Abstract
Glycosphingolipids (GSLs) are a family of bioactive lipids that in addition to their role in the regulation of structural properties of membrane bilayers have emerged as crucial players in many biological processes and signal transduction pathways. Rather than being uniformly distributed within membrane bilayers, GSLs are localized in selective domains called lipid rafts where many signaling platforms operate. One of the most important functions of GSLs, particularly ceramide, is their ability to regulate cell death pathways and hence cell fate. This complex role is accomplished by the ability of GSLs to act in distinct subcellular strategic centers, such as mitochondria, endoplasmic reticulum (ER) or lysosomes to mediate apoptosis, ER stress, autophagy, lysosomal membrane permeabilization and necroptosis. Hence better understanding the role of GSLs in cell death may be of relevance for a number of pathological processes and diseases, including neurodegeneration, metabolic liver diseases and cancer.
Keywords: Membrane microdomains, mitochondria, autophagy, ER stress, apoptosis, necroptosis, lysosomal membrane permeabilization
INTRODUCTION
Glycosphingolipids (GSLs) are ubiquitous components of membrane bilayers where they play key structural functions. However, in light of recent evidence during the past decade, the role of GSLs in biology has transcended from their classical function as regulators of biophysical properties of membrane bilayers to being considered key active players in many biological processes, signaling pathways and cell death regulation. This family of lipids consists of an sphingoid long chain base to which a fatty acid is N-acylated to form an amide bond at the C2 position, and various polar or non-polar head groups at the C1 position. Their characteristic association with cholesterol defines specific domains of membrane bilayers that exhibit unique physical properties where key signaling platforms operate in the regulation of important cell processes, including proliferation, sencescence, differentiation and death pathways. Among GSLs ceramide is the best characterized and most studied prototype due to its description as a second messenger in response to stress, apoptotic triggers, chemotherapy, inflammation and in the regulation of metabolism [1-4]. The metabolism to other derivatives with pro or antiapoptotic functions illustrate the complex role of GSLs and ceramide, in particular, in the regulation of apoptosis. Remarkably, while interaction of GSLs with mitochondria promotes the release of proapoptotic mitochondrial proteins to engage caspase activation, which underlies their best-studied role in triggering cell death, GSLs use other pathways and intracellular organelles to induce cell death. This versatility translates in the ability of GSLs to induce endoplasmic reticulum (ER) stress, autophagy, lysosomal membrane permeabilization (LMP) and necroptosis, which amplify the potential of GSLs as proapoptotic lipid players mediating the effect of stress, cytokines and the action of many antitumoral therapies, including ionizing radiation and chemotherapy [5]. In the present review, we summarize the regulation of GSLs metabolism and their diverse mechanisms of action in initiating cell death pathways.
GSLs GENERATION, METABOLISM AND ROLE IN CELL DEATH
De novo ceramide synthesis and regulation
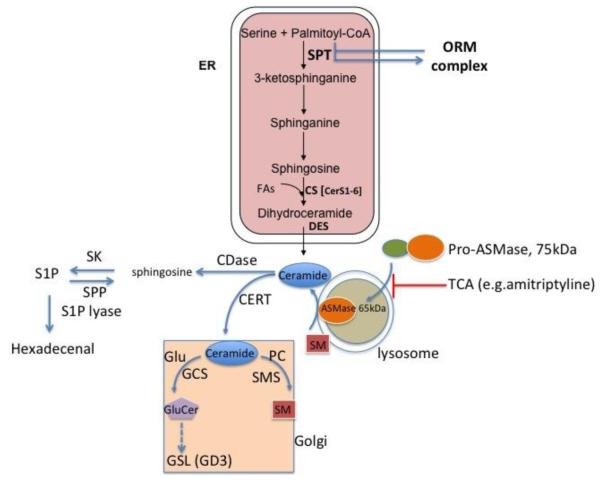
Ceramide is the most studied GSLs first characterized as a bioactive second lipid messenger. There are several pathways whereby cells can generate ceramide, which differ in kinetics and cellular localization [1, 5]. The synthesis of ceramide de novo starts in endoplasmic reticulum (ER), where the amino acid serine is conjugated with the fatty acid palmitoyl-CoA by serine palmitoyl transferase (SPT), the rate-limiting enzyme in this pathway (Figure 1). This reaction yields sphinganine (dihydrosphingosine), which is acylated by (dihydro)ceramide synthase (CerS) to dihydroceramide. The dehydrogenation of dihydroceramide catalyzed by dihydroceramide desaturase (DES) gives rise to ceramide. CerS not only acylate sphinganine but also catalyze the reacylation of sphingosine to ceramide in the salvage pathway. So far, six different CerS have been identified, which exhibit tissue specific expression and variable substrate selectivity, thereby providing the basis for tissue specific synthesis of ceramides with varying acyl chain lengths [1, 6, 7]. For instance, ceramide synthase CerC2 is widely expressed in tissues but in liver is the predominant isoform, which preferentially incorporates long chain C20–C24 acyl residues in ceramide, whereas CerS3 is predominantly expressed in skin and incorporates very long acyl chains up to C34:0 in the resulting ceramides. CerS5 is preferentially involved in the formation of C16 ceramide, while CerC6 shows a wider substrate selectivity yielding C14, C16, and C18 ceramide synthesis [8]. This diversity in synthesis implies that ceramides with different acyl chain lengths are generated in specific tissues and pathophysiological contexts. Despite this defined specific profile of ceramide synthesized by the different CerS, there are adaptive mechanisms that compensate the absence of specific ceramide species. Furthermore, an increase in a particular CerS may regulate a specific ceramide pool that may affect the integrity and function of individual cell compartments, such as lysosomes, ER or mitochondria. In this regard, deletion of CerS2 in mice results in a compensatory increase in the levels of C16 and sphinganine in the liver [9]. These changes in ceramide impact in increased hepatocyte apoptosis, mitochondrial dyfunction and mitochondrial generation of ROS, as well as proliferation that progresses to widespread formation of nodules of regenerative hepatocellular hyperplasia in aged mice. Moreover, progressive hepatomegaly and hepatocellular carcinoma (HCC) are observed in 10-months old CerS2 null mice [9]. Increased ceramide C16 generation by CerS6 or CerS2 haploinsufficiency impaired β-oxidation and sensitized to diet-induced steatosis and insulin resistance [10, 11].
Figure 1. Regulation and metabolism of GSLs.
Predominant mechanisms of ceramide generation include de novo synthesis in the ER or from SM hydrolysis by SMases. In particular, ASMase in endolysosomal compartment is produced from a pro inactive form by a proteolytic processing sensitive to TCA. Once generated, ceramide traffics to the Golgi where is transformed into GSLs and gangliosides or to SM. Ceramide can be deacylated into sphingosine by CDases, which is then phosphorylated to S1P and hydrolyzed to hexedecenal by S1P lyases.
Ceramide synthesis can be regulated by substrate availability and membrane lipid composition [12, 13]. The availability of the precursor palmitoyl-CoA enhances the de novo synthesis of ceramide in the ER. Consistent with this notion, free palmitate levels increase in obesity and metabolic syndrome and related disorders (i.e. nonalcoholic fatty liver disease, NAFLD) and parallels enhanced sphingolipid flux through the de novo pathway [14, 15]. Of relevance to NAFLD, the lipotoxicity of the saturated fatty acid palmitate is due, in part, to increased de novo ceramide synthesis because of the selectivity of SPT for this fatty acid, consistent with the correlation between concentrations of plasma levels of free fatty acids and ceramides as well as the increased ceramide content in the muscle and liver of volunteers subjected to intravenous infusion of saturated fatty acids [16-18].
Besides the regulation of de novo ceramide synthesis by availability of precursors, this process is controlled by the ORM family of proteins, first described in yeasts, via SPT inhibition [19]. Ceramide depletion signals the phosphorylation of ORM proteins by the protein kinase YPK1, which prevents ORM proteins from inhibiting the SPT enzyme complex in the ER, thus stimulating de novo ceramide synthesis [20]. YPK1 kinase activation is triggered by TORC-2, which becomes activated when membrane GSLs levels become low [21, 22]. As GSLs reduce membrane stress, this mechanism represents a homeostatic regulatory system for cellular membranes [23]. Although this fine-tune mechanism of GSLs regulation has been shown to operate only in yeasts, recent findings have described a similar process in mammalian cells, where three ORM proteins have also been implicated in the regulation of GSL biosynthesis [24]. Moreover, induction of SPT in HEK293 cells resulted in a striking increase in the levels of ORM proteins without change at the mRNA level [25]. Increased ORM protein expression required SPT activity since overexpression of a catalytically inactive SPT form had little effect. Significantly, increased ORM expression was prevented by myriocin as well as fumonisin inhibition of the CerS.
Ceramide generation by sphingomyelinases
In addition to ceramide generation by de novo synthesis, cells can generate ceramide by sphingomyelin (SM) hydrolysis due to the activation of sphingomyelinases (SMases). In response to many deleterious stimuli causing stress, apoptosis, chemotherapy and cell death, cells activate SMases leading to a rapid and transient release of ceramide in specific sites that engage particular signaling pathways [1, 5, 26, 27]. Several mammalian SMases have been characterized, which are classified according to their optimal pH (alkaline, neutral or acid). Neutral sphingomyelinase (NSMase) and acid sphingomyelinase (ASMase) are the most studied enzymes in ceramide generation, which have been involved in pathophysiological processes and disease. In this regard, NSMase-induced ceramide generation has been described as a critical lipid mediator in inflammatory diseases and X. laevis oocyte maturation [28, 29]. Moreover, ASMase has been characterized as a signaling intermediate in extrinsic cell death pathways and liver diseases [30-36]. The primary role of ASMase is to catalyze the formation of ceramide from SM primarily within the endo-lysosomal compartment. However, ASMase is secreted extracellularly through Golgi trafficking as a secretory ASMase (S-SMase) form [26, 27]. An important feature of the secreted ASMase and the endo-lysosomal form is their differential dependence on Zn2+ for proper function, with the former being Zn2+ dependent. Both isoforms derive from a proinactive form whose proteolytic processing within the C terminal leads to the maturation of the endosomal/lysosomal ASMase and the secretory form [37]. Another important feature is that the mature ASMase counterpart (65kDa) but not the pro-ASMase form is sensitive to tricyclic antidepressants such as desipramine/imipramine. The evidence supporting a role for ASMase as an important mediator of stress and apoptosis stimuli has derived from mice with genetic deletion of ASMase. In this regard, it has been shown that ASMase knockout mice are resistant to Fas or TNF-mediated liver failure, ischemia/reperfusion, hepatocyte apoptosis due to Cu2+ accumulation, characteristic of Wilson disease and diet-induced steatohepatitis [31, 33, 34, 35, 36, 38, 39], indicating that the ASMase/ceramide pathway is critical in liver pathophysiology.
Moreover, ASMase-induced ceramide generation occurs also at the plasma membrane in specific microdomains where it functions as a signaling platform that promotes death receptor clustering, resulting in the initiation of apoptosis. For instance, in Jurkat T lymphocytes and in primary hepatocytes CD95 capping and killing required ceramide generated from ASMase and hence CD95-triggered translocation of ASMase to the plasma membrane outer surface, enabled clustering of CD95 in sphingolipid-rich membrane rafts and apoptosis induction [40, 41]. Furthermore, ASMase functions upstream of the death-inducing signaling complex (DISC) to mediate CD95 clustering in ceramide-enriched membrane platforms, an event that is required for DISC formation [42]. Recent findings indicated that upon CD95 stimulation, ASMase activation and translocation to the plasma membrane required the t-SNARE protein syntaxin 4, as syntaxin 4 down regulation blocked ASMase translocation and activation triggered by CD95 preventing caspase activation and apoptosis [43].
Besides this function in apoptosis induction, a novel role for ASMase in neuroinflammatory diseases has been recently described involving vesicle shedding and microparticle release from glial cells and astrocytes [44]. Following activation of the ATP receptor P2X7 in glial cells, microparticle shedding is associated with rapid activation of ASMase, which moves to plasma membrane outer leaflet. ATP-induced shedding and IL-1β release are abolished in glial cultures from ASMase−/− mice.
Consistent with the physiological role of ASMase in hydrolyzing lysosomal SM, the deficiency of ASMase results in a lysosomal storage disorder (Niemann-Pick disease) characterized by accumulation of lysosomal SM in affected organs, primarily brain and liver. This effect is accompanied by a secondary increase in lysosomal cholesterol, which likely reflects the high affinity of SM to bind cholesterol resulting in decreased efflux/trafficking of cholesterol out of lysosomes [45, 46]. The impaired cholesterol trafficking out of lysosomes due to its sequestration by SM decreases cholesterol sterification by acyl-CoA:cholesterol acyltransferase (ACAT) and increases SREBP-2 proteolytic processing, contributing to the secondary hypercholesterolemia in ASMase knockout mice. Based upon these findings, it appears that cells use the SREBP pathway to achieve an optimal ratio of SM to cholesterol in membrane bilayers. As discussed below, the accumulation of lysosomal cholesterol due to ASMase deficiency impairs autophagy in hepatocytes and in mouse coronary arterial smooth muscle cells (CASMCs) [35, 47].
In addition to ASMase, acid ceramide (ACDase) also regulates lysosomal ceramide homeostasis. ACDase deficiency results in lysosomal ceramide accumulation and causes Farber disease, a rare autosomal recessive lysosomal storage disorder manifested early after birth characterized by arthritis, subcutaneous nodules, psychomotor retardation and hepatosplenomegaly. Although loss of function of ACDase is causally linked to Farber disease, the mechanism whereby lysosomal ceramide accumulation contributes to Farber phenotype remains essentially unknown. Complete knockout of Asah1, the gene encoding for ACDase, is embryonic lethal and ACDase reduction in mouse ovaries results in oocyte apoptosis, which precluded the generation of a viable model to study the pathogenesis of the disease. Introducing a single-nucleotide mutation identified in human Farber disease patients into the murine Asah1 gene allowed the generation of the first viable model of systemic ACDase deficiency [48]. ACDase deficiency in mice increased lysosomal ceramide, MCP-1 levels and infiltration of lipid-laden macrophages in several tissues. Importantly, a single injection of human ACDase-encoding lentivector in this model diminished the severity of the disease and increased life span, offering hope for this approach in Farber patients.
NSMase activation has also been shown to play a key role in apoptosis. Recent evidence has shown that NSMase cooperates with Bak and Bax to promote the mitochondrial pathway of apoptosis [49]. In addition, bacterial NSMase has been identified as a key component of the therapeutic effects of the probiotic formulation VSL#3 in inflammatory bowel disease due to the selective induction of apoptosis of activated mucosal immune cells, as the beneficial effects of VSL#3 were prevented by NSMase inhibition with GW48469 [28].
Ceramide metabolism and GSLs generation
Once generated ceramide can be transformed in other derivatives and complex GSLs by different pathways (Figure 1). The de novo synthesized ceramide in the ER can be transported to the Golgi by a non-vesicular mechanism involving the protein CERT, where it serves as the precursor for SM synthesis by SM synthases (SMS). SM synthesis involves the transfer of a phosphocholine head group from phosphatidylcholine (PC) to ceramide with concomitant production of diacylglycerol (DAG). SM is then distributed to different membrane bilayers and concentrate in specific domains in association with cholesterol in the so-called membrane rafts. There are two SMS, SMS1 and SMS2, which are localized in distinct cellular locations such as Golgi and plasma membrane, respectively [50, 51]. Besides SMS1 and SMS2, a SMS related protein (SMSr) has been described as an ER-resident transmembrane protein, which does not sythesize SM but exhibits a ceramide ethanolamine phosphatransferase activity involved in the synthesis of ceramide phosphoethanolamine. SMSr antagonizes cell death by regulating ceramide localization. For instance, SMSr silencing has been shown to induce the trafficking of ceramide to mitocondria leading to cell death [52]. Ceramide is the precursor of complex GSLs and gangliosides, a process that first requires the trafficking of ceramide to the Golgi, where ceramide in the presence of glucose is converted into glucosylceramide (GlcCer) in a reaction catalyzed by glucosylceramide synthase (GCS) (Figure 1). The addition of subsequent sialic acid residues to GlcCer generates complex GSLs and gangliosides. Non-vesicular transport of GlcCer from its site of synthesis (early Golgi) to distal Golgi is carried out by FAPP2 [53]. Ganglioside GM3 is one of the simplest GSLs and has a dual fate: its conversion into complex gangliosides or a substrate of ceramide generation upon detachment of its sugar unit by Neu3 [53]. Interestingly, fibroblasts overexpressing Neu3 exhibit reduced proliferation rate and higher basal number of apoptotic cells in comparison with wild-type cells, consistent with the increase ceramide content. The subsequent addition of sialic acids to GM3 generates ganglioside GD3 in a step catalyzed by GD3 synthase (Figure 1). Ganglioside GD3 is a bioactive lipid with a prominent role in cell death pathways primarily by targeting mitochondria. As discussed below, recent findings have clearly indicated a novel role for ganglioside GD3 in autophagy and in the regulation of lipid rafts microdomains essential for its role in cell death.
The predominant pathway of ceramide catabolism is its deacylation to sphingosine catalyzed by ceramidases, which is followed by the phosphorylation of sphingosine to sphingosine-1-phosphate (S1P) by sphingosine kinases (SKs) (Figure 1). Three ceramidase (CDase) isoforms with distinctive pH optimum, acid, alkaline and neutral have been described [54, 55]. Their relative contribution to cell death is dependent on the stimuli and the cell type studied. The role of neutral CDase in cell death regulation has been well characterized in the protection against cytokine-induced cell death in messangial cells [56]. On the other hand, the acidic isoform, ACDase, has been best explored in cancer biology and in the protection of cancer cells against chemotherapy due to formation of S1P from ceramide deacylation and subsequent phosphorylation of sphingosine [5, 57]. S1P plays an antiapoptotic role and is therefore viewed a prosurvival lipid due to a combined mechanism of action including the antagonism of ceramide-mediated cell death pathways from inside cells, and by activating proliferative pathways from outside the cell via uptake through specific G protein-coupled S1P receptors (S1PR1-5) [58-60]. Two SK isoforms (SK1 and SK2) are known, whose selective increase in different tumor cell types has been associated with cancer progression. For instance, increased levels of the SK1 has been reported in stomach, brain, lung, colon or breast cancers and a correlation between SK1 expression and tumor grade or decreased patient survival has been reported, suggesting S1P as potential target to control tumor growth and angiogenesis [59, 61]. SKI-II has been reported as a dual inhibitor of SK1 and SK2 and has been widely used to uncover the role of SKs and S1P in cell death. However, recent findings have shown that SKI-II is a non-competitive inhibitor of DES activity without changes in the expression of DES protein levels [62]. Moreover, unlike the SK1 specific inhibitor PF-543, SKI-II induced the accumulation of dihydroceramide and other metabolites, while both SKI-II and PF-543 reduced S1P levels. Consistent with this outcome, SKI-II, but not PF543, reduced cell proliferation with accumulation of cells in the G0/G1 phase. These findings imply that many of the effects of SKI-II are not due to the reduction of S1P but rather to the increase in dihydroceramides. Furthermore, S1P can then be irreversibly cleaved by the S1P-lyase to phosphoethanolamine and hexadecenal [63] or alternatively it can be dephosphorylated by sphingosine phosphatases (SPP) back to sphingosine and thereby recycled for ceramide formation in the salvage pathway [64]. Thus, the concerted actions of S1P-lyase, SPP and SK2, which are localized at the ER, can regulate intracelular levels of S1P and hence cell death, indicating that the regulation of these enzymes are critical determinants of cell fate and death pathways and that they may be interesting targets in a number of pathologies and cancer [65, 66].
VERSATILITY AND MECHANISMS OF GSLs IN CELL DEATH
Besides their recognized role as structural components of membrane bilayers, cumulative evidence from the last decade has changed our view of GSLs as mere regulators of membrane’s physical properties to emerge as bioactive lipids regulating multiple cell functions and, particularly, apoptosis. Apoptosis is a specific form of cell death characterized by biochemical events that ultimately lead to cell fragmentation into compact membrane-enclosed structures, called “apoptotic bodies” that are taken up by neighboring cells and phagocytes, that normally prevent inflammation and tissue damage [67]. The role of GSLs in inducing cell death is versatile involving the recruitment of major pathways such as mitochondrial targeting, ER stress, autophagy and necroptosis as well as the inactivation of survival processes.
Mitochondrial apoptosis
In addition to the cardinal role of mitochondria in energy generation, they also play a strategic function in the regulation of cell death, including apoptosis (caspase-dependent and independent) or necrosis. Apoptosis is induced via two main routes involving either the mitochondria (the intrinsic pathway) or the activation of death receptors (the extrinsic pathway). While ceramide has been shown to mediate death receptor-mediated apoptosis as outlined above, ceramide also regulates the intrinsic pathway, and hence modulates the essential core of apoptosis pathways. Pioneering work established the ability of ceramide to interact with components of the mitochondrial electron transport chain accounting for the stimulation of ROS, mitochondrial depolarization and mitochondrial dysfunction [68-69]. These effects contributed to the mitochondrial membrane permeabilization and release of cytochrome c into the cytosol leading to the apoptosome assembly and activation. In cell-free assays using purified rat liver mitochondria it was first shown that the addition of ceramide C2 induced ROS generation predominantly from the complex III of the mitochondrial electron carriers, thus contributing to the mitochondrial depolarization and dysfunction observed during cell death [68]. This outcome paralleled the findings of purified mitochondria from cells exposed to stress and apoptosis triggers, such as TNF, Fas, or UV irradiation [70]. These results imply that the stimulated ceramide levels induced by TNF/Fas traffic to mitochondria. Recent findings have provided alternative possibilities, including the in situ generation of ceramide in mitochondria. In line with this possibility, it has been reported that the enforced mitochondrial targeting of NSMase in MCF7 cells resulted in mitochondrial ceramide increase that caused cytochrome c release and apoptotic cell death [71]. The pathways of ceramide generation in these organelles involved a reverse ceramidase activity or a ceramide synthase, implying that mitochondria have the ability to generate ceramide de novo [72, 73]. These observations suggest the existence of an independent and highly regulated sphingolipid metabolism in mitochondria that contribute to the accumulation of ceramide in these organelles modulating cell death pathways. In addition to ceramide other GSLs, including ganglioside GD3, have been described to traffic to mitochondria to interact with electron chain components, causing ROS overgeneration and potentiation of calcium-mediated mitochondrial permeability transition (MPT) [74-77]. Although in resting hepatocytes most of the endogenous GD3 is present at the plasma membrane, in response to apoptotic stimuli (TNF, ionizing radiatio or exogenous ASMase) GD3 undergoes a redistribution that involved first its disappearance from the plasma membrane followed by its trafficking to mitochondria [78]. The colocalization of GD3 with mitochondria was preceded by its location in early/late endosomes via coordinated secretory/endocytic vesicular trafficking targeting mitochondria. Quite interestingly, GD3-7-aldehyde (GD3-7), a GD3 derivative, has been shown to induce mitochondrial swelling and depolarization that was blocked by cyclosporin A (CsA), supporting a critical role of the MPT during GD3-7-mediated apoptosis [79]. In contrast to GD3, GD3-7 induces channel formation in proteoliposomes containing adenosine nucleotide translocator (ANT), suggesting that ANT is a molecular target of GD3-7. As suggested and best described in T cell apoptosis, the trafficking of GSLs to mitochondria to promote mitochondrial outer membrane permeabilization (MOMP) and apoptosis may occur in specific raft-like microdomains where they interact with critical components and proapoptotic members of the Bcl-2 family to orchestrate MOMP leading to cytochrome release and apoptosome assembly [74].
Mitochondria are functionally and physically associated with heterotypic membranes, and the mitochondrial apoptosis pathway is regulated by members of the Bcl-2 family proteins, particularly Bax and Bak that control MOMP, cytochrome c release and apoptosis. The dissociation of heterotypic membranes from mitochondria inhibited Bak/Bax-dependent cytochrome c release. Recent evidence has shown that sphingolipid metabolism plays a key role in mitochondrial apoptosis by regulating Bax/Bak activation [48]. Furthermore, S1P and hexadecenal cooperated specifically with Bak and Bax, respectively, to induce MOMP and apoptosis. These findings suggest that sphingolipids cooperates with Bak and Bax to promote the mitochondrial pathway of apoptosis. Moreover, it has been demonstrate that ceramide generation in the mitochondrial outer membrane of mammalian cells upon irradiation forms a platform into which Bax inserts, oligomerizes and functionalizes as a pore, causing mitochondrial membrane permeabilization and apoptosis [80].
Besides the regulation of mitochondrial apoptosis, a novel function of ceramide in cell death has described the targeting of mitochondria to autophagosomes leading to massive mitophagy [81]. Treatment of human cancer cells with C(18)-pyridinium ceramide treatment or endogenous C(18)-ceramide generation by CerS1 expression caused autophagic cell death, independent of apoptosis. C(18)-ceramide-induced lethal autophagy by targeting of mitochondria to LC3B-II-containing autophagolysosomes (mitophagy) through direct interaction between ceramide and LC3B-II, resulting in Drp1-dependent mitochondrial fission, leading to inhibition of mitochondrial function and oxygen consumption. Quite interestingly, there has been evidence suggesting that fission proteins associate with mitochondrial raft-like domains in response to CD95-induced apoptosis in T cells [82]. Overall, the targeting of sphingolipids to specific microdomains of mitochondrial membrane emerges as an important factor regulating MOMP and apoptosis.
Endoplasmic Reticulum stress
Besides the central role of mitochondria in apoptosis, cell death is controlled by a complex molecular interplay of organelles [83]. The ER plays a critical homeostatic function in cells, mainly in the control of protein and lipid synthesis and traffic. Disruption in this physiological function leads to a specific stress response called the unfolded protein response (UPR) whose primary aim is to recover homeostasis. The molecular machinery involved in UPR includes three conserved pathways regulated by Ca2+ and the master chaperone GRP78/Bip. Early during UPR unfolded proteins causes dissociation of chaperone Grp78/Bip from ER resident kinases such as type-I ER transmembrane protein kinase (IRE1), the PKR like ER kinase (PERK) and the activating transcription factor6 (ATF6). The translational arm of the UPR reflects a short-term adaptation that reduces the load of newly synthesized polypeptides entering the ER lumen, while the transcriptional arm constitutes a long-term adaptation that increases the capacity of the organelle to handle unfolded proteins. Secondary adaptation signals include the activation of transcription factors ATF4 that regulate genes involved in aminoacid transport, glutathione (GSH) synthesis and resistance to oxidative stress [84]. However, excessive and prolonged ER stress triggers apoptosis due to the activation of specific intermediates, including transcription factor CHOP and caspase 12 [85]. ER membranes are rich in gangliosides and de novo ceramide synthesis occurs in the ER, implying that GSLs regulate UPR and ER stress. In this regard, accumulation of GluCer, which is the main storage product in Gaucher disease, increases Ca2+ mobilization from intracellular stores in cultured neurons via ryanodine receptor-mediated Ca2+ release from the ER [86]. Moreover, microsomes from the brain in murine Sandhoff disease model accumulate GM2 and show reduced rates of Ca2+ uptake due to reduced Ca2+-ATPase SERCA activity [87]. In addition to alterations in Ca2+ homeostasis, GM1 accumulation due to deficiency of lysosomal beta-galactosidase leads to upregulation of GRP78 and CHOP and activation of JNK2 and caspase-12 that contribute to neuronal cell death in a mouse model of GM1 gangliosidosis [88]. Increased de novo synthesized ceramide elicits an ER stress-dependent death mechanism in glioma cells in response to tetra-dydrocannabinol that is inhibited upon inhibition of SPT [89]. In this process, ceramide synthesized de novo upregulated the levels of p8 (also known as candidate of metastasis 1), leading to the activation of ATF-4 and stress-regulated protein tribble homolog 3 (TRB-3), and these events are prevented by SPT inhibition. Importantly, these effects were reproduced by ceramide C2 and blocked by pharmacological inhibition of the mitochondrial respiratory chain, supporting an ER-mitochondria cross-talk in ceramide-induced cell death. Interestingly, this ceramide-ER stress axis mediated cell death seems to be specific for transformed or malignant cancer cells but not normal cells. As with the putative role of mitochondrial raft-like domains in cell death, recent findings have shown that alterations of ER lipid rafts contributes to palmitate-induced lipotoxicity in pancreatic β cells [90]. Palmitate induced ER GSLs remodeling characterized by a loss of SM and disruption of ER lipid rafts led to ATF4 and CHOP induction and β cell death and these effects were prevented by GluCer synthase overexpression. Thus, these results suggest that loss of SM in the ER is a key event in initiating β cell lipotoxicity, which leads to disruption of ER lipid rafts, perturbation of protein trafficking, and initiation of ER stress.
Autophagy
Autophagy, a term derived from the Greek auto meaning “self” and phagein meaning “to eat”, is a complex cellular process conserved from yeasts to mammals that ensures the degradation and recycling of cellular components. Three modes of autophagy have been identified: macroautophagy, microautophagy, and chaperone-mediated autophagy. During the autophagic process, nonspecific or targeted cytoplasmic constituents are delivered to and degraded in the lysosome via the formation of double membrane structures that lengthen to form an autophagosome, which then fuses with lysosomes to form an autolysosome where cargo contents are degraded. Activated during nutrient starvation, autophagy is generally considered a protective mechanism by channelling celular components for degradation to supply energy. However, depending on the context, degree and/or duration autophagy can also culminate with death is the so-called autophagic cell death. Autophagic cell death is a distintive form of cell death known as type II programmed cell death, in which autophagy triggers cell death pathways [91]. The characteristics of autophagic cell death include its independence from apoptosis, and prevention by agents that suppress autophagy. Sustained autophagy-mediated cell death is caused by irreversible cellular atrophy and dysfunction due to the loss of cytosolic components and organelles. Moreover, autophagy can selectively destroy proteins involved in cellular defense and survival. For instance, autophagy has been shown to degrade catalase, which plays an important role in cellular antioxidant defense [92]. As critical components of bilayers, GSLs have been shown to regulate autophagy at various levels [93]. Ceramide has been implicated in the induction of autophagy by several mechanisms: stimulation of the phosphatase PP2A, which in turn blocks Akt activation and hence stimulate autophagy [94]. Moreover, nutrient deprivation induces an increase in ceramide, which then suppresses mTOR activity in a PP1/PP2A dependent manner [95]. In addition, ceramide suppresses amino acid transporters leading to AMPK-dependent autophagy induction [96] and enhances Beclin1 expression leading to autophagy induction, an effect that is prevented by SPT inhibition with myriocin [97]. Futhermore, ceramide-induced ER stress also contributes to autophagy induction [98]. Ablation of CerS2, which synthesizes long-chain ceramide species, results in increased compensatory ceramide forms of intermediate acyl lenght resulting in autophagy secondary to ER stress induction [99]. Interestingly, myristic acid oversupply led to increased ceramide levels via CerS5 resulting in autophagy and cardiomyocyte hypertrophy [100]. This finding is consistent with the emerging role of myristic acid in the stimulation of the de novo ceramide synthesis by stimulation of DES [101]. The final outcome of ceramide-mediated autophagy (protective or lethal) is cell type specific and stimuli dependent [102]. For example, treatment of renal carcinoma cells with low dose of anti-cancer drugs vorinostat and sorafenib has been shown to activate CD95 via ASMase stimulation and subsequent ceramide generation, which in turn, induced autophagy via increased ATG5 and consequently suppression of ATG5 increased sorafenib and vorinostat lethality [103]. Moreover, in the HCC cell line Hep3B treatment with ceramide C2 induced lethal autophagy by a mechanism involving JNK activation, which upregulated Beclin1 expression [104]. Consistent with the role of JNK, the JNK inhibitor SP600125 as well as Beclin1 silencing rescued Hep3B cells from ceramide-induced autophagic cell death.
Recent findings have provided evidence that ASMase promotes autophagy in different cell types at the level of fusion of lysosomes with autophagosomes. For instance, mouse CASMCs from ASMase null mice exhibit increased autophagsomes due to the defective autolysosome formation and enhanced CASMCs proliferation and atherosclerosis plaque formation [47]. In line with these findings, hepatocytes deficient in ASMase have also been shown to exhibit defects in autophagy characterized by increased LC3BII expression and p62 levels and decreased Atg7 expression [36]. As in CASMCs, hepatocytes from ASMase display increased lysosomal cholesterol accumulation secondary to the increased lysosomal SM content, which impairs the fusion of lysosomes with autophagosomes. ASMase can regulate autophagy via several mechanisms, including the regulation of the TRPLM1/lysosomal Ca2+/dynein axis by modulating microtubules and the trafficking of autophagosomes with lysosomes. Moreover, ceramide regulates lysosome fusion to cell plasma membranes, endosomes, phagogomes and other organelles while modulating cytoskeleton and microtubule assembly [105].
Besides ceramide, recent findings have revealed a previously unrecognized role for GD3 in autophagy by regulating autophagosome formation [106]. Following amino acid deprivation, ganglioside GD3 contributed to the biogenesis and maturation of autophagic vacuoles. Furthermore, ganglioside GD3 interacts with phosphatidylinositol 3-phosphate in inmature autophagosomes in association with LC3-II and in autolysosomes associated with LAMP1. Consistent with these findings knocking down ganglioside GD3 synthase impairs autophagy while exogenous ganglioside GD3 administration resumes autophagy. In addition to these effects, gangliosides have been shown to induce autophagic cell death in astrocytes by a mechanism dependent on ROS generation, inhibition of Akt/mTOR and activation of EK and formation of specific raft-like domains [107]. Ganglioside-induced cell death was abolished by knowdown of beclin-1/Atg-6 or Atg-7 gene expression of by 3-methyladenine, an autophagy inhibitor. These novel results suggest that gangliosides induce autophagy by multiple mechanisms, emerging as versatile lipids in the regulation of autophagy and autophagic cell death.
Lysosomal membrane permeabilization
Lysosomal membrane permeabilization (LMP) has been described as a pathway leading to apoptotic and non-apoptotic cell death, in part through the release of lysosomal proteases and recruitment of mitochondria. For instance, LMP has been described a key mechanism involved in saturated fatty acid-induced lipotoxicity of relevance in fatty liver disease [108]. Palmitic acid-induced LMP and release of lysosomal cathepsins preceded mitochondrial dysfunction, MOMP and apoptosis, effects that were prevented by blocking lysosomal cathepsin B. Accumulation of SM and cholesterol in lysosomes, characteristic of ASMase deficiency, impairs LMP and hence palmitic acid-induced apoptosis in primary hepatocytes [35]. Thus, these findings indicate that ASMase is essential for lysosomal stability by regulating lysosomal SM and cholesterol homeostasis. In line with these findings, there is increasing evidence indicating that tumor cell lysosomes are more fragile than normal lysosomes and are more susceptible to LMP and hence tumor demise. Recent studies described that ASMase inhibition emerges as a novel mechanism to destabilize cancer cell lysosomes to induce cell death [109]. Cationic amphiphilic drugs (CAD), including tricyclic antidepressants, antihistamines or calcium channel blockers, inhibit ASMase by interfering the binding of ASMase to its essential lysosomal cofactor, bis(monoacylglycerol)phosphate and induce LMP, tumor cell death, reduced tumor growth in vivo and revert multidrug resistance. Quite intriguingly, tumor cell death induction due to LMP by CAD occurred despite increase lysosomal SM, which unexpectedly was not accompanied by increased cholesterol accumulation in lysosomes of cancer cells. The mechanisms for the selective accumulation of SM without cholesterol increase in lysosomes of cancer cells is not currently known and do not appear to reflect the high affinity of SM for cholesterol normally seen in primary cells [45, 46]. While the direct effect of lysosomal SM in regulating LMP in cancer cells has not been addressed, there is emerging evidence showing that lysosomal cholesterol accumulation impairs LMP and that its extraction reverses this effect [35, 110]. Further understanding of the mechanisms involved in the fragility of tumor lysosomes and LMP is required. Interestingly, database search demonstrated a highly significant cancer-associated reduction in ASMase expression in several microarray studies comparing mRNA levels in tumors originating from gastrointestinal tract, liver, head and neck, kidney, pancreas, cervix, lung and brain, indicating that inhibiting ASMase may be a novel approach in cancer by promoting LMP. Although the role of lysosomal ceramide in LMP is controversial, recent findings have shown that increased lysosomal ceramide in response to TNF/cycloheximide is converted into sphingosine, which directly mediates LMP leading to lysosomal cathepsins release and these effects were abrogated upon ASMase and acid CDase silencing [111]. Futhermore, incubation of rat hepatoma cell line HTC with ceramide C2 failed to cause LMP and cathepsin release, further confirming that sphingosine derived from ceramide mediates TNF/cycloheximide cell death via LMP [111], in line with the known lysosomotropic ability of sphingosine to elicit LMP [112]. Thus, these findings show that unlike ceramide the accumulation of sphingosine in lysosomal is instrumental in LMP and cell death.
Ganglioside GD3 and the inactivation of survival pathways
The transcription factor NF-κB is a master regulator of innate immunity and inflammation. In addition, NF-κB activation is known to induce crucial antipapoptotic genes and survival pathways. In line with this indispensable role, mice deficient in the NF-κB transactivating subunit, RelA (p65) is embryonic lethal and mice die during mid-gestation from massive hepatocyte apoptosis [113, 114]. This essential role of NF-κB in cell protection is multifactorial and involves the induction of genes that antagonize apoptosis by preventing caspase activation and ROS generation, among others. The role of ceramide in NF-κB regulation is controversial, with some studies indicating that SM hydrolysis and ceramide generation were important events in NF-κB signaling, while others reported that increasing basal levels of ceramide or N-acetylsphingosine did not affect NF-κB activation [115-117]. In contrast to ceramide, ganglioside GD3 has been shown to block the activation of NF-κB by preventing the nuclear translocation of active DNA-binding competent κB members to the nuclei in response to TNF or ionizing radiation, hence disabling the induction of antiapoptotic genes [118, 119]. Moreover, the use of different GSLs derivatives indicated that the presence of N-fatty acyl sphingosine, a common features of both ceramide and GD3, is necessary for the ROS stimulating effect, while the presence of sugar residues in the backbone of ceramide is required for the prevention of the translocation of NF-κB to the nuclei, most likely by a mechanism that interferes with the nuclear localization signal of the κB complex members. The potential of this dual function of GD3 in apoptosis by stimulating mitochondrial ROS and blocking NF-κB transactivation has been shown in human hepatoma cells, which are resistant to cancer therapy [119]. However, the role of GD3 to inactivate survival pathways is modulated by the acetylation status of GD3, as acetylated GD3 has antiapoptotic properties [120]. Indeed, O-acetylation of GD3 has been shown to prevent its apoptotic effect, promoting the survival of lymphoblasts in childhood acute lymphoblastic leukaemia and glioblastoma cells [121, 122]. Consistent with this function, GD3 and 9-O-acetyl-GD3 are overexpressed in about 50% of invasive ductal breast carcinoma [123], although no relationship between GD3 expression vs 9-O-acetyl-GD3 expression and breast cancer progression has been established. To exploit the versatility of GD3 ganglioside in cell death, it has been shown that overexpression of GD3 synthase which increases the levels of GD3 from endogenous GM3, rendered hepatocellular carcinoma Hep3B cells susceptible to hypoxia-induced ROS generation by suppressing the hypoxia-mediated NF-κB activation via tyrosine kinase Src, and reduces tumor growth in vivo in Hep3B-GD3 xenografts [124]. Although these findings support a potential relevance of exploiting the dual role of GD3 in cell death as an anticancer strategy, the enforced expression of GD3 synthase in tumor cells signals its own 9-O-acetylation machinery, which may limit efficacy in induced tumor cell death [125].
GSLs and necroptosis
While apoptosis is one of the most intensively researched forms of programmed cell death, certain GSLs have been recently shown to elicit necroptosis, a programmed cell death independent of caspases. This programmed form of necrosis is dependent on protein serine-threonine kinases RIPK1 and RIPK3, which can be activated in response to various stimuli, including TNF receptors, Toll-like receptors, genotoxic stress and during virus infection [126]. While cleavage of RIPK1 and RIPK3 by caspase-8 prevents necroptosis and elicits apoptosis, caspase-8 inactivation promotes the engagement of RIPK1 and RIPK3 to the effector mechanisms of necroptosis [127]. Besides caspase-8, c-FLIP, a catalytically inactive homolog of caspase-8, which exits in two isoforms (c-FLIP long and short forms, c-FLIPL and c-FLIPS, respectively), plays also an essential role in necroptosis. The association of caspase-8 with c-FLIPS but not c-FLIPL allows the assembly of RIPK1 and RIPK3 thus promoting necroptosis. This form of cell death has been recently described in specific models of sphingolipid LSD such as Gaucher and Krabbe disease. Gaucher disease is an inherited metabolic disorder caused by mutations in the glucocerebrosidase gene GBA characterized by neurological symptons due to neuronal loss caused by the accumulation of monohexosylceramides, GluCer and glucosylsphingosine in the brain [128]. Using a mouse model of Gaucher disease due to the deficiency of GBA, it has been recently shown the absence of apoptotic cell death and caspase activation despite of the onset of overt neurodegeneration [129]. However, levels of RIPK1 and RIPK3 were elevated in brain of GBA null mice both in microglia and neurons correlating with neuroinflammation and neuronal cell death. Similar findings were observed upon administration of conduritol B epoxide (CBE), an irreversible inhibitor of glucocerebrosidase, resulting in increased levels of GluCer. While CBE caused neurodegeneration and shortened life span in Ripk3 heterozygous mice, the deficiency of Ripk3 caused sustained life extension. These findings indicating necroptosis in association with monohexosylceramides contrast with those found in another LSD, Niemann Pick type C disease (NPC), characterized by mutation in the endolysosomal NPC1 protein causing accumulation of cholesterol, and GSLs like GluCer predominantly in lysosomes. In Gaucher disease the accumulation of GluCer occurs in the ER contributing to the dysregulation of ER Ca2+ homeostasis and increased cytosolic Ca2+ levels. These findings indicate that in addition to the biochemical nature of the stored material in LSD the intracellular site of accumulation is an important determinant of whether GSLs induce apoptosis or necroptosis.
CONCLUDING REMARKS
The interest on GSLs in the scientific community has resurrected following their emergence as second messengers in signaling pathways and as crucial players in cell death regulation. A particular important scenario of this renewed attention involved the mitochondrial apoptosis pathway and its central stage of MOMP, which is controlled by the actions of members of the Bcl-2 family of proteins Bax and Bak. However, recent evidence identified two sphingolipids, especifically S1P and hexedecenal, as specific cofactors for Bax/Bak activation that lower the threshold for apoptosis-associated cytochrome c release [48]. The exposure of Bak and Bax to these sphingolipids requires the association of mitochondria with ER. These new data complement existing evidence of the interaction of ceramide and ganglioside GD3 with mitochondrial respiratory chain components as an important step in the committment of cell death. As an intrincate process regulated by complex molecular machinery cell death can be initiated by interactions of GSLs with different organelles, including mitochondria, ER or lysosomes. Therefore, by regulating the core of cell death GSLs play an important role in pathophysiology and disease. For instance, GSLs participate in cancer cell biology and metastasis, with involvement of predominant pathways regulating GSLs such as SMases, acid ceramidase, and ganglioside GD3. Given the function of ceramide as an effective cell death trigger recruiting diverse pathways and mechanisms, strategies that result in its accumulation may stand as an interesting approach to enhance the efficacy of current cancer therapy. Recent advances in nanotechnology have illustrated the feasibility of generating nanoliposomes that encapsulate hydrophobic compounds, like ceramide. In this regard, recent observations have shown that nanoliposomes containing ceramide C6 induced apoptosis and antagonize the Warburg effect in chronic lymphocytic leukemia and hepatocellular carcinoma cells [130, 131]. On the other hand, SMases are involved in a number of pathological processes and diseases. For instance, targeting ASMase may be of potential relevance in liver diseases due to its role in promoting hepatocellular apoptosis, liver fibrosis and steatohepatitis, [132] as well as Alzheimer’s disease [133]. In this regard, it has been recently described a novel mechanism of ASMase in Alzheimer’s disease involving impaired autophagy due to defective lysosomal biogenesis, indicating that partial ASMase inhibition may be of potential relevance in this devastating disease [134]. Thus, a further understanding of the cell biology of GSLs and their role in cell death may increase the opportunities of the impact of modulating GSLs metabolism in human diseases.
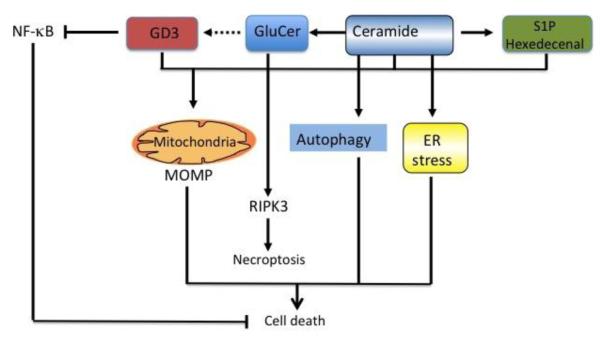
Figure 2. GSLs and mechanisms of cell death.
Schematic summary of predominant pathways of cell death caused by GSLs. GSLs induce apoptosis or necroptosis via interaction with mitochondria and activation of RIPK3, respectively. In addition, ceramide can induce ER stress and autophagy, further contributing to cell death. Besides the activation of these cell death pathways, some GSLs such as ganglioside GD3 inactivate survival pathways dependent on NF-κB activation.
ACKNOWLEDGMENTS
The work was supported by grants SAF-2011-23031, SAF-2012-34831 from Plan Nacional de I+D, Spain, the center grant P50-AA-11999 Research Center for Liver and Pancretic Diseases funded by NIAAA/NIH, a grant from Fundació Marató de TV3, La Mutua Madrileña, PI11/0325 (META) grant from the Instituto Salud Carlos III, and by the support of CIBEREHD.
REFERENCES
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 2.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 3.Chavez Jose a., Summers Scott a. A Ceramide-Centric View of Insulin Resistance. Cell Metabolism. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morales A, Lee H, Goñi F, Kolesnick R, Fernandez-Checa J. Sphingolipids and cell death. Apoptosis. 2007;12:923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 6.Mizutani Y, Kihara A, Chiba H, Tojo H, Igarashi Y. 2-Hydroxy ceramide synthesis by ceramide synthase family: enzymatic basis for the preference of FA chain length. J. Lipid Res. 2008;49:2356–2364. doi: 10.1194/jlr.M800158-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta. 2014;1841:671–681. doi: 10.1016/j.bbalip.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Progress in Lipid Research. 2012;51:50–62. doi: 10.1016/j.plipres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Pewzner-Jung Y, Brenner O, Braun S, Laviad EL, Ben-Dor S, Feldmesser E, Horn-Saban S, Amann-Zalcenstein D, Raanan C, Berkutzki T, Erez-Roman R, Ben-David O, Levy M, Holzman D, Park H, Nyska A, Merrill AH, Jr., Futerman AH. A Critical Role for Ceramide Synthase 2 in Liver Homeostasis: II. Insights into molecular changes leading to hepatopathy. J. Biol. Chem. 2010;285:10911–10923. doi: 10.1074/jbc.M109.077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Brönneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Blüher M, Krönke M, Brüning JC. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–86. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Öhman MK, Takeda K, Sugii S, Pewzner-Jung Y, Futerman AH, Summers SA. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–95. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J. Lipid Res. 2009:S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breslow DK, Weissman JS. Membranes in balance: Mechanisms of sphingolipid homeostasis. Mol. Cell. 2010;40:267–279. doi: 10.1016/j.molcel.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Invest. 2011;121:4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. Inhibition of Carnitine Palmitoyltransferase I Augments Sphingolipid Synthesis and Palmitate-induced Apoptosis. Journal of Biological Chemistry. 1997;272:3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 17.Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366–2373. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- 18.Watt MJ, Barnett AC, Bruce CR, Schenk S, Horowitz JF, Hoy AJ. Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia. 2012;55:2741–2746. doi: 10.1007/s00125-012-2649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, Weissman JS. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–1053. doi: 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roelants FM, Breslow DK, Muir A, Weissman JS, Thorner J. Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences. 2011;108:19222–19227. doi: 10.1073/pnas.1116948108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aronova S, Wedaman K, Aronov PA, Fontes K, Ramos K, Hammock BD, Powers T. Regulation of Ceramide Biosynthesis by TOR Complex 2. Cell Metabolism. 2008;7:148–158. doi: 10.1016/j.cmet.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickson RC. Thematic Review Series: Sphingolipids. New insights into sphingolipid metabolism and function in budding yeast. Journal of Lipid Research. 2008;49:909–921. doi: 10.1194/jlr.R800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, Walther TC, Loewith R. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat Cell Biol. 2012;14:542–547. doi: 10.1038/ncb2480. [DOI] [PubMed] [Google Scholar]
- 24.Siow DL, Wattenberg BW. Mammalian ORMDL proteins mediate the feedback response in ceramide biosynthesis. J Biol Chem. 2012;287:40198–204. doi: 10.1074/jbc.C112.404012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta SD, Gable K, Alexaki A, Chandris P, Proia RL, Dunn TM, Harmon JM. Expression of the ORMDLS, Modulators of Serine Palmitoyltransferase, is Regulated by Sphingolipids in Mammalian Cells. J Biol Chem. 2014 Nov 13; doi: 10.1074/jbc.M114.588236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canals D, Perry DM, Jenkins RW, Hannun YA. Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. British Journal of Pharmacology. 2011;163:694–712. doi: 10.1111/j.1476-5381.2011.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EL, Schuchman EH. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. The FASEB Journal. 2008;22:3419–3431. doi: 10.1096/fj.08-108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angulo S, Morales A, Danese S, Llacuna L, Masamunt MC, Pultz N, Cifone MG, De Simone C, Delgado S, Vila J, Panes J, Donskey C, Fernandez-Checa JC, Fiocchi C, Sans M. Probiotic sonicates selectively induce mucosal immune cells apoptosis through ceramide generation via neutral sphingomyelinase. PLoS One. 2011;6:e16953. doi: 10.1371/journal.pone.0016953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coll O, Morales A, Fernández-Checa JC, Garcia-Ruiz C. Neutral sphingomyelinase-induced ceramide triggers germinal vesicle breakdown and oxidant-dependent apoptosis in Xenopus laevis oocytes. Journal of Lipid Research. 2007;48:1924–1935. doi: 10.1194/jlr.M700069-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Marí M, Fernández-Checa JC. Sphingolipid signalling and liver diseases. Liver International. 2007;27:440–450. doi: 10.1111/j.1478-3231.2007.01475.x. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Ruiz C, Colell A, Mari M, Morales A, Calvo M, Enrich C, Fernandez-Checa JC. Defective TNF-α-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J. Clin. Invest. 2003;111:197–208. doi: 10.1172/JCI16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mari M, Colell A, Morales A, Paneda C, Varela-Nieto I, Garcia-Ruiz C, Fernandez-Checa JC. Acidic sphingomyelinase downregulates the liver-specific methionine adenosyltransferase 1A, contributing to tumor necrosis factor-induced lethal hepatitis. J. Clin. Invest. 2004;113:895–904. doi: 10.1172/JCI19852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin T, Genestier L, Pinkoski MJ, Castro A, Nicholas S, Mogil R, Paris F, Fuks Z, Schuchman EH, Kolesnick RN, Green DR. Role of Acidic Sphingomyelinase in Fas/CD95-mediated Cell Death. Journal of Biological Chemistry. 2000;275:8657–8663. doi: 10.1074/jbc.275.12.8657. [DOI] [PubMed] [Google Scholar]
- 34.Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, Lupescu A, Koka S, Eisele K, Klarl BA, Rubben H, Schmid KW, Mann K, Hildenbrand S, Hefter H, Huber SM, Wieder T, Erhardt A, Haussinger D, Gulbins E, Lang F. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med. 2007;13:164–170. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- 35.Fucho R, Martinez L, Baulies A, Torres S, Tarrats N, Fernandez A, Ribas V, Astudillo AM, Balsinde J, Garcia-Roves P, Elena M, Bergheim I, Lotersztajn S, Trautwein C, Appelqvist H, Paton AW, Paton JC, Czaja MJ, Kaplowitz N, Fernandez-Checa JC, Garcia-Ruiz C. Asmase Regulates Autophagy and Lysosomal Membrane Permeabilization and its Inhibition Prevents Early Stage Nonalcoholic Steatohepatitis. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez A, Matias N, Fucho R, Ribas V, Von Montfort C, Nuño N, Baulies A, Martinez L, Tarrats N, Mari M, Colell A, Morales A, Dubuquoy L, Mathurin P, Bataller R, Caballeria J, Elena M, Balsinde J, Kaplowitz N, Garcia-Ruiz C, Fernandez-Checa JC. ASMase is required for chronic alcohol induced hepatic endoplasmic reticulum stress and mitochondrial cholesterol loading. Journal of Hepatology. 2013;59:805–813. doi: 10.1016/j.jhep.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins RW, Idkowiak-Baldys J, Simbari F, Canals D, Roddy P, Riner CD, Clarke CJ, Hannun YA. A Novel Mechanism of Lysosomal Acid Sphingomyelinase Maturation: REQUIREMENT FOR CARBOXYL-TERMINAL PROTEOLYTIC PROCESSING. Journal of Biological Chemistry. 2011;286:3777–3788. doi: 10.1074/jbc.M110.155234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paris F, Grassmé H, Cremesti A, Zager J, Fong Y, Haimovitz-Friedman A, Fuks Z, Gulbins E, Kolesnick R. Natural Ceramide Reverses Fas Resistance of Acid Sphingomyelinase −/− Hepatocytes. Journal of Biological Chemistry. 2001;276:8297–8305. doi: 10.1074/jbc.M008732200. [DOI] [PubMed] [Google Scholar]
- 39.Llacuna L, Mari M, Garcia-Ruiz C, Fernandez-Checa JC, Morales A. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology (Hoboken, NJ, U. S.) 2006;44:561–572. doi: 10.1002/hep.21285. [DOI] [PubMed] [Google Scholar]
- 40.Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001;276:20589–96. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 41.Cremesti A, Paris F, Grassmé H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. J. Biol. Chem. 2001;276:23954–61. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- 42.Grassmé H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogen. 2003;22:5457–70. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- 43.Perrotta C, Bizzozero L, Cazzato D, Morlacchi S, Assi E, Simbari F, Zhang Y, Gulbins E, Bassi MT, Rosa P, Clementi E. Syntaxin 4 is required for acid sphingomyelinase activity and apoptotic function. J Biol. Chem. 2010;285:40240–51. doi: 10.1074/jbc.M110.139287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28:1043–54. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridgway ND. Interactions between metabolism and intracellular distribution of cholesterol and sphingomyelin. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2000;1484:129–141. doi: 10.1016/s1388-1981(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 46.Slotte JP. Sphingomyelin–cholesterol interactions in biological and model membranes. Chemistry and Physics of Lipids. 1999;102:13–27. doi: 10.1016/s0009-3084(99)00071-7. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Xu M, Pitzer A, Xia M, Boini K, Li P-L, Zhang Y. Control of autophagy maturation by acid sphingomyelinase in mouse coronary arterial smooth muscle cells: protective role in atherosclerosis. Journal of Molecular Medicine. 2014;92:473–485. doi: 10.1007/s00109-014-1120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alayoubi AM, Wang JC, Au BC, Carpentier S, Garcia V, Dworski S, El-Ghamrasni S, Kirouac KN, Exertier MJ, Xiong ZJ, Privé GG, Simonaro CM, Casas J, Fabrias G, Schuchman EH, Turner PV, Hakem R, Levade T, Medin JA. Systemic ceramide accumulation leads to severe and varied pathological consequences. EMBO Mol Me. 2013;5:827–42. doi: 10.1002/emmm.201202301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chipuk Jerry e., Mcstay Gavin p., Bharti A, Kuwana T, Clarke Christopher j., Siskind Leah j., Obeid Lina m., Green Douglas r. Sphingolipid Metabolism Cooperates with BAK and BAX to Promote the Mitochondrial Pathway of Apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SH, Seo GS, Park P-H, Choi J-Y, Park YN, Kim HK, Chae K-S, Sohn DH. Increased expression of O-acetyl disialoganglioside synthase during rat liver fibrogenesis relates to stellate cell activation. Biochem. Biophys. Res. Commun. 2003;303:954–961. doi: 10.1016/s0006-291x(03)00448-0. [DOI] [PubMed] [Google Scholar]
- 51.Huitema K, Van Den Dikkenberg J, Brouwers JFHM, Holthuis JCM. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vacaru AM, Tafesse FG, Ternes P, Kondylis V, Hermansson M, Brouwers JFHM, Somerharju P, Rabouille C, Holthuis JCM. Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER. J. Cell Biol. 2009;185:1013–1027. doi: 10.1083/jcb.200903152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D'angelo G, Polishchuk E, Tullio GD, Santoro M, Campli AD, Godi A, West G, Bielawski J, Chuang C-C, Van Der Spoel AC, Platt FM, Hannun YA, Polishchuk R, Mattjus P, De Matteis MA. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 54.Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2008;1781:424–434. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassler DF, Bell RM. Ceramidases: Enzymology and metabolic roles. Adv. Lipid Res. 1993;26:49–57. [PubMed] [Google Scholar]
- 56.Franzen R, Pautz A, Bräutigam L, Geisslinger G, Pfeilschifter J, Huwiler A. Interleukin-1β Induces Chronic Activation and de Novo Synthesis of Neutral Ceramidase in Renal Mesangial Cells. Journal of Biological Chemistry. 2001;276:35382–35389. doi: 10.1074/jbc.M102153200. [DOI] [PubMed] [Google Scholar]
- 57.Ramirez De Molina A, De La Cueva A, Machado-Pinilla R, Rodriguez-Fanjul V, Gomez Del Pulgar T, Cebrian A, Perona R, Lacal JC. Acid ceramidase as a chemotherapeutic target to overcome resistance to the antitumoral effect of choline kinase α inhibition. Curr. Cancer Drug Targets. 2012;12:617–624. doi: 10.2174/156800912801784811. [DOI] [PubMed] [Google Scholar]
- 58.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind JS, Spiegel S. Suppression of ceramide-mediated programmed cell death. 1996. [DOI] [PubMed]
- 59.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 60.Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 1997;3:1228–1232. doi: 10.1038/nm1197-1228. [DOI] [PubMed] [Google Scholar]
- 61.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 62.Cingolani F, Casasampere M, Sanllehi P, Casas J, Bujons J, Fabrias G. Inhibition of dihydroceramide desaturase activity by the sphingosine kinase inhibitor SKI II. Journal of Lipid Research. 2014 doi: 10.1194/jlr.M049759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Veldhoven PP. Sphingosine-1-phosphate lyase. Methods Enzymol. 2000;311:244–254. doi: 10.1016/s0076-6879(00)11087-0. [DOI] [PubMed] [Google Scholar]
- 64.Le Stunff H, Giussani P, Maceyka M, Lépine S, Milstien S, Spiegel S. Recycling of Sphingosine Is Regulated by the Concerted Actions of Sphingosine-1-phosphate Phosphohydrolase 1 and Sphingosine Kinase 2. Journal of Biological Chemistry. 2007;282:34372–34380. doi: 10.1074/jbc.M703329200. [DOI] [PubMed] [Google Scholar]
- 65.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular Cloning and Functional Characterization of a Novel Mammalian Sphingosine Kinase Type 2 Isoform. Journal of Biological Chemistry. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 66.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 67.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 68.García-Ruiz C, Colell A, Marí M, Morales A, Fernández-Checa JC. Direct Effect of Ceramide on the Mitochondrial Electron Transport Chain Leads to Generation of Reactive Oxygen Species: ROLE OF MITOCHONDRIAL GLUTATHIONE. Journal of Biological Chemistry. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 69.Gudz TI, Tserng K-Y, Hoppel CL. Direct Inhibition of Mitochondrial Respiratory Chain Complex III by Cell-permeable Ceramide. Journal of Biological Chemistry. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 70.Dai Q, Liu J, Chen J, Durrant D, Mcintyre TM, Lee RM. Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin. Oncogene. 2004;23:3650–3658. doi: 10.1038/sj.onc.1207430. [DOI] [PubMed] [Google Scholar]
- 71.Birbes H, Luberto C, Hsu Y-T, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem. J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. Molecular Cloning and Characterization of a Human Mitochondrial Ceramidase. Journal of Biological Chemistry. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 73.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem. J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Maria R, Rippo MR, Schuchman EH, Testi R. Acidic sphingomyelinase (ASM) is necessary for Fas-induced GD3 ganglioside-accumulation and efficient apoptosis of lymphoid cells. J. Exp. Med. 1998;187:897–902. doi: 10.1084/jem.187.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.García-Ruiz C, Colell A, París R, Fernández-Checa JC. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. The FASEB Journal. 2000;14:847–858. doi: 10.1096/fasebj.14.7.847. [DOI] [PubMed] [Google Scholar]
- 76.Kristal BS, Brown AM. Apoptogenic Ganglioside GD3 Directly Induces the Mitochondrial Permeability Transition. Journal of Biological Chemistry. 1999;274:23169–23175. doi: 10.1074/jbc.274.33.23169. [DOI] [PubMed] [Google Scholar]
- 77.Rippo MR, Malisan F, Ravagnan L, Tomassini B, Condo I, Costantini P, Susin SA, Rufini A, Todaro M, Kroemer G, Testi R. GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion. The FASEB Journal. 2000;14:2047–2054. doi: 10.1096/fj.99-1028com. [DOI] [PubMed] [Google Scholar]
- 78.García-Ruiz C, Colell A, Morales A, Calvo MA, Enrich C, Fernández-Checa JC. Trafficking of Ganglioside GD3 to Mitochondria by Tumor Necrosis Factor-α. Journal of Biological Chemistry. 2002;277:36443–36448. doi: 10.1074/jbc.M206021200. [DOI] [PubMed] [Google Scholar]
- 79.Brenner C, Kniep B, Maillier E, Martel C, Franke C, Röber N, Bachmann M, Rieber EP, Sandhoff R. GD3–7-aldehyde is an apoptosis inducer and interacts with adenine nucleotide translocase. Biochemical and Biophysical Research Communications. 2010;391:248–253. doi: 10.1016/j.bbrc.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 80.Sorice M, Mattei V, Matarrese P, Garofalo T, Tinari A, Gambardella L, Ciarlo L, Manganelli V, Tasciotti V, Misasi R, Malorni W. Dynamics of mitochondrial raft-like microdomains in cell life and death. Commun Integr Biol. 2012;5:217–9. doi: 10.4161/cib.19145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee H, Rotolo JA, Mesicek J, Penate-Medina T, Rimner A, Liao W-C, Yin X, Ragupathi G, Ehleiter D, Gulbins E, Zhai D, Reed JC, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS One. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ciarlo L, Manganelli V, Garofalo T, Matarrese P, Tinari A, Misasi R, Malorni W, Sorice M. Association of fission proteins with mitochondrial raft-like domains. Cell Death Differ. 2010;17:1047–58. doi: 10.1038/cdd.2009.208. [DOI] [PubMed] [Google Scholar]
- 83.Galluzzi L, Bravo-San Pedro JM, Kroemer G. Organelle-specific initiation of cell death. Nat Cell Biol. 2014;16:728–36. doi: 10.1038/ncb3005. [DOI] [PubMed] [Google Scholar]
- 84.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An Integrated Stress Response Regulates Amino Acid Metabolism and Resistance to Oxidative Stress. Molecular Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 85.Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 86.Lloyd-Evans E, Pelled D, Riebeling C, Bodennec J, De-Morgan A, Waller H, Schiffmann R, Futerman AH. Glucosylceramide and Glucosylsphingosine Modulate Calcium Mobilization from Brain Microsomes via Different Mechanisms. Journal of Biological Chemistry. 2003;278:23594–23599. doi: 10.1074/jbc.M300212200. [DOI] [PubMed] [Google Scholar]
- 87.Pelled D, Lloyd-Evans E, Riebeling C, Jeyakumar M, Platt FM, Futerman AH. Inhibition of Calcium Uptake via the Sarco/Endoplasmic Reticulum Ca2+-ATPase in a Mouse Model of Sandhoff Disease and Prevention by Treatment with N-Butyldeoxynojirimycin. Journal of Biological Chemistry. 2003;278:29496–29501. doi: 10.1074/jbc.M302964200. [DOI] [PubMed] [Google Scholar]
- 88.Tessitore A, Del P. Martin M, Sano R, Ma Y, Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM, D'azzo A. GM1-Ganglioside-Mediated Activation of the Unfolded Protein Response Causes Neuronal Death in a Neurodegenerative Gangliosidosis. Molecular Cell. 2004;15:753–766. doi: 10.1016/j.molcel.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 89.Carracedo A, Lorente M, Egia A, Blázquez C, García S, Giroux V, Malicet C, Villuendas R, Gironella M, González-Feria L, Piris MÁ, Iovanna JL, Guzmán M, Velasco G. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006;9:301–312. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Boslem E, Weir JM, Macintosh G, Sue N, Cantley J, Meikie PJ, Biden TJ. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic β-cells. J Biol Chem. 2013;288:26569–82. doi: 10.1074/jbc.M113.489310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gozuacik D, Kimchi A. Autophagy and cell death. Curr. Top. Dev. Biol. 2007;78:217–245. doi: 10.1016/S0070-2153(06)78006-1. [DOI] [PubMed] [Google Scholar]
- 92.Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, Lenardo M. Autophagic programmed cell death by selective catalase degradation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Y, Li S, Qin X, Hou W, Dong h, Yao L, Xiong L. The pleiotropic roles of sphingolipid signaling in autophagy. Cell Death Disease. 2014;5:e1245. doi: 10.1038/cddis.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schubert KM, Scheid MP, Duronio V. Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J. Biol. Chem. 2000;275:13330–13335. doi: 10.1074/jbc.275.18.13330. [DOI] [PubMed] [Google Scholar]
- 95.Edinger AL. Starvation in the midst of plenty: making sense of ceramide-induced autophagy by analyzing nutrient transporter expression. Biochem. Soc. Trans. 2009;37:253–258. doi: 10.1042/BST0370253. [DOI] [PubMed] [Google Scholar]
- 96.Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17402–17407. doi: 10.1073/pnas.0802781105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, Ghidoni R, Codogno P. Ceramide-mediated Macroautophagy Involves Inhibition of Protein Kinase B and Up-regulation of Beclin 1. Journal of Biological Chemistry. 2004;279:18384–18391. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- 98.Young MM, Kester M, Wang H-G. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. Journal of Lipid Research. 2013;54:5–19. doi: 10.1194/jlr.R031278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spassieva SD, Mullen TD, Townsend DM, Obeid LM. Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response. Biochemical Journal. 2009;424:273–283. doi: 10.1042/BJ20090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, Cowart LA. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Invest. 2012;122:3919–3930. doi: 10.1172/JCI63888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beauchamp E, Goenaga D, Le Bloch J, Catheline D, Legrand P, Rioux V. Myristic acid increases the activity of dihydroceramide Delta4-desaturase 1 through its N-terminal myristoylation. Biochimie. 2007;89:1553–61. doi: 10.1016/j.biochi.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 102.Jiang W, Ogretmen B. Autophagy paradox and ceramide. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2014;1841:783–792. doi: 10.1016/j.bbalip.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, Graf M, Rahmani M, Ryan K, Liu X, Spiegel S, Norris J, Fisher PB, Grant S, Dent P. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol. Ther. 2008;7:1648–1662. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li DD, Wang LL, Deng R, Tang J, Shen Y, Guo JF, Wang Y, Xia LP, Feng GK, Liu QQ, Huang WL, Zeng YX, Zhu XF. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 2009;28:886–98. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- 105.Zeidan YH, Jenkins RW, Hannun YA. Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. J Cell Biol. 2008;181:335–50. doi: 10.1083/jcb.200705060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matarrese P, Garofalo T, Manganelli V, Gambardella L, Marconi M, Grasso M, Tinari A, Misasi R, Malorni W, Sorice M. Evidence for the involvement of GD3 ganglioside in autophagosome formation and maturation. Autophagy. 2014;10:750–65. doi: 10.4161/auto.27959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hwang J, Lee S, Lee JT, Kwon TK, Kim DR, Kim H, Park HC, Suk K. Gangliosides induce autophagic cell death in astrocytes. Br. J. Pharmaco. 2010;159:586–603. doi: 10.1111/j.1476-5381.2009.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li ZZ, Berk M, Mcintyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology (Hoboken, NJ, U. S.) 2008;47:1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petersen Nikolaj h. T., Olsen Ole d., Groth-Pedersen L, Ellegaard A-M, Bilgin M, Redmer S, Ostenfeld Marie s., Ulanet D, Dovmark Tobias h., Lønborg A, Vindeløv Signe d., Hanahan D, Arenz C, Ejsing Christer s., Kirkegaard T, Rohde M, Nylandsted J, Jäättelä M. Transformation-Associated Changes in Sphingolipid Metabolism Sensitize Cells to Lysosomal Cell Death Induced by Inhibitors of Acid Sphingomyelinase. Cancer Cell. 2013;24:379–393. doi: 10.1016/j.ccr.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 110.Appelqvist H, Nilsson C, Garner B, Brown AJ, Kågedal K, Öllinger K. Attenuation of the Lysosomal Death Pathway by Lysosomal Cholesterol Accumulation. The American Journal of Pathology. 2011;178:629–639. doi: 10.1016/j.ajpath.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ullio C, Casas J, Brunk UT, Sala G, Fabriàs G, Ghidoni R, Bonelli G, Baccino FM, Autelli R. Sphingosine mediates TNFα-induced lysosomal membrane permeabilization and ensuing programmed cell death in hepatoma cells. Journal of Lipid Research. 2012;53:1134–1143. doi: 10.1194/jlr.M022384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Villamil Giraldo AM, Appelqvist H, Ederth T, Öllinger K. Lysosomotropic agents: impact on lysosomal membrane permeabilization and cell death. Biochem Soc Tran. 2014;42:1460–4. doi: 10.1042/BST20140145. [DOI] [PubMed] [Google Scholar]
- 113.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–70. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 114.Doi TS, Takahshi T, Taguchi O, Azuma T, Obata Y. NF-kappa B RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J Exp Med. 1997;185:953–61. doi: 10.1084/jem.185.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schütze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–76. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 116.Dbaibo GS, Obeid LM, Hannun YA. Tumor necrosis factor-alpha (TNF-alpha) signal transduction through ceramide. Dissociation of growth inhibitory effects of TNF-alpha from activation of nuclear factor-kappa B. J Biol Chem. 1993;268:17762–6. [PubMed] [Google Scholar]
- 117.Betts JC, Agranoff AB, Nabel GJ, Shayman JA. Dissociation of endogenous cellular ceramide from NF-kappa B activation. J Biol Chem. 1994;269:8455–8. [PubMed] [Google Scholar]
- 118.Colell A, García-Ruiz C, Roman J, Ballesta A, Fernández-Checa JC. Ganglioside GD3 enhances apoptosis by suppressing the nuclear factor-kB-dependent survival pathway. The FASEB Journal. 2001 doi: 10.1096/fj.00-0574fje. [DOI] [PubMed] [Google Scholar]
- 119.Paris R, Morales A, Coll O, Sánchez-Reyes A, García-Ruiz C, Fernández-Checa JC. Ganglioside GD3 Sensitizes Human Hepatoma Cells to Cancer Therapy. Journal of Biological Chemistry. 2002;277:49870–49876. doi: 10.1074/jbc.M208303200. [DOI] [PubMed] [Google Scholar]
- 120.Malisan F, Franchi L, Tomassini B, Ventura N, Condo I, Rippo MR, Rufini A, Liberati L, Nachtigall C, Kniep B, Testi R. Acetylation. 2002 doi: 10.1084/jem.20020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mukherjee K, Chava AK, Mandal C, Dey SN, Kniep B, Chandra S, Mandal C. O-acetylation of GD3 prevents its apoptotic effect and promotes survival of lymphoblasts in childhood acute lymphoblastic leukaemia. J. Cell. Biochem. 2008;105:724–734. doi: 10.1002/jcb.21867. [DOI] [PubMed] [Google Scholar]
- 122.Birks SM, Danquah JO, King L, Vlasak R, Gorecki DC, Pilkington GJ. Targeting the GD3 acetylation pathway selectively induces apoptosis in glioblastoma. Neuro-Oncology. 2011;13:950–960. doi: 10.1093/neuonc/nor108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cazet A, Groux-Degroote S, Teylaert B, Kwon K-M, Lehoux S, Slomianny C, Kim C-H, Le Bourhis X, Delannoy P. GD3 synthase overexpression enhances proliferation and migration of MDA-MB-231 breast cancer cells. Biological Chemistry. 2009;390:601–9. doi: 10.1515/BC.2009.054. [DOI] [PubMed] [Google Scholar]
- 124.Lluis JM, Llacuna L, Von MC, Barcena C, Enrich C, Morales A, Fernandez-Checa JC. GD3 synthase overexpression sensitizes hepatocarcinoma cells to hypoxia and reduces tumor growth by suppressing the cSrc/NF-kappaB survival pathway. PLoS One. 2009;4:e8059. doi: 10.1371/journal.pone.0008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen HY, Challa AK, Varki A. 9-O-Acetylation of Exogenously Added Ganglioside GD3: THE GD3 MOLECULE INDUCES ITS OWN O ACETYLATION MACHINERY. Journal of Biological Chemistry. 2006;281:7825–7833. doi: 10.1074/jbc.M512379200. [DOI] [PubMed] [Google Scholar]
- 126.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 127.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–6. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 128.Platt FM. Sphingolipids lysosomal storage disorders. Nature. 2014;510:68–75. doi: 10.1038/nature13476. [DOI] [PubMed] [Google Scholar]
- 129.Vitner EB, Salomon R, Farfel-Becker T, Meshcheriakova A, Ali M, Klein AD, Platt FM, Cox TM, Futerman AH. RIPK3 as a potential therapeutic target for Gaucher's disease. Nat Med. 2014;20:204–8. doi: 10.1038/nm.3449. [DOI] [PubMed] [Google Scholar]
- 130.Ryland LK, Fox TE, Liu X, Loughran TP, Kester M. Dysregulation of sphingolipid metabolism in cancer. Cancer Biol. Ther. 2011;11:138–149. doi: 10.4161/cbt.11.2.14624. [DOI] [PubMed] [Google Scholar]
- 131.Tagaram HRS, Divittore NA, Barth BM, Kaiser JM, Avella D, Kimchi ET, Jiang Y, Isom HC, Kester M, Staveley-O'carroll KF. Nanoliposomal ceramide prevents in vivo growth of hepatocellular carcinoma. Gut. 2011;60:695–701. doi: 10.1136/gut.2010.216671. [DOI] [PubMed] [Google Scholar]
- 132.Garcia-Ruiz C, Mato JM, Vance D, Kaplowitz N, Fernández-Checa JC. Acid sphingomyelinase-ceramide system in steatohepatitis: a novel target regulating multiple pathways. JHepatol. 2014 Oct 1; doi: 10.1016/j.jhep.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 133.Grimm MO, Zimmwe VC, Lehmann J, Grimm H, Hartmann T. The impact of cholesterol, DHA, and sphingolipids on Alzheimer's disease. Biomed Res Int. 2013:814390. doi: 10.1155/2013/814390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee JK, Jin HK, Park MH, Kim BR, Lee PH, Nakauchi H, Carter JE, He X, Schuchman EH, Bae JS. Acid sphingomyelinase modulates the autophagic process by controlling lysosomal biogenesis in Alzheimer's disease. J Exp Med. 2014;211:1551–70. doi: 10.1084/jem.20132451. [DOI] [PMC free article] [PubMed] [Google Scholar]