Abstract
AIM: To investigate the effect of secreted frizzled-related proteins (sFRPs) on CXC chemokine expression in human mesenchymal stem cells (hMSCs).
METHODS: CXC chemokines such as CXCL5 and CXCL8 are induced in hMSCs during differentiation with osteogenic differentiation medium (OGM) and may be involved in angiogenic stimulation during bone repair. hMSCs were treated with conditioned medium (CM) from L-cells expressing non-canonical Wnt5a protein, or with control CM from wild type L-cells, or directly with sFRPs for up to 10 d in culture. mRNA expression levels of both CXCL5 and CXCL8 were quantitated by real-time reverse transcriptase-polymerase chain reaction and secreted protein levels of these proteins determined by ELISA. Dose- (0-500 ng/mL) and time-response curves were generated for treatment with sFRP1. Signal transduction pathways were explored by western blot analysis with pan- or phosphorylation-specific antibodies, through use of specific pathway inhibitors, and through use of siRNAs targeting specific frizzled receptors (Fzd)-2 and 5 or the receptor tyrosine kinase-like orphan receptor-2 (RoR2) prior to treatment with sFRPs.
RESULTS: CM from L-cells expressing Wnt5a, a non-canonical Wnt, stimulated an increase in CXCL5 mRNA expression and protein secretion in comparison to control L-cell CM. sFRP1, which should inhibit both canonical and non-canonical Wnt signaling, surprisingly enhanced the expression of CXCL5 at 7 and 10 d. Dickkopf1, an inhibitor of canonical Wnt signaling prevented the sFRP-stimulated induction of CXCL5 and actually inhibited basal levels of CXCL5 expression at 7 but not at 10 d post treatment. In addition, all four sFRPs isoforms induced CXCL8 expression in a dose- and time-dependent manner with maximum expression at 7 d with treatment at 150 ng/mL. The largest increases in CXCL5 expression were seen from stimulation with sFRP1 or sFRP2. Analysis of mitogen-activated protein kinase signaling pathways in the presence of OGM showed sFRP1-induced phosphorylation of extracellular signal-regulated kinase (ERK) (p44/42) maximally at 5 min after sFRP1 addition, earlier than that found in OGM alone. Addition of a phospholipase C (PLC) inhibitor also prevented sFRP-stimulated increases in CXCL8 mRNA. siRNA technology targeting the Fzd-2 and 5 and the non-canonical Fzd co-receptor RoR2 also significantly decreased sFRP1/2-stimulated CXCL8 mRNA levels.
CONCLUSION: CXC chemokine expression in hMSCs is controlled in part by sFRPs signaling through non-canonical Wnt involving Fzd2/5 and the ERK and PLC pathways.
Keywords: CXC chemokines, Mesenchymal stem cell, Osteogenesis, Differentiation, Wnt signaling pathway, Frizzled-related protein, Frizzled receptors
Core tip: Chemokines have multiple functions during bone formation and fracture repair. The ELR+ chemokines classically have a role in blood vessel formation and were found to be stimulated by the non-canonical Wnt5a protein and also by soluble frizzled-related proteins (sFRPs) that are known inhibitors of both canonical and non-canonical Wnt signaling. This stimulation was mediated via the p44/42 extracellular signal-regulated kinase and phospholipase C pathways signaling through the non-canonical frizzled receptors 2 and 5. This is a newly identified role for the sFRPs in stimulation of ELR+ chemokines which may be involved in blood vessel formation during wound repair.
INTRODUCTION
Bone fracture repair proceeds through a series of sequential steps including an inflammatory phase resulting in recruitment and differentiation of mesenchymal stem cells (MSCs) into osteoblasts, restoration of blood supply, subsequent soft (cartilaginous, in the case of endochondral repair) and hard (bone, in both endochondral and intramembranous) callus formation, and ultimately remodeling of the new woven bone into lamellar bone. During the initial inflammatory stage, neutrophils, macrophages, and lymphocytes migrate to the wound, fight infectious organisms, scavenge tissue debris, and begin the process of granulation tissue formation[1]. Cytokines, chemokines, and growth factors released from these cells are necessary to initiate bone repair in the adult. The pro-inflammatory cytokine, tumor necrosis factor-α (TNF-α) is critical in both long bone fracture as well as intramembranous bone repair[2,3]. TNF-α can highly induce members of the CXC chemokine family via NF-κB signaling in osteoblasts[4]. CXC chemokines can be grouped as to whether or not they contain a Glu-Leu-Arg (ELR) motif. ELR+ CXC chemokines, such as CXCL8 (IL-8), are present during the inflammatory phase to serve as chemoattractants for neutrophils[5,6] and exhibit angiogenic activity[7-9]. Chemokines without the ELR sequence are anti-angiogenic[9].
Human MSCs (hMSCs) express CXCL8 mRNA[10-12] and it has been reported that TNF-α can prime hMSCs to upregulate production of several CXC chemokines (highest upregulation with CXCL5 and CXCL8) and induce hMSC migration[13]. In humans, CXCL8 is a ligand for both CXC receptor 1 (CXCR1) and CXCR2 whereas CXCL5 interacts solely with CXCR2. Angiogenesis in response to CXCL8 has only been associated with CXCR2 signaling[14-16]. We previously demonstrated that CXCL8 expression can be stimulated with dexamethasone treatment during osteoblastic differentiation[17] and by low extracellular pH[18] in hMSCs. We also demonstrated that secreted CXC chemokines induced angiogenic tube formation of a human microvascular endothelial cell line (HMEC-1)[17] consistent with in vitro angiogenesis.
The mouse CXC receptor (mCXCR) is functionally related to hCXCR2[19]. Mice lacking the mCXCR (mCXCR2-/-) have been described[20] and some healing[21] and bone[22-24] defects have been reported. A second murine CXCR (mCXCR1) has also been identified; although, it has no discernable defect phenotype when inactivated (Jackson Laboratory Stock #005820). We have shown by DEXA and micro computerized tomography analysis that the mCXCR2-/- mice (Jackson Laboratory Stock #002724) have an osteopenic phenotype with decreased trabecular bone volume, number, and thickness without any changes in bone formation and resorption indices[25]. However, bone quality was affected as femurs had reduced stiffness and a lower ultimate load breaking point[25]. There was also a reduction in the blood vessel density in the newly repaired bone in a cranial defect model[25]. During bone regeneration, ingrowth of blood vessels is required for endochondral bone formation[1]. These results suggest a potential coupling of mMSC differentiation, bone formation, and angiogenesis in response to mCXCR signaling.
The Wnt family of secreted glycoproteins is involved in differentiation of an assortment of tissues[26]. Wnts signal through specific seven transmembrane spanning G-protein coupled frizzled (Fzd) receptors via both canonical β-catenin signaling, and non-canonical Wnt/calcium and Wnt/planar cell polarity pathways[27,28]. The highly conserved and redundant nature of the Wnt/Fzd system (19 Wnts and 10 Fzd in humans) only adds to the complexity of this system and confusion as to its role in osteogenesis.
The canonical pathway is characterized by Wnt binding to both Fzd and LRP5/LRP6 co-receptors resulting in activation of Disheveled (Dsh) which inhibits glycogen synthase kinase 3β (GSK3β) phosphorylation. In the absence of Wnt binding, GSK3β phosphorylation ultimately results in β-catenin degradation, preventing its nuclear translocation for activation of target genes. In murine models, evidence suggests that canonical Wnt/β-catenin signaling is necessary for lineage commitment of pluripotent MSCs to osteochondroprogenitor cells, then to osteoprogenitor cells, and for differentiation to mature osteoblasts while suppressing both chondrogenesis and adipogenesis[29,30]. However, in hMSC models, β-catenin and canonical Wnt3a can negatively regulate the differentiation of MSCs into the skeletal precursor cells that precede the appearance of the osteochondroprogenitor cells[31-35]. Additionally, de Boer et al[36] reported a dose-response relationship in which lower levels of β-catenin stimulated hMSC proliferation while blocking adipogenesis; whereas, higher levels induced expression of alkaline phosphatase. The authors thus concluded that canonical Wnt/β-catenin signaling could initiate osteogenic differentiation in the human system[36].
Signal transduction through the non-canonical or β-catenin-independent Wnt pathways has also been shown to inhibit adipogenesis and chondrogenesis in MSC models and to stimulate osteogenesis[33,37-40] mediated through activation of phospholipase C (PLC), and then through activation of the calcium-calmodulin kinase, nuclear factor of activated T-cells (NFAT), and protein kinase C (PKC) pathways or through the mitogen-activated protein kinase (MAPK) and RhoA pathways. Non-canonical Wnt signaling through traditional canonical Wnt ligands, Wnt3a and Wnt7b, and the non-canonical Wnt ligand, Wnt4, can also lead to osteogenic differentiation in both murine and human MSCs models through the activation of PKC and/or MAPK pathways[33,41,42]. Levels of non-canonical Wnt5a are increased in the inflammatory environment during early fracture healing[43] and non-canonical signaling (Wnt4 and Wnt5a) can affect the transition from proliferative osteoprogenitors to mature osteogenic cells[44,45]. However, as with canonical signaling, there are conflicting reports as to whether non-canonical Wnt5a can induce osteogenesis[30,33].
Wnt antagonists which include secreted frizzled related proteins (sFRPs), that can inhibit both canonical and non-canonical Wnt signaling[46], or the canonical Wnt/β-catenin Dickkopf (Dkk) inhibitors may also contribute to osteoblast differentiation and mineralization[30,38,47]. sFRP1 knock-out mice exhibit increased trabecular bone mass due to reduced osteoblast and osteocyte apoptosis[48] suggesting that Wnt signaling is involved in bone formation. Additionally, long bone fracture healing is enhanced in sFRP knockout mice through canonical Wnt signaling as a consequence of MSCs directed to differentiate into osteoblasts rather than towards cartilage[49]. However, high sFRP1 levels expressed early in fracture repair in this model would suggest that both canonical and non-canonical Wnt signaling are inhibited in early callus formation. Wnt5a/5b expression was decreased in the sFRP1 knockouts; although, contrary to conventional thought, canonical Wnt7a and Wnt1 were elevated[49]. There have been other reports of sFRPs enhancing rather than inhibiting Wnt activity[50] through mechanisms which may involve: (1) sFRP-Wnt binding to each other and facilitating transport and binding of Wnts to Fzd receptors on distant cells; or (2) binding to both Wnt molecules and Fzd receptors simultaneously to activate downstream Fzd signaling[51]. Many repair processes are stimulated by sFRP2-Wnt interactions including the enhancement of vascular density during granulation tissue formation[52]; inhibition of cardiomyocyte apoptosis during cardiac repair[53]; establishment of MSC-endothelial and smooth muscle contacts to stabilize new blood vessel formation[54]; and stimulation of angiogenesis by sFRP1 (FzlA) independent of VEGF, bFGF2, or angiopoietin1[55].
A key observation made by several laboratories is that canonical Wnt/β-catenin signaling may be important in osteoblastogenesis through the cooperation of Wnt signaling with other known osteogenic factors such as BMP-2 and BMP-4[47,56]. Thus taken together, canonical Wnt/β-catenin signaling appears to be involved in determining a specific tissue fate of MSCs. However, further effects of Wnt signaling (both canonical and non-canonical) on osteogenic differentiation is dependent on several factors including: The species from which the MSCs are derived, the specific Wnt (and Fzd receptors) expressed, the stage of osteogenic differentiation, the amount of β-catenin available to translocate to the cell nucleus, and other biologically active molecules (e.g., growth factors) present in the MSC’s microenvironment[30,57]. These other factors could include the ELR+ CXC chemokines which are also elevated in the inflammatory phase of healing and which have been shown to be stimulated by non-canonical Wnt5a[58,59]. In this article, we report the observation that sFRP treatment of hMSCs leads to an increased expression of ELR+ CXCL5 and CXCL8 which may serve to attract MSCs to the wound or to couple angiogenesis to osteogenesis in the early phase of bone repair.
MATERIALS AND METHODS
Cell culture
hMSCs, growth supplements, and basal medium were purchased from LonzaWalkersville, Inc. (Walkersville, MD). hMSCs from several donors were used: 19 years old male (Lonza Lot #6F4393; race unknown); 20 years old Caucasian male (Lot #0000351482); 27 years old Black male (Lot #0000318006). Cells were grown in complete medium (HMSCGM) at 37 °C under 95% air/5% CO2 atmosphere and subcultured once a week at 60%-70% confluence.
For osteoblastic differentiation, hMSCs were treated every 3-4 d with osteogenic medium (OGM) consisting of complete growth medium with 50 mmol/L ascorbic acid-2-phosphate, 10 mmol/L β-glycerophosphate, and 10-7 mol/L dexamethasone (Sigma-Aldrich, St. Louis, MO).
Cells (passage 2-7) were plated at 5000-10000 cell/cm2 in HMSCGM and allowed to adhere for 4 h prior to exposure to OGM (n ≥ 3 for all experiments). Differentiation toward the osteoblastic lineage was monitored by detection of mRNA levels of the reporter gene alkaline phosphatase or by Alizarin Red staining for calcium at 28 d as previously described[17]. Qiagen RNeasy Miniprep columns (Qiagen, Inc., Valencia, CA) were used to isolate RNA at the specified time-points. In some experiments exogenous sFRPs (varying concentration from 0-500 ng/mL; PeproTech Inc., Rocky Hill, NJ), Dkk1 (50 ng/mL, PeproTech), and L-cell or Wnt5a-conditioned medium (CM) (1:1 mixture with HMSCGM) were added as needed. The PLC signal transduction inhibitor (U73122) and control (U73343) were used at 10 μmol/L (Calbiochem, San Diego, CA). Effects of siRNA inhibition of receptors were determined by transfection of hMSCs with siRNA (150 ng/mL) using the HiPerFect transfection reagent (Qiagen) followed by treatment of the cells with sFRPs for 48 h before gene expression analysis. siRNA were purchased from Qiagen and the nucleotide sequences indicated in Table 1. A scrambled oligonucleotide siRNA was used as a negative control.
Table 1.
siRNA oligonucleotide sequences
| Gene | Qiagen product name | Qiagen catalog No. | Human target sequence |
| siFZD2 | Hs_FZD2_5 | SI02757433 | CACGGTCTACATGATCAAATA |
| siFZD5 | Hs_FZD5_5 | SI02757650 | TAAGGTTGGCGTTGTAATGAA |
| siROR2 | Hs_ROR2_6 | SI00287525 | CTGGTGCTTTACGCAGAATAA |
| siScambled | Ctrl_Control_1 | SI03650325 | AATTCTCCGAACGTGTCACGT |
Quantitative reverse transcriptase-polymerase chain reaction
Relative mRNA levels of various genes were determined by real-time RT-PCR using the Opticon Continuous Fluorescence System (Bio-Rad Laboratories, Inc., Hercules, CA) and the SYBR Green RT-PCR kit (Qiagen). Primers used for RT-PCR are indicated in Table 2.
Table 2.
Reverse transcription-polymerase chain reaction primer sequences
| Gene | Human primer sequence |
| hCXCL5 | 5’GCTGGTCCTGCCGCTGCTGTG3’ |
| 5’GTTTTCCTTGTTTCCACCGTC3’ | |
| hCXCL8 | 5’GCCTTCCTGATTTCTGCAGC3’ |
| 5’TCCAGACAGAGCTCTCTTCC3’ | |
| 18S Ribosomal RNA | 5’CGGGTCATAAGCTTGCGTT3’ |
| 5’CCGCAGGTTCACCTACGG3’ | |
| FZD2 | 5’CCTCAAGGTGCCATCCTATCTCAG3’ |
| 5’GTGTAGCAGCCCGACAGAAAAATG3’ | |
| FZD5 | 5’CCTACCACAAGCAGGTGTCC3’ |
| 5’GGACAGGTTCTTCCTCGAAA3’ | |
| ROR2 | 5’TCCTTCTGCCACTTCGTGTTTCC3’ |
| 5’TGCTTGCCGTTCCTCTGTAATCC3’ |
PCR reactions were performed in triplicate. Reactions consisted of reverse transcription at 50 °C (30 min), inactivation at 95 °C (15 min); followed by 50 cycles of denaturing at 94 °C (15 s), annealing at 60 °C (30 s), and extension at 72 °C (30 s). Gene expression changes were calculated and normalized to 18S ribosomal levels and the reference time point using the 2-ΔΔC(T) method[60].
ELISA analysis
Secreted CXCL5 protein levels were determined with the human CXCL5/ENA-78 DuoSet (R and D Systems, Minneapolis, MN) after concentration of supernatants with microcon centrifugal filters (EMD Millipore Inc, Billerica, MA). Culture supernatant samples were compared to CXCL5 standard curves and were run in duplicate.
Western blot analysis
Cells were plated in 35 mm dishes and treated with OGM medium (7 d). sFRP1 (150 ng/mL) was added and cell lysates isolated at indicated time points in PhosphoSafe Extraction Reagent (EMD Chemicals, Gibbstown, NJ). Proteins were separated (SDS–PAGE), transferred to polyvinylidenedifluoride membrane, and probed with ERK-specific pan or phospho-antibodies (Cell Signaling Technology, Danvers, MA). Immunoreactive proteins were detected using the ECL kit (GE Healthcare Bio-sciences, Piscataway, NJ) and levels quantitated using AlphaView Software (ProteinSimple, San Jose, CA).
Statistical analysis
Data values are reported as mean ± SD. Statistical analysis (1-way ANOVA with the Bonferroni method for multiple comparisons between pairs or non-parametric Mann-Whitney t test) was performed using GraphPad Prism software. Differences from negative controls were considered to be statistically significant at the P < 0.05 level.
RESULTS
We had previously demonstrated that mRNA and protein for CXCL8 (IL-8) and CXCL1 (GROα)[17] were induced in hMSCs exposed to osteogenic differentiation medium (OGM) containing ascorbate-2-phosphate, β-glycerophosphate, and dexamethasone. To see if another angiogenic CXC chemokine, CXCL5 (ENA-78), was also induced by osteogenic differentiation, RNA from hMSCs treated with OGM was analyzed for CXCL5 expression levels. OGM treatment for 7 d stimulated CXCL5 mRNA levels approximately 8-fold (P < 0.01; Figure 1A). Dexamethasone alone (0.1 μmol/L) in the presence of proliferating medium (HMSCGM) increased CXCL5 mRNA by 5.5-fold (P < 0.01).
Figure 1.
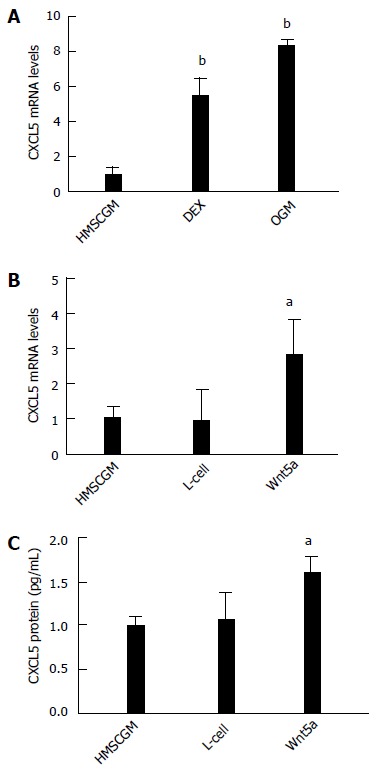
CXCL5 chemokine induction in human mesenchymal stem cells treated with osteogenic medium or non-canonical Wnt5a. A: CXCL5 mRNA levels are induced at 7 d in complete osteogenic medium or medium containing 0.1 μmol/L dexamethasone. Conditioned medium containing non-canonical Wnt5a induces (B) mRNA expression and (C) protein secretion. All values are mean ± SD. aP < 0.05; bP < 0.01 vs HMSCGM-treated group. HMSCGM: Human mesenchymal stem cell growth medium; DEX: Dexamethasone; OGM: Osteogenic medium; L-cell: Conditioned medium from L-cells; Wnt5a: Conditioned medium from L-cells expressing Wnt5a.
Non-canonical Wnt signaling has also been associated with osteogenic differentiation of hMSCs[32,61]. Since osteogenic differentiation using OGM or dexamethasone alone resulted in CXCL5 mRNA expression, we then explored if treatment of hMSC cells with non-canonical Wnt5a protein was able to stimulate the expression of CXCL5. hMSC cells were treated for 7 d with CM from L-cells overexpressing Wnt5a protein or control L-cell CM. CM containing Wnt5a induced the expression of CXCL5 mRNA 3-fold (P < 0.05) compared to the lack of stimulation of CXCL5 in both non-osteogenic medium (HMSCGM medium) or control L-cell medium (Figure 1B). CXCL5 protein secretion was also increased 1.5-fold (P < 0.05) above controls (Figure 1C).
We next sought to inhibit all Wnt signaling, both canonical and non-canonical Wnt signaling, using sFRPs. Surprisingly and unexpectedly, sFRP1 increased CXCL5 mRNA levels 3-fold (P < 0.01) at 7 d and approximately 8-fold (P < 0.01) at 10 d of culture in HMSCGM medium (Figure 2A). To see if canonical Wnt signaling inhibition was responsible for the unexpected stimulation of CXCL5, hMSCs were treated with Dkk1 which binds to the low density lipoprotein receptor related protein 6 (LRP6) to inhibit canonical signaling. Unlike sFRP-1, Dkk1 addition did not induce an increase in CXCL5 levels at 7 or 10 d and in fact significantly inhibited basal mRNA expression levels more than 50% (P < 0.01) at 7 d (Figure 2A). To see if the effect of sFRP1 on CXCL5 was unique amongst the other sFRP family members, sFRPs 2, 3, or 4 was each added separately to the medium (150 ng/mL) for 7 d and levels of CXCL5 protein secreted into the medium determined. All four sFRPs added independently significantly stimulated CXCL5 protein secretion 3-4-fold (P < 0.01) over un-stimulated vehicle control (Figure 2B).
Figure 2.
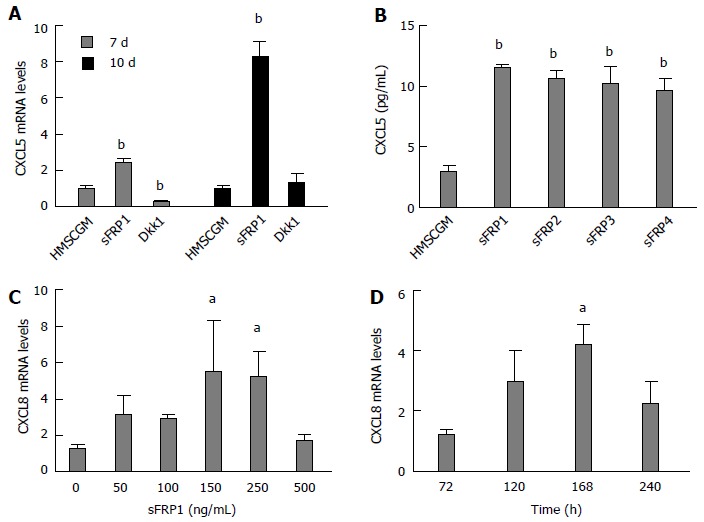
Secreted frizzled-related protein-stimulated expression of CXCL5 and CXCL8 in human mesenchymal stem cells. A: sFRP1, an inhibitor of canonical and non-canonical Wnt signaling, induces expression of CXCL5 mRNA. Dkk1, an inhibitor of canonical Wnt signaling does not induce CXCL5; B: All four of the sFRPs stimulated CXCL5 protein secretion as determined by ELISA analysis of cell supernatants. sFRP1 induces expression of CXCL8 mRNA in a dose- (C) and time- (D) dependent manner with maximum expression at 150 ng/mL and 7 d post-treatment. All values are mean ± SD. aP < 0.05; bP < 0.01 vs HMSCGM-treated group (A, B), vs untreated (C), or 3-d CXCL8 mRNA levels (D). HMSCGM: Human mesenchymal stem cell growth medium; sFRP: Secreted frizzled-related protein; Dkk1: Dickkopf-related protein 1.
We next tested if CXCL8 mRNA levels are also stimulated by sFRP1 treatment. sFRP1 treatment increased CXCL8 mRNA levels in a dose-dependent manner (Figure 2C). Maximum stimulation of CXCL8 mRNA expression (approximately 5-fold) was observed at a concentration of 150 ng/mL sFRP1 (P < 0.05). A time-course study of CXCL8 mRNA expression stimulated by sFRP1 (150 ng/mL) showed maximal expression levels (P < 0.05) between 5 and 7 d of culture (Figure 2D).
In an effort to explore the mechanism of the sFRP1-stimulated increase in both CXCL5 and CXCL8 expression, we characterized the phosphorylated state of the extracellular signal-regulated kinase (ERK) p44/42, a member of the MAPK pathway, since it has been previously reported that CXC ligand expression can be increased via MAPK activation[62-66]. p44/42 was shown to be phosphorylated maximally at 5 min in the presence of OGM and sFRP1, whereas maximal p44/42 phosphorylation occurred at 10 min in OGM alone (Figure 3).
Figure 3.
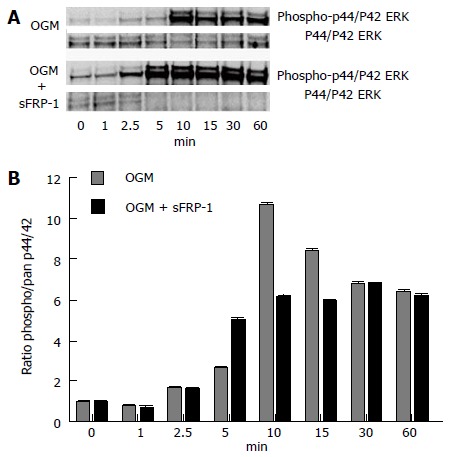
Phosphorylated state of mitogen-activated protein kinase p44/42 extracellular-signal-regulated kinases in response to secreted frizzled-related protein 1 stimulation. Western Blot time-course analysis of pan and phosphorylated states of the MAPK p44/42 ERK proteins (A); The ratio of phospho/pan p44/42 ERK reaches a maximum at 5 min in the presence of OGM and sFRP1 (B); whereas, in OGM alone the maximal ratio level is not reached until 10 min post stimulation (representative experiment). sFRP1: Secreted frizzled-related protein 1; MAPK: Mitogen-activated protein kinase; ERK: Extracellular-signal-regulated kinases; OGM: Osteogenic medium.
To see if other G-protein coupled signaling mechanisms could be involved in sFRP stimulation of CXCL8 mRNA expression, the PLC inhibitor, U73122, was added (10 μmol/L) to the HMSCGM medium and CXCL8 mRNA levels determined after 3 d of sFRP treatment. Both sFRP1 and sFRP2 in the presence of the inactive isomer, U73343, enhanced CXCL8 mRNA by approximately 3 to 5 fold (P < 0.05). However, U73122 prevented the increase in both sFRP1- and sFRP2-stimulated CXCL8 mRNA levels returning them back to HMSCGM control levels (Figure 4).
Figure 4.
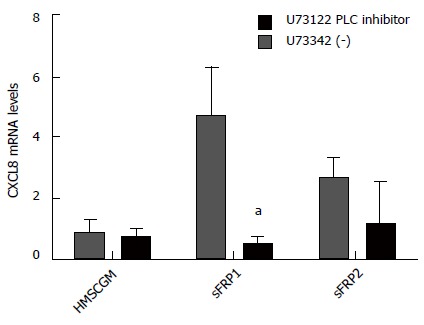
Modulation of secreted frizzled-related protein-stimulated induction of CXCL8 mRNA. sFRP1/2-stimulated CXCL8 mRNA expression is suppressed in the presence of the PLC inhibitor U73122 suggesting that signaling is via the Wnt/calcium non-canonical pathway (values are mean ± SD). aP < 0.05 vs HMSCGM-treated levels. sFRP1/2: Secreted frizzled-related protein 1/2; PLC: Phospholipase C.
Since Fzd receptors are G-protein coupled seven transmembrane receptors, it was further investigated if the sFRP stimulation of CXCL8 could be through interactions with specific frizzled receptors. Fzd2 and Fzd5 have been associated with non-canonical Wnt signaling[67,68]; whereas, Fzd7 is associated with canonical signaling[69]. sFRP1, 2 and 3 all stimulated CXCL8 mRNA levels in the presence of a scrambled siControl siRNA (7 to 10-fold for sFRP1 and sFRP2; 3-fold for sFRP3). In the presence of siRNA to Fzd2 and 5, the sFRP-stimulation was almost entirely inhibited back to baseline levels (P < 0.05; Figure 5A and B). siRNA to Fzl7 did not have any effect on sFRP1 or sFRP2 induction of CXCL8 mRNA (data not shown). The receptor tyrosine kinase-like orphan receptor-2 (RoR2) has been shown to be either a stand-alone receptor or co-receptor with Fzd in non-canonical Wnt signaling[70,71]. siRNA directed against RoR2 inhibited sFRP1-stimulated CXCL8 mRNA expression by approximately 65% but did not have an effect on sFRP2-stimulated CXCL8 mRNA expression (Figure 5C).
Figure 5.
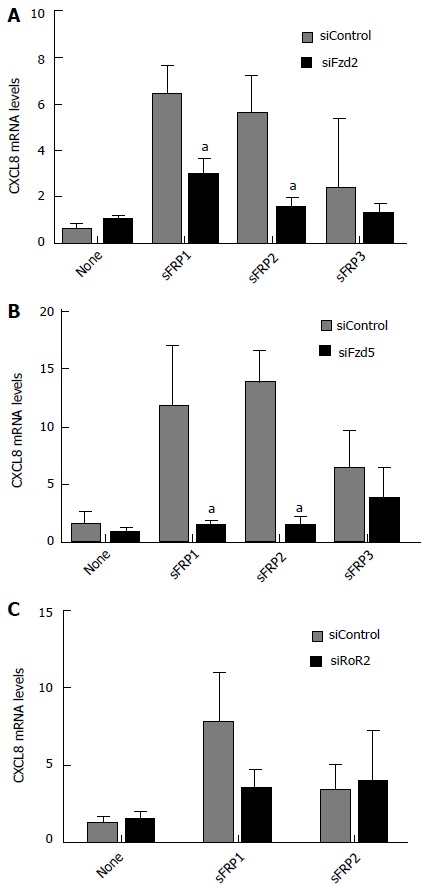
Effect of siRNA directed to frizzled receptors or receptor tyrosine kinase-like orphan receptor-2 on secreted frizzled-related protein-stimulated CXCL8 mRNA levels. sFRP-stimulated CXCL8 mRNA expression is inhibited in the presence of siRNAs directed toward non-canonical (A) Fzd2 and (B) Fzd5 or (C) the non-canonical Frizzled co-receptor RoR2. All values are mean ± SD. aP < 0.05 vs untreated siControl levels for each graph. sFRP1-3: Secreted frizzled-related protein 1-3; siFzd2/5: siRNA to the non-canonical frizzled receptor 2/5; RoR2: Receptor tyrosine kinase-like orphan receptor-2; siRoR2: siRNA to RoR2; siControl: Scrambled control siRNA.
DISCUSSION
sFRPs have been traditionally thought to act as Wnt signaling antagonists by binding to Wnt molecules and preventing them from binding to Fzd receptors thus inhibiting signal initiation[72]. Interaction of Wnts with Fzd receptors has been hypothesized to occur via interaction with the extracellular cysteine-rich domains (CRDs) found in Fzd receptors. sFRPs also contain N-terminal CRDs, but without the transmembrane domain characteristic of Fzd receptors[73], and it has been demonstrated that sFRP interaction with Wnts occurs through binding within this CRD[74,75]. Additionally, Bafico et al[75] found that at least one sFRP could also bind to a selected Fzd (e.g., Fzd6) and hypothesized that heteromeric complex of Fzd and sFRPs would render Fzd receptors nonfunctional.
However, there has been emerging evidence that sFRPs may not only function as Wnt antagonists or anti-morphogens but also serve as molecules that promote differentiation of specific tissues. It has been suggested that sFRPs may aid in Wnt protein distribution and signaling within tissues. Wnt proteins interact with heparin sulfate proteoglycans thereby limiting diffusion of the Wnt proteins within tissues. sFRPs may compete with this binding, enabling the Wnt-sFRP “transport” complexes that are formed to diffuse along a more extended gradient allowing for longer range Wnt signaling within the tissue[76] thereby aiding in tissue generation or differentiation. In one mouse model system, MSCs engineered to overexpress protein kinase B (also known as Akt) produce high levels of sFRP2 which can effectively limit cardiac muscle infarct size through the inhibition of cardiomyocyte apoptosis[53]. The increased sFRP2 led to increased levels of nuclear β-catenin thus enhancing canonical Wnt signaling and increased transcription of anti-apoptotic genes such as Birc1b and to a lesser extent Bcl2.
Others have reported links between non-canonical Wnt signaling and sFRP-mediated differentiation processes. Chung et al[77] reported that sFRP3 could increase osteoblast differentiation in the mouse pre-osteoblastic cell line, MC3T3-E1, by increasing alkaline phosphatase, osteocalcin, and promoting mineralization of MC3T3-E1 cultures. Endostatin, which promotes degradation of β-catenin independent of GSK3β, did not abrogate sFRP3-stimulated osteogenic differentiation suggesting that non-canonical Wnt signaling may be involved in the sFRP3 effect. Esteve et al[78] also reported that chick sFRP1 enhanced retinal differentiation by increasing the generation of retinal ganglion and photoreceptor cells independent of cell proliferation. This group noted that canonical Wnt-β-catenin signaling was not involved in this process; although, they did find that phosphorylation of GSK3β down-regulated its activity while promoting retinal cell differentiation. The authors were unclear if non-canonical Wnt signaling was involved in the sFRP1 findings at that time. In a subsequent communication, this group reported that chick sFRP1 binds to Fzd2 to stimulate axonal outgrowth from retinal neurons[79]. Furthermore, the action of sFRP1 on the retinal ganglion cells was dependent on cAMP and cGMP in a pertussis toxin-sensitive manner suggesting that sFRP1 acted as an agonist for Fzd2 non-canonical Wnt signaling.
Several reports have shown that sFRPs were involved in stimulating angiogenesis through canonical Wnt signaling but independent of VEGF signaling[54,55,80]. In studies utilizing MRL/MpJ mice, which have enhanced regenerative capacity, it was found that MRL/MpJ bone marrow MSCs showed decreased expression of cyclin D1, Sox2, and Axin2, which are target genes of canonical Wnt signaling. Concomitantly, sFRP2 and sFRP4 expression was found to be significantly up-regulated[52] in these cells. It was also reported that sFRP2 overexpression in mouse MSCs that were then injected into the cardiac peri-infarct area reduced infarct size and improved cardiac function similar to that seen when MRL/MpJ MSCs were injected. Of note, vascularization of granulation tissue was also enhanced by sFRP2 overexpression. This was also reported in another MRL/MpJMSC engraftment wound healing model[52] whereby sFRP2 overexpression in mouse MSCs increased levels of several angiogenic factors including FGF2 receptor, PDGF receptor beta, VEGF, and angiopoietins among others. While the authors concluded that the increased sFRP2 inhibited canonical Wnt signaling which may be related to the increased angiogenesis, non-canonical Wnt signaling was not examined. Furthermore, the expression of ELR+ CXC chemokines that are also angiogenic was not assessed.
There are a number of steps that occur in new blood vessel formation, several of which have been linked to sFRP1 signaling, including endothelial cell (EC) spreading, proliferation and migration, vascular channel formation, and blood vessel stabilization. sFRP1 has been shown to enhance angiogenesis in a chick chorioallantoic membrane model of angiogenesis and to increase blood vessel density in a tumor implantation model[55]. EC spreading was hypothesized to be a result of an interaction of sFRP1 with Fzd4 and Fzd7 thereby blocking Fzd activity and has also been shown to be independent of canonical Wnt-β-catenin signaling; although, this process still involving GSK3β upstream of Rac 1 signaling[80]. sFRP1 has also been shown to stimulate EC migration and chemotaxis in vitro, increase EC branching in capillary structures when cultured on Matrigel, and inhibit EC apoptosis[55]. EC and vascular smooth muscle cell proliferation are also inhibited as evidenced by slower entry into S-phase as well as decreased expression of the cell cycle components cyclin D1 and cdk4[81]. This latter inhibition of vascular cell proliferation appeared to be dependent on inhibition of canonical Wnt-β-catenin signaling but not MAPK signaling through ERK1/2. Vessel maturation and stabilization of EC channels by pericytes or MSCs were also enhanced by sFRP1 stimulated cell-cell interactions between MSCs and ECs or smooth muscle cells in a GSK3β-dependent manner. Interestingly, sFRP1 increased α-smooth muscle actin expression in MSCs suggesting differentiation of MSCs to pericytes which are involved with blood vessel stabilization. Furthermore, the localization of β-catenin at cell-cell junctions rather than intranuclear locations could further support a non-canonical Wnt signaling mechanism. Others have also reported that sFRP2 can also stimulate EC migration and tube formation as well as inhibit hypoxia-induced EC apoptosis through a non-canonical Wnt-calcium pathway involving an increase in NFATc3 nuclear translocation[82,83].
The mechanism of how sFRPs stimulate angiogenesis is currently unknown. sFRP1, which did not induce expression of the known angiogenic factors VEGF or FGF2, did increase expression of PDGF-BB which is involved in postnatal blood vessel maturation[84]. Expression of other angiogenic factors such as the ELR+ CXC chemokines could potentially be the result of sFRP1 actions. Indeed we are the first to report here that sFRPs are able to induce the ELR+ CXC chemokines CXCL5 and CXCL8 in hMSCs. Rauner et al[85] has shown that human bone marrow MSCs stimulated with pro-inflammatory factors (lipopolysaccharide or TNF-α) resulted in Wnt5a and RoR2 increases in mRNA and protein. The expression of the ELR+ CXC chemokines, CXCL1, CXCL2, and CXCL5, was also increased with Wnt5a treatment of these hMSCs. Additionally the CC chemokines, CCL2, CCL5, CCL7, and CCL19 were also upregulated, although, to a lesser extent than the CXC chemokines CXCL1 and CXCL5. Albers et al[59] also reported that Fzd9 knockout mice demonstrated an osteopenic phenotype caused by decreased bone formation which was unrelated to canonical Wnt signaling[59]. The presumed non-canonical Wnt regulation of bone mass in Fzd9-deficient mice was also shown to have significantly decreased CXCL5 expression. In these studies, treatment of wild type osteoblasts with Wnt5a showed a 12-fold increase in CXCL5 mRNA. In a fracture repair model in Fzd9-deficient mice, protein expression of CXCL5 and CCL2 in the healing callus was diminished in comparison to wild-type, and overall new bone in the Fzd9 knockout mice was reduced[86]. Most recently, Zhao et al[87] reported that in human dental pulp cells, non-canonical Wnt5a significantly induced CXCL8, CCL2, and CCL5 mRNA and protein expression, as well as increasing CXCL1 mRNA expression. CXCL5 expression was not tested in response to Wnt5a stimulation in this model.
The signaling mechanism(s) responsible for sFRPs induction of ELR+ CXC chemokine expression are unknown. Our results suggest that ELR+ CXC chemokine stimulation by sFRPs in human bone marrow-derived MSCs is via non-canonical Wnt signaling. MAPK, specifically though ERK1/2, and PLC pathways appear to play a role in sFRP stimulation of the ELR+ CXC chemokines. PLC signaling can be upstream of MAPK/ERK[88] and perhaps the non-canonical Wnt-calcium pathway is involved. Our findings that inhibition of sFRP-induced ELR+ CXC chemokine expression by PLC as well as demonstration of ERK phosphorylation upon sFRP stimulation of MSCs are consistent with potential sFRP signaling thru serpentine G protein-coupled receptors such as the Fzd receptors. Furthermore, our data demonstrating prevention of sFRP1-stimulated CXCL8 mRNA induction with siRNA-directed inhibition of the non-canonical Fzd2 and Fzd5 are also consistent with a role of sFRP-Fzd receptor interaction in ELR+ CXC chemokine genesis. How RoR2 either acting as a co-receptor with non-canonical Fzd receptors or independently fits in to the regulation of ELR+ chemokine expression is currently unknown. Since these angiogenic chemokines are expressed during the inflammatory phase of wound healing, these chemokines could contribute to several aspects of bone repair including attraction of additional MSCs to the site for differentiation or attraction of endothelial cells for generation of vascularized granulation tissue and stimulation of angiogenesis as we had previously demonstrated[17]. Thus, in addition to regulation of Wnt signaling as inhibitory substances, our study adds to a growing body of knowledge on the stimulatory functions of sFRPs. Specifically, a novel function of sFRPs in stimulating angiogenic chemokines can be envisioned that may aid in wound and bone repair.
COMMENTS
Background
Mesenchymal stem cells (MSCs) have the capability to differentiate into several cell types including adipocytes, chondrocytes, and osteoblasts and thus have high potential as treatment for repairing bone defects. This process requires the interaction of various growth factors, chemokines, and signaling pathways resulting in the necessary inflammatory, angiogenic, and osteogenic stages of bone repair.
Research frontiers
Although much attention has been placed on the role of the major angiogenesis proteins (vascular endothelial growth factor and fibroblast growth factors) and the Wnt system in bone repair, not much research has been conducted on the role of the ELR+ chemokines in this process. These chemokines also have important functions in inflammation and blood vessel formation and have been shown to be stimulated by non-canonical Wnt signaling and during osteogenic differentiation of MSCs.
Innovations and breakthroughs
In this report, the authors demonstrate that treatment of human MSC (hMSC) with the soluble frizzled-related proteins (sFRPs), which should inhibit both canonical and non-canonical Wnt signaling, actually stimulates the expression of the angiogenic CXC ELR+ chemokines CXCL5 and CXCL8. CXC ELR+ chemokine stimulation was mediated through the non-canonical frizzled receptors 2 (Fzd2) and Fzd5 Wnt receptors and the RoR2 co-receptor. This adds to the data suggesting non-canonical Wnt control of several bone formation processes through expression of the ELR+ chemokines and identifies a potential new role for the sFRPs in coupling ELR+ chemokine angiogenesis to bone repair.
Applications
Many recent reports have focused on the use of native or genetically engineered MSCs as a treatment to speed up or enhance the quality and mineralization of bone in wound and bone defect models. The ability of the sFRPs to stimulate ELR+ CXC chemokines detailed in this study may suggest another avenue for manipulation of bone formation pathways and may eventually lead to a therapeutic treatment to hasten bone healing and return bone strength back to pre-injury levels.
Terminology
MSCs: Multipotent stromal cells that can be differentiated into several cells types including cartilage (chondrocytes), bone (osteoblasts), fat (adipocytes) and muscle (myocytes); ELR+ CXC chemokines: Family of small cytokines secreted by cells and containing a Cys-X-Cys domain. CXC chemokines can be further divided into those with or without a Glu-Leu-Arg (ELR+) motif. ELR+ CXC chemokines are angiogenic. ELR- CXC chemokines are angiostatic. Wnt signaling: family of signaling molecules (Wnts) and Fzds that are important in many developmental pathways including cell fate, proliferation, and differentiation; sFRPs: Family of proteins that inhibit Wnt signaling by acting as soluble, decoy receptors preventing Wnt binding to Fzds.
Peer-review
The paper found that CXC chemokine expression in hMSC is controlled in part by sFRPs signalling through non-canonical Wnt involving Fzd2/5 and the ERK and PLC pathways. The results are interesting.
Footnotes
Supported by Merit Review Award from the United States, Department of Veterans Affairs Biomedical Laboratory Research and Development Service of the VA Office of Research and Development, No. I01BX000170.
Institutional review board statement: Not applicable.
Institutional animal care and use committee statement: Not applicable.
Conflict-of-interest statement: To the best of our knowledge, no conflict of interest exists. All authors are employees of the United States Federal Government and the work is part of their official duties.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 1, 2015
First decision: September 17, 2015
Article in press: November 25, 2015
P- Reviewer: Antonelli A, Kolonin MG S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci USA. 2011;108:1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Cruceta J, Graves BD, Einhorn TA. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169:285–294. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- 4.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt E, Toksoy A, Goebeler M, Debus S, Bröcker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 7.Bernardini G, Ribatti D, Spinetti G, Morbidelli L, Ziche M, Santoni A, Capogrossi MC, Napolitano M. Analysis of the role of chemokines in angiogenesis. J Immunol Methods. 2003;273:83–101. doi: 10.1016/s0022-1759(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 8.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 9.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 10.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–848. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Yoo KH, Choi KS, Choi J, Choi SY, Yang SE, Yang YS, Im HJ, Kim KH, Jung HL, et al. Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine. 2005;31:119–126. doi: 10.1016/j.cyto.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 14.Murdoch C, Monk PN, Finn A. Cxc chemokine receptor expression on human endothelial cells. Cytokine. 1999;11:704–712. doi: 10.1006/cyto.1998.0465. [DOI] [PubMed] [Google Scholar]
- 15.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 16.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff DS, Zhu JH, Makhijani NS, Kumar A, Yamaguchi DT. Angiogenic CXC chemokine expression during differentiation of human mesenchymal stem cells towards the osteoblastic lineage. J Cell Biochem. 2008;103:812–824. doi: 10.1002/jcb.21450. [DOI] [PubMed] [Google Scholar]
- 18.Bischoff DS, Zhu JH, Makhijani NS, Yamaguchi DT. Acidic pH stimulates the production of the angiogenic CXC chemokine, CXCL8 (interleukin-8), in human adult mesenchymal stem cells via the extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and NF-kappaB pathways. J Cell Biochem. 2008;104:1378–1392. doi: 10.1002/jcb.21714. [DOI] [PubMed] [Google Scholar]
- 19.Mihara K, Smit MJ, Krajnc-Franken M, Gossen J, Rooseboom M, Dokter W. Human CXCR2 (hCXCR2) takes over functionalities of its murine homolog in hCXCR2 knockin mice. Eur J Immunol. 2005;35:2573–2582. doi: 10.1002/eji.200526021. [DOI] [PubMed] [Google Scholar]
- 20.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 21.Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, Richmond A. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luan J, Furuta Y, Du J, Richmond A. Developmental expression of two CXC chemokines, MIP-2 and KC, and their receptors. Cytokine. 2001;14:253–263. doi: 10.1006/cyto.2001.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padovani-Claudio DA, Liu L, Ransohoff RM, Miller RH. Alterations in the oligodendrocyte lineage, myelin, and white matter in adult mice lacking the chemokine receptor CXCR2. Glia. 2006;54:471–483. doi: 10.1002/glia.20383. [DOI] [PubMed] [Google Scholar]
- 24.Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bischoff DS, Sakamoto T, Ishida K, Makhijani NS, Gruber HE, Yamaguchi DT. CXC receptor knockout mice: characterization of skeletal features and membranous bone healing in the adult mouse. Bone. 2011;48:267–274. doi: 10.1016/j.bone.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 27.Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- 28.Miller JR. The Wnts. Genome Biol. 2002;3:REVIEWS3001. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Boer J, Wang HJ, Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10:393–401. doi: 10.1089/107632704323061753. [DOI] [PubMed] [Google Scholar]
- 32.Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93:1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 33.Neth P, Ciccarella M, Egea V, Hoelters J, Jochum M, Ries C. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24:1892–1903. doi: 10.1634/stemcells.2005-0503. [DOI] [PubMed] [Google Scholar]
- 34.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 35.Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002;17:101–110. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- 36.de Boer J, Siddappa R, Gaspar C, van Apeldoorn A, Fodde R, van Blitterswijk C. Wnt signaling inhibits osteogenic differentiation of human mesenchymal stem cells. Bone. 2004;34:818–826. doi: 10.1016/j.bone.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Cho HH, Kim YJ, Kim SJ, Kim JH, Bae YC, Ba B, Jung JS. Endogenous Wnt signaling promotes proliferation and suppresses osteogenic differentiation in human adipose derived stromal cells. Tissue Eng. 2006;12:111–121. doi: 10.1089/ten.2006.12.111. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 39.Lou J, Tu Y, Li S, Manske PR. Involvement of ERK in BMP-2 induced osteoblastic differentiation of mesenchymal progenitor cell line C3H10T1/2. Biochem Biophys Res Commun. 2000;268:757–762. doi: 10.1006/bbrc.2000.2210. [DOI] [PubMed] [Google Scholar]
- 40.Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–4459. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- 41.Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 42.Lemonnier J, Ghayor C, Guicheux J, Caverzasio J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J Biol Chem. 2004;279:259–264. doi: 10.1074/jbc.M308665200. [DOI] [PubMed] [Google Scholar]
- 43.Hadjiargyrou M, Lombardo F, Zhao S, Ahrens W, Joo J, Ahn H, Jurman M, White DW, Rubin CT. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem. 2002;277:30177–30182. doi: 10.1074/jbc.M203171200. [DOI] [PubMed] [Google Scholar]
- 44.Chang J, Sonoyama W, Wang Z, Jin Q, Zhang C, Krebsbach PH, Giannobile W, Shi S, Wang CY. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–30948. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- 45.Olivares-Navarrete R, Hyzy SL, Hutton DL, Dunn GR, Appert C, Boyan BD, Schwartz Z. Role of non-canonical Wnt signaling in osteoblast maturation on microstructured titanium surfaces. Acta Biomater. 2011;7:2740–2750. doi: 10.1016/j.actbio.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 47.Vaes BL, Dechering KJ, van Someren EP, Hendriks JM, van de Ven CJ, Feijen A, Mummery CL, Reinders MJ, Olijve W, van Zoelen EJ, et al. Microarray analysis reveals expression regulation of Wnt antagonists in differentiating osteoblasts. Bone. 2005;36:803–811. doi: 10.1016/j.bone.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 49.Gaur T, Wixted JJ, Hussain S, O’Connell SL, Morgan EF, Ayers DC, Komm BS, Bodine PV, Stein GS, Lian JB. Secreted frizzled related protein 1 is a target to improve fracture healing. J Cell Physiol. 2009;220:174–181. doi: 10.1002/jcp.21747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- 51.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 52.Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamazière JM, Couffinhal T, Duplàa C. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells. 2008;26:2991–3001. doi: 10.1634/stemcells.2008-0372. [DOI] [PubMed] [Google Scholar]
- 55.Dufourcq P, Couffinhal T, Ezan J, Barandon L, Moreau C, Daret D, Duplàa C. FrzA, a secreted frizzled related protein, induced angiogenic response. Circulation. 2002;106:3097–3103. doi: 10.1161/01.cir.0000039342.85015.5c. [DOI] [PubMed] [Google Scholar]
- 56.Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:33–39. doi: 10.1007/s11154-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 57.Kim K, Pang KM, Evans M, Hay ED. Overexpression of beta-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol Biol Cell. 2000;11:3509–3523. doi: 10.1091/mbc.11.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 59.Albers J, Schulze J, Beil FT, Gebauer M, Baranowsky A, Keller J, Marshall RP, Wintges K, Friedrich FW, Priemel M, et al. Control of bone formation by the serpentine receptor Frizzled-9. J Cell Biol. 2011;192:1057–1072. doi: 10.1083/jcb.201008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Bhat RA, Seestaller-Wehr LM, Fukayama S, Mangine A, Moran RA, Komm BS, Bodine PV, Billiard J. The orphan receptor tyrosine kinase Ror2 promotes osteoblast differentiation and enhances ex vivo bone formation. Mol Endocrinol. 2007;21:376–387. doi: 10.1210/me.2006-0342. [DOI] [PubMed] [Google Scholar]
- 62.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- 63.Parhar K, Ray A, Steinbrecher U, Nelson C, Salh B. The p38 mitogen-activated protein kinase regulates interleukin-1beta-induced IL-8 expression via an effect on the IL-8 promoter in intestinal epithelial cells. Immunology. 2003;108:502–512. doi: 10.1046/j.1365-2567.2003.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J, Kartha S, Iasvovskaia S, Tan A, Bhat RK, Manaligod JM, Page K, Brasier AR, Hershenson MB. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am J Physiol Lung Cell Mol Physiol. 2002;283:L690–L699. doi: 10.1152/ajplung.00060.2002. [DOI] [PubMed] [Google Scholar]
- 65.Saatian B, Zhao Y, He D, Georas SN, Watkins T, Spannhake EW, Natarajan V. Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells. Biochem J. 2006;393:657–668. doi: 10.1042/BJ20050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim HJ, Byun SJ, Kim TY. Differential regulation of IGF-II-induced IL-8 by extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinases in human keratinocytes. Biochem Biophys Res Commun. 2004;317:276–284. doi: 10.1016/j.bbrc.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 67.Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 2010;29:41–54. doi: 10.1038/emboj.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kühl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 70.Green J, Nusse R, van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol. 2014;6:pii: a009175. doi: 10.1101/cshperspect.a009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 72.Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- 75.Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem. 1999;274:16180–16187. doi: 10.1074/jbc.274.23.16180. [DOI] [PubMed] [Google Scholar]
- 76.Mii Y, Taira M. Secreted Wnt “inhibitors” are not just inhibitors: regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev Growth Differ. 2011;53:911–923. doi: 10.1111/j.1440-169X.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 77.Chung YS, Baylink DJ, Srivastava AK, Amaar Y, Tapia B, Kasukawa Y, Mohan S. Effects of secreted frizzled-related protein 3 on osteoblasts in vitro. J Bone Miner Res. 2004;19:1395–1402. doi: 10.1359/JBMR.040412. [DOI] [PubMed] [Google Scholar]
- 78.Esteve P, Trousse F, Rodríguez J, Bovolenta P. SFRP1 modulates retina cell differentiation through a beta-catenin-independent mechanism. J Cell Sci. 2003;116:2471–2481. doi: 10.1242/jcs.00452. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez J, Esteve P, Weinl C, Ruiz JM, Fermin Y, Trousse F, Dwivedy A, Holt C, Bovolenta P. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat Neurosci. 2005;8:1301–1309. doi: 10.1038/nn1547. [DOI] [PubMed] [Google Scholar]
- 80.Dufourcq P, Leroux L, Ezan J, Descamps B, Lamazière JM, Costet P, Basoni C, Moreau C, Deutsch U, Couffinhal T, et al. Regulation of endothelial cell cytoskeletal reorganization by a secreted frizzled-related protein-1 and frizzled 4- and frizzled 7-dependent pathway: role in neovessel formation. Am J Pathol. 2008;172:37–49. doi: 10.2353/ajpath.2008.070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ezan J, Leroux L, Barandon L, Dufourcq P, Jaspard B, Moreau C, Allières C, Daret D, Couffinhal T, Duplàa C. FrzA/sFRP-1, a secreted antagonist of the Wnt-Frizzled pathway, controls vascular cell proliferation in vitro and in vivo. Cardiovasc Res. 2004;63:731–738. doi: 10.1016/j.cardiores.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Courtwright A, Siamakpour-Reihani S, Arbiser JL, Banet N, Hilliard E, Fried L, Livasy C, Ketelsen D, Nepal DB, Perou CM, et al. Secreted frizzle-related protein 2 stimulates angiogenesis via a calcineurin/NFAT signaling pathway. Cancer Res. 2009;69:4621–4628. doi: 10.1158/0008-5472.CAN-08-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siamakpour-Reihani S, Caster J, Bandhu Nepal D, Courtwright A, Hilliard E, Usary J, Ketelsen D, Darr D, Shen XJ, Patterson C, et al. The role of calcineurin/NFAT in SFRP2 induced angiogenesis--a rationale for breast cancer treatment with the calcineurin inhibitor tacrolimus. PLoS One. 2011;6:e20412. doi: 10.1371/journal.pone.0020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rauner M, Stein N, Winzer M, Goettsch C, Zwerina J, Schett G, Distler JH, Albers J, Schulze J, Schinke T, et al. WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J Bone Miner Res. 2012;27:575–585. doi: 10.1002/jbmr.1488. [DOI] [PubMed] [Google Scholar]
- 86.Heilmann A, Schinke T, Bindl R, Wehner T, Rapp A, Haffner-Luntzer M, Nemitz C, Liedert A, Amling M, Ignatius A. The Wnt serpentine receptor Frizzled-9 regulates new bone formation in fracture healing. PLoS One. 2013;8:e84232. doi: 10.1371/journal.pone.0084232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Y, Wang CL, Li RM, Hui TQ, Su YY, Yuan Q, Zhou XD, Ye L. Wnt5a promotes inflammatory responses via nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells. J Biol Chem. 2014;289:21028–21039. doi: 10.1074/jbc.M113.546523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]


