Abstract
Objective:
To improve current understanding of the mechanisms behind thalamic amnesia, as it is unclear whether it is directly related to damage to specific nuclei, in particular to the anterior or mediodorsal nuclei, or indirectly related to lesions of the mammillothalamic tract (MTT).
Methods:
We recruited 12 patients with a left thalamic infarction and 25 healthy matched controls. All underwent a comprehensive neuropsychological assessment of verbal and visual memory, executive functions, language, and affect, and a high-resolution structural volumetric MRI scan. Thalamic lesions were manually segmented and automatically localized with a computerized thalamic atlas. As well as comparing patients with controls, we divided patients into subgroups with intact or damaged MTT.
Results:
Only one patient had a small lesion of the anterior nucleus. Most of the lesions included the mediodorsal (n = 11) and intralaminar nuclei (n = 12). Patients performed worse than controls on the verbal memory tasks, but the 5 patients with intact MTT who showed isolated lesions of the mediodorsal nucleus (MD) only displayed moderate memory impairment. The 7 patients with a damaged MTT performed worse on the verbal memory tasks than those whose MTT was intact.
Conclusions:
Lesions in the MTT and in the MD result in memory impairment, severely in the case of MTT and to a lesser extent in the case of MD, thus highlighting the roles played by these 2 structures in memory circuits.
Amnesia following thalamic lesions was first described several decades ago, in particular following strokes,1–3 but the mechanisms underlying the memory impairment remain unclear. It is crucial to establish whether the impairment is directly related to damage to specific nuclei,4–6 and if so which ones, or whether it is indirectly related to lesion of the mammillothalamic tract (MTT), first described by Vicq d'Azyr in 1786, linking the hippocampus to the thalamus via a synapse in the mammillary body.7–10
As the anterior nucleus (AN) is connected with the hippocampus and the MTT,11–13 it was assumed to explain the memory impairment.3,14,15 The mediodorsal nucleus (MD) is another candidate owing to its dense connections with subhippocampal structures.4,5,12 The MTT runs through the medial thalamus.11 Therefore, anterior-medial thalamic strokes may also damage the MTT and cause memory impairment.4,11,16,17 An important caveat in previous studies is that many have relied on single or a few cases. Neuroimaging has been absent or of poor quality, and few studies have used a thalamic atlas fused with MRI scans to identify the damaged structures.2 Furthermore, because of the close vicinity of the AN, MD, and MTT, it is usually hard to say which structure is responsible for the memory impairment observed in thalamic stroke patients.13
The aim of this study was to assess the role of the AN, MD, and MTT in thalamic amnesia following a stroke.
METHODS
Standard protocol approvals, registrations, and patient consents.
All participants provided written informed signed consent to take part in this study, which was approved by the local institutional review board (Comité de Protection des Personnes Sud-Ouest et Outre-Mer no. 2-11-04).
Patients with single unilateral left ischemic thalamic stroke were recruited in the stroke units of Toulouse and Bordeaux university hospitals (France). All had brain infarcts observed on a diffusion-weighted MRI scan during the acute phase. They had no previously known neurovascular, inflammatory, or neurodegenerative diseases.
We performed this study prospectively, with one recruitment criterion: the presence of a first symptomatic left thalamic infarct, regardless of complaint or neurobehavioral report at onset. We focused on left thalamic strokes to build a homogenous group.
Following inclusion, all patients and healthy controls (matched for age and education level) underwent a clinical examination, a neuropsychological assessment, and an MRI scan (at least 3 months after stroke for patients). All the investigations took place in a single day and order.
Neuropsychological assessment.
We tested verbal memory (Free and Cued Selective Reminding Test [FCSRT],18 Logical Memory19), visual memory (Rey-Osterrieth Complex Figure,20 DMS4821), executive functions (Digit span and Spatial span,19 d2 test,22 Trail Making Test,23 Stroop,23 Symbol Search,24 literal and semantic lexical fluency,19 Similarities24), language (ExaDé naming test25), and affect (State-Trait Anxiety Inventory,26 Starkstein Apathy Scale,27 Beck Depression Inventory28). Handedness was assessed with the Edinburgh Handedness Inventory.29
MRI acquisition.
MRIs for the study were acquired on a 3T scanner (Philips Achieva; Best, the Netherlands). Thalamic lesions were documented using a 3D T2-weighted sequence (1 × 1 × 1-mm voxel size, echo time 337 ms, repetition time 8,000 ms, inversion time 2,400 ms, field of view 240 × 240 × 170, slice thickness 1 mm, slice number 170) and a 3D T1-weighted sequence (1 × 1 × 1-mm voxel size, echo time 8.1 ms, repetition time 3.7 ms, flip angle 8°, field of view 240 × 240 × 170, slice thickness 1 mm, slice number 170).
White matter lesions were quantified with the Fazekas and Schmidt score30 by 2 independent raters (L.D. and M.P.).
Nuclei volumes and lesion location.
Lesions were manually segmented on the native T1 images by 2 independent investigators (L.D. and P.E.) using MRIcron software.31 Native images and segmented lesions were normalized on the Montreal Neurological Institute (MNI) template using first linear (FMRIB Linear Image Registration Tool [FLIRT]; FMRIB Software Library [FSL]) then nonlinear transformation (FMRIB Nonlinear Image Registration Tool [FNIRT]; FSL). The volumes of the normalized lesions were automatically calculated for each participant using the Fsl.anat toolbox, and expressed in mm3. The Krauth et al.32 digital version of the Morel33 atlas of the thalamus, based on the MNI template, was implemented in FSL to automatically localize the lesions. The script allowed us to access the atlas via the FSL atlasquery function. We then calculated the volume (mm3) and the proportion of the normalized lesions in each nucleus per patient using the labeled volumes of the Morel atlas. We then grouped the nuclei together according to Morel's33 nomenclature: anterior group (anteroventral, anteromedial, anterodorsal, and lateral dorsal nuclei); medial group (mediodorsal parvocellular and magnocellular, intralaminar nuclei such as center median, central lateral, parafascicular, midline nuclei such as central medial, paraventricular, and habenula nuclei); lateral group (ventroposterior complex, ventral lateral posterior/anterior, and ventral anterior and ventral medial nuclei); posterior group (medial and lateral geniculate nuclei, posterior, suprageniculate/limitans, and lateral posterior and pulvinar nuclei); and reticular nucleus. The proportion of lesion outside the thalamus (expressed as %) was assessed. We used FIRST (FSL) for the automatic segmentation of the thalamus in native space. All lesions were overlapped and summed using the iCalc function in SPM8. The relevant Morel atlas sections and the thalamus mask were then superimposed on the summed lesions in order to illustrate the lesions' distribution.
MTT assessment.
As the MTT is a relatively small structure, we used 2 methods to assess its integrity. These were applied to both patients and controls, and both left and right MTTs. First, using the MTT label in the Morel atlas, we assessed the number of voxels that were damaged in the MTT. Second, we carried out a volumetric analysis after patients' and controls' MTTs had been manually segmented on T1 axial slices by 2 independent examiners (L.D. and P.E.) using MRIcron software. Segmentations with an interrater agreement below 70% were reviewed by the 2 raters together. We calculated the z scores of the patients' MTT volumes using data from the control group. MTTs with a z score 2.5 SD below the mean were deemed to be damaged.
Patients were grouped in the damaged MTT subgroup (dMTT) if at least one of the 2 methods indicated damage, and in the intact MTT subgroup (iMTT) if neither method indicated damage.
Statistical analysis.
Group comparisons were carried out using χ2, the nonparametric Mann-Whitney U test, and permutation tests.34 Correlations were realized using the nonparametric Spearman rho test. The significance threshold was set at p = 0.05. Modified kappa scores were calculated to assess interobserver reproducibility35 for Fazekas and Schmidt scores, volumes of the segmented lesions, and volumes of the MTTs.
RESULTS
Participants' characteristics.
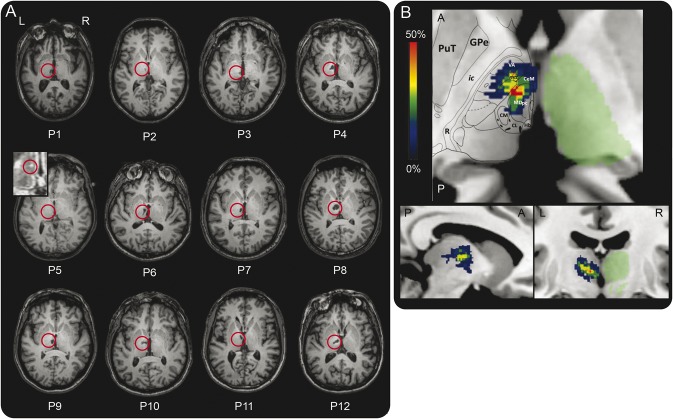
Between March 2012 and November 2013, we recruited 14 patients who had had a single left thalamic stroke. Two patients were excluded because of comorbidity: depressive syndrome that impaired cognitive performance (n = 1) and a lacuna visible in the T1 sequence in the acute phase but not in the study MRI (n = 1). The final sample therefore comprised 12 patients (P1–P12). All, except for 1 ambidextrous participant, were right-handed. Initial symptoms were visual (n = 4), motor (n = 5), sensory (n = 1), and, for all the patients, cognitive, with tip-of-the-tongue and paraphasia (n = 8), jargon aphasia (n = 1), verbal anterograde amnesia (n = 7), spatial memory impairment (n = 1), short-term memory loss (n = 1), and anosognosia (n = 3).17 Behavioral disturbances were also reported: emotional incontinence (n = 2), aggressiveness (n = 2), apathy (n = 1), and hyperactivity (n = 1). Details are provided in table e-1 on the Neurology® Web site at Neurology.org. The majority of the acute symptomatology significantly decreased in a few days or weeks, with the exception of amnesia. Patients were matched with 25 healthy participants for age and education level. The demographic details of both groups are provided in table 1. Patients' lesions on native T1 and overlap of lesions are shown in figure 1A (κ = 0.82).
Table 1.
Demographic details of patients, controls, and dMTT and iMTT subgroups
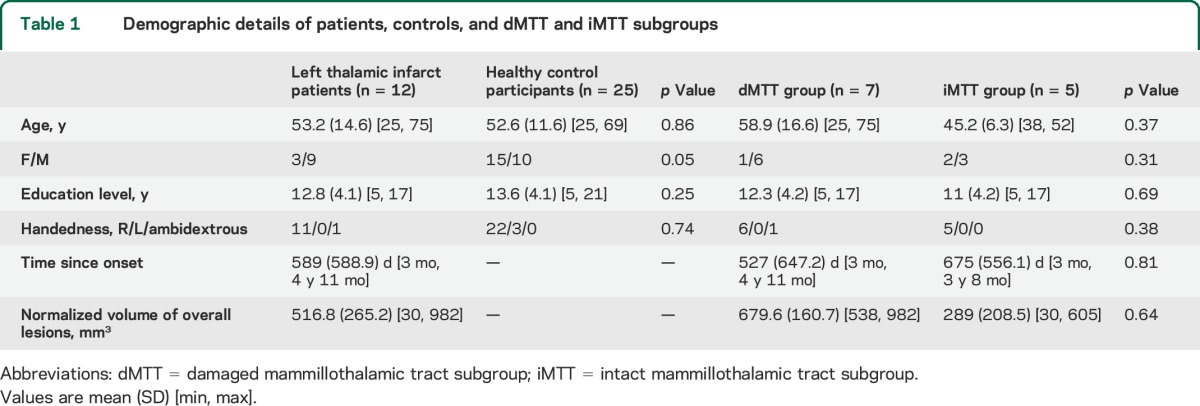
Figure 1. Lesions.
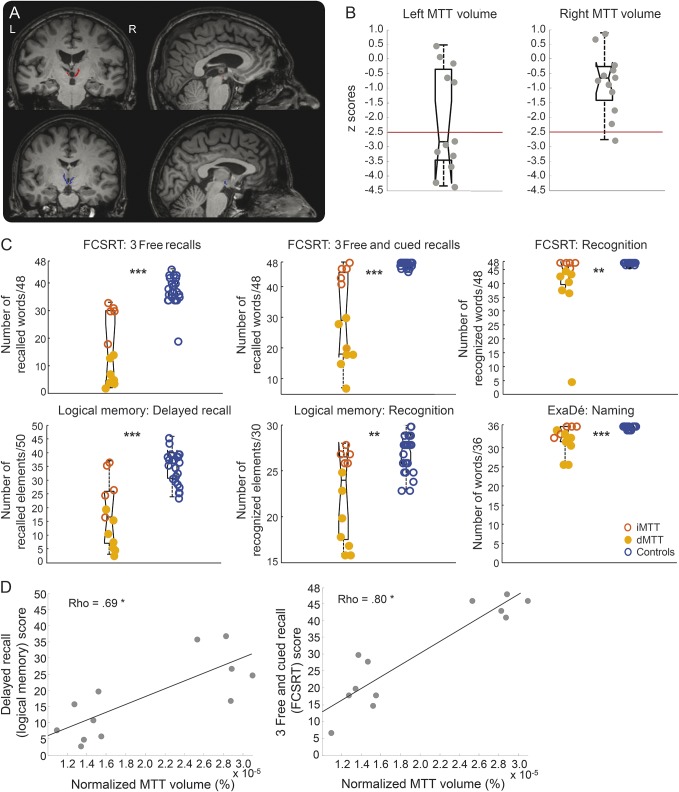
(A) T1 axial sections of the patients' native MRI. The red circles show infarcts. P5's lesion is scarcely visible on the image (lesion volume = 5 mm3). We therefore provide a zoom on the lesion on the fluid-attenuated inversion recovery sequence. (B) Overlap of the lesions across patients (% of patients, n = 12) in an axial view (top; A = anterior, P = posterior), sagittal view (bottom left; A = anterior, P = posterior), and coronal view (bottom right). A mask of the right thalamus (in green) obtained using the FIRST tool in FSL is provided for information. A slice of the Morel atlas featuring structures of interest is overlaid on the axial view. CeM = central medial; CL = central lateral; CM = centromedian; GPe = external globus pallidus; Hb = habenula; ic = internal capsule; MDpc = parvocellular part of the mediodorsal nucleus; mtt = mammillothalamic tract; PuT = putamen; R = reticular nucleus; VA = ventral-anterior.
Neuropsychological assessment.
Patients performed worse than controls on the verbal memory tests, including free recall, cued recall, and recognition (table 2). We found no difference regarding visual memory. Patients also exhibited a moderate dysexecutive syndrome. Spontaneous language was clinically normal, but moderate naming difficulties were observed at the group level on a confrontation naming test. There was no correlation between naming and memory performances in patients. Patients' and controls' affect did not differ. No behavioral disturbance was observed during assessment.
Table 2.
Results of the neuropsychological assessment
Neuroimaging analyses of the thalamic nuclei.
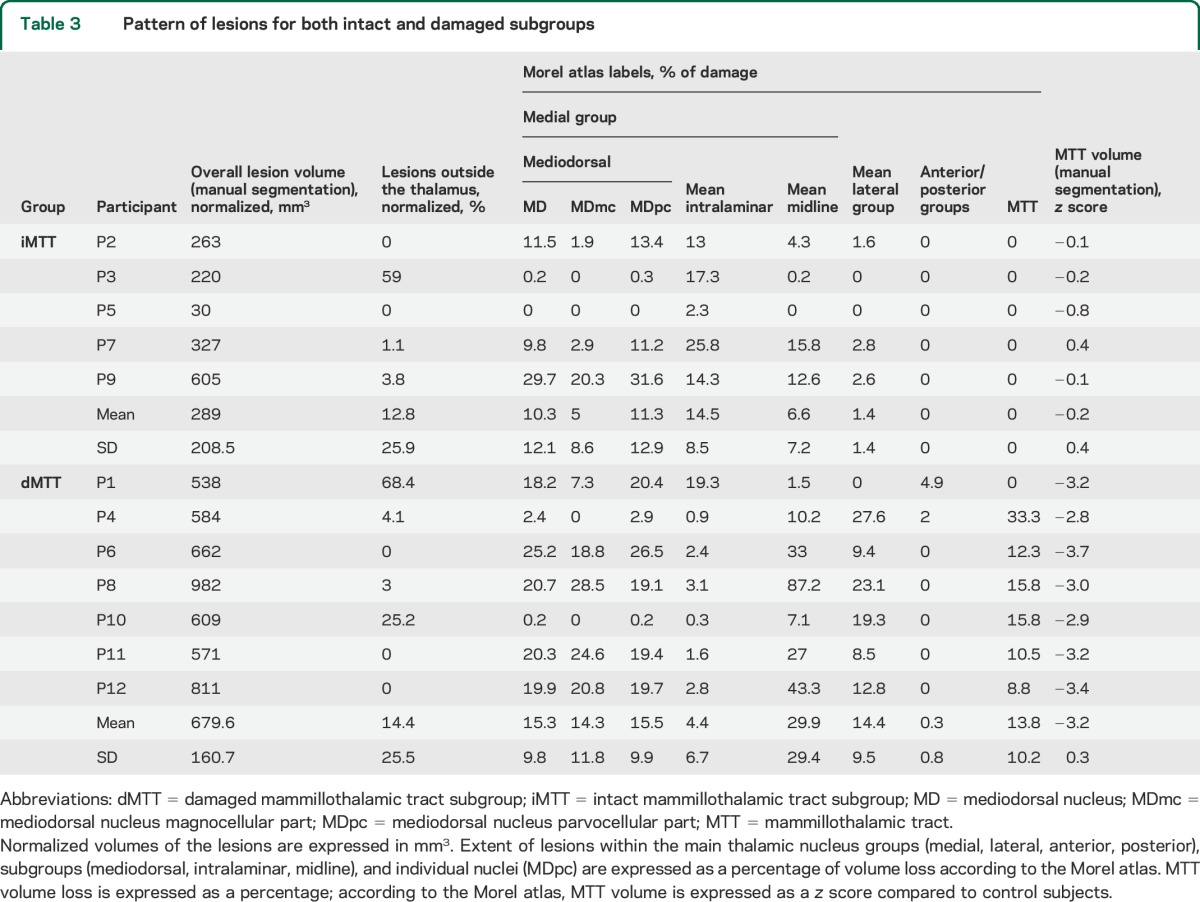
The Fazekas and Schmidt score was ≤2 for all patients and controls (κ = 0.8). Following manual segmentation of the lesions and identification of the nuclei using the Krauth et al.32 digital version of the Morel atlas, all patients were found to have a lesion in the medial group, especially in the parvocellular part of the mediodorsal nucleus (MDpc) (n = 11) and the intralaminar nuclei (n = 11) (figure 1B). Ten patients also had lesions in the lateral group. One patient had minor damage in the anterior group (1 mm3 in the anteromedial nucleus), and 1 had a small lesion in the posterior group (1 mm3 in the limitans nucleus) (table 3). The most severe damage (>10% of the nucleus damaged) was located in some areas of the medial group, such as the central medial (mean damage across patients: 28.2%, minimum 0.6%, maximum 76%) and paraventricular (13.1%, minimum 14.3%, maximum 85.7%) part of midline nuclei; parafascicular part of intralaminar nuclei (16.9%, minimum 1.1%, maximum 61.2%); and MDpc (13.7%, minimum 0.2%, maximum 31.6%); but also in nuclei of the lateral group, such as the ventral medial (19.3%, minimum 6.3%, maximum 100%), magnocellular ventral anterior (17.3%, minimum 4.1%, maximum 83.8%), and ventral lateral posterior (13%, minimum 2.3%, maximum 44.5%) (figure 1B). More than 20% of the lesions of P1, P3, and P10 were located outside the thalamus (in order of importance: brainstem, red nucleus, and white matter).
Table 3.
Pattern of lesions for both intact and damaged subgroups
Neuroimaging analyses of the MTT.
The analysis based on the Morel atlas indicated that 6 of the 12 patients had left MTT damage. The volumetric analysis (see example in figure 2A) showed a group-level difference between patients and controls for the left but not the right MTT (respectively p < 0.001 vs p > 0.1), with 7 of the 12 patients exhibiting severe atrophy of the left MTT (z scores < −2.5, figure 2B). These 7 patients included all 6 patients who had been identified as having a damaged MTT in the Morel atlas analysis. The Morel atlas analysis did not detect a MTT lesion in one patient (P1) exhibiting MTT atrophy. In subsequent analyses, these 7 patients were included in the dMTT and the remaining 5 in the iMTT. Details of the 2 subgroups are provided in tables 1 and 3.
Figure 2. MTTs in patients and controls, volumes of the MTTs, memory and language performance in groups (patients vs controls) and subgroups (dMTT vs iMTT), and MTT volume and memory performance.
(A) Illustration of mammillothalamic tracts (MTTs) in patients and controls. Segmentation of left and right MTTs in native brains of a patient (top, in red) and a control (bottom, in blue). (B) Volumes of the patients' MTTs. Left and right volumetric distribution in patients (z scores). (C) Memory and language performance in groups (patients vs controls) and subgroups (damaged MTT subgroup [dMTT] vs intact MTT subgroup [iMTT]). Intergroup comparisons of patients and controls. **p < 0.01. ***p < 0.001. Blue circles represent controls, empty orange circles represent iMTT patients, filled orange circles represent dMTT patients. (D) MTT volume and memory performance. Correlation between verbal memory performance (assessed here using the Logical Memory Test and Free and Cued Selective Reminding Test [FCSRT]) and MTT. *p < 0.05, 2-tailed.
Performance of dMTT and iMTT patients.
dMTT patients were severely impaired on all the verbal memory tasks, and moderately impaired on the ExaDé naming task, compared with both controls and iMTT patients (demographic data in table 1; see figure 2C and details in table e-2). By contrast, the iMTT patients were impaired on the verbal memory tasks, performing worse than controls only on the FCSRT word free recall task. The dMTT and iMTT subgroups did not differ significantly from controls on the rest of the assessment.
Whole-group correlations.
A significant correlation was observed between MTT volumes and verbal memory performances on the Logical Memory and FCRST tasks (figure 2D) but the distribution of MTT lesions was bimodal. By contrast, no correlation was found between total MD volume loss and memory performance (rho = −0.24, p > 0.05). We found that overall lesion volume correlated significantly with FCRST scores (rho = −0.74*), but not with delayed recall performances on the Logical Memory test (rho = −0.52).
DISCUSSION
In this study, we found that patients with left thalamic infarcts experienced severe verbal memory impairment compared with a group of healthy matched controls.1,2,13 Unexpectedly, none of the patients had a significant lesion in the AN. A subgroup of patients showed isolated lesions of the MD (n = 5) and a memory deficit mainly impairing recall, suggesting that MD lesions alone can result in amnesia. Patients with MTT damage exhibited more severe memory impairment than those whose MTT was intact.
Only one patient had a lesion in the AN (P4 lesion: 1 mm3). This was an unexpected finding. Current neuroanatomical models of memory suggest that thalamic memory impairment may be due to damage located in the AN because of its links with the hippocampus via the mammillary bodies and the fornix.10–14 Moreover, studies have reported verbal memory impairment after left AN infarction.3,15 One possible explanation for the scarcity of AN lesions in our study is that we used a neuroimaging thalamic atlas, allowing more precision than in previous studies, where AN lesions may have been overestimated. Besides, tuberothalamic strokes occur less often than paramedian ones and one third of the population has no tuberothalamic artery.17 This may decrease the overall risk for AN stroke.
Still, all our patients exhibited a pattern of memory impairment similar to that usually observed in the unilateral hippocampal lesion, including impaired verbal recall and recognition memory without confabulations or false alarms.
A recent study of 7 patients with lesions in the left MD (with no MTT damage) found that they had memory impairment.16 However, this study's outcome is questionable because 3 of the patients also had lesions in the AN, and 3 had bilateral lesions. In the present study, 5 patients presented a MD lesion but no MTT damage, constituting the largest pure group of patients with isolated and quantitatively measured MD lesions that has ever been reported. Neuropsychological results showed that these patients exhibited memory impairment. Nevertheless, we did not observe any correlation between MD volume loss and memory performance. Several studies have highlighted the MD's probable role in memory,4,5,16 showing that MD lesions affect recall,6,16 although the role of this nucleus in recognition memory remains unproven.12 This subgroup exhibited impaired recall of single items (words that had to be learned), but preserved recall of complex relational material (stories). No recognition memory impairment was observed. This may appear intriguing at first sight, considering that the Aggleton and Brown model predicts a recognition memory impairment related to impaired familiarity when the MD is damaged. This nucleus does indeed have links with subhippocampal structures, especially the perirhinal cortex.11,12,36 However, our recognition memory tasks may have been insufficiently sensitive to reveal such impairment. Interestingly, the perirhinal cortex is also thought to be critical for single-item memory such as single words, which may explain why our patients were more impaired on this type of material.37,38 Overall, therefore, these results suggest that MD lesions alone result in amnesia, limited to a certain type of material.
Our subgroup of patients with a damaged MTT performed worse not just than controls, but also than patients whose thalamic infarcts had not affected their MTT (whereas both subgroups exhibited MD damage). Our results therefore confirm previous findings—often case studies—about the MTT's critical role in memory,2,8,9,11,12,36 and are in accordance with a review of 35 studies of 60 patients with a thalamic infarct that concluded that amnesic syndrome only occurs in cases of MTT damage.7
Since each nucleus has its own pattern of connectivity,12,36 we may attribute thalamic amnesia to a disconnection between the AN and the mammillary bodies owing to MTT damage. This could explain the severe recall impairment observed in the dMTT subgroup. In other words, the pattern of memory impairment we observed in this patient subgroup may be explained by a subcortical disconnection via lesions of the MTT. In support of the view that the pattern of connectivity is critical in memory impairment following thalamic lesions, the MD is linked to the prefrontal cortex via a thalamofrontal pathway.36 This network featuring the prefrontal cortex could be involved in the executive-attention functions supporting encoding and retrieval, possibly explaining the moderate pattern of memory impairment we observed in our patients with isolated MD lesions.1 However, no correlation between MD damage and executive performance was observed in this iMTT subgroup.
One possibility worth discussing is that the pattern of verbal memory impairment observed in our patients was due to language dysfunction more than to memory proper. Overall, however, our patients exhibited few language difficulties (i.e., no aphasia and little or no difficulty finding words in spontaneous speech). During confrontation naming, occasional errors produced were tip-of-the-tongue and semantic paraphasia. This corroborates classical reports of thalamic aphasia and precisely the adynamic aphasia after paramedian thalamic stroke.17,39 The mechanisms underlying thalamic aphasia are thought to be related to an executive impairment (deficit of selective attention, inhibition, switching).39 The neuropsychological results of our patients' group suggest that the response times were impaired on executive tasks while accuracy was generally preserved. Assessing whether the moderate lexical difficulties we observed are related to an impairment of the lexicon proper or lexical selection, and how this may affect recall, remains complex. Furthermore, the MD nucleus is not always associated with language impairment although paramedian artery strokes can lead to aphasia.17,40 Finally, no correlation was found between naming and memory performance. It is worth mentioning that 3 patients (P1, P3, P10) had >25% of their lesions outside the thalamus, in the surrounding structures. However, their cognitive profile did not differ clinically.
Our findings highlight the roles played by MTT and MD in memory showing that lesions in these structures cause a severe amnesia in the case of MTT and to a lesser extent memory impairment in the case of MD damage.1,2,5,8,9
This study supports an updated view of the role of the MTT and MD in memory12 suggesting that a disconnection of memory networks due to lesions to either a white matter tract (MTT) or to a specific nucleus (MD) may produce thalamic amnesia.40
Supplementary Material
ACKNOWLEDGMENT
The authors thank Gabor Székely for sharing his digital atlas of the human thalamus; Jonathan Curot, Aicha Lyoubi, and Marie Lafuma for neurologic examinations; Gabriel Besson, Djilali Adel, Thomas Busigny, and Marlene Poncet for conceptual and technical support; Hélène Gros, Nathalie Vayssière, Lucette Foltier, and Jean-Pierre Désirat of the MRI technical platform (Inserm UMR825); and the participants.
GLOSSARY
- AN
anterior nucleus
- dMTT
damaged mammillothalamic tract subgroup
- FCSRT
Free and Cued Selective Reminding Test
- FSL
FMRIB Software Library
- iMTT
intact mammillothalamic tract subgroup
- MD
mediodorsal nucleus
- MDpc
parvocellular part of the mediodorsal nucleus
- MNI
Montreal Neurological Institute
- MTT
mammillothalamic tract
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
L. Danet: study concept and design, acquisition, analysis, and interpretation of data, writing of the manuscript. E. Barbeau: study concept and design, critical revision of the manuscript for important intellectual content. P. Eustache: independent examiner for MTT and lesions segmentation. M. Planton: independent examiner for Fazekas & Schmidt scoring. N. Raposo: critical revision of the manuscript for important intellectual content. I Sibon: critical revision of the manuscript for important intellectual content. J.-F. Albucher: critical revision of the manuscript for important intellectual content. F. Bonneville: critical revision of the manuscript for important intellectual content. P. Péran: study concept and design, imaging data analysis. J. Pariente: study concept and design, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Van Der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HBM, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus: a study of 22 cases with localised lesions. Neuropsychologia 2003;41:1330–1344. [DOI] [PubMed] [Google Scholar]
- 2.Cipolotti L, Husain M, Crinion J, et al. The role of the thalamus in amnesia: a tractography, high-resolution MRI and neuropsychological study. Neuropsychologia 2008;46:2745–2758. [DOI] [PubMed] [Google Scholar]
- 3.Ghika-Schmid F, Bogousslavsky J. The acute behavioral syndrome of anterior thalamic infarction: a prospective study of 12 cases. Ann Neurol 2000;48:220–227. [PubMed] [Google Scholar]
- 4.Zoppelt D, Koch B, Schwarz M, Daum I. Involvement of the mediodorsal thalamic nucleus in mediating recollection and familiarity. Neuropsychologia 2003;41:1160–1170. [DOI] [PubMed] [Google Scholar]
- 5.Pergola G, Güntürkün O, Koch B, Schwarz M, Daum I, Suchan B. Recall deficits in stroke patients with thalamic lesions covary with damage to the parvocellular mediodorsal nucleus of the thalamus. Neuropsychologia 2012;50:2477–2491. [DOI] [PubMed] [Google Scholar]
- 6.Von Cramon DY, Hebel N, Schuri U. A contribution to the anatomical basis of thalamic amnesia. Brain 1985;108:993–1008. [DOI] [PubMed] [Google Scholar]
- 7.Van Der Werf YD, Witter MP, Uylings HBM, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia 2000;38:613–627. [DOI] [PubMed] [Google Scholar]
- 8.Edelstyn NMJ, Mayes AR, Denby C, Ellis SJ. Impairment in material-specific long-term memory following unilateral mediodorsal thalamic damage and presumed partial disconnection of the mammillo-thalamic tract. J Neuropsychol 2012;6:119–140. [DOI] [PubMed] [Google Scholar]
- 9.Carlesimo GA, Serra L, Fadda L, Cherubini A, Bozzali M, Caltagirone C. Bilateral damage to the mammillo-thalamic tract impairs recollection but not familiarity in the recognition process: a single case investigation. Neuropsychologia 2007;45:2467–2479. [DOI] [PubMed] [Google Scholar]
- 10.Papez JW. A proposed mechanism of emotion. Arch Neurol Psychol 1937;38:725–743. [Google Scholar]
- 11.Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci 1999;22:425–489. [PubMed] [Google Scholar]
- 12.Aggleton JP, Dumont JR, Warburton EC. Unraveling the contributions of the diencephalon to recognition memory: a review. Learn Mem 2011;18:384–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlesimo GA, Lombardi MG, Caltagirone C. Vascular thalamic amnesia: a reappraisal. Neuropsychologia 2011;49:777–789. [DOI] [PubMed] [Google Scholar]
- 14.Vann SD, Tsivilis D, Denby CE, et al. Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc Natl Acad Sci USA 2009;106:5442–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishio Y, Hashimoto M, Ishii K, et al. Multiple thalamo-cortical disconnections in anterior thalamic infarction: Implications for thalamic mechanisms of memory and language. Neuropsychologia 2014;53:264–273. [DOI] [PubMed] [Google Scholar]
- 16.Tu S, Miller L, Piguet O, et al. Accelerated forgetting of contextual details due to focal medio-dorsal thalamic lesion. Front Behav Neurosci 2014;8:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003;34:2264–2278. [DOI] [PubMed] [Google Scholar]
- 18.Van der Linden M, GREMEM members. l'Évaluation des Troubles de la Mémoire [in French]. Marseilles: Solal; 2004. [Google Scholar]
- 19.Wechsler D. MEM-III, Échelle Clinique de Mémoire de Wechsler, 3rd ed Paris: Editions du Centre de Psychologie Appliquée; 2001. [Google Scholar]
- 20.Rey A, Vaivre-Douret L. Figure de Rey. Paris: Editions du Centre de Psychologie Appliquée; 1960. [Google Scholar]
- 21.Barbeau E, Tramoni E, Joubert S, et al. Evaluation de la mémoire de reconnaissance visuelle: normalisation d'une nouvelle épreuve en choix forcé (DMS48) et utilité en neuropsychologie clinique [in French]. In: Ergis AM, Gely-Nergeot MC, Van der Linden M, eds. Les Troubles de la Mémoire dans la Maladie d'Alzheimer. Marseilles: Solal; 2004. [Google Scholar]
- 22.Brickenkamp R, Zillmer E. The D2 Test of Attention. Cambridge: Hogrefe and Huber; 1998. [Google Scholar]
- 23.Godefroy O, GREFEX members. Fonctions Exécutives et Pathologies Neurologiques et Psychiatriques [in French]. Marseilles: Solal; 2008. [Google Scholar]
- 24.Wechsler D. Manual for the Wechsler Adult Intelligence Scale–III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 25.Bachy-Langedock N. Batterie d'Examen des Troubles de la Dénomination (ExaDé). Brussels: Editions du Centre de Psychologie Appliquée; 1989. [Google Scholar]
- 26.Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) (“Self-Evaluation Questionnaire”). Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 27.Starkstein SE, Leentjens AFG. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry 2008;79:1088–1092. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 29.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 30.Kapeller P, Barber R, Vermeulen RJ, et al. Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke 2003;34:441–445. [DOI] [PubMed] [Google Scholar]
- 31.Rorden C, Karnath H, Bonilha L. Improving lesion–symptom mapping. J Cogn Neurosci 2007;19:1081–1088. [DOI] [PubMed] [Google Scholar]
- 32.Krauth A, Blanc R, Poveda A, Jeanmonod D, Morel A, Székely G. A mean three-dimensional atlas of the human thalamus: generation from multiple histological data. Neuroimage 2010;49:2053–2062. [DOI] [PubMed] [Google Scholar]
- 33.Morel A. Stereotactic Atlas of the Human Thalamus and Basal Ganglia, 2nd ed New York: Informa Healthcare; 2007. [Google Scholar]
- 34.Ernst MD. Permutation methods: a basis for exact inference. Stat Sci 2004;19:676–685. [Google Scholar]
- 35.Chakravarty MM, Sadikot AF, Germann J, Hellier P, Bertrand G, Collins DL. Comparison of piece-wise linear, linear, and nonlinear atlas-to-patient warping techniques: analysis of the labeling of subcortical nuclei for functional neurosurgical applications. Hum Brain Mapp 2009;30:3574–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pergola G, Suchan B. Associative learning beyond the medial temporal lobe: Many actors on the memory stage. Front Behav Neurosci 2013;7:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbeau EJ, Didic M, Felician O, Tramoni E. Pure progressive amnesia: an atypical amnestic syndrome? Cogn Neuropsychol 2006;23:1230–1247. [DOI] [PubMed] [Google Scholar]
- 38.Barbeau EJ, Pariente J, Felician O, Puel M. Visual recognition memory: a double anatomo-functional dissociation. Hippocampus 2011;21:929–934. [DOI] [PubMed] [Google Scholar]
- 39.Crosson B. Thalamic mechanisms in language: a reconsideration based on recent findings and concepts. Brain Lang 2013;126:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex 2008;44:1037–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.