Abstract
The adult hippocampus generates functional dentate granule cells (GCs) that release glutamate onto target cells in the hilus and cornus ammonis (CA)3 region, and receive glutamatergic and γ-aminobutyric acid (GABA)ergic inputs that tightly control their spiking activity. The slow and sequential development of their excitatory and inhibitory inputs makes them particularly relevant for information processing. Although they are still immature, new neurons are recruited by afferent activity and display increased excitability, enhanced activity-dependent plasticity of their input and output connections, and a high rate of synaptogenesis. Once fully mature, new GCs show all the hallmarks of neurons generated during development. In this review, we focus on how developing neurons remodel the adult dentate gyrus and discuss key aspects that illustrate the potential of neurogenesis as a mechanism for circuit plasticity and function.
A new granule cell developing in the adult hippocampus faces the challenge of properly integrating into an established network. The process takes a few weeks and is characterized by several distinct stages.
Why is there neurogenesis in the dentate gyrus of the adult hippocampus and not in many other cortical regions of the mammalian brain with a high demand for plasticity? There are two sides to this question. One is the mechanistic aspect, which relates to understanding which cellular and molecular substrates allow the subgranular zone of the dentate gyrus to act as a discrete neurogenic niche in which adult neural stem cells can give rise to neurons, whereas other areas cannot support neurogenesis under physiological (nonpathological) conditions. These topics are discussed in depth in the literature (Beckervordersandforth et al. 2015; Choe et al. 2015; Götz et al. 2015; Kuhn 2015). The other side to this question focuses on how neurogenesis might influence hippocampal function, taking into account the function of the dentate gyrus as a whole, the properties of the network in which newly generated neurons are incorporated, and the function these new neurons might undertake.
Evidence accumulated over the last two decades has shown that dentate granule cells (GCs) are the only neuronal type generated in the adult hippocampus. A new GC developing in the adult hippocampus faces the challenge of properly integrating in a complex network and processing information with functional relevance. Neurogenesis begins when radial glia-like (RGL) neural stem cells of the subgranular zone exit the quiescent stage and become amplifying neural progenitor cells that finally divide to adopt a neuronal fate. There are several hallmarks that can be distinguished as almost discrete events of neuronal maturation: establishment of neuronal polarity and migration, γ-aminobutyric acid (GABA)ergic synapse formation onto apical dendrites, glutamatergic synaptogenesis, circuit integration, followed by the final steps of maturation and refinement. Completing this process in the adult mammalian brain requires several weeks. In this article, we will go in depth into this process to understand how neuronal development provides a unique mechanism of plasticity that will ultimately determine the influence of adult-born neurons on information processing in the hippocampus.
STRUCTURE AND CIRCUITS OF THE DENTATE GYRUS
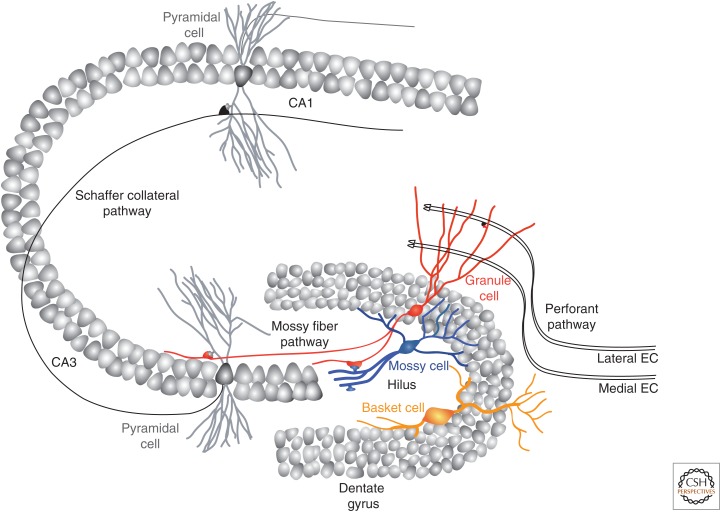
The dentate gyrus forms a V-shaped structure embedded into the curved cornus ammonis (CA), which itself is composed of CA3, CA2, and CA1 regions of the hippocampus (Fig. 1). It can be subdivided into three layers. The GC layer (GCL) is the middle stratum, formed by six to eight layers of densely packed cell bodies of GCs, the principal neurons of the dentate gyrus. The GCL is divided into the suprapyramidal and infrapyramidal blades, reflecting the portions of the layer located above or below the CA3 region, and the tip of the V-shaped structure is known as the apex or the crest (Amaral et al. 2007). GCs display an inverted cone-shaped tree of spiny apical dendrites that project through the “stratum moleculare” (molecular layer), the most superficial layer of the dentate gyrus. This layer mainly contains axons of the perforant pathway that originate from the entorhinal cortex and form synaptic contacts with dendrites of GCs. It also contains GABAergic interneurons and afferent fibers of inputs extrinsic to the dentate gyrus. The innermost layer is the hilus, also called polymorphic layer, which contains the axons of GCs as well as GABAergic and glutamatergic interneurons, the most abundant of which are the mossy cells. The border between the GCL and the hilus is the subgranular zone, a region in which adult neurogenesis occurs, which contains neural stem cells and progenitor cells, as well as the bodies of GABAergic basket cells.
Figure 1.
Circuits of the hippocampus. Schematic view of a transversal slice through the hippocampus depicting the dentate gyrus, cornus ammonis (CA)3, and CA1. The principal cells located in densely packed layers are synaptically connected via the so-called trisynaptic circuit. The main entry site for this circuit is the perforant path, composed of axons originating from lateral and medial entorhinal cortex (EC) and contacting the outer and medial molecular layer, respectively. Granule neurons then project mossy fibers to CA3 pyramidal neurons, which then project Schaffer collaterals to CA1 pyramidal neurons. Other input to granule neurons include interneurons located in the molecular layer and in the hilus, principally composed of basket and mossy cells.
Input Pathways
The dentate gyrus is the main entry point of the classical trisynaptic hippocampal network, which comprises a lamellar set of unidirectional connections connecting the entorhinal cortex to the dentate gyrus, the dentate gyrus to the CA3, the CA3 to the CA1, and the CA1 to the entorhinal cortex. It should be kept in mind, however, that the so-called trisynaptic network is not entirely linear, because the perforant pathway also impinges directly onto CA3 pyramidal cells, thus bypassing the dentate gyrus. Inputs to the dentate gyrus bring sensory information from the cortex that will ultimately lead to the production of episodic memories. The major excitatory/glutamatergic input to the dentate gyrus enters through the perforant pathway from the entorhinal cortex. Distinct areas of the entorhinal cortex project toward and innervate different dendritic portions of GCs. The medial entorhinal cortex (layer II, with some contribution of layers III, V, and VI) targets the middle third of the dendritic tree of GCs, whereas projections from the lateral entorhinal cortex contact the outer third of those dendrites (van Strien et al. 2009). GC dendrites of the inner third of the molecular layer are mostly innervated by associational and commissural fibers that arise from glutamatergic mossy cells of the ipsilateral and contralateral hilus (Frotscher et al. 1991; Buckmaster et al. 1992). Because presynaptic glutamatergic terminals contact postsynaptic dendritic spines, the number of spines may serve as an estimate of the total number of glutamatergic inputs of a given neuron. GCs have a total dendritic tree length of between 2800 to 3500 µm, depending on whether they are located in the infra- or suprapyramidal blades, and a dendritic spine density of ∼2 spines/µm (as evaluated by confocal microscopy). Thus, there are ∼6000 to 7000 glutamatergic terminals impinging onto each GC (Desmond and Levy 1985; Claiborne et al. 1990).
GCs also receive inputs from several types of GABAergic interneurons (Amaral 1978; Han et al. 1993; Freund and Buzsaki 1996; Houser 2007; Hosp et al. 2013). These include the basket cells that are located in the subgranular zone and contact primarily the cell bodies and axon initial segments of GCs, as well as their proximal dendrites in the molecular layer. In addition to releasing GABA, they also express the calcium-binding protein parvalbumin and are named after the basket-shaped axonal plexus they form around the cell bodies of GCs. Two main types of GABAergic interneurons reside in the hilus. The hilar perforant path-associated (HIPP) cells, some of which express the peptide somatostatin) that project to the outer two-thirds of the molecular layer, and the hilar commissural-associational pathway-related (HICAP) cells that project to the inner third of the molecular layer. In the molecular layer, molecular layer perforant path-associated (MOPP) cells project to the outer two thirds of the molecular layer, whereas the axo-axonic cells innervate the initial segments of granule neurons axons.
The dentate gyrus also receives extrinsic input from structures lying outside the hippocampus. The presubiculum and the parasubiculum project glutamatergic fibers to the second third of the molecular layer. The septal nuclei (mainly the medial septal nucleus and the diagonal band of Broca) project GABAergic fibers to the hilus and cholinergic fibers to the inner third of the molecular layer. The supramammillary area projects glutamatergic fibers (some of which also colocalize with substance P) to the most proximal dendritic portions of GCs. The locus coeruleus also projects noradrenergic fibers to the dentate gyrus, mainly into the hilus. Dopaminergic projections from the ventral tegmental area also innervate the hilus, as do the serotonergic projections from the raphe nucleus, in addition to their innervation of the subgranular zone.
Output Pathways
GCs project their axons (mossy fibers) through the stratum lucidum, across the hilus, reaching the CA3 region and contact pyramidal cells with their characteristically large and irregularly shaped mossy fiber terminals. Mossy fiber synapses are sparse; each CA3 pyramidal cell is contacted by fewer than 50 GCs (Amaral et al. 1990). However, mossy fiber terminals are very effective at activating their pyramidal target cells (Henze et al. 2002). Mossy fibers also have collaterals (∼6–7 per fiber) that innervate mossy cells and GABAergic interneurons through thin terminals (Acsady et al. 1998). Each GC possesses ∼160 to 200 axonal varicosities along its collaterals and ∼20 mossy fiber terminals. Of these, the mossy fiber–GABA interneuron–CA3 pyramidal cell disynaptic circuit underlies a potent feedforward inhibition that regulates the excitability of the CA3 area (Torborg et al. 2010). Individual mossy fiber terminals are morphologically distinct depending on the nature of the target cell, but also display distinct functional features in terms of transmission and plasticity (McBain 2008).
DEVELOPMENT AND MATURATION OF ADULT-BORN DENTATE GCs
Methods Used to Investigate the Maturation of Adult-Born Dentate GCs
Over the past decade, the functional properties of newly generated GCs have been mostly investigated using retroviral-labeling techniques combined with electrophysiological recordings performed in acute slices (van Praag et al. 2002). The most commonly used oncoretroviruses, derived from the Moloney murine leukemia virus, can be delivered directly into the dentate gyrus of anesthetized adult rodents through stereotaxic injection, and transduce dividing progenitor cells (Tashiro et al. 2006). Retroviruses can only enter the nucleus and integrate into the host genome during the prophase–prometaphase transition, when the nuclear envelope breaks down and, therefore, they effectively transduce fast-dividing neural progenitor cells. Fluorescent reporters and other transgenes of interest can be thus readily expressed in the progeny of neural progenitor cells. Expression of fluorescent reporters in the progeny of neural stem cells has enabled the characterization of the development, maturation, and integration of adult-generated GCs at the morphological and functional levels (Ge et al. 2006; Laplagne et al. 2006; Zhao et al. 2006; Toni et al. 2007).
Other methods, such as fluorescent reporters expressed under specific promoters in transgenic mice (such as the pro-opiomelanocortin [POMC]-green fluorescent protein [GFP] or the doublecortin [DCX]–GFP mice), have also contributed substantially to understanding the structure and function of newborn GCs (Couillard-Despres et al. 2006; Overstreet-Wadiche et al. 2006a). These methods allow GCs to be readily identified under fluorescence microscopy in live or chemically fixed tissue. Confocal and electron microscopy in chemically fixed sections has been used extensively to characterize the expression of early and late neuronal markers, and also to create complete morphological reconstructions of GCs at different developmental stages. Whole-cell patch clamp recordings have been used to describe the physiology of new neurons, from basic membrane properties to synaptic inputs and outputs throughout development. Used alone or in combination, these methods enabled great advances in our understanding of the maturation of adult-born GCs and their integration into the hippocampal circuit.
The Subgranular Zone and the Transition from Neural Stem Cell to Postmitotic Neuron
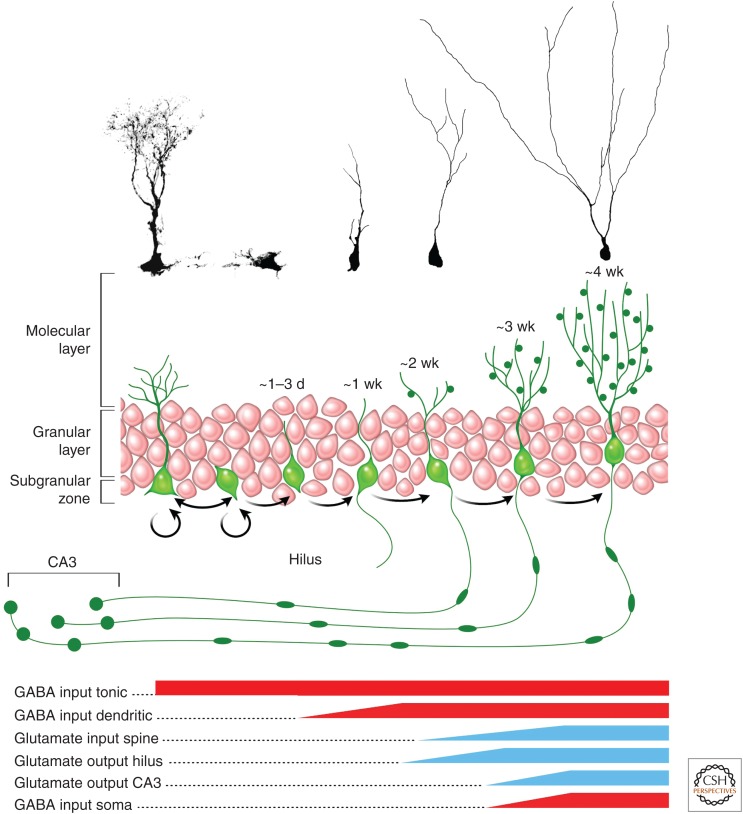
Adult-born GCs originate from neural stem cells that are located in the subgranular zone. This occurs through several intermediate steps, which can be characterized using marker expression (Kronenberg et al. 2003) and clonal analysis (Fig. 2) (Bonaguidi et al. 2011). Neural stem cells have a radial morphology, with an apical process extending through the GCL and branching into the molecular layer. They are often referred to as RGL cells or type 1 cells and express markers, such as nestin, sox2, and glial fibrillary acidic protein (GFAP). RGL cells rarely divide, but when they do, they self-renew and give rise to intermediate progenitor cells (IPCs) (Bonaguidi et al. 2012). IPCs are also located in the subgranular zone, have short processes, and proliferate rapidly. IPCs include type 2a cells that still express nestin and sox2, and type 2b cells that express tbr2 but not nestin. Type 2 cells then give rise to type 3 cells, which no longer express tbr2, but instead express the immature neuronal markers DCX and Prox1. They have a short vertical process and are already committed to the neuronal lineage. Finally, type 3 cells differentiate into GCs, which, on maturation, express the neuronal marker calbindin, extend an axon toward the CA3 region of the hippocampus and dendrites into the molecular layer.
Figure 2.
Developmental stages of adult-born granule cells (GCs). Schematic representation and confocal micrographs of the different stages of maturation, from stem cell to mature neuron. The bottom panel indicates the approximate timeline of the major input and output (based on data in Toni and Sultan 2011). CA, Cornus ammonis; GABA, γ-aminobutyric acid.
The functional integration of newborn neurons is not expected to have functional consequences for the hippocampal network before the formation of the first output synapses, which occurs by the end of the second week after division. However, at earlier time points in the development of newborn neurons, synaptic-like inputs play a significant role; indeed, even RGL cells express GABA and glutamate receptors (Tozuka et al. 2005; Wang et al. 2005). Although synaptic currents are absent from RGL cells, GABAergic axon terminals from parvalbumin-expressing neurons are found in close proximity, and their activity induces a tonic response in RGL cells, leading to their quiescence. Single-cell deletion of the γ2 subunit of the GABA receptor induces RGL cells to rapidly exit quiescence and begin symmetrical division. Conversely, optogenetic stimulation of parvalbumin axons reduces their rate of division (Song et al. 2012). Later in the developmental process, IPCs, although still dividing, receive immature GABAergic input from parvalbumin interneurons that can quickly mature on intense neuronal activity and promote the cell survival (Song et al. 2012). Thus, at these early stages, synaptic input couples network activity to the regulation of progenitor/stem-cell proliferation.
Early Postmitotic Neurons
GCs born in the adult hippocampus follow a pattern of morphological and functional maturation that closely resembles what occurs in new neurons during embryonic development, although it occurs at a much slower pace (Jones et al. 2003; Espósito et al. 2005; Overstreet-Wadiche et al. 2006a). During the first few days, postmitotic neurons remain in the proliferative (subgranular) zone and begin to display a polarized shape, with neurites that begin to extend in a bipolar fashion parallel to the GCL. These young neurons display very high input resistance (several gigaohms), because of the low density of K+ channels in the plasma membrane, and express early neuronal markers, such as the microtubule-associated protein DCX and polysialic acid neural cell-adhesion molecule (PSA-NCAM) (Schmidt-Hieber et al. 2004; Espósito et al. 2005; Ge et al. 2006). Although they lack synaptic inputs, they do express GABAA and glutamate (both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA] and N-methyl-d-aspartate [NMDA]) receptors as well as voltage-dependent Na+ and K+ channels at a low density. Thus, depolarizing steps elicit immature action potentials (single spikes with small amplitude and long duration) in current clamp recordings.
Similar to what occurs in the developing brain, GABAA-mediated currents in immature GCs of the adult dentate gyrus are depolarizing because of the reverse Cl− gradient (Ben Ari 2002; Overstreet-Wadiche et al. 2005; Tozuka et al. 2005). Intracellular Cl− regulation in neurons depends primarily on the balance between the activity of the Na+/K+/Cl− cotransporter NKCC1 that drives Cl− influx, and the Cl−/K+ cotransporter KCC2 that drives Cl− extrusion (Blaesse et al. 2009). Although the expression of NKCC1 in immature neurons is high, KCC2 levels are low, leading to a high intracellular Cl− concentration and, in turn, a depolarized equilibrium potential for Cl−. Thus, GABAA receptor activation in young neurons (including immature adult-born GCs) induces Cl− efflux with the consequent membrane depolarization, which also triggers Ca2+ influx through voltage-gated Ca2+ channels. As neurons mature, KCC2 levels increase and GABA becomes hyperpolarizing. In adult-born GCs, this transition seems to occur throughout the initial 4 wk of development, such that by the fourth week GABA becomes hyperpolarizing (Ge et al. 2006; Karten et al. 2006), acting as a bona fide inhibitory transmitter. The depolarizing effects of GABA are required for proper maturation and synaptic integration of new GCs (Ge et al. 2006).
As development proceeds through the second week, GCs continue to express immature neuronal markers (DCX/PSA-NCAM), display gigaohm input resistance, and increase the density of voltage-gated Na+ and K+ channels that support the maturation of membrane excitability. This is the period of maximal migration in which GCs establish their final position within the inner half of the GCL (Kempermann et al. 2003; Espósito et al. 2005; Mathews et al. 2010). They begin to acquire a polarized neuronal morphology, extending their axons through the hilus toward the CA3 region, and their dendrites through the molecular layer (Sun et al. 2013). GABAergic terminals begin to make the first functional synapses onto the dendrites of new GCs. Presynaptic GABA release elicits postsynaptic currents (PSCs) with slow kinetics that exert a depolarizing effect (Espósito et al. 2005; Overstreet-Wadiche et al. 2005, 2006a; Tozuka et al. 2005; Ge et al. 2006). One important population of GABAergic neurons responsible for the initial contacts with slow kinetics are the neurogliaform interneurons, whose connectivity with adult-born GCs is initially sparse and increases several-fold as GCs mature (Markwardt et al. 2011).
The Onset of Neuronal Activity
When GCs enter the third week, their cell bodies become positioned within the GCL and extend spineless dendrites through the molecular layer. This stage corresponds to the onset of glutamatergic synaptogenesis, when perforant path terminals begin to establish contacts onto the naked dendrites of newborn cells. Synaptogenesis has been characterized in detail using both morphological and electrophysiological approaches performed in retrovirally labeled GCs and in POMC transgenic mice (Espósito et al. 2005; Ge et al. 2006; Zhao et al. 2006; Toni et al. 2007). The mechanisms that trigger glutamatergic synaptogenesis onto new GCs have recently been tackled. Chancey and collaborators have shown that immature GCs bear NMDA receptors only—containing (silent) synapses that can be converted into NMDA/AMPA (active) synapses after coincident GABA-mediated depolarization and NMDA receptor activation by presynaptic glutamate release (Chancey et al. 2013). This work also showed that synaptic “unsilencing” can be induced in mice by a 2-h exposure to an enriched environment in a manner that is dependent on depolarizing GABAergic activity, revealing that a brief behavioral experience can produce the fast functional integration of new GCs into the network; an outstanding level of circuit plasticity. It remains to be understood whether or not all glutamatergic synaptogenesis requires activity-dependent unsilencing or perhaps some active synapses may form spontaneously.
From the initial moment of excitatory synapse formation at 2 wk of age, glutamatergic synaptogenesis onto developing GCs takes several weeks. As GCs mature, dendrites grow and branch, and spine density also increases, generating a continuous “demand” of active terminals (Espósito et al. 2005; Ge et al. 2006; Zhao et al. 2006; Toni et al. 2007; Piatti et al. 2011; Sun et al. 2013). This morphological development is accompanied by a substantial increase in the amplitude of postsynaptic responses that can be visualized on activation of the perforant pathway in acute slices. The maximal size of excitatory postsynaptic responses is reached after ∼6 wk (Laplagne et al. 2006; Mongiat et al. 2009). The integration of new neurons results in the formation of new networks and the modification of preexisting ones. Three-dimensional analyses of dendritic protrusions of new neurons at the electron microscopic level indicate that, instead of synapsing with new axonal terminals, neurons primarily connect to preexisting terminals, as evidenced by the presence of a mature dendritic spine on the contacted terminals (Toni et al. 2007). These axon terminals are thus defined as multiple synapse boutons, as they are contacted by two postsynaptic partners. As new GCs grow older, the proportion of multiple synapse boutons decreases, suggesting that they are transitory structures formed while new spines replace older spines, thus restructuring the network they formed before the generation of the new GC.
The velocity of maturation is also a highly dynamic process. It has been long known that electroconvulsive seizures increase the rate of neuronal proliferation and accelerate the process of neuronal development and maturation in the adult hippocampus (Parent et al. 1997; Overstreet-Wadiche et al. 2006b). These initial findings, obtained in models of brain disorders, have in fact revealed a set of complex mechanisms that control many aspects of adult neurogenesis under physiological conditions. Both proliferation and maturation are accelerated by running, which increases electrical activity in the hippocampus (van Praag et al. 1999; Piatti et al. 2011). It is likely that other conditions also modulate maturation in an activity-dependent manner. As an example, spatial learning has been recently shown to exert a remarkable effect in dendritic remodeling (Tronel et al. 2010).
Thus, by residing in the middle of the dentate gyrus, RGL cells and their progeny are strategically positioned at the crossroads of several feedforward and feedback network loops. As a consequence, every step of maturation, from stem-cell division to synaptic integration, is prone to modulation by neuronal activity and is therefore highly responsive to behavioral or pathological interference (reviewed in Kempermann 2015; Kuhn 2015; Lucassen et al. 2015).
Activity-Dependent Synaptic Plasticity
During this period of intense functional synaptogenesis, glutamatergic synapses onto new GCs are highly prone to activity-dependent plasticity, a mechanism that has long been implicated in learning and memory (Neves et al. 2008). The synapse between perforant path terminals and new GCs displays a low threshold for the induction of long-term potentiation (LTP). Work from several laboratories has shown that conditions that would normally elicit little or no plasticity in synapses onto mature GCs can potentiate inputs onto young GCs: (1) weak stimuli that elicit coincident pre- and postsynaptic activity are sufficient to induce LTP (Schmidt-Hieber et al. 2004); (2) LTP induction can be achieved leaving GABAergic inhibition intact (Wang et al. 2000; Snyder et al. 2001; Ge et al. 2008; Massa et al. 2011; Li et al. 2012); and (3) the levels of synaptic potentiation are largely increased (Ge et al. 2007). In this latter study, it is nicely shown that synaptic plasticity is enhanced only in excitatory inputs to neurons between 4 and 6 wk of age. After 7 wk, the level of plasticity in newborn GCs becomes similar to that of mature GCs. Activity-dependent plasticity is also sensitive to neuromodulators. For instance, dopamine can decrease not only the size of postsynaptic glutamatergic responses but also the extent of LTP in immature neurons (Mu et al. 2011).
Different synaptic and network mechanisms seem to contribute to the enhancement in activity-dependent synaptic plasticity observed in immature GCs. The key mechanism that has been described so far is the switch in the NMDA receptor composition that occurs during neuronal maturation. Immature GCs express NR2B-containing NMDA receptors, which display a high affinity for CaMKII and are responsible for the increased LTP levels (Snyder et al. 2001; Barria and Malinow 2005; Ge et al. 2007). As neurons mature, they switch toward NR2A-containing NMDA receptors that present lower affinity for CaMKII and, consequently, decreased levels of LTP expression. Interestingly, the enhanced plasticity seen for excitatory GC inputs has recently been found for the outputs of newborn GCs, at the level of mossy fibers (see below) (Gu et al. 2012). It is still unclear whether such enhanced plasticity is required for tailoring the connectivity of immature GCs that are being integrated into the network, or whether plasticity of input synapses onto new GCs is, in itself, a mechanism involved in processing and/or storage of incoming information.
FUNCTIONAL PROPERTIES AND CONNECTIVITY NEW GCs AT THE MATURE STAGE
New GCs develop and mature for several weeks to reach a fully mature neuronal phenotype (Fig. 2). Most evidence indicates that new GCs reach a plateau for morphological and functional development between the fourth and sixth weeks of age. At that stage, these neurons display a dendritic spine density of ∼2 spines/μm (Zhao et al. 2006), a total length of dendrites of ∼1500 µm, and a primary axonal length of 2 mm, with ∼10 axonal boutons/mm (Sun et al. 2013). However, discrete morphological parameters continue to mature up to 3 months, such as the number of presynaptic vesicles and contacted spines per mossy fiber terminal (Toni et al. 2008).
For a long time, it has been hypothesized that adult-born GCs might acquire functional properties that are unique and different compared with those of neurons born in development for several reasons. Adult neurogenesis occurs in a radically different environment, neuronal maturation in the adult hippocampus occurs at a much slower pace, and neural progenitor cells might express a different program. To understand whether all mature GCs ultimately belong to the same functional population or whether adult-born neurons maintain a distinctive functional phenotype, double retroviral labeling was used to distinguish between neurons generated in the developing and those generated in the adult dentate gyrus (Laplagne et al. 2006, 2007). A retrovirus expressing GFP was delivered to label neural progenitor cells dividing during early postnatal development. A second retroviral injection was then performed during adulthood to express a red fluorescent protein (RFP) in adult-born neurons of the same hippocampus. Electrophysiological recordings in acute slices were used to carry out a quantitative comparison of GABAergic inputs from different origins (dendritic and somatic), as well as glutamatergic inputs from the medial and lateral perforant paths. Remarkably, all mature GCs (regardless of their birth date) displayed GABAergic and glutamatergic inputs of similar kinetics and strength, and showed similar firing responses to evoked glutamatergic excitation. The only exception to the similarity is that GCs generated during embryonic development seemed to display lower values for input resistance, which might in turn decrease their overall activity levels (Laplagne et al. 2007). Therefore, developmental and adult neurogenesis produces neurons that acquire similar functional properties. However, as discussed below, adult-born neurons may become engaged in circuit activity while still immature, at which time they display unique functional properties.
Data on input connectivity obtained by electrophysiological recordings has been further substantiated by anatomical studies using retrograde monosynaptic tracing with pseudotyped rabies virus. This method allows tracing connections between specific types of neurons and their first-order presynaptic partners (Wickersham et al. 2007). To search for the presynaptic connectome of adult-born GCs, Vivar and colleagues (2012) and Deshpande and colleagues (2013) combined retroviral transduction of neural progenitor cells with the rabies virus approach. Retroviral vectors were used to transduce progenitor cells of the adult dentate gyrus with an avian receptor for the envelope protein EnvA, the rabies virus glycoprotein G (which allows retrograde transsynaptic viral transfer to presynaptic neurons), and a fluorescent reporter. Subsequent delivery of the EnvA-pseudotyped rabies virus with another fluorescent reporter was used to assemble the rabies virus in new GCs carrying the EnvA receptor and glycoprotein G (the starter neuron), allowing the virus to jump and label first-order presynaptic partners. Consistent with previous functional studies, hilar GABAergic interneurons were found among the most abundant presynaptic partners at early developmental stages (within the first 2 wk), followed by molecular layer interneurons that seemed to appear somewhat later. GABA neurons included basket, HIPP and MOPP cells, which remained connected long after the maturation of new GCs (Li et al. 2013). Intriguingly, hilar mossy cells were also found to target new GCs only at early developmental time points (1–2 wk). After ∼3 wk, glutamatergic neurons from the medial and lateral entorhinal cortex, the most well-characterized input to GCs, established their connectivity, together with cholinergic neurons, from the medial septum and diagonal band of Broca.
Recent work by Berninger and colleagues has revealed that the quantity and quality of input connections to adult-born GCs can be defined by experience, but only within a critical period of GC development encompassed within the second and the sixth weeks (Bergami et al. 2015). Exposure to enriched environment during the 4-wk critical period produced a transient increase in GABAergic interneurons innervating new GCs and a permanent enhancement in innervation from the entorhinal cortex, which might be a result of the increased levels of synaptic potentiation described for 4-wk-old GCs (Ge et al. 2007).
OUTPUT OF ADULT-BORN GCs: AXONS, SYNAPSES, AND NEUROTRANSMITTERS
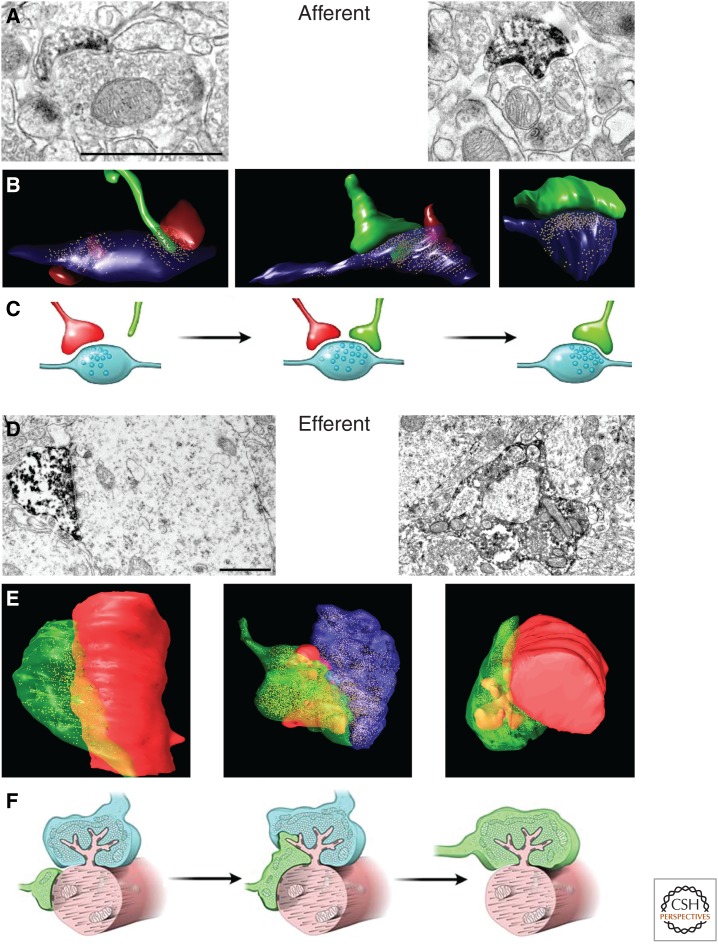
Functional integration in the dentate gyrus network depends not only on establishing appropriate afferent connections that will allow new GCs to integrate an excitatory signal to elicit a spike, but also on the capability to transmit the encoded information onto postsynaptic target cells. Axons can be observed in the hilar region and proximal CA3 area (CA3c) during the second week of development (Fig. 3). They arrive at the more distal CA3 area (CA3a) by the early days of the third week and reach a plateau in their growth during the fourth week, with the primary axon reaching a maximum length of ∼1.8 mm (Zhao et al. 2006; Sun et al. 2013). It is interesting that, although axonal growth proceeds at a relatively fast pace (an average of ∼70 µm/day over 3 wk), morphological maturation of presynaptic terminals is a slow process. For instance, cross-sectional areas of presynaptic terminals onto hilar interneurons reach mature values by the fourth week, and terminals impinging onto CA3 pyramidal cells seem to require even longer periods (Faulkner et al. 2008; Toni et al. 2008). In fact, more than 8 wk are needed for new terminals to reach sizes and densities that are similar to those of mature GCs. This relatively slow maturation may be caused by the synaptic rearrangements induced by the formation of new mossy fiber terminals, which may interfere with preexisting ones (Fig. 3). During synapse formation in the CA3 area, several mossy fiber terminals contact thorny excrescences that are already contacted by other mossy fibers (Toni et al. 2008). Such a mechanism resembles the synaptic competition occurring between axons at the developing neuromuscular synapse and may represent a mechanism of activity-dependent refinement of the connectivity of newborn neurons (Buffelli et al. 2003). Thus, maturation of the connectivity of newborn neurons induces modifications in the connectivity of preexisting neurons and may contribute to the pruning of older, perhaps less-effective synapses.
Figure 3.
Synaptogenesis in new GCs. (A–C) Afferent synapses from perforant path axons. (A) Electron micrographs of dark immunolabeled dendritic spines from new GCs contacting a multiple synapse bouton (left) or a single synapse bouton (right). (B) Three-dimensional reconstructions from serial-section electron micrographs of dendritic protrusions from new GCs (in green). Left panel: filopodia approaching and making a physical contact with a synapse. Middle panel: dendritic spine synapsing with a multiple synapse bouton. Right panel: dendritic spine synapsing with a single synapse bouton. (C) Schematic representation of the hypothesis on the development of dendritic spine connectivity: Left panel: filopodia from new GCs (green) grow preferentially toward preexisting synapses. On stabilization, some filopodia transform into dendritic spines, thereby forming multiple synapse boutons (middle panel). Over maturation time, multiple synapse boutons transform into single-synapse boutons, probably through the retraction of the preexisting spine. Green, dendritic protrusion from new GCs; red, dendritic spine from other GCs; blue, axonal boutons (based on Toni and Sultan 2011). (D–F) Efferent synapses on mossy-fiber terminals in the CA3. (D) Electron micrographs of immunolabeled mossy fiber terminals from new GCs at 17 dpi (left panel) and 30 dpi (right panel). Note the thorny excrescence protruding into the terminal on the right panel. (E) Three-dimensional reconstructions of terminals from new GCs synapsing directly on the dendrite (left panel), with a shared thorny excrescence with another, nonimmunolabeled GCs (middle panel) or with an individual thorny excrescence (right panel). Green, terminal from a new GC; red, dendrite from a CA3 pyramidal neuron; blue, terminal from a nonimmunolabeled GC. (F) Schematic depicting the hypothetical development of output connectivity from new GCs. Left panel: the terminal of a new GC synapses first with the dendrite of a CA3 pyramidal cell. Middle panel: on maturation (30 dpi), some thorny excrescences are shared between new and preexisting terminals. Right panel: after 75 dpi, all terminals from new GCs synapse with individual thorny excrescences (based on Toni and Sultan 2011). Scale bars, 1 µm.
The question of whether mossy fiber terminals of newborn cells establish functional synapses onto target neurons was approached using optogenetics, namely, retroviral transduction of newborn GCs with the light-activated cation channel channelrhodopsin-2 (ChR2) (Boyden et al. 2005; Li et al. 2005). Specific expression of ChR2 in the progeny of dividing progenitor cells allowed the selective activation of labeled newborn GCs, by delivery of blue light onto hippocampal slices, while performing whole-cell recordings in putative postsynaptic cells (Toni et al. 2008). This approach revealed that fully mature newborn GCs make functional glutamatergic synapses onto hilar interneurons, mossy cells, and CA3 pyramidal cells, and excitatory postsynaptic responses were observed in pyramidal cells as early as 2 to 3 wk after retroviral delivery (Gu et al. 2012). The demonstration that glutamatergic inputs elicit spiking of adult-born GCs (Laplagne et al. 2006), and that optogenetic stimulation elicits glutamate release from mossy fibers, indicate that mature adult-born GCs neurons are fully integrated in the hippocampal network and can, presumably, process information in a functionally appropriate fashion.
ChR2 expression was also used to investigate activity-dependent plasticity (specifically, LTP) of mossy fiber synapses onto CA3 pyramidal cells. Gu and colleagues (2012) implanted an optical fiber in the dentate gyrus region to deliver optical stimulation and recorded field excitatory postsynaptic potentials in the CA3 region in vivo. They elicited θ burst stimulation of newborn GCs at 3, 4, and 8 wk of age and recorded excitatory postsynaptic potential field activity before and after the induction of LTP. They observed that 4-wk-old neurons displayed the highest sensitivity to LTP induction, in terms of both the percent of neurons displaying LTP and the extent of LTP expression. These results showed that the output of new GCs is highly sensitive to LTP induction within the same time window when excitatory inputs to new GCs are most sensitive to the same type of plasticity. Thus, newly generated GCs seem to be able to tune the strength of specific excitatory inputs and outputs to their level of activity. This might determine the number and identity of those connections that will be strengthened by the activity itself, and will ultimately determine which of those synapses will remain or disappear.
Optogenetic and chemogenetic approaches were recently used to compare the feedforward and feedback networks activated by 4- versus 8-wk-old (mature) GCs (Temprana et al. 2015). It was found that new GCs recruit powerful feedback inhibition onto the GCL and feedforward inhibition onto CA3 pyramidal cells. It is, however, remarkable that new neurons control distal feedforward networks at the younger stages, but they can only activate substantial feedback inhibition after they become mature. The functional implications of such a delay in the establishment of proximal networks are discussed in the next section.
WHAT IS UNUSUAL ABOUT ADULT-BORN GCs?
The previous section highlights that, on reaching maturation, adult-born GCs become fully integrated in the preexisting network (inputs and outputs) in a manner that is similar to all other GCs generated during development. Consequently, new GCs appear ready to process incoming information. For some time, the idea that the adult hippocampus would continue to generate neurons throughout life and produce cells with no special or differential characteristics has remained puzzling. We discuss below recent findings that have contributed to change this view.
In recent years, the role of adult-born GCs in the dentate gyrus network has been interrogated in different manners. Focal X-ray irradiation was used to eliminate new GCs from the dentate gyrus (up to 10-wk-old neurons). Subsequent in vivo electrophysiological recordings in anesthetized mice were then used to assess the impact of their loss on dentate gyrus activity (Lacefield et al. 2010). Responses to perforant path activation were reduced in mice lacking new GCs, indicating that this neuronal population contributes to overall dentate activity. Interestingly, the absence of new GCs increased the amplitude of spontaneous γ-frequency bursts, suggesting that new GCs might somehow disorganize recurrent activity in the dentate gyrus.
Work of several laboratories has shown that developing GCs of the adult dentate gyrus display electrophysiological properties that are different from mature GCs in terms of their increased excitability, reduced GABAergic inhibition, and weak excitatory connectivity (Schmidt-Hieber et al. 2004; Espósito et al. 2005; Overstreet-Wadiche et al. 2005, 2006a,b; Couillard-Despres et al. 2006; Ge et al. 2006; Karten et al. 2006; Stocca et al. 2008). It was then investigated whether new GCs acquire information-processing capabilities before reaching a fully mature developmental stage. Studies in acute slices showed that immature GCs with high input resistance can trigger action potentials in response to small current steps that would not activate mature neurons because of their low input resistance (Mongiat et al. 2009). In regard to the activation of perforant path axons, immature GCs display weak excitatory inputs owing to the low number of afferent synapses. Yet, those few glutamatergic contacts are sufficient to trigger action potentials in a substantial proportion of immature GCs by the third developmental week. These experiments were done in the presence of GABAA receptor antagonists to isolate excitatory inputs and monitor the intrinsic neuronal properties of new neurons. Notably, the increased membrane resistance was sufficient to compensate for the weak afferent connectivity. The mechanisms underlying such compensation include the rapid development of voltage-gated Na+ and K+ channels and the delayed expression of the inward rectifier K+ conductance that decreases membrane resistance (Mongiat et al. 2009). Thus, the extent of afferent excitation and intrinsic excitability would allow adult-born GCs at 3 to 4 wk of age to contribute to information processing.
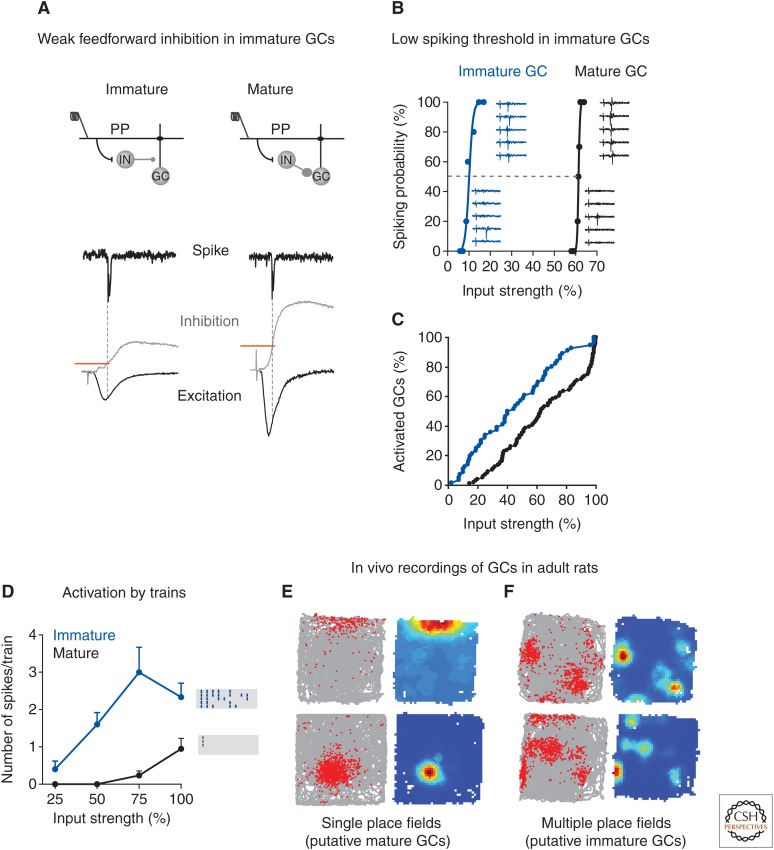
To investigate whether immature GCs are capable of information processing, the activation of 4-wk-old neurons and mature GCs were compared (Fig. 4) (Li et al. 2012; Marin-Burgin et al. 2012). Activation (spiking) in response to stimulation of the perforant path was assessed using Ca2+ imaging to monitor entire neuronal populations and loose patch recordings of individual cells in acute hippocampal slices. For any given stimulus, the proportion of active neurons was substantially higher for immature than for mature GCs. Weak stimuli primarily activated immature neurons. The mechanism underlying the difference in activation threshold involved feedforward GABAergic inhibition. Whole-cell recordings showed that the excitation/inhibition balance at the time of spiking is higher in immature cells owing to the delayed development of perisomatic GABAergic inhibition (Espósito et al. 2005). Thus, neuronal activity in the dentate gyrus is biased toward the immature population of principal neurons that bypass inhibitory control, while the activation of mature neurons is limited by inhibition. The observed functional properties of immature GCs suggest that activity reaching the dentate gyrus could undergo differential decoding through immature neuronal cohorts that are highly responsive and integrative and, in parallel, through a large population of mature GCs with sparse activity and high input specificity.
Figure 4.
Functional significance of immature granule cells (GCs). (A–D) Experiments in acute hippocampal slices. (A) A stimulating electrode was placed in the perforant pathway. Stimulation of the medial perforant path (mPP) evoked monosynaptic excitation and disynaptic inhibition via γ-aminobutyric acid (GABA)ergic interneurons (INs) onto GCs. Spiking and the underlying synaptic currents were recorded in individual cells by loose patch followed by whole cell. (B) Example curves of spiking probability versus input strength with example traces. (C) Cumulative distributions of input strength required to elicit 50% spiking probability (activated cells). (D) Activation of GCs evoked by trains and raster plots depicting spikes recorded in response 10 pulses at 10 Hz (five trials/neuron). (From Marin-Burgin et al. 2012; modified, with permission, from The American Association for the Advancement of Science © 2012.) (E,F) In vivo single unit recording in the rat dentate gyrus. Examples of cells with single (E) and multiple (F) place fields. Rate maps and plots showing spikes (Kunze et al. 2009) from the cell superimposed on the rat’s trajectory (gray) are shown in the left and right columns. For the rate maps, blue represents no firing and red represents peak firing (data kindly provided by J.P. Neunuebel and J.J. Knierim).
It is interesting to note that a recent study by Neunuebel and Knierim (2012) found that there are at least two classes of neurons in the dentate gyrus in freely moving rats. One class, which they suggest corresponds to mature GCs, fires very sparsely, with a small number of active cells firing at single locations within an environment like CA1 and CA3 place cells. The second class, which they suggest might correspond to newborn GCs and/or hilar mossy cells, fires more promiscuously, with a high fraction of cells active in an environment and with multiple spatial subfields. Although this latter profile would nicely reflect the low-activation threshold displayed by immature GCs, this possibility is yet to be investigated.
CONCLUDING REMARKS
Adult-born GCs develop very slowly, over several weeks. This is in striking contrast to the few days that are required to reach maturation during perinatal development. Thus, developing GCs dwell at different stages for long intervals, allowing them to sense information while they remain immature, and become substrates of intense activity-dependent synaptic remodeling. Their delayed coupling to inhibitory networks make them particularly sensitive to incoming signals and also suggests that they might contribute to information encoding once they become mature, when they can increase the threshold for activation of neighbor cells. From this point of view, adult neurogenesis may work as a mechanism to continuously generate pools of new neurons with an unusual capacity to tailor their connectivity based on current experience. The thousands of new cells added every day will contribute to new cohorts of neurons that are ready to sense hippocampal inputs that will make their connectivity unique. Understanding how different stages of GC development contribute to discrimination of similar inputs, a basic function of the dentate gyrus, is the next challenge in this field.
ACKNOWLEDGMENTS
We thank Jonathan Moss for critical comments on the manuscript and Jamie Simon and Georgina Davies-Sala for artwork. This work was funded by grants from the National Institutes of Health (NIH) (Fogarty International Research Collaboration Award [FIRCA]), the Howard Hughes Medical Institute (Senior International Research Grant), and the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2010, Argentina) to A.F.S., and from the Swiss National Science Foundation (PP00P3-150753) to N.T. A.F.S. is a principal investigator from the Argentine Research Council (CONICET).
Footnotes
Editors: Fred H. Gage, Gerd Kempermann, and Hongjun Song
Additional Perspectives on Neurogenesis available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. 1998. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci 18: 3386–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. 1978. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol 182: 851–914. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Ishizuka N, Claiborne B. 1990. Neurons, numbers and the hippocampal network. Prog Brain Res 83: 1–11. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P. 2007. The dentate gyrus: Fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 163: 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. 2005. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48: 289–301. [DOI] [PubMed] [Google Scholar]
- *.Beckervordersandforth R, Zhang C-L, Lie DC. 2015. Transcription factor-dependent control of adult hippocampal neurogenesis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ari Y. 2002. Excitatory actions of GABA during development: The nature of the nurture. Nat Rev Neurosci 3: 728–739. [DOI] [PubMed] [Google Scholar]
- Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Gobel J, Yang SM, Conzelmann KK, Schinder AF, Götz M, et al. 2015. A critical period for experience-dependent remodeling of adult-born neuron connectivity. Neuron 85: 710–717. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. 2009. Cation-chloride cotransporters and neuronal function. Neuron 61: 820–838. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. 2011. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145: 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Song J, Ming GL, Song H. 2012. A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr Opin Neurobiol 22: 765–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. 2005. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Strowbridge BW, Kunkel DD, Schmiege DL, Schwartzkroin PA. 1992. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus 2: 349–362. [DOI] [PubMed] [Google Scholar]
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. 2003. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature 424: 430–434. [DOI] [PubMed] [Google Scholar]
- Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, Overstreet-Wadiche LS. 2013. GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci 33: 6614–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Choe Y, Pleasure SJ, Mira H. 2015. Control of adult neurogenesis by short-range morphogenic signaling molecules. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. 1990. Quantitative, three-dimensional analysis of granule cell dendrites in the rat dentate gyrus. J Comp Neurol 302: 206–219. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Karl C, Lindemann G, Schmid P, Aigner R, Laemke J, Bogdahn U, Winkler J, Bischofberger J, et al. 2006. Targeted transgene expression in neuronal precursors: Watching young neurons in the old brain. Eur J Neurosci 24: 1535–1545. [DOI] [PubMed] [Google Scholar]
- Deshpande A, Bergami M, Ghanem A, Conzelmann KK, Lepier A, Götz M, Berninger B. 2013. Retrograde monosynaptic tracing reveals the temporal evolution of inputs onto new neurons in the adult dentate gyrus and olfactory bulb. Proc Natl Acad Sci 110: E1152–E1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. 1985. Granule cell dendritic spine density in the rat hippocampus varies with spine shape and location. Neurosci Lett 54: 219–224. [DOI] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. 2005. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 25: 10074–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, et al. 2008. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci 105: 14157–14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. 1996. Interneurons of the hippocampus. Hippocampus 6: 347–470. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Seress L, Schwerdtfeger WK, Buhl E. 1991. The mossy cells of the fascia dentata: A comparative study of their fine structure and synaptic connections in rodents and primates. J Comp Neurol 312: 145–163. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. 2006. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. 2007. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Sailor KA, Ming GL, Song H. 2008. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol 586: 3759–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Götz M, Nakafuku M, Petrik D. 2015. Neurogenesis in the developing adult brain—Similarities and key differences. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S. 2012. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci 15: 1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZS, Buhl EH, Lorinczi Z, Somogyi P. 1993. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci 5: 395–410. [DOI] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsaki G. 2002. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci 5: 790–795. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Struber M, Yanagawa Y, Obata K, Vida I, Jonas P, Bartos M. 2013. Morpho-physiological criteria divide dentate gyrus interneurons into classes. Hippocampus 24: 189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR. 2007. Interneurons of the dentate gyrus: An overview of cell types, terminal fields and neurochemical identity. Prog Brain Res 163: 217–232. [DOI] [PubMed] [Google Scholar]
- Jones SP, Rahimi O, O’Boyle MP, Diaz DL, Claiborne BJ. 2003. Maturation of granule cell dendrites after mossy fiber arrival in hippocampal field CA3. Hippocampus 13: 413–427. [DOI] [PubMed] [Google Scholar]
- Karten YJ, Jones MA, Jeurling SI, Cameron HA. 2006. GABAergic signaling in young granule cells in the adult rat and mouse dentate gyrus. Hippocampus 16: 312–320. [DOI] [PubMed] [Google Scholar]
- *.Kempermann G. 2015. Activity dependency and aging in the regulation of adult neurogenesis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. 2003. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 130: 391–399. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. 2003. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467: 455–463. [DOI] [PubMed] [Google Scholar]
- *.Kuhn HG. 2015. Control of cell survival in adult neurogenesis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze A, Congreso MR, Hartmann C, Wallraff-Beck A, Huttmann K, Bedner P, Requardt R, Seifert G, Redecker C, Willecke K, et al. 2009. Connexin expression by radial glia-like cells is required for neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci 106: 11336–11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. 2010. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus 22: 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Espósito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. 2006. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol 4: e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Kamienkowski JE, Espósito MS, Piatti VC, Zhao C, Gage FH, Schinder AF. 2007. Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis. Eur J Neurosci 25: 2973–2981. [DOI] [PubMed] [Google Scholar]
- Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. 2005. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci 102: 17816–17821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Aimone JB, Xu X, Callaway EM, Gage FH. 2012. Development of GABAergic inputs controls the contribution of maturing neurons to the adult hippocampal network. Proc Natl Acad Sci 109: 4290–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Stam FJ, Aimone JB, Goulding M, Callaway EM, Gage FH. 2013. Molecular layer perforant path-associated cells contribute to feed-forward inhibition in the adult dentate gyrus. Proc Natl Acad Sci 110: 9106–9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lucassen PJ, Oomen CA, Naninck EFG, Fitzsimons CP, van Dam AM, Czeh B, Korosi A. 2015. Regulation of adult neurogenesis and plasticity by (early) stress, glucocorticoids, and inflammation. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Burgin A, Mongiat LA, Pardi MB, Schinder AF. 2012. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science 335: 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt SJ, Dieni CV, Wadiche JI, Overstreet-Wadiche L. 2011. Ivy/neurogliaform interneurons coordinate activity in the neurogenic niche. Nat Neurosci 14: 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa F, Koehl M, Wiesner T, Grosjean N, Revest JM, Piazza PV, Abrous DN, Oliet SH. 2011. Conditional reduction of adult neurogenesis impairs bidirectional hippocampal synaptic plasticity. Proc Natl Acad Sci 108: 6644–6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews EA, Morgenstern NA, Piatti VC, Zhao C, Jessberger S, Schinder AF, Gage FH. 2010. A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis. J Comp Neurol 518: 4479–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ. 2008. Differential mechanisms of transmission and plasticity at mossy fiber synapses. Prog Brain Res 169: 225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat LA, Espósito MS, Lombardi G, Schinder AF. 2009. Reliable activation of immature neurons in the adult hippocampus. PLoS ONE 4: e5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Zhao C, Gage FH. 2011. Dopaminergic modulation of cortical inputs during maturation of adult-born dentate granule cells. J Neurosci 31: 4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. 2012. Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus. J Neurosci 32: 3848–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV. 2008. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat Rev Neurosci 9: 65–75. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. 2005. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol 94: 4528–4532. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. 2006a. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci 26: 2326–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. 2006b. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci 26: 4095–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. 1997. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17: 3727–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Davies-Sala MG, Esposito MS, Mongiat LA, Trinchero MF, Schinder AF. 2011. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci 31: 7715–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. 2004. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429: 184–187. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. 2001. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol 85: 2423–2431. [DOI] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, et al. 2012. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489: 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Song J, Olsen RHJ, Sun J, Ming G-L, Song H. 2015. Neuronal circuitry mechanisms regulating adult mammalian neurogenesis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocca G, Schmidt-Hieber C, Bischofberger J. 2008. Differential dendritic Ca2+ signalling in young and mature hippocampal granule cells. J Physiol 586: 3795–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GJ, Sailor KA, Mahmood QA, Chavali N, Christian KM, Song H, Ming GL. 2013. Seamless reconstruction of intact adult-born neurons by serial end-block imaging reveals complex axonal guidance and development in the adult hippocampus. J Neurosci 33: 11400–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Zhao C, Gage FH. 2006. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat Protoc 1: 3049–3055. [DOI] [PubMed] [Google Scholar]
- Temprana SG, Mongiat LA, Yang SM, Trinchero MF, Alvarez DD, Kropff E, Giacomini D, Beltramone N, Lanuza GM, Schinder AF. 2015. Delayed coupling to feedback inhibition during a critical period for the integration of adult-born granule cells. Neuron 85: 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Sultan S. 2011. Synapse formation on adult-born hippocampal neurons. Eur J Neurosci 33: 1062–1068. [DOI] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. 2007. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci 10: 727–734. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. 2008. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 11: 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Nakashiba T, Tonegawa S, McBain CJ. 2010. Control of CA3 output by feedforward inhibition despite developmental changes in the excitation–inhibition balance. J Neurosci 30: 15628–15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. 2005. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47: 803–815. [DOI] [PubMed] [Google Scholar]
- Tronel S, Fabre A, Charrier V, Oliet SH, Gage FH, Abrous DN. 2010. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc Natl Acad Sci 107: 7963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. 1999. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. 2002. Functional neurogenesis in the adult hippocampus. Nature 415: 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. 2009. The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci 10: 272–282. [DOI] [PubMed] [Google Scholar]
- Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H. 2012. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun 3: 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. 2000. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol 42: 248–257. [PubMed] [Google Scholar]
- Wang LP, Kempermann G, Kettenmann H. 2005. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci 29: 181–189. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. 2007. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53: 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG Jr, Ming GL, Gage FH. 2006. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]