Abstract
Chronic lymphocytic leukemia is an indolent disorder with an increased infectious risk remaining one of the main causes of death. Development of therapies with higher safety profile is thus a challenging issue. Docosahexaenoic acid (DHA, 22:6) is an omega-3 fatty acid, a natural compound of normal cells, and has been shown to display antitumor potency in cancer. We evaluated the potential in vitro effect of DHA in primary CLL cells. DHA induces high level of in vitro apoptosis compared to oleic acid in a dose-dependent and time-dependent manner. Estimation of IC50 was only of 4.813 µM, which appears lower than those reported in solid cancers. DHA is highly active on CLL cells in vitro. This observation provides a rationale for further studies aiming to understand its mechanisms of action and its potent in vivo activity.
Key words: Chronic lymphocytic leukemia, docosahexaenoic acid
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western countries. CLL cells are in a low proliferative state but their in vivo apoptosis is notably reduced by the influence of the BCR signaling, bcl-2 family protein overexpression and a supportive microenvironment. In spite of highly effective treatment options, all patients will eventually relapse.
Essential polyunsaturated fatty acids (FA) are important biocompounds with structural and functional roles as in inflammation in normal cells. Their availability depends on external supply, mammalian cells being deficient in n-3 desaturase. They are divided into two main types: the omega-6 series, derived from linoleic acid (LA, 18:2, n-6) present in plant oils, and the omega-3 series, derived from alphalinolenic acid (ALA, 18:3, n-3), e.g. docosahexaenoic acid (DHA, 22:6) present in algal and fish oils.
Omega-3 FA including DHA have consistently been shown to induce apoptosis and/or enhance sensitivity to chemotherapy of various solid tumor cells, both in vitro and in vivo, targeting various mechanisms involved in cancer (lipid raft disruption, signaling, oxidative stress).1-5 Among omega-3 FA, DHA has displayed the highest antitumor potency.6
Some reports showed that omega-3 FA can enhance the sensitivity of myeloid leukemia cell lines and lymphoid B-cell lines to anti-cancer drugs.7,8 A recent report showed that an omega-3 FA in vivo supplement in CLL patients can lead to an increase of their in vitro sensitivity to doxorubicin, fludarabine and cyclophosphamide.9 However, no controlled in vitro evaluation of the direct and specific effect of DHA has been so far documented in primary CLL cells. We investigated the potential apoptotic effect of DHA on Ramos B-cell line and primary B-cells issued from 10 CLL patients to assess its antitumor potency, when compared to OA, in a time and dose-dependent manner.
Materials and Methods
Cell culture
The Burkitt lymphoma-derived Ramos B-cell line (ATCC, CRL-1923) was cultured at a density between 0.5×106 and 1×106 cells/mL in RPMI 1640, supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 125 U/mL penicillin, 125 lg/mL streptomycin, in 5% CO2 at 37°C. Peripheral blood samples were collected from 10 previously untreated CLL patients after obtaining informed consents according to the declaration of Helsinki. Briefly, mononuclear cells were separated using Ficoll-Plaque PLUS (GE Healthcare, Little Chalfont, UK) and purified by magnetic negative immunoselection [B Cell isolation Kit (B-CLL), Miltenyi Biotec] according to the manufacturer’s instructions. Tumor purity was over 90% after CD5/CD19 immunostaining. B-CLL cells were cultured at 5×106 cells/mL in RPMI 1640, in the same above-mentioned conditions.
Reagents
Oleic acid, a monounsaturated omega-9 FA (OA, 18:1, n-9), was used as a control for the evaluation of the specific effect of DHA. Both OA and DHA were purchased from Sigma-Aldrich (Saint Louis, MO, USA) and stored after dilution in absolute ethanol at the concentration of 30 mM at -20°C in glass tube to avoid adsorption on plastic. Fludarabine (Sigma-Aldrich) was stored after dilution in Dimethylsulfoxide at -20°C. Dimethylsulfoxide (vehicle) was used as negative control.
Apoptosis assays
Apoptosis was quantified by Annexin V-fluorescein isothiocyante/7-Aminoactinomycin D staining (Beckman Coulter, Milan, Italy) followed by flow cytometry analysis according to the manufacturer’s instructions using FC500 (Beckman Coulter). Experiments are duplicated. IC50 was determined by constructing a dose-response curve and by analyzing the results with the GraphPad Prism5 software.
Statistical analysis
Analyses were performed using the GraphPad Prism5 software. Data are shown as mean ± standard error of mean. Comparisons were assessed by the Mann-Whitney test. Statistical significance was defined as P<0.05.
Results
Effects of docosahexaenoic on apoptosis of the RAMOS cell line
As a preliminary experiment, we first checked the potential effect of DHA on the Ramos B-cell line. We observed that DHA 10 µM induced a high rate of apoptosis (annexin-V positive cells) compared to OA 10 µM as a negative control either at 24-hr or 48-hr of exposure (Figure 1). When comparing percentage of viable cells at 24-hr, this difference was significant (7% vs 29.1%, N=4, P=0.03).
Figure 1.
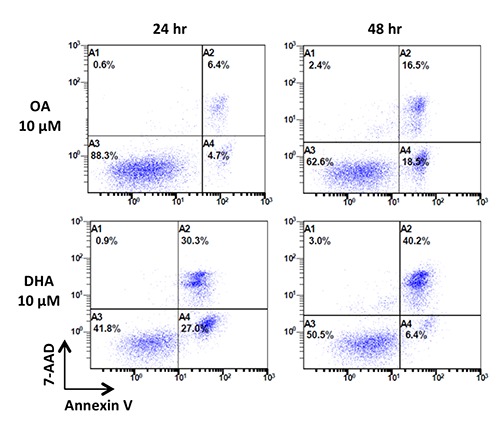
Effects of OA 10 µM and DHA 10 µM on apoptosis of the Ramos cell line (representative experiment). Ramos cells viability was assessed by Annexin V-FITC/7-AAD immunostaining after exposure to OA and DHA at 10 µM for 24 and 48 hours. Cells in the lower left quadrant (A3) correspond to viable cells (Annexin V-negative and 7AAD-negative), cells in the lower right quadrant (A4) correspond to early apoptotic cells (Annexin V-positive and 7AAD-negative), while cells in the upper quadrant (A2) correspond to late apoptotic cells (Annexin V-positive and 7AAD-positive). OA, oleic acid; DHA, docosahexaenoic acid.
Effects of docosahexaenoic on apoptosis of primary B-chronic lymphocytic leukemia cells
Efficacy of DHA to induce apoptosis was assessed on 10 purified primary CLL cells samples in cell culture after 24-hr exposure to fludarabine 3.2 µM, OA 10 µM or DHA 10 µM. As expected, fludarabine, as a positive control, induced significant apoptosis at 24-hr with a lower percentage of viable cells (25.2%) compared to vehicle (59.9%) (P=0.0175). DHA induced a higher proportion of apoptotic cells compared to OA (viable cells = 13.5 vs 64% respectively, P=0.0003) (Figure 2).
Figure 2.
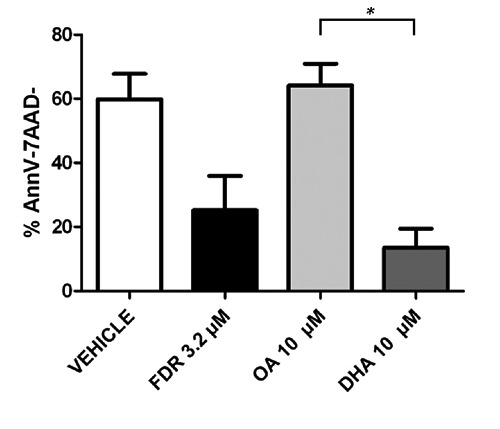
Effects of OA 10 µM and DHA 10 µM on apoptosis of primary CLL cells (10 samples). CLL cells viability was assessed by Annexin V-FITC/7-AAD immunostaining after exposure to OA and DHA 10 µM for 24 hours. Fludarabine 3.2 µM as positive control for apoptosis of primary CLL cells. Viable cells assessed by % of both Annexine V and 7-AAD negative are shown as mean ± standard error of mean. Statistical comparisons were performed using the Mann-Whitney test.*, P=0.0003; AnnV-7AAD-, Annexine V-negative and 7AAD-negative; FDR, fludarabine; OA, oleic acid; DHA, docosahexaenoic acid.
We then evaluated the induction of apoptosis by DHA in CLL cells by studying the effect of various doses and various durations of exposure. DHA and OA were first used at 0.1 µM, 1 µM, 10 µM and 100 µM for 24 hours. The dose-response curve revealed that DHA induced significant apoptosis compared to OA in a dose-dependent manner and determined the 24-hr IC50 of DHA at 4813 µM (Figure 3A). Finally, DHA 10 µM also induced apoptosis in a time-dependent manner as shown in Figure 3B while the largest proportion of the cells died before 24-hr.
Figure 3.
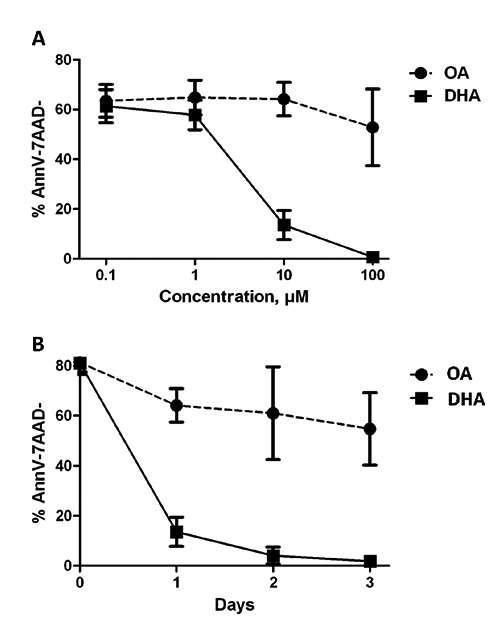
Effects of dose (A) and time (B) on DHA-induced apoptosis in primary CLL cells (10 samples). CLL cells viability was assessed by Annexin V-FITC/7-AAD immunostaining after exposure to DHA at concentrations ranging from 0,1 µM to 100 µM for 24 hours (A) and after exposure to DHA 10 µM for 24, 48 and 72 hours (panel B). Viable cells assessed by % of both Annexine V and 7-AAD negative are shown as mean ± standard error of mean. AnnV-7AAD-, Annexine V-negative and 7AAD-negative; OA, oleic acid; DHA, docosahexaenoic acid.
Discussion
While highly effective combinations are now available, CLL remains an incurable disease. Conversely, such treatments are associated with an increased risk of infections, known as the main cause of death in CLL. Development of therapies with higher safety is thus a challenging issue.
Omega-3 FAs are natural compounds of normal cells leading to expect a good safety profile. Besides their role in nutrition, evidence of their role in cancer cell biology is increasing. CLL is an indolent disease and subsequently a good model for the evaluation of omega-3 FA as candidate anti-cancer drugs, alone or in combination with other therapies.
Our results demonstrate that DHA induces a strong apoptosis of primary CLL cells compared to OA in the same range as fludarabine at 3.2 µM, which was the dose commonly used both in vitro and in vivo. OA, an omega-9 FA did not induce significant apoptosis compared to vehicle and could be a decent negative control for the assessment of omega-3 FA efficacy. There is a crucial need for the use of negative control in assessment of drug-related efficacy in CLL, CLL cells undergoing spontaneous apoptosis in cell culture.
Previous studies showed that DHA can induce apoptosis or/and growth arrest of various cell lines models. Our study highlights that DHA is also active in primary B-CLL cells. Moreover this activity seems to be more important in primary CLL cells than in any other previously evaluated tumor cells. We estimated DHA-related IC50 at 4813 µM which appeared to be lower than in AML cell lines (IC50=74 µM),7 in breast cancer cell lines (IC50=173 µM)4 or in colon cancer cell lines (IC50=46.8 µM).5 These results are in line with a previous study showing that lowest IC50 for DHA was observed in lymphoid cell lines (IC50=5.2 µM).3
Other studies have proposed mechanisms for DHA induced cell death in lymphoid cells. Firstly, it has been shown that DHA is able to increase lipid peroxidation and generation of reactive oxygen species in B-cell lines.8 Moreover, a previous study proposed to evaluate the potential effect of a dietary omega-3 supplementation for CLL patients in early stages.9 The analyses of lymphocytes issued from these patients demonstrated a decrease of the activation of NF B after omega-3 consumption.9 This action may however result from off-tumor effects in the setting of such in vivo experiments. Indeed it has been demonstrated that DHA exerts anti-inflammatory effects after linking to the G protein-coupled receptor 120 in macrophages.10 Our analyses were needed to highlight a direct apoptosis effect of DHA on primary CLL cells, which is independent of other cooperating cells. Further studies are warranted to confirm similar downstream events in primary cells than those observed in cell lines.
References
- 1.Hardman WE, Avula CP, Fernandes G, et al. Three percent dietary fish oil concentrate increased efficacy of doxorubicin against MDA-MB 231 breast cancer xenografts. Clin Cancer Res 2001;7:2041-9. [PubMed] [Google Scholar]
- 2.Serini S, Trombino S, Oliva F, et al. Docosahexaenoic acid induces apoptosis in lung cancer cells by increasing MKP-1 and down-regulating p-ERK1/2 and p-p38 expression. Apoptosis 2008;13:1172-83. [DOI] [PubMed] [Google Scholar]
- 3.Ding WQ, Vaught JL, Yamauchi H, et al. Differential sensitivity of cancer cells to docosahexaenoic acid-induced cytotoxicity: the potential importance of down-regulation of superoxide dismutase 1 expression. Mol Cancer Ther 2004;3:1109-17. [PubMed] [Google Scholar]
- 4.Cao W, Ma Z, Rasenick MM, et al. N-3 polyunsaturated fatty acids shift estrogen signaling to inhibit human breast cancer cell growth. PLoS One 2012;7:e52838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danbara N, Yuri T, Tsujita-Kyutoku M, et al. Conjugated docosahexaenoic acid is a potent inducer of cell cycle arrest and apoptosis and inhibits growth of colo 201 human colon cancer cells. Nutr Cancer 2004;50:71-9. [DOI] [PubMed] [Google Scholar]
- 6.Kafrawy O, Zerouga M, Stillwell W, et al. Docosahexaenoic acid in phosphatidylcholine mediates cytotoxicity more effectively than other omega-3 and omega-6 fatty acids. Cancer Lett 1998;132:23-9. [DOI] [PubMed] [Google Scholar]
- 7.Chiu LCM, Wong EYL, Ooi VEC. Docosahexaenoic acid modulates different genes in cell cycle and apoptosis to control growth of human leukemia HL-60 cells. Int J Oncol 2004;25:737-44. [PubMed] [Google Scholar]
- 8.Fahrmann JF, Hardman WE. Omega 3 fatty acids increase the chemo-sensitivity of B-CLL-derived cell lines EHEB and MEC-2 and of B-PLL-derived cell line JVM-2 to anti-cancer drugs doxorubicin, vincristine and fludarabine. Lipids Health Dis 2013;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahrmann JF, Ballester OF, Ballester G, et al. Inhibition of nuclear factor kappa B activation in early-stage chronic lymphocytic leukemia by omega-3 fatty acids. Cancer Invest 2013;31:24-38. [DOI] [PubMed] [Google Scholar]
- 10.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sen- doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]


