure, socioeconomic status (SES), and parents' ages.
Diagnosis
At each assessment, youth and parents participated in semistructured interviews using the K-SADS-PL KDRS and KMRS to assess youth's depressive and manic symptoms, respectively (Chambers et al. 1985; Kaufman et al. 1997; Axelson et al. 2003). Using information from these measures, CYC was diagnosed using Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria (American Psychiatric Association 1994) BP-NOS was diagnosed using the LAMS/COBY definition: Elated or irritable mood plus ≥2 (≥3, if mood is only irritable) associated manic symptoms, clear change in functioning with impairment, duration of ≥4 hours within a 24 hour period and totaling ≥4 cumulative lifetime days, not meeting criteria for BP-I/II.
KDRS and KMRS ratings are “filtered;” that is, symptoms are only rated if they are associated with and occur during a mood episode. Therefore, symptomatic behavior not associated with the episode is excluded. KDRS scores summed the following: Depressed mood, irritability, guilt, negative self-image, anhedonia, fatigue, difficulty concentrating, psychomotor agitation, psychomotor retardation, insomnia, hypersomnia, anorexia, increased appetite, and suicidal ideation. KMRS sums included: Elation, irritability, mood lability, decreased need for sleep, racing thoughts/flight of ideas, unusually energetic, increased goal-directed activity/motor hyperactivity, grandiosity, pressured/increased speech, poor judgment, distractibility, hallucinations, and delusions. The Children's Interview for Psychiatric Syndromes– Child and Parent Forms (ChIPS/P-ChIPS), a reliable and valid measure also administered at screen, assessed DSM-IV non-mood diagnoses (Fristad et al. 1998a,b,c; Teare et al. 1998a,b; Weller et al. 1999a,b; 2000). A study physician or nurse practitioner completed a physical examination and medical history at screen.
Symptom severity
The 17-item Children's Depression Rating Scale-Revised (CDRS-R) and the 11-item Young Mania Rating Scale (YMRS), both semistructured interviews, were administered at each assessment. They provide “unfiltered” ratings, or ratings of symptom severity as it presented in the past 2 weeks, regardless of the presence of a mood episode. Both are reliable and valid measures (Young et al. 1978; Poznanski et al. 1984; Fristad et al. 1992, 1995; Youngstrom et al. 2003). (For a thorough discussion of “filtered” versus “unfiltered” ratings, see Yee et al. 2014).
IQ
The Kaufman Brief Intelligence Test – 2nd Edition (KBIT-2), a standardized test estimating cognitive ability (Kaufman and Kaufman 2004), was administered at screen to determine study eligibility.
Ω3 Safety and acceptability
When enrolled, parents were made aware of potential Ω3 side effects (e.g., fishy burps, upset stomach, nausea, diarrhea) and asked to report any concerns to the study coordinator at or between assessments. At each interview, parents completed a side effects questionnaire about changes in the child's health since the last interview and the severity of any side effects on a scale from 0 (absent) to 6 (severe).
Ω3 supplementation
OmegaBrite (www.omegabrite.com; Las Vegas, NV) provided study capsules. Families received a pill organizer at each visit containing capsules and daily multivitamin/mineral tablets to standardize nutritional levels. No other nutritional supplements were permitted the month prior to or during study treatment. Ω3 groups received two 500 mg Ω3 capsules (350 mg EPA, 50 mg DHA; 100 mg other Ω3) twice daily for a total daily dose of 2000 mg of Ω3 (1400 mg EPA, 200 mg DHA; 400 mg other). PBO groups received two capsules twice daily matched for odor and appearance. At each visit, parents were asked to inform study staff of any discarded pills to ensure accurate adherence calculations (capsules consumed/ capsules instructed to consume).
IF-PEP
Families who randomized into IF-PEP attended two sessions weekly (one child session in which the parent participated at the beginning and end and one parent-only session), each typically 45–50 minutes in length. Therapists (four post-doctoral clinicians) used the treatment manual and received training and weekly supervision with a Co-PI (MAF). Parents and children received workbooks containing session content (outlined in Table 1), activity worksheets, and between-session projects (Fristad et al. 2011a,b). Recorded therapy sessions were rated for fidelity to core PEP concepts. Child session fidelity was 75%, parent session fidelity was 76%, and overall fidelity was 75%, indicating that, on average, therapists were moderately to highly adherent.
Table 1.
Individual-Family Psychoeducational Psychotherapy (IF-PEP) Sessions
| Session | Parent content | Child content |
|---|---|---|
| 1 | Childhood mood disorders and symptoms | Childhood mood disorders and symptoms |
| 2 | Medication classes and indications, monitoring effectiveness and adverse effects | Treatments, being an active member of the treatment team, and “Naming the Enemy” (differentiating personality from symptoms) |
| 3 | Mental health and school services | Healthy habits: Sleeping, eating, and exercising (choose 1 or 2) |
| 4 | Identifying and improving negative family cycles | Building a “Tool Kit” to manage symptoms |
| 5 | Problem-solving and basic coping skills | “Thinking, Feeling, Doing”: Understanding the connection among thoughts, feelings, and actions |
| 6 | Revisit school and mental health treatment (optional) | “Stop, Think, Plan, Do, Check”: Developing problem-solving skills |
| 7 | Meeting with school personnel (optional) | Healthy habits: Sleeping, eating, and exercising (choose 1 or 2) |
| 8 | Nonverbal and verbal communication skills | Nonverbal communication skills |
| 9 | Symptom management and family preservation skills | Verbal communication skills |
| 10 | Sibling session: Psychoeducation, support for sibling (optional) | Review and graduate |
| In the Bank | Up to four more sessions to review content, address comorbidities or crises | |
See Fristad et al. (2011b).
Study blind
Participants, independent evaluators, therapists, and study staff who had contact with families were blinded to participants' PBO versus Ω3 status. Staff not directly involved with study families filled pill organizers. Independent evaluators were additionally blinded to psychotherapy condition. One Co-PI (MAF) supervised the therapists whereas the other (LEA) remained blind to therapy condition and completed all consensus conferences with independent evaluators occurring after randomization.
Data analytic plan
Univariate ANOVA and χ2 tests analyzed sample screen characteristics by treatment group. Linear mixed effects (LME) models were fit to each of the main outcome variables (KDRS, KMRS, CDRS-R, YMRS) using the full intent-to-treat sample to examine treatment group differences in mood trajectories. Intercepts and slopes were modeled as random effects. Fixed effects were treatment group (contrast coded relative to PBO+AM), time since randomization, and group × time. Additional LME models were run with planned contrasts of combined treatment versus Ω3 or PEP monotherapy as well as contrasts comparing the groups who received Ω3 (Ω3 + PEP and Ω3 + AM groups) with those who received PBO (PBO + PEP and PBO + AM groups) and PEP (Ω3 + PEP and PBO + PEP groups) versus AM (Ω3 + AM and PBO + AM groups). Effect sizes were calculated with treatment group × time slopes using methods described by Feingold (Feingold 2009). Analyses were conducted using IBM SPSS Statistics version 22, with α < 0.05 as the cutoff for statistical significance. Corrections were not made for the multiple planned comparisons, as this was a pilot study.
Results
Study sample
The sample was 57% male and primarily white (74%) with a mean age of 10.2 ± 2.2 years. Most parents were biological parents (83%), female (96%), and middle aged (mean = 38.8 ± 7.4 years); families were lower to middle SES (39% Medicaid status; 35% ≤ $40,000 household income). All participants had comorbidity; the most frequent comorbid diagnoses were anxiety (83%), ADHD (74%), and disruptive behavior disorders (65%). Of the demographic and clinical characteristics examined (Table 2), only child sex differed by group, with a greater proportion of boys in PBO and AM (100%) than PBO and PEP (14.3%), χ2(2) = 11.37, p < 0.05.
Table 2.
Participant Characteristics by Treatment Group
| Total (n = 23) | Ω3+PEP (n = 5) | Ω3+AM (n = 5) | PBO+PEP (n = 7) | PBO+AM (n = 6) | |
|---|---|---|---|---|---|
| Screen characteristics | Mean ± SD or n (column %) | ||||
| Child age | 10.2 ± 2.2 | 9.9 ± 1.7 | 10.3 ± 2.5 | 9.6 ± 1.8 | 10.9 ± 3.0 |
| Child sex: Malea | 13 (56.5) | 4 (80.0) | 2 (40.0) | 1 (14.3) | 6 (100.0) |
| Child race | |||||
| White | 17 (73.9) | 3 (60.0) | 3 (60.0) | 6 (85.7) | 5 (83.3) |
| Black/African American | 3 (13.0) | 1 (20.0) | 1 (20.0) | 0 | 1 (16.7) |
| Asian | 1 (4.3) | 1 (20.0) | 0 | 0 | 0 |
| Bi/multiracial | 2 (8.7) | 0 | 1 (20.0) | 1 (14.3) | 0 |
| Child insurance: Medicaid | 9 (39.1) | 0 | 3 (60.0) | 3 (42.9) | 3 (50.0) |
| Parent age | 38.8 ± 7.4 | 42.8 ± 5.1 | 38.8 ± 9.7 | 36.0 ± 5.2 | 37.8 ± 8.8 |
| Parent relationship | |||||
| Biological parent | 19 (82.6) | 4 (80.0) | 4 (80.0) | 6 (85.7) | 5 (83.3) |
| Adoptive parent | 2 (8.7) | 1 (20.0) | 1 (20.0) | 0 | 0 |
| Grandparent | 2 (8.7) | 0 | 0 | 1 (14.3) | 1 (16.7) |
| Parent sex: Female | 22 (95.7) | 4 (80.0) | 5 (100.0) | 7 (100.0) | 6 (100.0) |
| Family structure | |||||
| Married parents | 7 (30.4) | 3 (60.0) | 1 (20.0) | 2 (28.6) | 1 (16.7) |
| Single parent | 10 (43.4) | 2 (40.0) | 4 (80.0) | 2 (28.6) | 2 (33.3) |
| Step family | 3 (13.0) | 0 | 0 | 1 (14.3) | 2 (33.3) |
| Other | 3 (13.0) | 0 | 0 | 2 (28.6) | 1 (16.7) |
| Household income ($) | |||||
| < 20,000 | 5 (21.7) | 0 | 2 (40.0) | 2 (28.6) | 1 (16.7) |
| 20,000–40,000 | 3 (13.0) | 0 | 1 (20.0) | 2 (28.6) | 0 |
| 40,000–60,000 | 4 (17.4) | 1 (20.0) | 1 (20.0) | 1 (14.3) | 1 (16.7) |
| 60,000–80,000 | 4 (17.4) | 3 (60.0) | 0 | 1 (14.3) | 0 |
| 80,000–100,000 | 4 (17.4) | 1 (20.0) | 0 | 0 | 3 (50.0) |
| > 100,000 | 3 (13.0) | 0 | 1 (20.0) | 1 (14.3) | 1 (16.7) |
| Comorbid disorders | |||||
| Anxiety disorder | 19 (82.6) | 5 (100.0) | 3 (60.0) | 6 (85.7) | 5 (83.3) |
| ADHD | 17 (73.9) | 5 (100.0) | 2 (40.0) | 5 (71.4) | 5 (83.3) |
| DBD | 15 (65.2) | 4 (80.0) | 3 (60.0) | 4 (57.1) | 4 (66.7) |
| Number of diagnoses | 4.3 ± 1.1 | 4.8 ± 0.4 | 3.6 ± 1.5 | 4.0 ± 0.8 | 4.8 ± 1.0 |
| Full scale IQ | 104.4 ± 16.7 | 105.0 ± 20.8 | 96.2 ± 15.5 | 112.4 ± 14.0 | 101.5 ± 17.1 |
| Study completer: Yes | 19 (82.6) | 5 (100.0) | 4 (80.0) | 5 (71.4) | 5 (83.3) |
| Psychotropics taken during trial | |||||
| ADHD medication | 6 (26.1) | 2 (40.0) | 1 (20.0) | 1 (14.3) | 2 (33.3) |
| Sleep aid | 5 (21.7) | 3 (60.0) | 1 (20.0) | 0 | 1 (16.7) |
Child sex differed by group with a greater proportion of boys in PBO+AM than PBO+PEP, χ2(2) = 11.37, p < 0.05.
Ω3, omega-3; ADHD, attention-deficit/hyperactivity disorder; AM, active monitoring; DBD, disruptive behavior disorder; IQ, intelligence quotient; PBO, placebo; PEP, individual-family psychoeducational psychotherapy.
Adherence, attrition, and acceptability
Participant adherence to study capsules was high: 97% Ω3 and PEP, 89% Ω3 and AM, 92% PBO and PEP, and 89% PBO and AM. These group differences were nonsignificant (p > 0.05). Families randomized to IF-PEP completed 15.8 ± 4.0 sessions of the 17 regular sessions.
All 23 randomized participants remained in the study through week 4; 19 (83%) completed the 12 week trial; group differences in attrition were nonsignificant (Fig. 1).
Reports of most physical complaints (i.e., constipation, diarrhea, stomachache, appetite increase/decrease, belching, fishy breath) were low (possible range: 0–6: mean ± SD = 0.5 ± 0.6) and similar for Ω3 and placebo at each time point. However, at week 12, Ω3 groups were more likely to report nausea (n = 4; 44%) than placebo groups (n = 0), χ2(1) = 5.63, p = 0.018. Among those reporting nausea at week 12, two parents rated nausea as moderate and two rated it as mild (both these children had physical illnesses at their week 12 visit).
Unfortunately, a psychotherapy evaluation form was inadvertently not included at the beginning of the study. When it was added, families were given the questionnaire at their final assessment and asked to mail it back to study staff. The return rate was low; therefore, data are not available on acceptability of IF-PEP. Informal feedback, however, coupled with excellent attendance for 90% of those assigned to IF-PEP, suggests that families valued their therapy sessions or at least found them palatable.
Intent-to-treat analyses
Combined therapy and monotherapy versus no active therapy
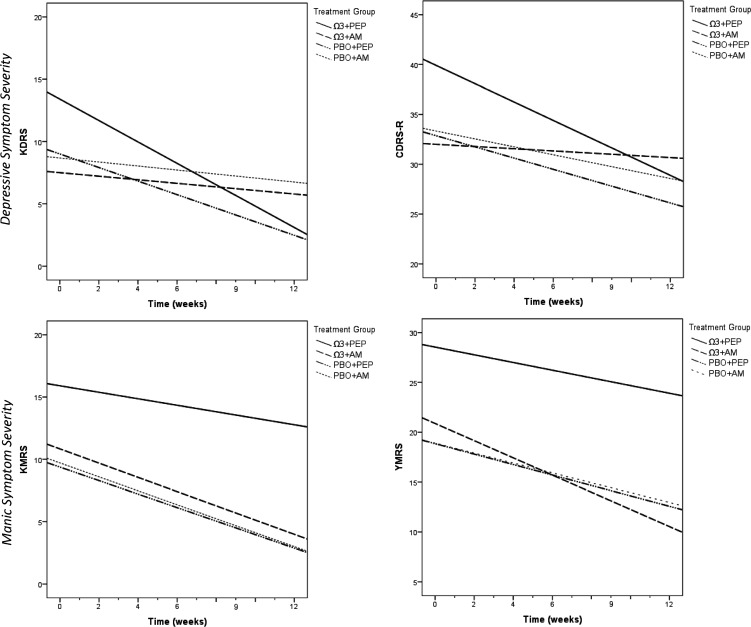
Participants in the combined therapy group demonstrated greater improvement on the KDRS than those in the PBO and AM group (p = 0.01), a large effect (d = 1.70). Treatment effects on CDRS-R trajectories were nonsignificant, however the effect size was large (d = 0.81). Manic symptoms declined in all groups, but group differences were not significant. Although neither monotherapy demonstrated statistically significant superiority to PBO and AM on any outcome, Ω3 monotherapy yielded a large effect size for YMRS trajectories (d = 0.86) but not for the KMRS or depression symptom measures; and PEP monotherapy yielded a large effect size for the KDRS (d = 0.92) but not for the CDRS-R or manic symptom measures (Table 3 and Fig. 2).
Table 3.
Results of the Linear Mixed-Effects Models of the Effect of PEP and Ω3 on Mood
| Measure | Parameter | Estimate | df | t | p value |
|---|---|---|---|---|---|
| KDRS | Intercept | 8.65 | 40.45 | 4.76 | <0.001 |
| Treatment group (reference: PBO+AM) | |||||
| Combined | 4.80 | 38.00 | 1.81 | 0.078 | |
| Ω3 monotherapy | −1.18 | 39.50 | −0.44 | 0.660 | |
| PEP monotherapy | 0.34 | 39.06 | 0.14 | 0.891 | |
| Time (weeks) | −0.16 | 105.08 | −0.82 | 0.413 | |
| Treatment group × time | |||||
| Combined × time | −0.707 | 103.39 | −2.63 | 0.010 | |
| Ω3 monotherapy × time | −0.04 | 104.20 | −0.14 | 0.887 | |
| PEP monotherapy × time | −0.38 | 104.60 | −1.43 | 0.155 | |
| CDRS-R | Intercept | 16.37 | 34.85 | 5.61 | <0.001 |
| Treatment group | |||||
| Combined | 6.60 | 33.00 | 1.55 | 0.131 | |
| Ω3 monotherapy | −1.44 | 34.12 | −0.33 | 0.741 | |
| PEP monotherapy | −0.56 | 33.81 | −0.14 | 0.889 | |
| Time | −0.42 | 104.46 | −1.47 | 0.144 | |
| Treatment group × time | |||||
| Combined × time | −0.51 | 102.96 | −1.30 | 0.197 | |
| Ω3 monotherapy × time | 0.19 | 103.62 | 0.47 | 0.639 | |
| PEP monotherapy × time | −0.12 | 103.93 | −0.31 | 0.758 | |
| KMRS | Intercept | 9.63 | 32.06 | 4.57 | <0.001 |
| Treatment group | |||||
| Combined | 6.30 | 29.83 | 2.06 | 0.048 | |
| Ω3 monotherapy | 1.31 | 31.30 | 0.42 | 0.676 | |
| PEP monotherapy | −0.21 | 30.97 | −0.08 | 0.941 | |
| Time | −0.61 | 26.75 | −2.05 | 0.050 | |
| Treatment group × time | |||||
| Combined × time | 0.34 | 25.23 | 0.82 | 0.419 | |
| Ω3 monotherapy × time | −0.05 | 26.85 | −0.12 | 0.906 | |
| PEP monotherapy × time | 0.04 | 28.25 | 0.11 | 0.916 | |
| YMRS | Intercept | 18.89 | 27.57 | 7.26 | <0.001 |
| Treatment group | |||||
| Combined | 9.71 | 26.06 | 2.56 | 0.017 | |
| Ω3 monotherapy | 2.02 | 27.08 | 0.53 | 0.603 | |
| PEP monotherapy | −0.13 | 26.83 | −0.04 | 0.971 | |
| Time | −0.52 | 24.88 | −1.76 | 0.091 | |
| Treatment group × time | |||||
| Combined × time | 0.12 | 23.24 | −0.30 | 0.768 | |
| Ω3 monotherapy × time | −0.45 | 24.57 | −1.04 | 0.310 | |
| PEP monotherapy × time | 0.03 | 26.19 | 0.07 | 0.942 | |
Ω3, omega-3; AM, active monitoring; CDRS-R, Children's Depression Rating Scale-Revised; KDRS, Kiddie Depression Rating Scale; KMRS, Kiddie Mania Rating Scale; PBO, placebo; PEP, individual-family psychoeducational psychotherapy; YMRS, Young Mania Rating Scale.
FIG. 2.
The course of mood symptoms by treatment group across the 12 week trial of IF-PEP and Ω3 for subsyndromal bipolar disorder. Ω3, omega-3; AM, active monitoring; CDRS-R, Children's Depression Rating Scale-Revised; KDRS, Kiddie Depression Rating Scale; KMRS, Kiddie Mania Rating Scale; PBO, placebo; IF-PEP, individual-family psychoeducational psychotherapy; YMRS, Young Mania Rating Scale.
Combined therapy versus monotherapy
Planned contrasts examining the effect of combined therapy relative to each monotherapy indicated that youth in combined therapy improved significantly more than youth in Ω3 monotherapy on the KDRS, t(102.56) = 2.40, p = .018, but not on the CDRS-R or manic symptom measures. There were no significant benefits of combined therapy over PEP monotherapy on any outcome measure.
Ω3 versus placebo
Planned contrasts of both groups who received Ω3 (combined and Ω3 monotherapy groups) versus groups who received placebo (PEP monotherapy and PBO and AM) were nonsignificant. There were small to medium effects of Ω3 on the KDRS (d = 0.48), CDRS-R (d = 0.20) and YMRS (d = 0.29).
IF-PEP versus active monitoring
Planned contrasts of PEP (combined and PEP monotherapy) versus active monitoring (Ω3 monotherapy and PBO and AM) found a significant benefit of PEP on the KDRS, t(105.72) = 2.68, p = 0.009, d = 1.24. CDRS-R results were nonsignificant (p = 0.164) though the effect was medium (d = 0.63). There was no significant impact of PEP versus AM on manic symptoms.
Discussion
This study investigated the efficacy of Ω3 and IF-PEP alone and combined in youth with BP-NOS/CYC. Both Ω3 and IF-PEP appeared to be acceptable to families. Study capsule adherence and therapy attendance were both high and attrition was relatively low. Despite considerable study demands (twice-weekly therapy sessions for the PEP groups, four capsules daily, six 1.5-hour assessments after a 4–6 hour screen assessment), 83% of participants completed the trial and all participated for at least 4 weeks. Ω3 was well tolerated, with only one physical concern (nausea) at one time point reported with greater frequency in the Ω3 groups than in the PBO groups, severity was mild to moderate and several of the children with nausea had concurrent physical illnesses. Two families assigned to IF-PEP did not complete their sessions; both were completely lost to follow-up as opposed to simply not completing their psychotherapy component.
Combined therapy was associated with greater improvement in depressive symptoms than PBO and AM. IF-PEP demonstrated medium to large effects on depressive symptoms compared with AM. Additionally, combined therapy was more effective than Ω3 monotherapy, but not more effective than IF-PEP monotherapy. Together, these findings suggest that the effects of combined therapy and IF-PEP alone on depressive symptoms may be comparable.
Whereas all participants experienced some decline in manic symptoms over the course of the study, the treatments investigated did not significantly improve manic symptoms relative to PBO and AM. Two planned comparisons yielded effect sizes ranging from small (Ω3 vs. placebo) to large (Ω3+AM vs. PBO+AM) on the YMRS, the unfiltered measure of manic symptoms, but not on the KMRS, the filtered measure. This may indicate potential benefits of Ω3 for co-occurring problems such as inattention, hyperactivity, and aggressive behavior, which are assessed on the unfiltered YMRS and have been reported to improve with Ω3 in prior studies (Sinn and Bryan 2007; Sorgi et al. 2007). It is of note that group differences in depression trajectories were primarily on KDRS scores, a filtered rating of depression, rather than the CDRS-R (which is more likely to capture symptoms of comorbid conditions), possibly because PEP was designed to specifically treat mood, and the KDRS is a more precise measure of depression.
For all four outcome measures, children in the no-active-therapy group improved to some degree. This might reflect normal waxing and waning of mood symptoms; it might also reflect the impact of regular contact with attentive study staff. Allowing 1.5 hours for each assessment, families would have had 9 hours of contact with evaluators over the 12 week trial, averaging 45 minutes of regularly scheduled attentive interactions per week. This is in keeping with prior research that demonstrated the therapeutic effect of assessments (Poston and Hanson 2010).
This is the first controlled trial examining combined treatment in youth with BP-NOS or CYC. These youth often have highly complex clinical presentations and currently, there are no evidence-based treatment guidelines for these subsyndromal presentations of BP in youth. Therefore, this pilot study addresses a significant public health concern by identifying potentially beneficial treatments for these youth. IF-PEP is a manualized treatment (Fristad et al 2011c) with workbooks available for parents and children (www.moodychildtherapy.com); therefore, it is easily accessible to community clinicians. Although community-based effectiveness data are not available for IF-PEP, an effectiveness trial for the multifamily model (MF-PEP) has demonstrated PEP's acceptability, feasibility, and sustainability in the community (MacPherson et al. 2014). Results of this pilot trial suggest that a larger trial is feasible and warranted.
Limitations and future directions
These results should be interpreted in light of the study's primary limitation, which is its small sample size. A larger multisite trial to accumulate the needed sample would provide more rigorous evaluation of these interventions, particularly testing any potential benefits of combined therapy over monotherapy. Further, relatively low baseline manic symptom severity made it difficult to detect differential treatment effects for manic symptoms. Additionally, it is not known what EPA:DHA ratio is of most benefit to children or adults with mood disorders. The 7:1 ratio of EPA to DHA used in this study on recommendation of lipid experts is different from the 2.5:1 ratio naturally found in fish. Also, results may not be generalizable to fish oil supplements that do not utilize a 7:1 EPA:DHA ratio. A future study should compare effects of different ratios. Lastly, previous trials of biological and psychosocial interventions for mood disorders in youth have demonstrated increased benefits of intervention at longer-term follow-up assessments (Fristad 2006; The TADS Team 2007; Fristad et al. 2009); long-term follow-up data are currently being collected. Future analyses will also examine potential mediators and moderators of treatment to assist with more individualized treatment planning.
Conclusions
This pilot study supports IF-PEP, alone and in combination with Ω3, as promising treatments for depressive symptoms of subsyndromal BPSD in youth. There may also be benefits to Ω3 for reducing manic symptoms. IF-PEP and Ω3 are safe and palatable treatments that provide a favorable risk/benefit ratio compared with existing pharmacotherapy. A large multisite trial is warranted.
Clinical Significance
Given the absence of published, evidence-based treatment guidelines for BP-NOS/CYC, these results are a significant contribution to the field. Few psychosocial or biological interventions have demonstrated efficacy in treating subsyndromal BP in children and adolescents. Both IF-PEP and Ω3 were well tolerated. Analyses indicate that IF-PEP and Ω3 supplementation may be efficacious interventions for this population.
Acknowledgments
We thank the staff who collected data and provided therapy, the families who participated, and OmegaBrite, who provided study capsules.
Disclosures
Dr. Fristad receives royalties from American Psychiatric Press, CFPSI, and Guilford Press. Dr. Young, Mr. Vesco, Mr. Nader, Mr. Healy, Dr. Gardner, and Ms. Wolfson have no disclosures. Dr. Arnold has received research funding from Curemark, Forest, Lilly, Neuropharm, Novartis, Noven, Shire, and YoungLiving (as well as the National Institutes of Health [NIH] and Autism Speaks); has consulted with or been on advisory boards for Gowlings, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Roche, Seaside Therapeutics, Sigma Tau, Shire, and Tris Pharma; and has received travel support from Noven.
References
- Aman MG, Mcdougle CJ, Scahill L, Handen B, Arnold LE, Johnson C, Stigler KA, Bearss K, Butter E, Swiezy NB: Medication and parent training in children with pervasive developmental disorders and serious behavior problems: Results from a randomized clinical trial. J Am Acad Child Adolesc Psychiatry 48:1143–1154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Amminger GP, Berger GE, Schäfer MR, Klier C, Friedrich MH, Feucht M: Omega-3 fatty acids supplementation in children with autism: A double-blind randomized, placebo-controlled pilot study. Biol Psychiatry 61:551–553, 2007 [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE: Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry 67:146–154, 2010 [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N: A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol 13:463–470, 2003 [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M: Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry 63:1139–1148, 2006 [DOI] [PubMed] [Google Scholar]
- Axelson DA, Birmaher B, Strober MA, Goldstein BI, Ha W, Gill MK, Goldstein TR, Yen S, Hower H, Hunt JI, Liao F, Iyengar S, Dickstein D, Kim E, Ryan ND, Frankel E, Keller MB: Course of subthreshold bipolar disorder in youth: Diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry 50:1001–1016 e3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M: Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry 63:175–183, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers WJ, Puig–Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M: The assessment of affective disorders in children and adolescents by semistructured interview: test-retest reliability of the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present Episode Version. Arch Gen Psyhiatry 42: 696–702, 1985 [DOI] [PubMed] [Google Scholar]
- Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL: Reduced mania and depression in juvenile bipolar disorder associated with long-chain [omega]-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr 63: 1037–40, 2009 [DOI] [PubMed] [Google Scholar]
- Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, Muldoon MF: Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett 421:209–212, 2007 [DOI] [PubMed] [Google Scholar]
- Correll CU: Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: A systematic review and pooled analysis of short-term trials. J Am Acad Child Adolesc Psychiatry 46:687–700, 2007 [DOI] [PubMed] [Google Scholar]
- DelBello MP, Adler CM, Whitsel RM, Stanford KE, Strakowski SM: A 12-week single-blind trial of quetiapine for the treatment of mood symptoms in adolescents at high risk for developing bipolar I disorder. J Clin Psychiatry 68:789–795, 2007 [DOI] [PubMed] [Google Scholar]
- Feingold A: Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods 14:43–53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, Frazier TW, Axelson D, Ryan N, Demeter CA, Gill MK, Fields B, Depew J, Kennedy SM, Marsh L, Rowles BM, McCue Horwitz S: Characteristics of children with elevated symptoms of mania: The Longitudinal Assessment of Manic Symptoms (LAMS) study. J Clin Psychiatry 71:1664–1672, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhaker C, Heiser P, Hennighausen K, Herpertz–Dahlmann B, Holtkamp K, Mehler–Wex C, Rauh R, Remschmidt H, Schulz E, Warnke A: Clinical drug monitoring in child and adolescent psychiatry: Side effects of atypical neuroleptics. J Child Adolesc Psychopharmacol 16:308–316, 2006 [DOI] [PubMed] [Google Scholar]
- Fristad MA: Psychoeducational treatment for school-aged children with bipolar disorder. Dev Psychopathol 18:1289–1306, 2006 [DOI] [PubMed] [Google Scholar]
- Fristad MA, Cummins J, Verducci JS, Teare M, Weller E, Weller RA: Study IV: Concurrent validity of the DSM-IV revised Children's Interview for Psychiatric Syndromes (ChIPS). J Child Adolesc Psychopharmacol 8:227–236, 1998a [DOI] [PubMed] [Google Scholar]
- Fristad MA, Glickman AR, Verducci JS, Teare M, Weller EB, Weller RA: Study V: Children's Interview for Psychiatric Syndromes (ChIPS): Psychometrics in two community samples. J Child Adolesc Psychopharmacol 8:237–245, 1998b [DOI] [PubMed] [Google Scholar]
- Fristad MA, Goldberg–Arnold JS: Individual-family Psychoeducational Psychotherapy (IF-PEP): Parent Workbook. Columbus, OH: CFPSI Press; 2011a [Google Scholar]
- Fristad MA, Goldberg–Arnold JS, Gavazzi SM: Multifamily psychoeducation groups (MFPG) for families of children with bipolar disorder, Bipolar Disord 4:254–262, 2002 [DOI] [PubMed] [Google Scholar]
- Fristad MA, Goldberg–Arnold JS, Gavazzi SM: Multi-family psychoeducation groups in the treatment of children with mood disorders, J Marital Fam Ther 29:491–504, 2003 [DOI] [PubMed] [Google Scholar]
- Fristad MA, Goldberg–Arnold JS, Leffler J: Individual-Family Psychoeducational Psychotherapy (IF-PEP): Child Workbook. Columbus, OH: CFPSI Press; 2011b [Google Scholar]
- Fristad MA, Goldberg–Arnold JS, Leffler J: Psychotherapy for Children with Bipolar and Depressive Disorders. New York: Guilford Press; 2011c [Google Scholar]
- Fristad MA, MacPherson HA: Evidence-based psychosocial treatments for child and adolescent bipolar spectrum disorders. J Clin Child Adolesc Psychol 43:339–355, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristad MA, Teare M, Weller EB, Weller RA, Salmon P: Study III: Development and concurrent validity of the Children's Interview for Psychiatric Syndromes—parent version (P-ChIPS). J Child Adolesc Psychopharmacol 8:221–226, 1998c [DOI] [PubMed] [Google Scholar]
- Fristad MA, Verducci JS, Walters K, Young ME: Impact of multifamily psychoeducational psychotherapy in treating children aged 8 to 12 years with mood disorders. Arch Gen Psychiatry 66:1013–1020, 2009 [DOI] [PubMed] [Google Scholar]
- Fristad MA, Weller EB, Weller RA: The Mania Rating Scale: Can it be used in children? A preliminary report. J Am Acad Child Adolesc Psychiatry 31:252–257, 1992 [DOI] [PubMed] [Google Scholar]
- Fristad MA, Weller RA, Weller EB: The Mania Rating Scale (MRS): Further reliability and validity studies with children. Ann Clin Psychiatry 7:127–132, 1995 [DOI] [PubMed] [Google Scholar]
- Geller B, Luby JL, Joshi P, Wagner KD, Emslie G, Walkup JT, Axelson DA, Bolhofner K, Robb A, Wolf DV, Riddle MA, Birmaher B, Nusrat N, Ryan ND, Vitiello B, Tillman R, Lavori P: A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents, Arch Gen Psychiatry 69:515–528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracious BL: An RCT of Flax Oil in Children and Adolescents with Bipolar Disorder. Chicago: National Institute of Mental Health (NIMH) Pediatric Bipolar Conference; 2006 [Google Scholar]
- Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR: Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder, Bipolar Disord 12:142–154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D, Axelson D, Demeter C, Findling RL, Fristad MA, Kowatch RA, Youngstrom EA, Horwitz SM, Arnold LE, Frazier TW, Ryan N, Gill MK, Hauser–Harrington JC, Depew J, Rowles BM, Birmaher B: Phenomenology of bipolar disorder not otherwise specified in youth: A comparison of clinical characteristics across the spectrum of manic symptoms. Bipolar Disord 15:240–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AM, Buckley JD, Murphy KJ, Howe PR: Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors, Am J Clin Nutr 85:1267–1274, 2007 [DOI] [PubMed] [Google Scholar]
- Joshi G, Petty C, Wozniak J, Faraone SV, Doyle R, Georgiopoulos A, Hammerness P, Walls S, Glaeser B, Brethel K, Yorks D, Biederman J: A prospective open-label trial of quetiapine monotherapy in preschool and school age children with bipolar spectrum disorder. J Affect Disord 136:1143–1153, 2012 [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL: Kaufman Brief Intelligence Test. San Antonio: Pearson; 2004 [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kowatch RA, Strawn JR, Sorter MT: Clinical trials support new algorithm for treating pediatric bipolar mania: 4 atypical antipsychotics are first-line therapy, based on current evidence. Curr Psychiatry 8:19–34, 2009 [Google Scholar]
- Maayan L, Correll CU: Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 21:517–535, 2011 [DOI] [PubMed] [Google Scholar]
- MacPherson HA, Leffler JM, Fristad MA: Implementation of multi-family psychoeducational psychotherapy for childhood mood disorders in an outpatient community setting. J Marital Fam Ther 40:193–211, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J, Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA 292:807–820, 2004 [DOI] [PubMed] [Google Scholar]
- McClellan J, Kowatch R, Findling RL, The Work Group on Quality Issues: Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 46:107–125, 2007 [DOI] [PubMed] [Google Scholar]
- McNamara RK, Nandagopal JJ, Strakowski SM, DelBello MP: Preventative strategies for early-onset bipolar disorder: Towards a clinical staging model. CNS Drugs 24:983–996, 2010 [DOI] [PubMed] [Google Scholar]
- Mendenhall AN, Fristad MA, Early TJ: Factors influencing service utilization and mood symptom severity in children with mood disorders: Effects of multifamily psychoeducation groups (MFPGs), J Consult Clin Psychol 77:463–473, 2009 [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group: A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 56:1073, 1999a [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group: Moderators and mediators of treatment response for children with Attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 56:1088, 1999b [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group: National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: Changes in effectiveness and growth after the end of treatment. Pediatrics 113:762–769, 2004a [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group: National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics 113:754–761, 2004b [DOI] [PubMed] [Google Scholar]
- Perlis RH, Dennehy EB, Miklowitz DJ, Delbello MP, Ostacher M, Calabrese JR, Ametrano RM, Wisniewski SR, Bowden CL, Thase ME, Nierenberg AA, Sachs G: Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: Results from the STEP-BD study. Bipolar Disord 11:391–400, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston JM, Hanson WE: Meta-analysis of psychological assessment as a therapeutic intervention. Psychol Assess 22:203–212, 2010 [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R: Preliminary studies of the reliability and validity of the Children's Depression Rating Scale. J Am Acad Child Psychiatry 23:191–197, 1984 [DOI] [PubMed] [Google Scholar]
- Sarris J, Mischoulon D, Schweitzer I: Omega-3 for bipolar disorder: Meta-analyses of use in mania and bipolar depression, J Clin Psychiatry 73:81–86, 2012 [DOI] [PubMed] [Google Scholar]
- Sinn N, Bryan J: Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J Dev Behav Pediatr 28:82–91, 2007 [DOI] [PubMed] [Google Scholar]
- Sorgi P, Hallowell EM, Hutchins HL, Sears B: Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutr J 6:16, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teare M, Fristad MA, Weller EB, Weller RA, Salmon P: Study I: Development and criterion validity of the Children's Interview for Psychiatric Syndromes (ChIPS). J Child Adolesc Psychopharmacol 8:205–211, 1998a [DOI] [PubMed] [Google Scholar]
- Teare M, Fristad MA, Weller EB, Weller RA, Salmon P: Study II: Concurrent validity of the DSM-III-R Children's Interview for Psychiatric Syndromes (ChIPS). J Child Adolesc Psychopharmacol 8: 213–219, 1998b [DOI] [PubMed] [Google Scholar]
- The TADS Team: The Treatment of Adolescents with Depression Study (TADS): Long-term effectiveness and safety concerns. Arch Gen Psychiatry 64:1132–1143, 2007 [DOI] [PubMed] [Google Scholar]
- Van Meter A, Youngstrom EA, Demeter C, Findling RL: Examining the validity of cyclothymic disorder in a youth sample: replication and extension. J Abnorm Child Psychol 41:367–378, 2013 [DOI] [PubMed] [Google Scholar]
- Van Meter AR, Youngstrom EA, Findling RL: Cyclothymic disorder: A critical review. Clin Psychol Rev 32:229–243, 2012 [DOI] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B: Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 359:2753–2766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller EB, Weller RA, Fristad MA, Rooney MT, Schecter J: Children's Interview for Psychiatric Syndromes (ChIPS). J Am Acad Child Adolesc Psychiatry 39:76–84, 2000 [DOI] [PubMed] [Google Scholar]
- Weller EB, Weller RA, Rooney MT, Fristad MA: Children's Interview for Psychiatric Syndromes (ChIPS). Washington, DC: American Psychiatric Press, Inc.; 1999a [DOI] [PubMed] [Google Scholar]
- Weller EB, Weller RA, Rooney MT, Fristad MA: Children's Interview for Psychiatric Syndromes– Parent Version (P-ChIPS). Washington, DC: American Psychiatric Press, Inc.; 1999b [Google Scholar]
- West AE, Weinstein SM, Peters AT, Katz AC, Henry DB, Cruz RA, Pavuluri MN: Child- and family-focused cognitive-behavioral therapy for pediatric bipolar disorder: A randomized clinical trial. J Am Acad Child Adolesc Psychiatry 53:1168–1178, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Cluettebrown J, Laposata M: Omega-3 fatty acid monotherapy for pediatric bipolar disorder: A prospective open-label trial. Eur Neuropsychopharmacol 17: 440–47, 2007 [DOI] [PubMed] [Google Scholar]
- Young C, Martin A: Omega-3 fatty acids in mood disorders: An overview. Rev Bras Psiquiatria 25:184–187, 2003 [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–435, 1978 [DOI] [PubMed] [Google Scholar]
- Youngstrom EA, Gracious BL, Danielson CK, Findling RL, Calabrese J: Toward an integration of parent and clinician report on the Young Mania Rating Scale. J Affect Disord 77:179–190, 2003 [DOI] [PubMed] [Google Scholar]