Abstract
Hepatic encephalopathy (HE) is one of the worst complications of liver disease and can be greatly influenced by nutritional status. Ammonia metabolism, inflammation and muscle wasting are relevant processes in HE pathophysiology. Malnutrition worsens the prognosis in HE, requiring early assessment of nutritional status of these patients. Body composition changes induced by liver disease and limitations superimposed by HE hamper the proper accomplishment of exams in this population, but evidence is growing that assessment of muscle mass and muscle function is mandatory due to the role of skeletal muscles in ammonia metabolism. In this review, we present the pathophysiological aspects involved in HE to support further discussion about advantages and drawbacks of some methods for evaluating the nutritional status of cirrhotic patients with HE, focusing on body composition.
Keywords: Hepatic encephalopathy, Liver cirrhosis, Malnutrition, Anthropometry, Muscle strength, Electric impedance, Nutrition assessment, Dual-energy X-ray absorptiometry
Core tip: Ammonia metabolism, inflammation and muscle wasting are relevant processes in hepatic encephalopathy (HE) pathophysiology and malnutrition worsens the prognosis in this condition, requiring early assessment of nutritional status in these patients. Body composition changes induced by liver disease and limitations superimposed by HE make difficult to accomplish exams properly in this population, but there is a growing evidence that assessment of muscle mass and muscle function is mandatory due to the role of skeletal muscles in ammonia metabolism. In this article, we review HE pathophysiology and discuss the main methods of nutritional assessment, suggesting the best approaches in HE patients.
INTRODUCTION
Hepatic encephalopathy (HE), a neurological dysfunction affecting primarily the brain, is caused by acute or chronic liver insufficiency and/or by the presence of portosystemic shunting. This syndrome embraces distinct forms of neurological symptoms from subclinical presentations to hepatic coma[1]. Overt HE afflicts 30%-45% of cirrhotic patients, whereas minimal HE may affect more than half of the cases with advanced cirrhosis[2-4]. The estimated incidence in decompensated cirrhosis is 8% per year and the majority of patients with HE episodes are received in emergency care services[5]. In an observational study, the mean hospitalization length of these patients was between 5.7 and 7.1 d[2].
Among cirrhosis complications, HE mortality is higher than the rate caused by variceal bleeding or ascites. After overt episodes, death rates vary between 42% and 64% in one year according to HE grade[6-8]. The relevance of this condition was demonstrated even in patients without overt HE, for whom a mortality index combining electroencephalogram findings and the model of end-stage liver disease score (MELD) had a higher accuracy for predicting 12- and 18-mo survival than the MELD score alone[9].
The role of nutrition in HE physiopathology is well established, and nutritional treatment is an important step in order to improve quality of life (QOL) and survival of patients with cirrhosis and HE. Since HE is a late complication of advanced liver disease, it is not surprising that cirrhotic patients with HE are susceptible to nutrition disorders[10]. Hence, there are many reasons that nutrition and HE cause a great impact on each other.
Ammonia metabolism is probably the most studied of the mechanisms responsible for the nutritional effects on HE, because ammonia production is highly influenced by dietary components. Consequently, many nutritional treatments have been studied in order to modulate ammonia production, to increase survival rates and to improve QOL of cirrhotic patients stricken by HE.
Nutrition status is directly associated with survival of cirrhotic patients[11-14]. Moreover, when these patients also have HE this association can be even more important. Although there are many studies focusing on the nutrition role in cirrhosis, this article specifically aimed to discuss practical nutritional strategies that can be applied to evaluate the body composition of HE patients. With the purpose of giving pathophysiological support to this discussion, the main factors related to HE etiology are presented as ammonia production, inflammation, and muscle wasting - whereas other factors are briefly addressed.
PATHOPHYSIOLOGY OF HE AND NUTRITIONAL STATUS INFLUENCE ON THE MAIN PROCESSES INVOLVED
The pathophysiology of HE is multifactorial and there is a general agreement that ammonia and inflammation act synergistically to cause astrocyte swelling and brain edema[15]. Since body composition affects ammonia metabolism and has a clear impact on cirrhosis and HE, the main pathophysiological point discussed herein is ammonia metabolism.
Ammonia metabolism
Even though ammonia levels are not strictly proportional to neurological impairment, ammonia is of great relevance in HE. High blood ammonia levels are not sufficient to establish the diagnosis of HE in cirrhotic patients; however, a normal value requires diagnostic reevaluation[1]. It means that ammonia blood levels are still valuable when they are related to additional clinical information, but isolated ammonia levels should never be considered sufficient to make a diagnosis. It is also important to consider that the values are influenced by physical activity, the source of the blood collected (arterial or venous), the prandial status and the diet pattern before the test.
In humans, there are three main sources of ammonia: The intestines, the kidneys and the muscles. The total daily production in adults is around 1000 mmol of ammonia[16]. The kidney production is increased in cirrhotic patients during hemodynamic disturbances[17]. The role of intestines in ammonia production is fairly the most frequently cited aspect in prior studies about HE, making the colon one of the main treatment targets.
Studies on non-absorbable disaccharides were the initial evidence that orally ingested substances could be useful in HE treatment. Lactulose and lactitol are the most studied among them in clinical trials of HE treatment. Lactulose is a good example of how a non-absorbable component could be relevant in this setting. Beyond the cathartic property, achieved by increasing intestinal transit, lactulose is also converted to lactic acid and acetic acid, which have the potential of converting ammonia to ammonium. Another interesting effect of disaccharides is that the colon acidification favors the proliferation of nonammoniagenic bacteria[18]. According to this point of view, lactulose also has a prebiotic property, and these effects could be achieved, at least in part, by nutritional treatments.
Colonic production of ammonia is a target in HE treatment, but it is important to notice that three systems are able to convert ammonia into other substances. The liver is the chief organ, and urea synthesis is the main metabolic route for ammonia clearance in normal conditions[19]. Skeletal muscles are a secondary system that can convert ammonia to glutamine, as is the brain tissue. Given the osmotic effect of glutamine, continuous conversion of ammonia to glutamine inside the astrocytes can cause cellular edema. Astrocytes constitute a significant part of the cerebral volume, so any degree of edema in these cells can be relevant to the brain and can lead to decreased excitatory neurotransmission, which is a characteristic feature of HE[20].
Accordingly, in advanced cirrhosis, the liver can be insufficient to accomplish the ammonia clearance as needed. Hence, the only way to avoid glutamine formation inside the neural cells is to convert ammonia to glutamine in skeletal muscles. Conversely, cirrhosis is a catabolic disease, and patients with advanced cirrhosis are commonly affected by muscular wasting, which decreases the amount of muscle tissue available to make this conversion. One of the main reasons for this catabolic state is that changes in the cirrhotic liver impair the glucose metabolism, so that when these patients are in a fasting state they have to use muscular proteins as an energy source. In many of these patients, HE arises as a late complication, developed when their muscles have been affected for a long duration, leading to muscle depletion and malnutrition. It can be difficult to counteract this process in HE patients, and prevention is clearly the best choice to preserve muscle mass in this setting.
It is estimated that nearly half of the total ammonia can be metabolized in muscles through glutamine synthesis. However, glutamine synthesis and ammonia uptake are low during rest[19]. Some studies have hypothesized that in the rest state the muscle might not be significant to ammonia metabolism in healthy subjects, but it could be more important in liver disease[20]. Since muscle is the best means to increase ammonia clearance in cirrhotic patients with HE, physical activity could be very relevant in this setting. However, the role of muscle activity as a way to prevent HE was not verified in clinical trials.
Due to the role of muscle mass in cirrhotic patients, especially those with HE, early nutritional assessment must be performed properly. The early nutritional diagnosis must be followed by an appropriate dietary treatment in order to reduce the process of muscle wasting related to this liver disease. Even so, ammonia metabolism is not the only aspect that should be considered.
Inflammation
The inflammatory process is another significant issue in HE pathophysiology that can also be a target for different forms of treatment, including nutritional strategies. Inflammatory cytokines affect the blood-brain barrier and increase the ammonia diffusion in astrocytes[21]. Cirrhosis is characterized by subclinical increasing of inflammatory cytokines, but it can be difficult to show the correlation between HE and inflammation through peripheral blood cytokine tests[22]. In a double-blind randomized trial comparing the effects of two antibiotics, our group showed for the first time that hospitalization length was associated with C reactive protein levels during HE treatment. This finding illustrates the inflammation impact not only when the patient arrives at the hospital but also during HE treatment[23].
The level of inflammation products found through fecal calprotectin tests is higher in patients with cirrhosis and HE, indicating that the gastrointestinal tract (GIT) is the main source of inflammation in these patients[24]. A further study suggested that there is a global mucosal-immune interface change in these patients that affects the entire GIT[25]. Cirrhosis is often associated to slow gastrointestinal transit and mucosal edema, allowing the passage of bacterial products through the epithelial barrier. These products keep a continuous flow of bacterial particles through the portal vein, promoting the release of tumor necrosis factor inside the liver and causing a long-lasting cellular damage into the organ[26].
Obviously, nutritional management is not sufficient to avoid inflammation caused by acute infections, and reducing this sustained inflammatory state in cirrhotic patients is a challenge. However, when the main source of inflammation is the GIT, nutritional treatments can be useful to limit this process. Cirrhotic patients with HE are frequently stricken by small intestinal bacterial overgrowth, which leads to alterations in intestinal microbiota profile[27,28]. In this context, probiotics, prebiotics and symbiotics ingested can increase GIT transit and alter the microbiota involved in GIT inflammation to achieve a better bacterial profile, similar to the effects obtained by lactulose.
To demonstrate this effect in a clinical trial, Liu et al[27] evaluated the results obtained by a symbiotic preparation on minimal HE in patients with cirrhosis. They found that the symbiotic treatment favored the fecal proliferation of Lactobacilli, thus reducing the amount of the previous bacterial strains, which were more associated to inflammation. This modulation of GIT microbiota was concomitant to a remarkable decrease in serum ammonia and a significant improvement of HE in half of the subjects. Remarkably, the symbiotic treatment was also associated with endotoxemia reduction, improving the Child-Pugh class in nearly 50% of cases. Treatment with fermentable fiber alone was similarly helpful to many patients. Thus, the authors concluded that treatment with symbiotics or fermentable fiber would constitute an alternative to lactulose for the management of minimal HE in cirrhotic patients[27].
Other mechanisms involved in HE and also in different types of encephalopathy
Besides the fact that ammonia and inflammation are the most appraised components involved in HE pathophysiology, the evidence supporting the role of oxidative stress and hyponatremia in HE is growing. The increase of blood-brain barrier permeability related to hyponatremia and oxidative stress has been associated to HE development[17]. Hyponatremia in cirrhotic patients is often caused by fluid retention, leading to hypervolemic (dilutional) hyponatremia. Conventional therapy in this setting is a challenge because fluid restriction and loop diuretics are frequently inefficacious[29]. It has been considered as a third hit in HE pathophysiology, because it can worsen the cerebral edema caused by hyperammonemia and inflammation. The management of these conditions in the emergency room is beyond the scope of this review, but nutritional strategies can be convenient in order to avoid or correct some electrolytic disturbances.
Electrolytes and fluid imbalances are commonly associated with several illnesses and have a great impact in cirrhosis and HE. Potassium and zinc deficits can be a consequence of restricted diets, and some enriched supplements can be useful for avoiding or treating them. Hypokalemia should be promptly corrected in patients with HE because it increases ammonia production and excretion by the kidneys[30]. Additionally, zinc deficiency is not rare in HE, and the early diagnosis should be done in this setting[31]. It should always be suspected in the presence of HE associated with malnutrition, especially when a poor diet is maintained for a long duration, as in the case of severe alcohol addiction. In theory, the lack of dietary proteins could be a contributing factor, because they are a significant source of zinc. Zinc deficiency can lead to HE, diarrhea, muscle cramps and skin lesions, complications that can be avoided by the prompt initiation of the disturbance correction.
Many HE patients have high manganese levels, possibly reproducing the effects of hepatocellular failure, as well as impaired biliary flow and the existence of porto-systemic venous shunts[32]. Manganese accumulation in brain tissue is found in severe cases of HE and acquired hepatocerebral degeneration[33,34]. Other electrolyte imbalances found in patients with cirrhosis and HE include hypocalcaemia and hypomagnesaemia, but their role in HE is not so clear. Finally, these patients present severe amino acid imbalances that lead to depletion in branched chain amino acids, which can be supplemented to achieve improvement in HE[35,36].
NUTRITIONAL STATUS IN HE
Among cirrhotic patients, 75% of those who develop HE have moderate to severe malnutrition, which affects their energy reserves and muscle mass[1]. Due to the muscular involvement in ammonia metabolism, malnutrition is associated with a higher incidence of HE[37-39]. The best means to define malnutrition in cirrhosis is protein calorie malnutrition, in which both lean and fat tissue can be depleted[39,40]. Although this reduction in both tissues is recognized as cachexia, the predominant loss of muscle mass in cirrhosis suggests that sarcopenia, or loss of skeletal muscle mass, is the first nutritional deficiency[40].
Concurrently, overweight has been cited in cirrhotic patients as another matter of concern. Berzigotti et al[41] evaluated 161 cirrhotic patients over a mean follow-up of 59 mo or until cirrhosis decompensation. The incidence of complications in patients was 15% in those with normal body mass index (BMI), 31% in those overweight and 43% in obese patients. In cirrhosis, obesity can be associated with loss of muscle mass, an ambiguous state of excess adipose tissue and muscle wasting denominated sarcopenic obesity, which has accumulated risks for each of the two phenotypes of body composition[41-44].
Thus, the presence of cirrhosis and HE affects nutritional status by many mechanisms, as described in Table 1.
Table 1.
Possible causes of malnutrition in patients with cirrhosis and hepatic encephalopathy
| Possible causes | Clinical manifestation |
| Reduced ingestion of foods | Anorexia |
| Early satiety | |
| Ascites | |
| Confusion and/or excessive somnolence | |
| Frequent hospitalizations | |
| Impaired absorption of | Alterations in enterohepatic circulation |
| nutrients | Impaired biliary excretion |
| Small intestinal bacterial overgrowth | |
| Portosystemic shunts | |
| Metabolic disturbances | Protein hypercatabolism/BCAA depletion |
| Decreased glycogen stores and gluconeogenesis | |
| Insulin resistance and enhanced ketogenesis | |
| Increased lipolysis and fatty acid oxidation | |
| Other factors | Restricted diets (e.g., low sodium diets) |
| Protein loss during large volume paracentesis | |
| Abdominal distention during lactulose therapy |
BCAA: Branched chain amino acids.
As HE is frequently associated with advanced cirrhosis and this combination of severe conditions can have a substantial effect on food ingestion, the effects are clear in the clinical setting. In a multicentric trial assessing cirrhotic inpatients with jaundice, the spontaneous caloric intakes in the control group were approximately 20-25 kcal/kg per day, considered by the authors to constitute more than expected[45]. A large study aimed to document the impact of malnutrition and nutritional practice in 396 cirrhotic inpatients registered the caloric intake as 35.1 ± 10.0 kcal/kg per day in Child A, 29.0 ± 7.3 kcal/kg per day in Child B and 24.0 ± 8.0 kcal/kg per day in Child C subjects. Of note, changes in dietary and protein intake during hospitalization were related to mortality[46]. In a study of 60 outpatients, in which the majority of whom had compensated liver disease, the caloric intake was between 24 and 40 kcal/kg per day[47]. Another study assessing 300 cirrhotic outpatients obtained a mean value of 32 kcal/kg per day[48]. Unfortunately, the HE rates are not clearly documented, so it is difficult to identify the food intake patterns in this condition.
To analyze this question, our group assessed 60 outpatients with cirrhosis and HE. The majority of them had grade 1 HE (34 patients), while 23 presented minimal HE and only two subjects had grade 2 HE. The mean caloric ingestion was 20.5 ± 8.61 kcal/kg per day (unpublished data). These values were clearly below the recommendations for cirrhotic patients with HE, which range from 35 to 40 kcal/kg per day[32,49]. It is difficult to know whether the presence of HE is the main reason for the disagreement between our data and other results of caloric ingestion obtained in studies that did not evaluate only patients with HE, given the potential relevance of many other differences between the populations assessed. To clarify this hypothesis, we encourage researchers to implement new studies of caloric ingestion in HE patients to contribute with additional data on this topic.
Given all the mechanisms that influence malnutrition in HE, there are no doubts that it is necessary to evaluate the nutritional status of cirrhotic patients who develop HE. However, it can be a challenge because patients with HE often have altered body composition, including variations in fluid balance and protein catabolism induced by the liver disease[1,32,50,51]. Moreover, they can be particularly difficult to evaluate through exams requiring extensive patient collaboration.
Some authors have suggested that changes in water homeostasis and compartmentalization can exist even before fluid accumulation is detected, and when these patients have ascites, pleural effusion or edema their evaluation via traditional methods can be even less accurate for assessing body composition[52,53]. Additionally, changes in lean mass and fat ratio can also reduce the accuracy of some methods of nutritional evaluation, as in sarcopenic, obese and also sarcopenic obese patients[41,43,44]. These alterations in body composition require more attention to assess patients with advanced liver disease, which can affect some measures obtained by the traditional methods.
Finally, metabolic changes lead to lessening in protein and fat reserves in 50%-75% of cirrhotic patients[1,50]. Furthermore, the degree of depletion in these reserves is related to prognosis and is a risk factor for developing HE, thus necessitating the evaluation of body composition in this population[39].
METHODS TO EVALUATE THE NUTRITIONAL STATUS OF CIRRHOTIC PATIENTS WHO DEVELOPED HE
Detailed body composition assessment is essential in HE patients. It is the first step to define the pattern of tissue loss and to establish nutritional treatment strategies[54]. Therefore, nutritional assessment of cirrhotic patients with HE must combine a good dietary history, body composition data and laboratory exams[1,32,49,55]. In this review, we will focus on body composition evaluation.
Most techniques to assess body composition are focused on differentiating fat mass from fat-free mass, while some methods presume that fat-free mass has constant characteristics, such as hydration fraction and density. Thus, techniques such as anthropometry and bioelectrical impedance could lead to over- or under-estimation of body composition findings when these assumptions are invalid[56]. Despite that, anthropometric and bioelectrical impedance data were associated with prognosis in cirrhotic patients, and their potential lack of accuracy is difficult to quantify.
Herein, we analyze the usefulness of the most widely utilized techniques in the assessment of nutritional status in cirrhotic patients who developed HE, presenting some of the advantages and drawbacks of each method. Given that patients with severe HE can be difficult to evaluate through exams requiring extensive patient collaboration, we suggest some of them that could be suitable in a practical scenario, adding more complex techniques in the initial evaluation and/or to bring more accuracy. Table 2 summarizes some of the findings in this setting.
Table 2.
Advantages and disadvantages of the main methods of nutritional assessment for cirrhotic patients with hepatic encephalopathy
| Method | Advantages | Disadvantages |
| SGA | Quick application | Requires patient comprehension and collaboration |
| Low cost | Subjectivity (the only objective measure used is weight) | |
| Can identify patients under risk of malnutrition | Can underestimate malnutrition | |
| upon hospital arrival | Cannot be used as a follow-up method | |
| Can be applied in hospital rooms | ||
| Anthropometry | Quick application | Some measures (body weight, body mass index, AC, TSF) can be highly influenced by water retention and overweight/obesity |
| Low cost | Interobserver variation decreases the data reproducibility | |
| Demands little collaboration | Can underestimate malnutrition | |
| Can be applied in hospital rooms | ||
| Some measures (CAMA, MAMC, APMT) are less influenced by water retention and overweight/obesity | ||
| MAMC is widely recommended for liver disease patients | ||
| MAMC and TSF are associated with outcomes in cirrhotic patients and are related to the presence of HE | ||
| Handgrip strength | Quick application | Cannot identify muscle wasting anatomically |
| Low cost | Is not so suitable for evaluating cirrhotic women, because skeletal | |
| Can be applied in hospital rooms | muscle function correlates with muscle mass only in men | |
| Identify impaired muscle function | ||
| Is not influenced by either water retention or overweight/obesity | ||
| Is an independent predictor of cirrhosis decompensation | ||
| Bioelectrical impedance analysis | Quick application | Controversial applicability in patients with fluid retention |
| Can be applied in hospital rooms when portable equipment is used | Requires patient removal to the equipment room when non-portable equipment is used | |
| PA and BCM are associated with outcomes in cirrhotic patients | Can underestimate malnutrition | |
| Dual-energy X-ray absorptiometry | Adequate accuracy to identify muscle depletion | High cost |
| Excellent reproducibility | Requires patient removal to the equipment room | |
| Can also identify bone mass reduction as a screening tool | Exposure to ionizing radiation makes routine use less attractive as a follow up method | |
| Gives detailed analyses of body composition (segmental results), obtaining measures that have prognostic impact in cirrhotic patients | ||
| FFMI is an independent predictor of HE | ||
| AMMI can be used to diagnose sarcopenia | ||
| Computed tomography scan | Adequate accuracy to identify muscle depletion | High cost |
| Excellent reproducibility | Requires patient removal to the equipment room | |
| Can be performed retrospectively from images previously obtained | Exposure to ionizing radiation makes routine use less attractive as a follow-up method | |
| Can also identify hepatic nodules, portosystemic shunts and other abnormalities | ||
| Skeletal muscle thickness in cross-sectional images has prognostic impact in cirrhotic patients | ||
| L3 SMI can be used to diagnose sarcopenia |
The bedside techniques are presented at the top and can be valuable even in conditions of restricted access to technology. The more complex methods are shown at the bottom, and should also be used when technology is unrestrained, providing more accuracy. Methods used only for research purposes and those not applied to patients with hepatic encephalopathy are not included. AC: Arm circumference; AMMI: Appendicular muscle mass index; APMT: Adductor pollicis muscle thickness; BCM: Body cell mass; CAMA: Corrected arm muscle area; FFMI: Fat-free mass index; HE: Hepatic encephalopathy; L3 SMI: Third lumbar vertebrae skeletal muscle index; MAMC: Mid-arm muscle circumference; PA: Phase angle; TSF: Triceps skinfold; SGA: Subjective Global Assessment.
Subjective Global Assessment
Subjective Global Assessment (SGA) is one of the most widely used methods to evaluate nutritional status in patients during their hospital stay. It gathers information about food intake, weight changes, gastrointestinal symptoms and physical examinations, which are intended to evaluate subcutaneous fat, muscular atrophy, edema and ascites[57].
Although SGA is useful as a screening tool to be applied upon hospital arrival, it is not sufficient to evaluate cirrhotic patients with HE on account of some methodological limitations. First, SGA depends on personal information that can be difficult to obtain from patients with cognitive impairment or somnolence. Second, the only anthropometric measure utilized is the body weight, which is often changed by ascites and edema[50,51]. Therefore, the guidelines of the International Society for HE and Nitrogen Metabolism (ISHEN) highlight that SGA can underestimate malnutrition occurrence in cirrhotic patients and does not predict outcome accurately[32].
Anthropometry
Anthropometric measurements are objective methods to evaluate the nutritional status. They are rapid, non-invasive and low-cost techniques, specifically suited to assess somatometric characteristics. These measurements have been considered the most useful procedures to assess nutritional status in cirrhotic patients[58]. Nonetheless, they also have limitations when applied in patients with HE and cirrhosis. For instance, ascites and edema can influence body weight and BMI values, underestimating the prevalence of malnutrition among these patients[51]. Although there is a specific classification of BMI for cirrhotic patients that categorizes the presence of ascites, this reference is not fully applied in clinical trials, and presents a clear limitation of having a single cutoff value to diagnose malnourished patients, precluding the diagnosis of eutrophic or overweight patients[59]. Most studies still use the dry weight, which is estimated by subtracting the weight of ascites and edema that is known from information obtained during clinical assessment, weight values formerly registered, ascites volume drained and references previously established[32].
Skinfolds and body circumferences are less affected by water retention than BMI. Skinfolds are mainly used to estimate the body fat. Fiore et al[60], evaluating 40 cirrhotic subjects without overt fluid retention, found that the percentage of body fat assessed by skinfolds had a difference of less than 5% in comparison to the values obtained by dual-energy X-ray absorptiometry (DEXA). The authors evaluated body composition measures in patients who did not present any degree of edema or ascites. Moreover, most subjects had compensated cirrhosis (Child-Pugh A/B/C = 24/16/0) while the rate of patients with HE is not mentioned in the article, hampering extrapolation of the findings to patients with HE and/or more advanced liver disease.
Two of the most recommended measures to evaluate the nutritional status of cirrhotic patients with HE are triceps skinfold (TSF) and mid-arm muscle circumference (MAMC)[55]. MAMC is calculated through TSF and mid-arm circumference (AC). In a study of 212 cirrhotic inpatients monitored for 2 years, the authors suggested that MAMC and TSF could be included in the Child-Pugh classification in order to improve the predictive accuracy of this score, although the prognostic power of TSF was lower than that of MAMC[11]. Another study of 300 subjects showed that MAMC values were expressively more affected in male than in female cirrhotic patients, while loss of fat deposits based on TSF was more significant in females[48]. In a study evaluating 143 patients before liver transplantation, the authors considered MAMC to be the best reliable anthropometric tool[61]. In a study of 102 patients submitted to orthotopic liver transplantation (OLT), AC and TSF were used to categorize patients according to the values before OLT; those below the 25th percentile had an increased incidence of hepatic tests result abnormalities, suggesting a higher incidence of complications in this group[62].
A method in which MAMC and BMI were combined with SGA information as a new way to evaluate the nutritional status of cirrhotic patients was previously validated[63]. The proposed algorithm showed prognostic value in this population and we encourage its use as a screening method for patients in their hospital admission. However, there is no information whether this algorithm could be useful during the patient follow-up, or whether variations in weight/BMI could impair the accuracy found by the authors. Thus, a simple and precise technique for assessing malnutrition in cirrhotic individuals is not available yet[64].
Another anthropometric measure that may be used for diagnosing muscle wasting in cirrhotic patients is the adductor pollicis muscle thickness (APMT). APMT is a simple tool, with little influence from such body composition changes as edema, ascites and overweight. It has been applied to evaluate the nutritional status in normal populations as well as surgical, renal failure and cancer patients[65-68]. However, we have not found studies specially aimed to evaluate cirrhotic patients.
Specific data of anthropometric measurements in patients with HE were presented in a study of 300 cirrhotic patients (200 men and 100 women). The authors confirmed that the prevalence of overt HE during hospitalization was significantly higher in patients with muscle depletion assessed by MAMC and TSF, as well as in those with decreased muscle strength, showing the relevance of anthropometric measures and muscle strength in HE[39]. Of note, the diagnosis of muscle wasting and fat store depletion was based on MAMC and TSF < 5th percentile, respectively, in relation to normal values.
In a comprehensive review about nutrition in HE, the authors suggested that parameters not affected by ascites or edema include MAMC, AC, and TSF. They also proposed that the diagnosis of malnutrition in advanced liver disease could be done when MAMC and/or TSF values were lower than the 5th percentile in individuals aged 18-74 years, using the 10th percentile for those aged more[64].
Indeed, these measurements were the most widely used for a long time, so there is more evidence that they can be used in cirrhotic patients. Even without such a well-known scientific basis, we suggest that corrected arm muscle area (CAMA) and APMT should also be documented in these patients as supplementary data. Both could be useful to identify muscle wasting, which is extremely relevant for cirrhotic patients with HE. CAMA is calculated through AC and TSF values. As the others measurements proposed by Bémeur et al[64], CAMA and APMT are also easy to perform, given that they do not require much cooperation from patients. These advantages are important in cases with somnolence and/or confusion. Additionally, we recommend that the diagnosis of malnutrition in cirrhotic patients with HE should not be based only on anthropometry, unless it is the only method available.
Since anthropometric classifications were not based on cirrhotic patients, it is difficult to establish cutoff values according to the aforementioned studies because most of these values were obtained from standard measures for healthy subjects in different populations. Likewise, other limitations must be considered: The inter-observer disagreement in some procedures, the variations in skin compressibility and hydration, and the fact that in many cases the overweight can preclude the diagnosis of cirrhosis-related muscle loss. Anthropometric measurements should be confirmed by a skilled professional in order to increase their reliability[58]. Therefore, whether evaluations of cirrhotic patients with HE are based only on anthropometry, these issues can be a significant drawback. Given the severe disease found in this population, other methods should be used to support anthropometric findings whenever possible to provide greater accuracy for the data obtained.
Bioelectrical impedance analysis
Bioelectrical impedance analysis (BIA) measures are safe and relatively accurate for estimating fat mass and fat-free mass. The water component of the body is appraised according to the capacity of the body to conduct an electrical current[69]. Thus, the basic principle of BIA is that electrical conduction is faster through water and slower through fat tissue due to the resistance imposed by fat deposits, thus estimating the percentages of fat and fat-free tissues. The procedure takes less than 2 min to measure body electrical conductivity and resistance (impedance). This resistance value is then applied to the determination of total body water.
BIA has been applied in evaluations of cirrhotic patients for many years[70-77]. Among the results obtained, phase angle (PA) and body cell mass (BCM) deserve particular attention.
PA values are decreased in advanced cirrhosis. Furthermore, a prior study confirmed that PA was associated with muscle strength and muscle mass in cirrhotic patients. Moreover, a phase angle value less than or equal to 5.4 degrees was predictor of reduced survival[72]. Of note, Peres et al[75] found that the median PA was 4.17° (3.19°-7.42°) in a HE subpopulation, expressively worse compared to cirrhotic individuals in which HE was not present (5.04°).
BCM is a lean tissue compartment that is diminished in protein-calorie malnutrition. It estimates the body cellular elements, and has been considered one of the best nutritional references for appraising metabolic pathways like protein turnover and energy expenditure[58]. Thus, low BCM is often observed in advanced liver disease.
Analyzing 150 potential candidates for OLT equally divided into two categories (validation group and study group), Selberg et al[78] showed that hypermetabolism and low BCM proportion (< 35% of body weight) were related to short survival. Of note, when the authors compared the subjects who died with those who survived, HE and edema severity were expressively worse in the former group, and CAMA measures were correlated with BCM[78]. Figueiredo et al[79] evaluated 69 cirrhotic patients using SGA, anthropometry, handgrip strength, lab tests and dual-energy X-ray absorptiometry. Most of the methods applied were weakly associated with BCM measurements, but the authors showed that handgrip strength and MAMC values obtained in this study were the most sensitive markers of depleted BCM. Combining the cutoff points of 30 kg obtained by handgrip strength test and 23 cm obtained by MAMC, the authors showed that these methods could be used together in order to identify BCM depletion accurately in most patients[79].
Müller et al[80] in a study with 123 cirrhotic patients found that post-transplantation mortality was higher in patients with low BCM. The authors concluded that hypermetabolism was not present in all patients, but could be a cause of malnutrition, thus contributing to worsen prognosis[80].
As BIA results can be altered by physical activity, dehydration, diuretic use, fluid retention, as well as liquid and food ingestion before the test, the usage of this method for assessing cirrhotic patients has been controversial[56,81-84]. According to a prior study, total body water was comparable in compensated cirrhosis and in non-cirrhotic subjects, but was higher in patients with ascites[52]. Other studies assessing the changes caused by ascites have shown that fluid overload can be a cause of imprecision in BIA results[82,85,86].
In contrast, another research group postulated that ascites exerts an irrelevant influence on BIA measures since chest and abdomen together represent only 11% or less of the body resistance, showing that BCM appraised by BIA was associated with the same measure evaluated by the total body potassium count, regardless the presence of ascites[87]. However, it must be noticed that 72% of the subjects used diuretics.
Ascites should be viewed as a marker of fluid overload and not as a simple liquid accumulation. Accordingly, the instillation of 2-3 L of fluid into the peritoneal cavity has minimal effect on total body resistivity in patients submitted to peritoneal dialysis, and assessments of fat mass and fat-free mass do not vary significantly whether these patients are dry or filled[88,89]. According to ascites pathophysiology, it is a clinical sign that the patient presents diffuse water retention and not only in the abdomen. The controversial point is how much ascites can change BIA results.
To estimate this effect in cirrhotic patients with HE, we evaluated 50 patients with HE that were submitted to BIA and DEXA exams to compare the percentage of fat mass obtained in each exam (non-published data). The median of their Child-Pugh classification scores was 8 (Child-Pugh A/B/C = 11/33/6), 32% of them had ascites and 38% had edema. The mean fat mass according to BIA and DEXA exams were 29.3% ± 7.7% and 29.8% ± 7.1%, respectively. Pearson's r was used to test the correlation and Bland-Altman analysis to evaluate the agreement between the fat percentages obtained by BIA and DEXA[90]. Pearson correlation indicated good agreement (P = 0.0000882 and r = 0.526). However, we found some discrepancies when the differences were observed on the scatter graph and through Bland-Altman analysis (Figure 1). The results obtained by DEXA seem to be similar to the values previously found in cirrhotic patients without fluid retention (29.6 ± 9.2)[60]. Even so, it is important to notice that DEXA exams can also be affected by fluid alterations, as addressed below in this review[54,91].
Figure 1.
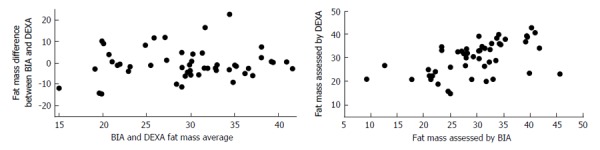
Bland-Altman analysis and scatter graph of fat mass percentages obtained from 50 cirrhotic patients with hepatic encephalopathy. Despite the correlation obtained between the two methods, the differences were also significant. DEXA: Dual-energy X-ray absorptiometry; BIA: Bioelectrical impedance analysis.
These limitations and controversial issues must be pondered when a cirrhotic patient with HE has been evaluated, because the precision of BIA results could be affected by all the body changes induced by the liver disease. Nevertheless, when there is not a single sign of fluid retention that could be clinically detected, BIA can be used for predicting total body water in cirrhotic patients, but precision is somewhat worse than in normal conditions[92]. Taking in account these limitations, when more accurate methods are not available and the patient has no signals of fluid overload, we consider that BIA results are still valuable in cirrhotic patients with HE because phase angle and BCM convey prognostic information[72]. Finally, multi-frequency BIA should be preferred in order to achieve more accurate results.
Handgrip strength
There is a growing interest in muscle activity evaluation for nutrition assessment. Walking tests and other tests requiring extensive collaboration could not be fully applied in patients stricken by severe illnesses like HE. Thus, handgrip strength (HS) can be a good option for patients with low-grade HE. Muscle strength is closely related to muscle activity, which is another valuable parameter for cirrhotic patients with HE, because HE tends to decrease physical activity.
A probable reason for decreased muscle activity in these patients is that hyperammonemia is related to fatigue, which is frequently reported in HE[19,93,94]. As previously mentioned in this review, in healthy adults the muscle role in ammonia clearance is minimal during rest, so the physical activity can be very important for ammonia metabolism. Accordingly, a previous study postulated that walking at least 5000 steps daily could be recommended for patients with compensated cirrhosis[95]. Although techniques of muscle activity evaluation are not considered to be methods of nutrition assessment, the information obtained would be of great value in patients with HE and more studies are needed in this setting.
HS has been suggested as a good indicator of nutritional status in liver disease patients by several guidelines[1,32,49]. The test is simple and a significant advantage is that the grip strength value is an independent predictor of cirrhosis decompensation[38,79,96,97]. As commented above, Merli et al[39] found that the prevalence of overt HE was higher in patients with decreased HS, suggesting a connection between strength and HE.
However, according to the ISHEN Consensus, skeletal muscle function is well associated with muscle mass only in men, and handgrip dynamometry would not be a reliable tool for appraising nutritional status in cirrhotic women. The degree of muscle loss caused by liver disease is different in each gender[32].
DEXA
The DEXA exam has received special attention because it is widely used to validate the results of body composition obtained by other methods, such as anthropometry and BIA. The procedure is based on measurement of body composition according to a model dividing the body elements in bone, fat, lean and bone-free lean masses, which can be distinct according to the energy photons passing through the body[98]. Additionally, radiation exposure is minimal with this technique[54,99]. The current guidelines recommend DEXA as a specific method for the diagnosis of malnutrition in liver diseases[1,32,49].
The DEXA exam allows assessment of fat-free mass and lean mass, which are technically different. Fat-free mass is composed by non-fat constituents of the body, including a small part of the adipose tissue. On the other hand, lean mass is composed by non-adipose constituents, which includes the lipids found in the nervous tissue and in cell membranes[100].
Riggio et al[101] evaluated 22 cirrhotic patients without ascites and 16 matched healthy controls using the DEXA exam. The authors found that the patterns of soft tissue loss in cirrhotic patients vary according to the gender. In women, the fat stores are more reduced, while lean tissue is maintained, as in early starvation. In men, the loss of lean tissue is more prominent, as seen under conditions of stress[101]. This finding could explain the poor correlation between muscle mass and muscle strength in cirrhotic women, making HS not so adequate for evaluating cirrhotic women according to the ISHEN Consensus[32]. Furthermore, it is in agreement with the finding of a prior study in which MAMC values were dramatically more affected in male than in female cirrhotic patients, while loss of fat deposits based on TSF was more significant in females, as formerly presented in this review[48].
Another study employed DEXA to evaluate 53 cirrhotic subjects, of whom only 30 seemed to be free of fluid retention. Results obtained by DEXA were compared with measurements from total body potassium, BIA and skinfold anthropometry. The authors found a good association between total body fat obtained from anthropometry and DEXA, regardless the existence of ascites. Even so, they found some discrepancy between DEXA and the other methods[102].
According to a prior review about the role of DEXA in the evaluation of cirrhotic patients, one of the main advantages of this exam is the high reproducibility of the measurements, with fat variations of less than 1% in normal individuals. Accuracy errors are minor, amounting to 1.5% for quantifying lean mass. Additionally, the findings estimate the body compartments properly. Even so, a disadvantage is that fluid imbalances can change the X-ray passage, leading to an inappropriate appraisal of the lean mass[54].
DEXA allows obtainment of the ratio of the lean mass content divided by the square of the height, which is named fat-free mass index (FFMI). In a study of 108 cirrhotic liver transplant candidates, muscle wasting detected by FFMI was an independent predictor of HE[103]. Estimating the content of body segments (upper and lower limbs and trunk), DEXA can also be used to calculate the appendicular muscle mass index (AMMI), which is obtained by dividing the sum of appendicular muscle mass of the four members (free of fat and bone tissue) by the square of the height[100,104]. The AMMI provides a precise estimation of muscle mass because it does not use bone density, which varies with age, ethnicity, and response to drugs[100]. It also disregards the trunk mass, which is commonly affected by fluid retention in cirrhotic patients.
According to AMMI values, the diagnosis of sarcopenia can be made when individuals present less than 2 standard deviations from the healthy adults. The cutoff points were established as 7.26 kg/m2 and 5.45 kg/m2 for men and women, respectively[43,44,104]. In the presence of sarcopenia associated with fat mass percentages higher than 27% for men or higher than 38% for women, the diagnosis of sarcopenic obesity can be made[44,104,105]. AMMI is supposed to be very useful in studies about cirrhosis because it is not altered by fluid retention nor overweight status, focusing only on the fat-free mass[95,106].
Additionally, DEXA screening is also important for assessing bone mass before the incidence of fractures in patients with cirrhosis, because bone mass reduction is frequent among them. Thus, AMMI and body composition analysis can be obtained in the same exam performed to evaluate the bone mass.
Computed tomography scan
Recently, there has been a growing interest in employing a computed tomography (CT) scan to assess cirrhosis-related lean mass depletion. One of the reasons is that cirrhotic patients are commonly submitted to this exam to evaluate liver nodules or other alterations previously detected by ultrasonography screening exams. In addition, CT can also be used to evaluate portosystemic shunts in HE patients in order to plan hemodynamic corrections, and another advantage is that CT allows obtainment of information on abdominal muscles that are not commonly accessible. In theory, it could be possible to assess body composition retrospectively from images previously registered. For instance, in a recent CT-based study to assess nutritional status of cirrhotic patients who are candidates for OLT, the authors commented that the CT scan is often implemented in such patients to appraise the blood vessels distribution into the liver, to study the biliary tree and for hepatocellular carcinoma screening[106]. Furthermore, another aim of employing CT to evaluate body composition is that the transversal muscle thickness is associated with prognosis in these patients[107,108].
The measures are recorded by a single cross-sectional image at the level of the third lumbar vertebra or between the vertebrae L3 and L4[106,109]. Skeletal muscle tissue can be identified among other tissues by density limits: A cutoff of 35 Hounsfield units (HU) is used to distinct muscle and fat, whereas the maximum of 150 HU is used to separate muscle and bones, although the former limit can vary between the studies[91,106].
Thus, the exam allows calculating the third lumbar vertebra skeletal muscle index (L3 SMI), established as the muscle area contained in this axial plane divided by the square of the height. The cutoffs for sarcopenia in cirrhotic patients are 42 cm2/m2 and 50 cm2/m2 for women and men, respectively[110].
Hanai et al[109], in a retrospective study of 130 cirrhotic patients submitted to CT exams, found that sarcopenia was significantly associated with mortality. Of interest, the authors suggested that the use of branched-chain amino acids could improve survival of such patients, although the latter finding should be evaluated in prospective studies[109].
The possibility of measurements using previous exams make this method very attractive as a means of bringing more precision to the evaluations of body composition in cirrhotic patients. However, the use of ionizing radiation, the costs and the fact that the patient needs to be removed to the CT equipment are considerable limitations, especially when applied to patients with HE and/or CT is proposed to be repeated during the patient follow-up.
To facilitate choosing appropriately among the methods presented above according to the HE grade, Figure 2 summarizes some of the main points discussed about each method in relation to its advantages and limitations.
Figure 2.
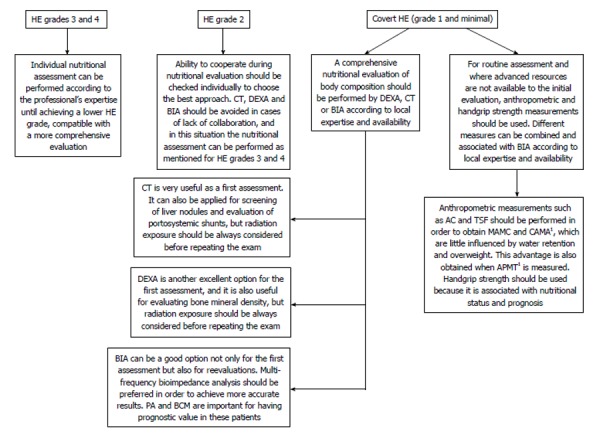
Proposal for choosing the best approaches during nutritional assessment of cirrhotic patients with hepatic encephalopathy. 1APMT and CAMA are also associated with nutritional status in many conditions, but were not well evaluated in patients with cirrhosis and HE. AC: Arm circumference; APMT: Adductor pollicis muscle thickness; BCM: Body cell mass; BIA: Bioelectrical impedance analysis; CAMA: Corrected arm muscle area; CT: Computed tomography scan; DEXA: Dual-energy X-ray absorptiometry; HE: Hepatic encephalopathy; MAMC: Mid-arm muscle circumference; PA: Phase angle; TSF: Triceps skinfold.
Other methods of nutritional assessment
In addition to the aforementioned methods for the assessment of body composition, other multi-compartmental techniques have been applied to cirrhotic patients, especially for scientific research.
Strauss et al[111] analyzed the usefulness of DEXA compared with a multi-compartmental model as a gold standard technique for evaluating body composition in 198 cirrhotic patients. The fat-free mass in this model was calculated adding bone content obtained from DEXA plus body water quantified by D2O dilution and whole body protein quantified by in vivo neutron activation analysis (IVNAA). Consequently, fat mass was obtained subtracting fat-free mass from body weight. DEXA showed good accuracy and proved to be a suitable technique to evaluate fat-free mass and fat mass. The only restriction was that DEXA could not provide details on the water amount inside of the fat-free mass. It is noteworthy that these patients presented overhydrated fat-free mass in both sexes, especially in women[111].
Figueiredo et al[112], in a study of 79 cirrhotic patients and 17 controls, compared nutritional evaluation based on anthropometry, SGA, albumin and lymphocytes with a multi-compartmental model combining DEXA and methods of dilution spaces (bromide and deuterium). The multi-compartment model was composed by total bone mineral mass, total body fat, extracellular water and body cell mass. Accordingly, the sum of these four variables corresponded to the body weight. The authors found that the two-compartment assessment was not sufficiently accurate to diagnose malnutrition nor to estimate the severity of this complication in cirrhotic patients[112].
The guidelines from the European Society for Clinical Nutrition and Metabolism in liver disease published in 1997 and 2006 encourage the use of total body potassium count, IVNAA and isotope dilution for accurate quantification of changes in body composition in cirrhotic patients[49,113]. The ISHEN consensus also recommends the usage of magnetic resonance imaging. However, the guidelines warn that while these methods are not biased by hepatic impairment and fluid imbalance, they can involve radiation and can be difficult to repeat during the patient follow up[32,49,113]. Additionally, specific studies employing these other methods in HE patients are still lacking.
Finally, in spite of the usage of methods based on laboratory tests to estimate the degree of liver impairment of cirrhotic patients with HE, they are inadequate to assess their nutritional status. For instance, in such patients the creatinine height index is less accurate, because muscle wasting can reduce creatinine levels whereas renal impairment can increase them[114].
CONCLUSION
HE is associated with many pathophysiological changes induced by liver disease and influenced by nutrition, such as ammonia accumulation and muscle wasting. Likewise, nutritional status has a big impact on outcomes of HE patients. The alterations in body composition induced by liver cirrhosis and the limitations superimposed by HE hamper the obtainment of an accurate nutritional assessment of these patients by a simple test. Fluid retention, metabolic alterations and depletion in muscle and fat deposits should be carefully investigated, whereas sufficient knowledge on the advantages and drawbacks of the main methods used in this population is essential to achieve consistent results by combining different techniques to assemble detailed data. Furthermore, for each method applied, it is important to recognize which measures are more trustworthy and which should be interpreted with caution due to possible bias. Prior studies in cirrhotic populations indicated that some anthropometric, BIA, DEXA and CT data convey prognostic information, whereas other measures remain controversial when obtained in patients with fluid overload. Most of these studies in cirrhotic patients did not mention the HE rate among the subjects included, so that specific data from patients with HE are scarce.
Knowledge on HE pathophysiology and evidence of the prognostic impact of muscle wasting on cirrhosis make muscle mass and muscle function some of the main points to evaluate in cirrhotic patients with HE. Ideally, a comprehensive assessment should gather information on food intake, anthropometric data not influenced by fluid overload, measurement of lean mass and tests of muscle function. The addition of complex techniques to measure body composition in HE patients increases the need for patient collaboration, the time spent, the demand for health professionals and the cost, but is useful to validate bedside measurements and should be encouraged. According to the local availability of each method and the patient condition, nutritional evaluation should be frequent during the patient follow-up, but the need to remove patients to the equipment room, the exposure to ionizing radiation and the costs can make the repetition of some of these methods less attractive. Therefore, some techniques can be combined to offer HE patients the safest and most cost-effective options, while maintaining the best accuracy in each nutritional evaluation.
Footnotes
P- Reviewer: Montagnese S, Sargsyants N S- Editor: Kong JX L- Editor: A E- Editor: Liu SQ
Supported by São Paulo Research Foundation (FAPESP), CAPES and PROPe/UNESP, Nos. 2013/15121-8 and 2013/11761-2.
Conflict-of-interest statement: No potential conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 6, 2015
First decision: September 8, 2015
Article in press: December 14, 2015
References
- 1.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 2.Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25 Suppl 1:3–9. doi: 10.1111/j.1746-6342.2006.03215.x. [DOI] [PubMed] [Google Scholar]
- 3.Bass NM. Review article: the current pharmacological therapies for hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25 Suppl 1:23–31. doi: 10.1111/j.1746-6342.2006.03218.x. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42 Suppl:S45–S53. doi: 10.1016/j.jhep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Moriwaki H, Shiraki M, Iwasa J, Terakura Y. Hepatic encephalopathy as a complication of liver cirrhosis: an Asian perspective. J Gastroenterol Hepatol. 2010;25:858–863. doi: 10.1111/j.1440-1746.2010.06242.x. [DOI] [PubMed] [Google Scholar]
- 6.Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890–895. doi: 10.1016/S0168-8278(99)80144-5. [DOI] [PubMed] [Google Scholar]
- 7.Stewart CA, Malinchoc M, Kim WR, Kamath PS. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease. Liver Transpl. 2007;13:1366–1371. doi: 10.1002/lt.21129. [DOI] [PubMed] [Google Scholar]
- 8.Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 9.Montagnese S, De Rui M, Schiff S, Ceranto E, Valenti P, Angeli P, Cillo U, Zanus G, Gatta A, Amodio P, et al. Prognostic benefit of the addition of a quantitative index of hepatic encephalopathy to the MELD score: the MELD-EEG. Liver Int. 2015;35:58–64. doi: 10.1111/liv.12490. [DOI] [PubMed] [Google Scholar]
- 10.Yun BC, Kim WR. Hyponatremia in hepatic encephalopathy: an accomplice or innocent bystander? Am J Gastroenterol. 2009;104:1390–1391. doi: 10.1038/ajg.2009.287. [DOI] [PubMed] [Google Scholar]
- 11.Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G, Caregaro L. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17:445–450. doi: 10.1016/S0899-9007(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 12.Fialla AD, Israelsen M, Hamberg O, Krag A, Gluud LL. Nutritional therapy in cirrhosis or alcoholic hepatitis: a systematic review and meta-analysis. Liver Int. 2015;35:2072–2078. doi: 10.1111/liv.12798. [DOI] [PubMed] [Google Scholar]
- 13.Ney M, Abraldes JG, Ma M, Belland D, Harvey A, Robbins S, Den Heyer V, Tandon P. Insufficient Protein Intake Is Associated With Increased Mortality in 630 Patients With Cirrhosis Awaiting Liver Transplantation. Nutr Clin Pract. 2015;30:530–536. doi: 10.1177/0884533614567716. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Margáin A, Macías-Rodríguez RU, Duarte-Rojo A, Ríos-Torres SL, Espinosa-Cuevas Á, Torre A. Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: a prospective cohort study. Dig Liver Dis. 2015;47:309–314. doi: 10.1016/j.dld.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Waghray A, Waghray N, Kanna S, Mullen K. Optimal treatment of hepatic encephalopathy. Minerva Gastroenterol Dietol. 2014;60:55–70. [PubMed] [Google Scholar]
- 16.Walker V. Ammonia metabolism and hyperammonemic disorders. Adv Clin Chem. 2014;67:73–150. doi: 10.1016/bs.acc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–447. doi: 10.1016/j.jhep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Al Sibae MR, McGuire BM. Current trends in the treatment of hepatic encephalopathy. Ther Clin Risk Manag. 2009;5:617–626. doi: 10.2147/TCRM.S4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson DJ, Smeeton NJ, Watt PW. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog Neurobiol. 2010;91:200–219. doi: 10.1016/j.pneurobio.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Cooper AJ. The role of glutamine synthetase and glutamate dehydrogenase in cerebral ammonia homeostasis. Neurochem Res. 2012;37:2439–2455. doi: 10.1007/s11064-012-0803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7:515–525. doi: 10.1038/nrgastro.2010.116. [DOI] [PubMed] [Google Scholar]
- 22.Wunsch E, Koziarska D, Milkiewicz M, Naprawa G, Nowacki P, Hartleb M, Milkiewicz P. In patients with liver cirrhosis, proinflammatory interleukins correlate with health-related quality of life irrespective of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2013;25:1402–1407. doi: 10.1097/MEG.0b013e328365a447. [DOI] [PubMed] [Google Scholar]
- 23.Romeiro FG, da Silva Yamashiro F, Américo MF, Corá LA, Silva GF, Miranda JR, Caramori CA. Erythromycin versus neomycin in the treatment of hepatic encephalopathy in cirrhosis: a randomized double-blind study. BMC Gastroenterol. 2013;13:13. doi: 10.1186/1471-230X-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alempijević T, Štulić M, Popovic D, Culafic D, Dragasevic S, Milosavljevic T. The role of fecal calprotectin in assessment of hepatic encephalopathy in patients with liver cirrhosis. Acta Gastroenterol Belg. 2014;77:302–305. [PubMed] [Google Scholar]
- 25.Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, Unser A, Thacker LR, Sanyal AJ, Kang DJ, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62:1260–1271. doi: 10.1002/hep.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nolan JP. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology. 2010;52:1829–1835. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849–855. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 29.John S, Thuluvath PJ. Hyponatremia in cirrhosis: pathophysiology and management. World J Gastroenterol. 2015;21:3197–3205. doi: 10.3748/wjg.v21.i11.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abu Hossain S, Chaudhry FA, Zahedi K, Siddiqui F, Amlal H. Cellular and molecular basis of increased ammoniagenesis in potassium deprivation. Am J Physiol Renal Physiol. 2011;301:F969–F978. doi: 10.1152/ajprenal.00010.2011. [DOI] [PubMed] [Google Scholar]
- 31.Yang SS, Lai YC, Chiang TR, Chen DF, Chen DS. Role of zinc in subclinical hepatic encephalopathy: comparison with somatosensory-evoked potentials. J Gastroenterol Hepatol. 2004;19:375–379. doi: 10.1111/j.1440-1746.2003.03281.x. [DOI] [PubMed] [Google Scholar]
- 32.Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, Uribe M, Vilstrup H, Morgan MY. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58:325–336. doi: 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- 33.Romeiro FG, Américo MF, Yamashiro FS, Caramori CA, Schelp AO, Santos AC, Silva GF. Acquired hepatocerebral degeneration and hepatic encephalopathy: correlations and variety of clinical presentations in overt and subclinical liver disease. Arq Neuropsiquiatr. 2011;69:496–501. doi: 10.1590/S0004-282X2011000400017. [DOI] [PubMed] [Google Scholar]
- 34.Maffeo E, Montuschi A, Stura G, Giordana MT. Chronic acquired hepatocerebral degeneration, pallidal T1 MRI hyperintensity and manganese in a series of cirrhotic patients. Neurol Sci. 2014;35:523–530. doi: 10.1007/s10072-013-1458-x. [DOI] [PubMed] [Google Scholar]
- 35.Gluud LL, Dam G, Les I, Córdoba J, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2015;2:CD001939. doi: 10.1002/14651858.CD001939.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Zhu GQ, Shi KQ, Huang S, Wang LR, Lin YQ, Huang GQ, Chen YP, Braddock M, Zheng MH. Systematic review with network meta-analysis: the comparative effectiveness and safety of interventions in patients with overt hepatic encephalopathy. Aliment Pharmacol Ther. 2015;41:624–635. doi: 10.1111/apt.13122. [DOI] [PubMed] [Google Scholar]
- 37.Kalaitzakis E, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Jalan R, Björnsson E. Malnutrition and diabetes mellitus are related to hepatic encephalopathy in patients with liver cirrhosis. Liver Int. 2007;27:1194–1201. doi: 10.1111/j.1478-3231.2007.01562.x. [DOI] [PubMed] [Google Scholar]
- 38.Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23:982–989. doi: 10.1097/MEG.0b013e32834aa4bb. [DOI] [PubMed] [Google Scholar]
- 39.Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, Lattanzi B, Riggio O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 40.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berzigotti A, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555–561. doi: 10.1002/hep.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everhart JE, Lok AS, Kim HY, Morgan TR, Lindsay KL, Chung RT, Bonkovsky HL, Ghany MG; HALT-C Trial Group. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology. 2009;137:549–557. doi: 10.1053/j.gastro.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeejeebhoy KN. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: overlap of clinical features. Curr Opin Clin Nutr Metab Care. 2012;15:213–219. doi: 10.1097/MCO.0b013e328352694f. [DOI] [PubMed] [Google Scholar]
- 44.Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Dupont B, Dao T, Joubert C, Dupont-Lucas C, Gloro R, Nguyen-Khac E, Beaujard E, Mathurin P, Vastel E, Musikas M, et al. Randomised clinical trial: enteral nutrition does not improve the long-term outcome of alcoholic cirrhotic patients with jaundice. Aliment Pharmacol Ther. 2012;35:1166–1174. doi: 10.1111/j.1365-2036.2012.05075.x. [DOI] [PubMed] [Google Scholar]
- 46.Campillo B, Richardet JP, Scherman E, Bories PN. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition. 2003;19:515–521. doi: 10.1016/S0899-9007(02)01071-7. [DOI] [PubMed] [Google Scholar]
- 47.Roongpisuthipong C, Sobhonslidsuk A, Nantiruj K, Songchitsomboon S. Nutritional assessment in various stages of liver cirrhosis. Nutrition. 2001;17:761–765. doi: 10.1016/S0899-9007(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 48.Carvalho L, Parise ER. Evaluation of nutritional status of nonhospitalized patients with liver cirrhosis. Arq Gastroenterol. 2006;43:269–274. doi: 10.1590/S0004-28032006000400005. [DOI] [PubMed] [Google Scholar]
- 49.Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, Ferenci P, Holm E, Vom Dahl S, Müller MJ, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25:285–294. doi: 10.1016/j.clnu.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 50.Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol. 2012;10:117–125. doi: 10.1016/j.cgh.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 51.Moctezuma-Velázquez C, García-Juárez I, Soto-Solís R, Hernández-Cortés J, Torre A. Nutritional assessment and treatment of patients with liver cirrhosis. Nutrition. 2013;29:1279–1285. doi: 10.1016/j.nut.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 52.McCullough AJ, Mullen KD, Kalhan SC. Measurements of total body and extracellular water in cirrhotic patients with and without ascites. Hepatology. 1991;14:1102–1111. doi: 10.1002/hep.1840140626. [DOI] [PubMed] [Google Scholar]
- 53.Oldroyd B, Bramley PN, Stewart SP, Simpson M, Truscott JG, Losowsky MS, Smith MA. A four-compartment model to determine body composition in liver cirrhosis. Basic Life Sci. 1993;60:221–224. doi: 10.1007/978-1-4899-1268-8_51. [DOI] [PubMed] [Google Scholar]
- 54.Madden AM, Morgan MY. The potential role of dual-energy X-ray absorptiometry in the assessment of body composition in cirrhotic patients. Nutrition. 1997;13:40–45. doi: 10.1016/S0899-9007(97)90877-7. [DOI] [PubMed] [Google Scholar]
- 55.Chadalavada R, Sappati Biyyani RS, Maxwell J, Mullen K. Nutrition in hepatic encephalopathy. Nutr Clin Pract. 2010;25:257–264. doi: 10.1177/0884533610368712. [DOI] [PubMed] [Google Scholar]
- 56.Morgan MY, Madden AM, Jennings G, Elia M, Fuller NJ. Two-component models are of limited value for the assessment of body composition in patients with cirrhosis. Am J Clin Nutr. 2006;84:1151–1162. doi: 10.1093/ajcn/84.5.1151. [DOI] [PubMed] [Google Scholar]
- 57.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 58.Matos C, Porayko MK, Francisco-Ziller N, DiCecco S. Nutrition and chronic liver disease. J Clin Gastroenterol. 2002;35:391–397. doi: 10.1097/00004836-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Campillo B, Richardet JP, Bories PN. Validation of body mass index for the diagnosis of malnutrition in patients with liver cirrhosis. Gastroenterol Clin Biol. 2006;30:1137–1143. doi: 10.1016/s0399-8320(06)73491-1. [DOI] [PubMed] [Google Scholar]
- 60.Fiore P, Merli M, Andreoli A, De Lorenzo A, Masini A, Ciuffa L, Valeriano V, Balotta MT, Riggio O. A comparison of skinfold anthropometry and dual-energy X-ray absorptiometry for the evaluation of body fat in cirrhotic patients. Clin Nutr. 1999;18:349–351. doi: 10.1016/S0261-5614(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 61.Guex E, Cheseaux M, Bertrand PC, Piquet MA, Bouvry S, Pilon N, Halkic N, Pascual M, Roulet M. [Prevalence of undernutrition in 143 patients before liver transplantation. The Lausanne experience between 1997 and 2005] Rev Med Suisse. 2008;4:927–930. [PubMed] [Google Scholar]
- 62.Harrison J, McKiernan J, Neuberger JM. A prospective study on the effect of recipient nutritional status on outcome in liver transplantation. Transpl Int. 1997;10:369–374. doi: 10.1111/j.1432-2277.1997.tb00931.x. [DOI] [PubMed] [Google Scholar]
- 63.Morgan MY, Madden AM, Soulsby CT, Morris RW. Derivation and validation of a new global method for assessing nutritional status in patients with cirrhosis. Hepatology. 2006;44:823–835. doi: 10.1002/hep.21358. [DOI] [PubMed] [Google Scholar]
- 64.Bémeur C, Desjardins P, Butterworth RF. Role of nutrition in the management of hepatic encephalopathy in end-stage liver failure. J Nutr Metab. 2010;2010:489823. doi: 10.1155/2010/489823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lameu EB, Gerude MF, Campos AC, Luiz RR. The thickness of the adductor pollicis muscle reflects the muscle compartment and may be used as a new anthropometric parameter for nutritional assessment. Curr Opin Clin Nutr Metab Care. 2004;7:293–301. doi: 10.1097/00075197-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 66.de Oliveira CM, Kubrusly M, Mota RS, Choukroun G, Neto JB, da Silva CA. Adductor pollicis muscle thickness: a promising anthropometric parameter for patients with chronic renal failure. J Ren Nutr. 2012;22:307–316. doi: 10.1053/j.jrn.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Bering T, Maurício SF, Silva JB, Correia MI. Nutritional and metabolic status of breast cancer women. Nutr Hosp. 2015;31:751–758. doi: 10.3305/nh.2015.31.2.8056. [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez MC, Pureza Duarte RR, Orlandi SP, Bielemann RM, Barbosa-Silva TG. Adductor pollicis muscle: A study about its use as a nutritional parameter in surgical patients. Clin Nutr. 2015;34:1025–1029. doi: 10.1016/j.clnu.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Thomas BJ, Cornish BH, Ward LC. Bioelectrical impedance analysis for measurement of body fluid volumes: a review. J Clin Eng. 1992;17:505–510. doi: 10.1097/00004669-199211000-00016. [DOI] [PubMed] [Google Scholar]
- 70.Pirlich M, Schütz T, Spachos T, Ertl S, Weiss ML, Lochs H, Plauth M. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology. 2000;32:1208–1215. doi: 10.1053/jhep.2000.20524. [DOI] [PubMed] [Google Scholar]
- 71.Cabré E, Gassull MA. Nutritional aspects of liver disease and transplantation. Curr Opin Clin Nutr Metab Care. 2001;4:581–589. doi: 10.1097/00075197-200111000-00020. [DOI] [PubMed] [Google Scholar]
- 72.Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86:509–516. doi: 10.1007/s00421-001-0570-4. [DOI] [PubMed] [Google Scholar]
- 73.Norman K, Kirchner H, Lochs H, Pirlich M. Malnutrition affects quality of life in gastroenterology patients. World J Gastroenterol. 2006;12:3380–3385. doi: 10.3748/wjg.v12.i21.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernandes SA, Bassani L, Nunes FF, Aydos ME, Alves AV, Marroni CA. Nutritional assessment in patients with cirrhosis. Arq Gastroenterol. 2012;49:19–27. doi: 10.1590/S0004-28032012000100005. [DOI] [PubMed] [Google Scholar]
- 75.Peres WA, Lento DF, Baluz K, Ramalho A. Phase angle as a nutritional evaluation tool in all stages of chronic liver disease. Nutr Hosp. 2012;27:2072–2078. doi: 10.3305/nh.2012.27.6.6015. [DOI] [PubMed] [Google Scholar]
- 76.Kalafateli M, Triantos C, Tsochatzis E, Michalaki M, Koutroumpakis E, Thomopoulos K, Kyriazopoulou V, Jelastopulu E, Burroughs A, Lambropoulou-Karatza C, et al. Adipokines levels are associated with the severity of liver disease in patients with alcoholic cirrhosis. World J Gastroenterol. 2015;21:3020–3029. doi: 10.3748/wjg.v21.i10.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liboredo JC, Vilela EG, Ferrari Mde L, Lima AS, Correia MI. Nutrition status and intestinal permeability in patients eligible for liver transplantation. JPEN J Parenter Enteral Nutr. 2015;39:163–170. doi: 10.1177/0148607113513465. [DOI] [PubMed] [Google Scholar]
- 78.Selberg O, Böttcher J, Tusch G, Pichlmayr R, Henkel E, Müller MJ. Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997;25:652–657. doi: 10.1002/hep.510250327. [DOI] [PubMed] [Google Scholar]
- 79.Figueiredo FA, Dickson ER, Pasha TM, Porayko MK, Therneau TM, Malinchoc M, DiCecco SR, Francisco-Ziller NM, Kasparova P, Charlton MR. Utility of standard nutritional parameters in detecting body cell mass depletion in patients with end-stage liver disease. Liver Transpl. 2000;6:575–581. doi: 10.1053/jlts.2000.9736. [DOI] [PubMed] [Google Scholar]
- 80.Müller MJ, Lautz HU, Plogmann B, Bürger M, Körber J, Schmidt FW. Energy expenditure and substrate oxidation in patients with cirrhosis: the impact of cause, clinical staging and nutritional state. Hepatology. 1992;15:782–794. doi: 10.1002/hep.1840150507. [DOI] [PubMed] [Google Scholar]
- 81.Madden AM, Morgan MY. A comparison of skinfold anthropometry and bioelectrical impedance analysis for measuring percentage body fat in patients with cirrhosis. J Hepatol. 1994;21:878–883. doi: 10.1016/S0168-8278(94)80253-X. [DOI] [PubMed] [Google Scholar]
- 82.Wu TJ, Huang JJ, Lin CY. Effects of fluid retention on the measurement of body composition using bioelectric impedance. J Formos Med Assoc. 1994;93:939–943. [PubMed] [Google Scholar]
- 83.Panella C, Guglielmi FW, Mastronuzzi T, Francavilla A. Whole-body and segmental bioelectrical parameters in chronic liver disease: effect of gender and disease stages. Hepatology. 1995;21:352–358. doi: 10.1002/hep.1840210214. [DOI] [PubMed] [Google Scholar]
- 84.Lehnert ME, Clarke DD, Gibbons JG, Ward LC, Golding SM, Shepherd RW, Cornish BH, Crawford DH. Estimation of body water compartments in cirrhosis by multiple-frequency bioelectrical-impedance analysis. Nutrition. 2001;17:31–34. doi: 10.1016/S0899-9007(00)00473-1. [DOI] [PubMed] [Google Scholar]
- 85.Guglielmi FW, Contento F, Laddaga L, Panella C, Francavilla A. Bioelectric impedance analysis: experience with male patients with cirrhosis. Hepatology. 1991;13:892–895. doi: 10.1002/hep.1840130515. [DOI] [PubMed] [Google Scholar]
- 86.Zillikens MC, van den Berg JW, Wilson JH, Swart GR. Whole-body and segmental bioelectrical-impedance analysis in patients with cirrhosis of the liver: changes after treatment of ascites. Am J Clin Nutr. 1992;55:621–625. doi: 10.1093/ajcn/55.3.621. [DOI] [PubMed] [Google Scholar]
- 87.Sörös P, Böttcher J, Weissenborn K, Selberg O, Müller MJ. Malnutrition and hypermetabolism are not risk factors for the presence of hepatic encephalopathy: a cross-sectional study. J Gastroenterol Hepatol. 2008;23:606–610. doi: 10.1111/j.1440-1746.2007.05222.x. [DOI] [PubMed] [Google Scholar]
- 88.Böhm D, Odaischi M, Beyerlein C, Overbeck W. Total body water: changes during dialysis estimated by bioimpedance analysis. Infusionstherapie. 1990;17 Suppl 3:75–78. doi: 10.1159/000222560. [DOI] [PubMed] [Google Scholar]
- 89.Kurtin PS, Shapiro AC, Tomita H, Raizman D. Volume status and body composition of chronic dialysis patients: utility of bioelectric impedance plethysmography. Am J Nephrol. 1990;10:363–367. doi: 10.1159/000168151. [DOI] [PubMed] [Google Scholar]
- 90.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 91.Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061–8071. doi: 10.3748/wjg.v20.i25.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zillikens MC, van den Berg JW, Wilson JH, Rietveld T, Swart GR. The validity of bioelectrical impedance analysis in estimating total body water in patients with cirrhosis. J Hepatol. 1992;16:59–65. doi: 10.1016/S0168-8278(05)80095-9. [DOI] [PubMed] [Google Scholar]
- 93.Malaguarnera M, Vacante M, Giordano M, Pennisi G, Bella R, Rampello L, Malaguarnera M, Li Volti G, Galvano F. Oral acetyl-L-carnitine therapy reduces fatigue in overt hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2011;93:799–808. doi: 10.3945/ajcn.110.007393. [DOI] [PubMed] [Google Scholar]
- 94.Banister EW, Cameron BJ. Exercise-induced hyperammonemia: peripheral and central effects. Int J Sports Med. 1990;11 Suppl 2:S129–S142. doi: 10.1055/s-2007-1024864. [DOI] [PubMed] [Google Scholar]
- 95.Hayashi F, Matsumoto Y, Momoki C, Yuikawa M, Okada G, Hamakawa E, Kawamura E, Hagihara A, Toyama M, Fujii H, et al. Physical inactivity and insufficient dietary intake are associated with the frequency of sarcopenia in patients with compensated viral liver cirrhosis. Hepatol Res. 2013;43:1264–1275. doi: 10.1111/hepr.12085. [DOI] [PubMed] [Google Scholar]
- 96.Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. doi: 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 97.Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257–1266. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]
- 98.Kohrt WM. Body composition by DXA: tried and true? Med Sci Sports Exerc. 1995;27:1349–1353. [PubMed] [Google Scholar]
- 99.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 100.Bosy-Westphal A, Müller MJ. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease--there is need for a unified definition. Int J Obes (Lond) 2015;39:379–386. doi: 10.1038/ijo.2014.161. [DOI] [PubMed] [Google Scholar]
- 101.Riggio O, Andreoli A, Diana F, Fiore P, Meddi P, Lionetti R, Montagnese F, Merli M, Capocaccia L, De Lorenzo A. Whole body and regional body composition analysis by dual-energy X-ray absorptiometry in cirrhotic patients. Eur J Clin Nutr. 1997;51:810–814. doi: 10.1038/sj.ejcn.1600490. [DOI] [PubMed] [Google Scholar]
- 102.Bramley P, Oldroyd B, Stewart S, Simpson M, Truscott J, Losowsky M, Smith M. Body composition analysis in liver cirrhosis. The measurement of body fat by dual energy X-ray absorptiometry in comparison to skinfold anthropometry, bioelectrical impedance and total body potassium. Basic Life Sci. 1993;60:211–214. doi: 10.1007/978-1-4899-1268-8_48. [DOI] [PubMed] [Google Scholar]
- 103.Kalaitzakis E, Josefsson A, Castedal M, Henfridsson P, Bengtsson M, Andersson B, Björnsson E. Hepatic encephalopathy is related to anemia and fat-free mass depletion in liver transplant candidates with cirrhosis. Scand J Gastroenterol. 2013;48:577–584. doi: 10.3109/00365521.2013.777468. [DOI] [PubMed] [Google Scholar]
- 104.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 105.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 106.Giusto M, Lattanzi B, Albanese C, Galtieri A, Farcomeni A, Giannelli V, Lucidi C, Di Martino M, Catalano C, Merli M. Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol. 2015;27:328–334. doi: 10.1097/MEG.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 107.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173, 173.e1. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 108.Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J, Moreau R, Vilgrain V, Valla D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151–1157. doi: 10.1016/j.jhep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 109.Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193–199. doi: 10.1016/j.nut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 110.Montano-Loza AJ, Meza-Junco J, Prado CMM, Tandon P, Bain VG, Ma M, Beaumont C, Esfandiari N, Sawyer MB, Baracos VE. New cutoff values for sarcopenia for predicting 6-months mortality in cirrhotic patients. J Hepatol. 2013;58:S95. doi: 10.1016/S0168-8278(13)60223-8. [DOI] [Google Scholar]
- 111.Strauss BJ, Gibson PR, Stroud DB, Borovnicar DJ, Xiong DW, Keogh J. Total body dual X-ray absorptiometry is a good measure of both fat mass and fat-free mass in liver cirrhosis compared to “gold-standard” techniques. Melbourne Liver Group. Ann N Y Acad Sci. 2000;904:55–62. doi: 10.1111/j.1749-6632.2000.tb06421.x. [DOI] [PubMed] [Google Scholar]
- 112.Figueiredo FA, Perez RM, Freitas MM, Kondo M. Comparison of three methods of nutritional assessment in liver cirrhosis: subjective global assessment, traditional nutritional parameters, and body composition analysis. J Gastroenterol. 2006;41:476–482. doi: 10.1007/s00535-006-1794-1. [DOI] [PubMed] [Google Scholar]
- 113.Plauth M, Merli M, Kondrup J, Weimann A, Ferenci P, Müller MJ. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43–55. doi: 10.1016/S0261-5614(97)80022-2. [DOI] [PubMed] [Google Scholar]
- 114.Francoz C, Prié D, Abdelrazek W, Moreau R, Mandot A, Belghiti J, Valla D, Durand F. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: impact on the model for end-stage liver disease score. Liver Transpl. 2010;16:1169–1177. doi: 10.1002/lt.22128. [DOI] [PubMed] [Google Scholar]


