Abstract
Microglia, the major myeloid cells of the central nervous system (CNS) are implicated in physiologic processes and in the pathogenesis of several CNS disorders. Since their initial description early in the 20th century, our ability to identify and isolate microglia has significantly improved and new research is providing insight into the functions of these cells in sickness and in health. Here, we review recent advances in our understanding of the role of microglia in physiological and pathological processes of the CNS with a focus on multiple sclerosis and Alzheimer’s disease. Because of the prominent roles CX3CR1 and its ligand fractalkine played in bringing about these advances, we discuss the physiological and pathological roles of microglia as viewed from the CX3CR1–fractalkine perspective, providing a unique viewpoint. Based on the most recent studies of molecular profiling of microglia, we also propose a molecular and functional definition of microglia that incorporates the properties attributed to these cells in recent years.
Insights into neuronal–microglial cross talk in development, physiology, aging, and disease have come from studies of fractalkine (made by neurons) and its receptor CX3CR1 (expressed by microglia), and the gene expression profiles of these cells.
A BRIEF HISTORY OF MICROGLIA
Microglia, the major myeloid cells of the central nervous system (CNS), have long been implicated in physiologic and pathologic processes. The 20th century brought several major breakthroughs in our ability to identify and isolate these cells. In the past decade, new research has emerged providing evidence and insight into the functions of these cells in sickness and in health.
The first breakthroughs in research on microglia were presented in a series of publications, nearly a century ago, by Pío del Río-Hortega. In an article published in 1918, del Río-Hortega described a method for staining microglia and was therefore able to distinguish these cells from other neighboring cells of the neural milieu (del Río-Hortega 1918). In a subsequent article published the following year, del Río-Hortega described microglia as the “third element” in the CNS, he also emphasized their phagocytic capacity (del Río-Hortega 1919). Additional work performed by del Río-Hortega indirectly described microglial plasticity and discussed the ability of these cells to change in response to stimuli (del Río-Hortega 1920). He further went on to describe the regional distribution of microglia and their heterogeneity (del Río-Hortega 1921). Since then, scientists investigating microglia using progressively sophisticated technologies have largely confirmed and built on del Río-Hortega’s initial observations.
After del Río-Hortega’s initial findings, it took another 50 years for the second wave of breakthroughs in microglia research to occur. Development of antibodies to various innate immune cell proteins identified markers for microglia expressed in situ such as F4/80 (Hume et al. 1983), Fc receptors, and complement receptor 3 (Perry et al. 1985). Subsequently, Giulian and Baker (1986) described a novel method to isolate and culture microglia from neonatal rodent brains. The ability to culture these cells led to a large number of in vitro investigations to define their functions. Cultured microglia were found to be capable of phagocytosis and lysozyme production (Zucker-Franklin et al. 1987), and respond to purinergic stimulation (Walz et al. 1993) and to β-amyloid (Meda et al. 1995; El Khoury et al. 1996). This approach also led to identification of novel markers for these cells such as Iba1 (Imai et al. 1996).
The availability of new markers for microglia provided tools to begin to investigate the role of these cells in various CNS disorders. Presence of these cells in senile plaques in Alzheimer’s disease (AD) was confirmed using specific monoclonal antibodies to α1 chymotrypsin, lysozyme (Rozemuller et al. 1986), and HLA-DR (McGeer et al. 1987). Microglia-expressing MHC II antigens were also identified in multiple sclerosis (MS) brains (Cuzner et al. 1988).
The third era of breakthroughs in microglia research started with the generation of a transgenic knockin mouse expressing green fluorescent protein (GFP) driven by the promoter for the chemokine receptor CX3CR1 (Jung et al. 2000). Because CX3CR1 is exclusively expressed on microglia in the CNS, GFP was expressed only in these cells, allowing investigators to visualize microglia without the need for immunohistochemistry or special staining. This mouse line ushered in a new line of investigations exploring the roles of microglia in various physiological and pathological processes.
The ability to visualize microglia without the need for immunolabeling and the development of novel two-photon in vivo imaging methodologies provided unprecedented views into the role of these cells in vivo. In a landmark publication, Nimmerjahn et al. (2005) showed that the morphologically “resting” microglia are highly active, continually surveying their microenvironment with extremely motile processes and protrusions; they also showed that focal injury to the brain provoked immediate and focal activation of microglia, switching their behavior from patrolling to shielding of the injured site. At the same time, Davalos and colleagues (2005) reported identical findings and showed further that the rapid process–extension response to laser lesion was mediated by ATP binding to the purinergic receptor P2ry12 (Haynes et al. 2006). Additional studies, discussed in the following pages, used the CX3CR1 GFP mice (or improved transgenic versions of these mice) and two-photon in vivo imaging to further explore the role of microglia in synaptic pruning and remodeling, and in various disease models including experimental autoimmune encephalitis (EAE), a model of MS, mouse models of amyloid deposition and tauopathies in AD, and models of ischemic injury.
The fourth era of breakthroughs in microglia research began when methods to isolate microglia from adult mice were developed (Hickman et al. 2008), leading to several landmark findings in 2013. With the increased recognition of the important roles microglia could play in physiological and pathological processes, and because of the multiple similarities and differences between these cells and other mononuclear phagocytes, it became crucial to develop a more accurate definition of microglia that distinguishes them from other cells in the CNS as well as other mononuclear phagocytes such as monocytes and macrophages. Over the past year, using methodologies such as direct RNA sequencing (RNASeq) (Chiu et al. 2013; Hickman et al. 2013), and microarrays (Beutner et al. 2013; Butovsky et al. 2014), four groups independently identified expression signatures of microglia that help define these cells and determine their unique transcriptional characteristics.
One further notable advance in understanding microglia came gradually over decades, so cannot conveniently be summarized in a single breakthrough. Microglial ontogeny was implied by del Río-Hortega as being mesenchymal. However, unequivocal evidence for this proposal was not forthcoming until 1996, when Maki and coworkers (McKercher et al. 1996) showed that mice lacking transcription factor PU.1 were devoid of microglia. In the same time frame, Alliot and colleagues (1991, 1999) conducted a series of descriptive studies and proposed that microglia originate in the yolk sac, enter brain rudiment after E8 in rodents and establish the adult microglial population through vigorous in situ proliferation. Ajami and colleagues followed in 2007, by showing that microglia self-renew in the CNS without a blood-monocyte contribution. In 2010, Ginhoux and colleagues (2010) performed a definitive fate mapping confirming Pessac’s hypothesis. Two years later, Geissmann (Schulz et al. 2012) showed the c-myb independence of microglia and other tissue macrophages, conclusively establishing that microglial maintenance did not rely on progeny of hematopoietic stem cells. With these insights came the ability to identify microglial survival or maintenance factors including colony stimulating factor (CSF)-1 and interleukin (IL)-34 (Greter et al. 2012; Wang et al. 2012), both produced in the CNS by neurons during development and both ligands for the CSF-1 receptor. Additionally, transcription factors such as interferon regulatory factor (IRF)-8 could be shown essential for microglial phenotypes by Kierdorf and Prinz (2013).
In the following pages, we will review recent advances in our understanding of the role of microglia in physiological and pathological processes of the CNS. Because of the prominent roles CX3CR1 and its ligand fractalkine played in bringing about these advances, and because fractalkine-induced, CX3CR1-mediated signaling appears to regulate microglial behavior in a number of CNS disorders including AD and MS, we will discuss the physiological roles of microglia as viewed from the CX3CR1–fractalkine perspective, providing a unique viewpoint. Based on the most recent studies of molecular profiling of microglia, we will also propose a molecular and functional definition of microglia that incorporates the properties attributed to these cells in recent years.
PHYSIOLOGICAL ROLES OF MICROGLIA VIEWED FROM THE FRACTALKINE-CX3CR1 PERSPECTIVE
Fractalkine (CX3CL1) and its receptor (CX3CR1) have assumed a prominent and unique role in CNS development, homeostasis, and disease within a relatively short time. The most salient and well-established neurobiological role of this ligand/receptor pair is defined in large part by their cellular sources. Fractalkine is made by neurons and the receptor is expressed by microglia. Therefore, fractalkine/receptor interactions constitute a neuron–microglial signaling system. Simply stated, effects of neuronal fractalkine mediated by microglial fractalkine receptor provide a capsule view of neuron–microglial cross talk throughout development, physiology, aging, and disease.
Early History of Fractalkine and Its Receptor
Fractalkine is a chemokine, albeit unusual. Chemokines were identified by virtue of mediating leukocyte subtype-specific chemotaxis in vitro in 1987 (Ransohoff 2005). Four years later, cloning of the first chemokine receptor cDNA (Horuk 1994) showed that chemokine receptors belong to the superfamily of G protein–coupled receptors (GPCRs), and subsequent work showed that chemokine receptors comprise a GPCR subclass signaling through Gαi. Genomic analysis assigned most chemokines and chemokine receptors to a restricted set of chromosomal loci, indicating their status as a coherent genetic and functional family (Charo and Ransohoff 2006). At present, humans and mice possess approximately 50 chemokines and 20 chemokine receptors. Two large structurally defined families, termed CC and CXC chemokines, include more than 90% of all chemokines. In primary structures of CC chemokines (CCL[ligand]1–28), two positionally conserved cysteines are side by side, whereas CXC (CXCL1–16) chemokines possess an intervening residue between the cysteines. CC chemokines signal almost without exception only to CC chemokine receptors (CCR[receptor]1–12), and CXC chemokines show a similar preference for a corresponding set of CXC chemokine receptors (CXCR1-7) (Rot and von Andrian 2004). Ligand–receptor relationships in the chemokine universe are complex. Chemokine receptors can respond to as many as 10 ligands, whereas individual chemokines can signal to as many as three receptors.
Virtually all chemokines are small (10–14 kDa) secreted peptides, transcribed rapidly and abundantly on demand with short-lived messages and quickly cleared by degradation of protein and message as well as receptor-mediated uptake and disposal of the peptides (Cardona et al. 2008).
From their initial identification until 1998, chemokines were viewed mainly in the context of inflammation and immunity because of their potent, selective action toward leukocyte populations. Deletion of chemokine receptor CXCR4 from the mouse genome upended this view of chemokine physiology, showing prominent defects in multiple tissues, including abnormal localization of numerous neuronal populations (Ma et al. 1998; Zou et al. 1998). These results ushered in an era of chemokine neurobiology, with fractalkine constituting a conspicuous element.
Fractalkine was identified by molecular cloning in 1997 (Bazan et al. 1997) and showed two unusual features. First, the primary translation product was not secreted and showed a single-pass transmembrane region, long mucin stalk, and amino-terminal chemokine ectodomain. Second, the canonical cysteine residues showed a CXXXC (CX3C) motif. In 2014, fractalkine and fractalkine receptor remain the sole members of their chemokine family. No other CX3C chemokines have been discovered and only one additional membrane tethered chemokine (CXCL16) has emerged. Fractalkine/fractalkine receptor appear, for practical purposes, to constitute a monogamous ligand/receptor system. One other signaling CX3CR1 ligand was reported (CCL26) but follow-up has been scant so its biological significance is uncertain (Nakayama et al. 2010).
Fractalkine’s unusual name deserves a comment. Apparently the lead author was reminded of fractals, in which a repeating subunit recapitulates the whole, by fractalkine’s primary structural characteristics.
Fractalkine was quickly shown to be expressed by endothelial cells, regulated in part by inflammatory cytokines. Initial studies showed that fractalkine signaled to receptor-bearing cells (mainly monocytes and natural killer [NK] cells) in two distinct ways. Either membrane fractalkine activated membrane fractalkine receptor in a contact-dependent fashion, or the chemokine domain, released by proteolysis, could function as a soluble ligand. The transmembrane expression of fractalkine at endothelial apical surfaces suggested a means of signaling to nearby leukocytes, and soon it was shown that fractalkine/receptor interactions could arrest leukocytes under flow without involvement either of pertussis toxin-sensitive G proteins or leukointegrins (Fong et al. 1998; Haskell et al. 1999, 2000).
Subsequent research showed that the soluble chemokine domain, mounted at the terminus of the mucin stalk, signaled by G protein–dependent interactions and mobilized calcium but failed to activate integrins, a property in contrast to virtually all soluble chemokines. These experiments, performed nearly 20 years ago, may be worth revisiting for guidance as we seek to determine mechanisms by which neuronal fractalkine signals to microglia, both through contact-dependent interactions and at a distance using soluble fractalkine. In addition to Gαi-mediated reduction of cAMP, chemokine receptors signal to elevate cytosolic calcium, activate mitogen-activated protein (MAP) kinases, and stimulate PI3K-Akt pathways. β-Arrestin recruitment and consequent receptor internalization, at times with additional signaling outputs, also characterizes chemokine receptors. These canonical, generic pathways provide a platform for detailed characterization, which remains to be completed for practically all chemokine receptors in their native cellular contexts. Only then will selective cellular responses to chemokines become susceptible to therapeutic manipulation, beyond blockade or agonism.
For soluble fractalkine, structural studies indicate that its receptor-binding properties differ substantially from those generally reported for chemokines (Mizoue et al. 1999), suggesting that an account of fractalkine/receptor signaling may well be useful for translational applications. Membrane fractalkine also showed distinct properties, as substitution of other chemokine domains at the end of the mucin stalk did not produce chimeric molecules that could support leukocyte arrest under flow (Haskell et al. 2000). Further, the fractalkine chemokine domain’s crystal structure showed an unusually compact globular domain, suggesting a mechanistic explanation for the chemokine’s distinct functional properties (Hoover et al. 2000). Outcomes of soluble versus membrane fractalkine signaling modes differ in relevant disease models of neurodegeneration. Despite extensive descriptive cell-biology investigation of membrane and soluble fractalkine, relatively little is known of differential downstream signaling cascades.
Fractalkine/Receptor in the Nervous System
Soon after its discovery, it became clear that fractalkine might participate in neurobiological processes. Database inspection showed that a large majority of expressed sequence tags (ESTs) for fractalkine and its receptor came from CNS sources, providing a rough estimate of relative tissue expression levels. Elegant in situ hybridization (Harrison et al. 1998) showed a highly provocative expression pattern. Fractalkine was produced by neurons, with the receptor restricted to microglia, and it was presciently predicted that neuron–microglial cross talk might be supported by this cellular distribution pattern. Surprisingly, fractalkine’s expression pattern throughout the remainder of the body was starkly different, being mainly on endothelium. Subsequent work confirmed that fractalkine derives from neurons in the CNS and that the cerebral vasculature is an outlier in its clear lack of fractalkine expression (Sunnemark et al. 2005).
Within a year, reports came suggesting that fractalkine exerted unexpected effects toward microglia, especially considering that chemokines were largely deemed inflammatory elements, which orchestrated accumulation of immune cells and then promoted their effector properties. Instead, fractalkine protected microglia from Fas-mediated cell death by altering the phosphorylation of apoptotic regulators such as Bcl-2 and Bad (Boehme et al. 2000). Others showed that neutralizing fractalkine antibodies nearly doubled microglial production of tumor necrosis factor (TNF) in response to lipopolysaccharide (LPS) and exerted the same magnitude of effect on microglial toxicity toward cocultured hippocampal neurons. In an important follow-up experiment (Zujovic et al. 2001), this group showed in vivo that intracerebroventricular (ICV) installation of fractalkine antibodies greatly increased the inflammatory response to ICV TNF-α. Interestingly, co-injection of fractalkine along with TNF-α had no effect at all, strongly suggesting that tonic soluble fractalkine signaling was not a limiting factor for fractalkine-mediated neuroprotection. The cumulative interpretation of these studies was that fractalkine tempered microglial responses to cardinal inflammatory stimuli. Further, without the moderating effects exerted by fractalkine, the microglial reactions to these stimuli could potentially be neurotoxic.
Other research implied greater complexity; in particular, neurons in vitro and in an in vivo stroke model responded to excitotoxic stimuli by increased proteolytic release of fractalkine (Chapman et al. 2000) with no change in mRNA level. In other cells, it was shown that proteases of the A disintegrin and metalloprotease (ADAM) family were responsible both for tonic (Hundhausen et al. 2003) and inducible (Garton et al. 2001; Tsou et al. 2001) fractalkine release. Because metalloproteases were also implicated in fractalkine release from neurons (Chapman et al. 2000), it seems plausible that these same factors mediate its proteolysis from some neuronal cells in hippocampus and cortex. Subsequent work showed that cathepsin S, a distinct protease (Clark et al. 2009), was required to generate soluble fractalkine from rat dorsal horn neurons.
Fractalkine signaling was also implicated in indirect neuroprotection. One particularly lucid series of experiments showed that neuronal fractalkine elicited adenosine release from microglia, activating survival pathways in excitotoxicity-challenged neurons via adenosine A1 receptors (Lauro et al. 2008, 2010). Initial findings in vitro were extended in vivo to a permanent focal ischemia model (Cipriani et al. 2011) and were enriched by showing that fractalkine signaling also supported glutamate uptake by astrocytes (Catalano et al. 2013).
New In Vivo Tools Bring New Insights
In 2000 came the report of a genetic model for fractalkine receptor, in which the CX3CR1 coding region had been replaced with GFP using homologous recombination, yielding mice in which cells transcribing the fractalkine receptor gene expressed GFP in the heterozygous (GFP/+) or homozygous knockout (GFP/GFP) state (Jung et al. 2000). These mice showed no immediately evident phenotype in either CNS or peripheral immune responses (Jung et al. 2000). As one example, the brain-stem microglial morphological response to peripheral facial nerve crush, an established model for inducing microgliosis (Graeber et al. 1998), was not affected in fractalkine receptor knockout mice. Results were confirmed in other genetic models of fractalkine receptor deficiency (Haskell et al. 2001; Tripp et al. 2001).
At about the same time, mice lacking fractalkine were generated (Cook et al. 2001; Soriano et al. 2002), enabling investigators to ask if ligand and receptor-knockout mice would show compatible phenotypes, an essential confirmation step for studies using these monogamous ligand/receptor genetic models.
CX3CR1gfp mice showed distinct advantages for monitoring microglial dynamics in vivo using two-photon imaging. As described above, these mice were used to establish the remarkably ceaseless movements of microglial processes (Davalos et al. 2005; Nimmerjahn et al. 2005) along with their lightning-like responses to microscopic foci of injury (Davalos et al. 2005). Thereafter, the concept of “resting microglia” became progressively obsolete. In initial experiments, mice deficient for fractalkine showed smaller stroke volume after transient focal ischemia (Soriano et al. 2002), suggesting that fractalkine/fractalkine receptor inflammatory signaling worsened tissue damage.
Fractalkine Receptor Signaling Illuminates Roles of Microglia in Neurodevelopment
It has become clear that parenchymal microglia enter the CNS parenchyma early (∼E10.5 in mice) during development (Ransohoff and Cardona 2010) and that their proliferation and maintenance depend in part on factors elaborated from neurons. After seminal documentation (Tremblay et al. 2010; Schafer et al. 2012) that microglia mediate complement-dependent synaptic pruning in some regions of the CNS, it was pertinent to ask whether fractalkine signaling contributed to regulating this process, as fractalkine receptor was shown present on microglial progenitors from their first entry into parenchyma (Mizutani et al. 2012). Results of examining synaptic maturation in the hippocampus of fractalkine receptor-deficient animals indicated a substantial delay in CX3CR1 knockouts, potentially caused by transient reduction in microglial numbers (Paolicelli et al. 2011; Ransohoff and Stevens 2011). Further, CX3CR1-deficient mice showed severely altered social interaction coupled to defective network interactions in adulthood (Paolicelli and Gross 2011; Zhan et al. 2014).
By analogy with macrophages, microglia may carry out two major functions during development: phagocytosis and trophic-factor production. Phagocytosis is exemplified by synaptic pruning. Factor production also occurs during development and is pivotal for certain neuronal populations. Layer V cortical neurons show decreased survival during the first postnatal week in fractalkine receptor deficient mice, because of reduced microglial production of insulin-like growth factor (IGF)-1 (Ueno et al. 2013).
Microglia Play a Role in Learning-Dependent Synapse Remodeling
With advancement in Cre-Lox technologies, it was only a matter of time before the properties of CX3CR1 as a receptor expressed uniquely on microglia were used to target microglia and/or specific genes in microglia to understand their physiological function. Using CX3CR1 CreER mice expressing tamoxifen-inducible Cre recombinase that allow for specific expression of the diphtheria toxin receptor in microglia, Parkhurst et al. (2013) were able to specifically deplete microglia from the brain following diphtheria toxin administration. Mice depleted of microglia showed deficits in multiple learning tasks and a significant reduction in motor-learning-dependent synapse formation. More interesting is that Cre-dependent removal of brain-derived neurotrophic factor (BDNF) from microglia recapitulated one salient effect of microglia depletion: impaired motor plasticity. Further, microglial BDNF increased neuronal tropomyosin-related kinase receptor B phosphorylation, a key mediator of synaptic plasticity. These findings suggest that microglia may have important physiological functions in learning and memory by promoting learning-related synapse formation through BDNF signaling. A detailed discussion of the role of fractalkine-CX3CR1 signaling in synapse development and pruning can be found in Schafer and Stevens (2015).
MICROGLIA IN CNS DISORDERS
Significant advances have been made in understanding the roles of microglia in normal brain functioning. In contrast, understanding the role of microglia in CNS disorders has proven more difficult. Brain pathology is frequently associated with disruption of the blood–brain barrier (BBB) and recruitment of peripheral blood monocytes. These cells, although significantly different from microglia, share several functional and transcriptional characteristics with microglia, making it more difficult to dissect their roles from those of microglia. However, the new emerging technologies described above, which allow specific targeting of microglial genes, could make such a task slightly easier. We will focus here on MS and AD, two major disorders of the CNS.
Microglia in MS
It is quite possible that the first description of microglia in the brain and spinal cord of a patient with MS was made by Carl Froininann who showed that cells (likely microglia) changed their morphology in CNS tissues from a 22-yr-old MS patient (Froininann 1878). However, despite of decades of research, the exact role of microglia in MS has not been clarified. In animal models of MS, macrophages and microglia have been reported to exert detrimental functions, including toxicity to neurons and oligodendrocyte precursor cells, release of proteases, production of inflammatory cytokines and reactive oxygen and nitrogen species, and recruitment and reactivation of T lymphocytes. Many studies, however, have also reported beneficial roles of macrophages and microglia, including roles in axonal regeneration, promotion of remyelination, clearance of inhibitory myelin debris, and the release of neurotrophic factors (Rawji and Yong 2013). A main point of confusion is that investigators have used microglia and macrophages interchangeably. Adding to the conceptual chaos, in EAE, a model for the inflammatory aspects of MS (Huang et al. 2006), fractalkine/receptor interactions mediate recruitment of disease-suppressing NK cells (Huang et al. 2006), which was shown subsequently to be a dominant effect (Garcia et al. 2013) over the effects of fractalkine/receptor interactions on microglia. The fog may be beginning to dissipate, however, on the role of microglia in EAE. Recently, using a CX3CR1-Cre mouse model similar to the one described by Parkhurst et al. (2013) above, Goldmann and colleagues (2013) found that conditional deletion of the transforming growth factor (TGF)-β-activated kinase 1 (TAK-1) in microglia only, not in neuroectodermal cells, suppressed EAE disease, significantly reduced CNS inflammation and diminished axonal and myelin damage by cell-autonomous inhibition of the NF-κB, JNK, and ERK-1/2 pathways. These results strongly suggest that microglia may promote tissue injury in EAE. Some caveats apply however. It is not entirely certain when the TAK-1 deletion from microglia exerts its effects. Further, it remains conceivable that the transient peripheral deletion of TAK-1 from all CX3CR1+ cells (an eccentricity of this genetic model) may play some role in the findings.
Another set of informative data came from examining dual-knockin mice in which Ccr2 was disrupted by insertion of red fluorescent protein (RFP), and the cross of these mice with CX3CR1gfp animals yielded animals in which both Ly6Chi and Ly6clo monocytes were labeled, as well as the microglia (Fig. 1). Studies at EAE onset revealed that macrophages derived from Ly6Chi monocytes initiated demyelination and made inflammatory mediators. In contrast, microglia did not associate with undisrupted axoglial units and showed an expression profile of repressed metabolism (Yamasaki et al. 2014). It will be useful to integrate the finding from this study with those in which TAK-1 was deleted from microglia. It is reasonable to speculate that microglial activities, which promote EAE, take place before disease onset.
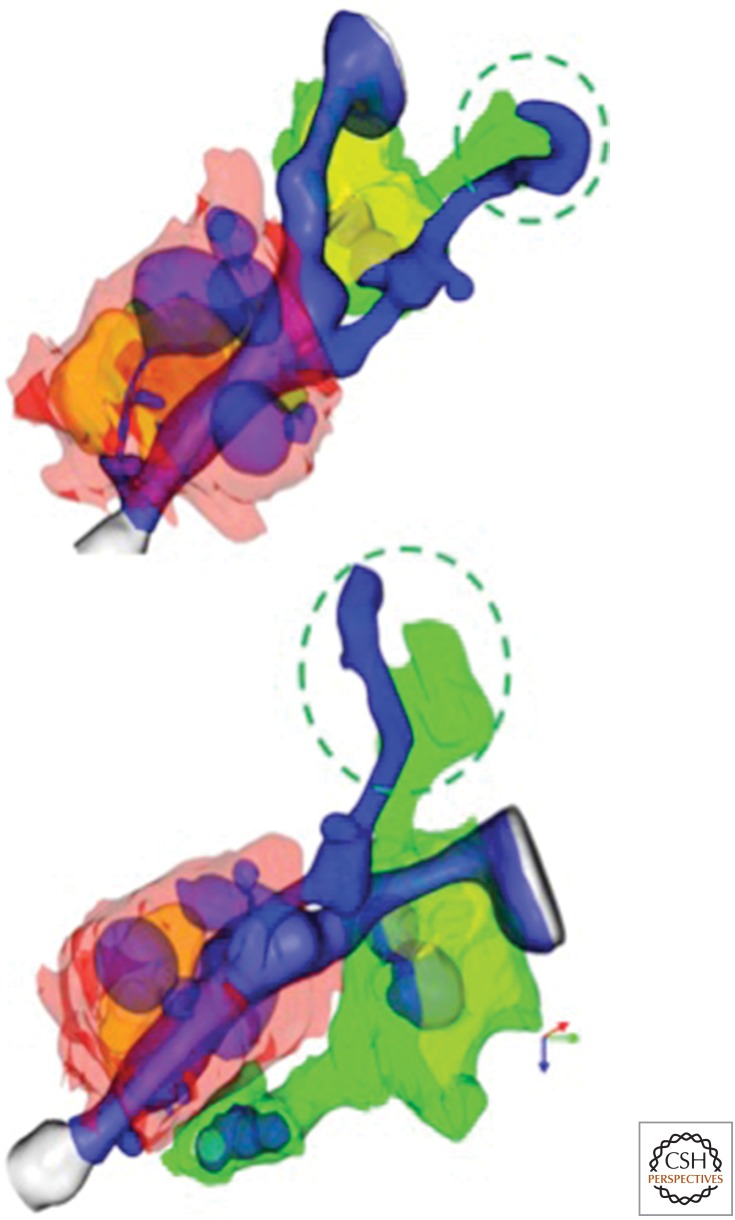
Figure 1.
Microglia begin to clear myelin debris at EAE onset. Three-dimensional reconstructions from serial block face scanning EM of spinal cord tissues of a mouse at day of EAE onset show a large flap of myelin debris (blue) engaged by a microglial process (green). A nearby monocyte (red cytosol; golden nucleus) enwraps a myelin internode and is actively removing myelin. Both monocyte and microglial cell (with yellow nucleus) contain myelin inclusions (blue), although those of the monocyte are larger and more numerous.
Finally, of course, the relevance of these observations to MS remains to be seen.
Microglia in AD
There is definitive evidence that AD is associated with significant accumulation of microglia in senile plaques (Fig. 2). Work over the past decade, however, suggests that, in addition to microglia, other mononuclear phagocytes, such as perivascular macrophages and circulating monocytes, may also be involved.
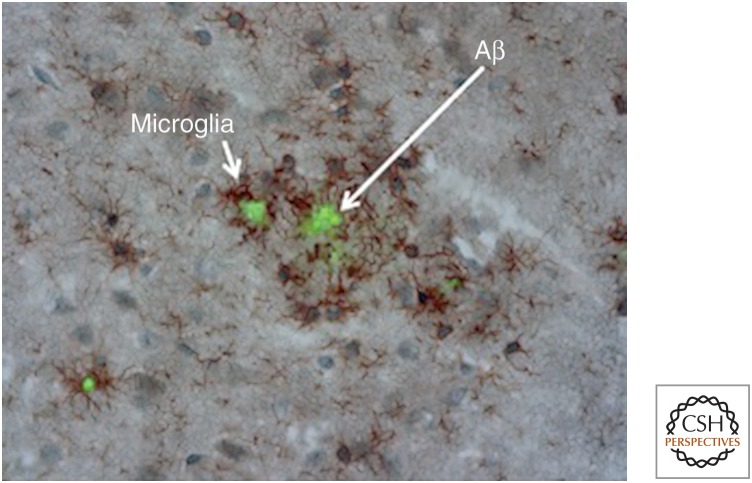
Figure 2.
β-Amyloid (Aβ) deposits are surrounded by microglia. Microglia, stained here in brown for CD11b, cluster around sites of Aβ deposition, stained in green with thioflavin S, in a mouse model of cerebral amyloid deposition.
Microglia are two- to fivefold more concentrated around plaques in AD patients and mouse models of this disease (Frautschy et al. 1998) and express major histocompatibility complex II and markers of inflammation (Tooyama et al. 1990; McGeer and McGeer 2010). β-Amyloid (Aβ) activates neonatal mouse microglia in vitro to produce reactive nitrogen species and TNF-α (Meda et al. 1995), a process that is mediated in part by binding Aβ to a CD36-TLR4-TLR6 receptor complex on microglia (El Khoury et al. 2003; Stewart et al. 2010). Aβ also activates the NLRP3 inflammasome in microglia leading to neurodegeneration (Heneka et al. 2013).
Although we have some insight into how microglia change following interaction with Aβ, understanding how microglia accumulate at sites of Aβ deposition has proven to be more difficult, and hinted at a role for peripheral monocytes in AD. Transgenic mice expressing the human Aβ protein precursor with the Swedish mutation (APP Tg2576) and deficient in the chemokine receptor CCR2 (APP-CCR2−/−) have reduced numbers of monocytes in the brain early in the disease process, before formation of senile plaques. This reduction in the number of monocytes was associated with increased mortality and higher Aβ levels exclusively around blood vessels in the brain, suggesting that early monocyte accumulation promotes the clearance of perivascular Aβ and protects mice from Aβ toxicity early in the disease process (El Khoury et al. 2007). The process of Aβ clearance is at least, in part, mediated by phagocytosis via the scavenger receptor SCARA1 (El Khoury et al. 1996; Frenkel et al. 2013).
Perivascular Aβ deposits are found in a high percentage of AD patients and are a hallmark of cerebral amyloid angiopathy. Because microglia express little CCR2 (Mizutani et al. 2012), these findings raise the possibility that accumulation of microglia at parenchymal sites of Aβ deposition involves a separate process that does not require CCR2, unlike what happens at perivascular sites of Aβ deposition. This is indirectly supported by results showing that selective depletion of perivascular macrophages exacerbates perivascular Aβ deposition, and stimulation of their turnover reduces perivascular load (Hawkes and McLaurin 2009). The distinction becomes less critical if, as proposed by Kumar-Singh and colleagues (2005), all Aβ deposits develop initially around blood vessels and then these vessels are obliterated leading to formation of the classic plaques. The latter possibility is supported by experiments in which adoptively transferred monocytes home to senile plaques in a mouse model of AD (Lebson et al. 2010).
Regardless of whether Aβ clearance is the task of microglia or recruited perivascular monocytes, the ability of microglia to clear Aβ appears to be reduced with disease progression. Expression of microglial Aβ phagocytic receptors and Aβ-degrading enzymes in a mouse model of amyloid deposition in AD is significantly reduced (Hickman et al. 2008), suggesting that accumulation of Aβ is in part the result of a failure of microglia to adequately clear this toxic peptide.
Additional insight into the role of microglia in AD came from analyzing the role of CX3CR1 in mouse models of amyloid deposition and tauopathy. CX3CR1-deficient animals show enhanced Aβ clearance (Lee et al. 2010; Liu et al. 2010), with a prominent gene-dosage effect. Increased IL-1β is also present in the CNS of the fractalkine receptor knockout animals, and the phenotype resembles that of mice overexpressing IL-1β (Shaftel et al. 2007). In mice with tau pathology, absence of CX3CR1 substantially worsens disease progression, neuropathologically, biochemically, and behaviorally (Bhaskar et al. 2010). It is plausible that increased microglial production of IL-1β is responsible both for enhanced Aβ clearance and for worsened tau pathology (Prinz et al. 2011).
Such findings raise the interesting question of whether soluble fractalkine (sFKN) and membrane-tethered fractalkine (mFKN) are functionally distinct. The corollary question is whether contact-dependent interactions between neurons and microglia via fractalkine/receptor interactions support the same functions as does signaling at a distance through cleaved fractalkine. Studies in the periphery suggested distinct functions of the two-fractalkine isoforms, as mFKN rescued survival of Ly6Clo monocytes, whereas sFKN reversed a morphological defect in CX3CR1+ gut dendritic cells (Kim et al. 2011). Use of recombinant adeno-associated virus (AAV) vectors to deliver fractalkine isoforms showed that sFKN (but not mFKN) reversed the severely augmented toxicity of MPTP for substantia nigra dopamine neurons (Morganti et al. 2012). This observation was extended to a therapeutic paradigm by using these AAV vectors to overexpress sFKN in a fractalkine-sufficient model of genetic tau pathology (Nash et al. 2013), with substantial benefit. However, the same approach was neither beneficial nor deleterious in an amyloid deposition model (Nash et al. 2013), compatible with earlier results using the LPS challenge (Zujovic et al. 2001).
Because recent findings suggest that tau pathology may occur following Aβ deposition, and based on the above discussion, we propose that the role of microglia in AD evolves with disease progression. Microglia initially interact with Aβ possibly in an attempt to clear it. But the continuous accumulation of Aβ also leads to induction of inflammatory cytokines that reduce the Aβ clearance ability of microglia and lead to tau pathology, initiating a self-perpetuating loop that culminates in worsening disease (see Table 1). This is reminiscent of the sequence of events that occur in atherosclerosis, another degenerative disease of aging. Monocytes are initially recruited to sites of deposition of modified lipoproteins, likely in an attempt to clear them. Continued accumulation of such lipoproteins overwhelms the ability of the monocytes to clear them and transforms these cells into foam cells that contribute to disease progression. Of interest, scavenger receptors that appear to mediate the interactions with Aβ in AD also mediate the interactions of monocytes with modified lipoproteins in atherosclerosis.
Table 1.
The neuroimmune system plays beneficial and detrimental roles in Alzheimer’s disease
| Potential beneficial roles | Potential detrimental roles |
|---|---|
| Aβ phagocytosis Release of Aβ-degrading enzymes Clearance of debris and degenerating neurons Release of neurotropic factors |
Release of inflammatory cytokines Release of reactive oxygen and nitrogen species |
In addition to their roles in MS and AD, microglia have been implicated in a number of neurological and neuropsychiatric disorders (El Khoury 2010). For example, microglia in a mouse model of amyotrophic lateral sclerosis (ALS) have a distinct molecular signature (Chiu et al. 2013). A defect in the microglial/myeloid gene Hoxb8 is associated with compulsive grooming similar to behavior in humans with obsessive–compulsive disorder (Chen et al. 2010). Another example is Rett syndrome, an X-linked autism spectrum disorder. In a mouse model of Rett syndrome, normal microglia can arrest the pathology and attenuate disease symptoms (Derecki et al. 2012).
TOWARD A MOLECULAR DEFINITION OF MICROGLIA
It is evident from the above discussion that the ability to distinguish microglia from other cells of the CNS as well as from other mononuclear phagocytes is crucial to understanding the functions of these cells in physiological and pathological processes. Development of methods to isolate microglia from adult animals with high purity and yield as well as recent advances in approaches to define the transcriptomes of various cells by RNASeq and microarrays allowed four independent groups to define the transcriptomes of adult mouse microglia and make these data publicly available in 2013 (Beutner et al. 2013; Chiu et al. 2013; Hickman et al. 2013; Butovsky et al. 2014). These results showed that, although microglia share several important transcripts with other mononuclear phagocytes, they also have significant differences. Analysis of these datasets shows that P2ry12 and P2ry13, Tmem119, Gpr34, Siglech, Trem2, and CX3CR1 are among the transcripts uniquely expressed in microglia compared with other mononuclear phagocytes. Macrophage-enriched genes include fibronectin, the chemokine Cxcl13, and the endothelin B receptor.
Analysis of these datasets also allowed correlation between microglial gene expression and function. For example, a cluster of genes termed “sensome” was introduced that defines the genes used by microglial to sense their environment (Hickman et al. 2013), a major function of microglia described using two-photon imaging (Davalos et al. 2005; Nimmerjahn et al. 2005). This cluster includes 100 genes that constitute the microglial toolset for sensing changes in the brain’s milieu and define the apparatus that microglia use to perform various homeostatic functions including sensing of chemokines and cytokines, purinergic molecules, inorganic substances, changes in pH and amino acids. Defining the microglial sensome under physiological conditions, establishes a baseline to which we can compare and identify changes that occur in this sensome under pathological conditions. Highlighting the importance of these datasets, two members of the microglial sensome, TREM2 and CD33, have been found to be independent risk factors for late onset AD (Jonsson et al. 2012; Bradshaw et al. 2013; Guerreiro et al. 2013).
Another important result in these datasets is the finding that resident microglia express high levels of several antimicrobial peptides not previously known to be expressed on these cells (Hickman et al. 2013). These include Camp, the cathelicidin-related antimicrobial peptide and Ngp, the neutrophilic granule protein. The high level of expression of these peptides indicates a high level of readiness by the normal “quiescent” resident microglia to perform its innate host defense function in the absence of adaptive immune molecules and cells. These findings further support the concept that there is no “resting” microglia, and that these cells are highly dynamic cells important for surveillance, protection, and housekeeping of the neural milieu.
CONCLUSIONS
It is an exciting time to study microglia. Based on the rapid pace of discovery in the past decade, and the continued development of new technologies, it is highly likely that the next decade will bring our level of understanding of these cells on par to our levels of understanding of other innate immune cells in the periphery and allow us to harness such knowledge to define the role of these cells in normal and pathological processes and to possibly target these cells for diagnosis and/or treatment of various CNS disorders. To fully define the exact roles of microglia in these disorders, experiments mapping the gene expression profiles of microglia and other neuroimmune cells in mouse models and patient samples and at multiple stages of various CNS disorders need to be performed (El Khoury 2010).
Footnotes
Editors: Ben A. Barres, Marc R. Freeman, and Beth Stevens
Additional Perspectives on Glia available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. 2007. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10: 1538–1543. [DOI] [PubMed] [Google Scholar]
- Alliot F, Lecain E, Grima B, Pessac B. 1991. Microglial progenitors with a high proliferative potential in the embryonic and adult mouse brain. Proc Natl Acad Sci 88: 1541–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. 1999. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 117: 145–152. [DOI] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. 1997. A new class of membrane-bound chemokine with a CX3C motif. Nature 385: 640–644. [DOI] [PubMed] [Google Scholar]
- Beutner C, Linnartz-Gerlach B, Schmidt SV, Beyer M, Mallmann MR, Staratschek-Jox A, Schultze JL, Neumann H. 2013. Unique transcriptome signature of mouse microglia. Glia 61: 1429–1442. [DOI] [PubMed] [Google Scholar]
- Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT. 2010. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme SA, Lio FM, Maciejewski-Lenoir D, Bacon KB, Conlon PJ. 2000. The chemokine fractalkine inhibits Fas-mediated cell death of brain microglia. J Immunol 165: 397–403. [DOI] [PubMed] [Google Scholar]
- Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A, Rosenkrantz LL, Imboywa S, Lee M, Von Korff A, et al. 2013. CD33 Alzheimer’s disease locus: Altered monocyte function and amyloid biology. Nat Neurosci 16: 848–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. 2014. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci 17: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, Hu T, Ransohoff RM. 2008. Scavenging roles of chemokine receptors: Chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood 112: 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano M, Lauro C, Cipriani R, Chece G, Ponzetta A, Di Angelantonio S, Ragozzino D, Limatola C. 2013. CX3CL1 protects neurons against excitotoxicity enhancing GLT-1 activity on astrocytes. J Neuroimmunol 263: 75–82. [DOI] [PubMed] [Google Scholar]
- Chapman GA, Moores K, Harrison D, Campbell CA, Stewart BR, Strijbos PJ. 2000. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J Neurosci 20: RC87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. 2006. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354: 610–621. [DOI] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR. 2010. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell 141: 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP, Muratet M, Carroll MC, Levy S, Tavazoie S, et al. 2013. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 4: 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani R, Villa P, Chece G, Lauro C, Paladini A, Micotti E, Perego C, De Simoni MG, Fredholm BB, Eusebi F, et al. 2011. CX3CL1 is neuroprotective in permanent focal cerebral ischemia in rodents. J Neurosci 31: 16327–16335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Yip PK, Malcangio M. 2009. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J Neurosci 29: 6945–6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DN, Chen SC, Sullivan LM, Manfra DJ, Wiekowski MT, Prosser DM, Vassileva G, Lira SA. 2001. Generation and analysis of mice lacking the chemokine fractalkine. Mol Cell Biol 21: 3159–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzner ML, Hayes GM, Newcombe J, Woodroofe MN. 1988. The nature of inflammatory components during demyelination in multiple sclerosis. J Neuroimmunol 20: 203–209. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8: 752–758. [DOI] [PubMed] [Google Scholar]
- del Río-Hortega Pd. 1918. Noticia de un nuevo y fácil método para la coloración de la neuroglia y el tejido conjuntivo [A new and easiest approach for staining glia and connective tissue]. Trab Lab Inv Biol 15: 367–378. [Google Scholar]
- del Río-Hortega Pd. 1919. El “tercer elemento” de los centros nerviosos. Poder fagocitario y mivilidad de la microglía [The “third element” of the central nervous system. Phagocytic power of microglia]. Bol Soc Esp Biol 8: 62–82. [Google Scholar]
- del Río-Hortega Pd. 1920. La microglía y su transformación en células en bastoncito y cuerpos granuloadiposos [Transformation in the microglial cell bodies]. Trab Lab Inv Biol 18: 37–82. [Google Scholar]
- del Río-Hortega Pd. 1921. Histogénesis y evolución normal, éxodo y distrubución regional de la microglía [Evolution and regional distribution of microglia]. Mem Real Soc Esp Hist Nat 11: 213–268. [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG, Kipnis J. 2012. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature 484: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J. 2010. Neurodegeneration and the neuroimmune system. Nat Med 16: 1369–1370. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. 1996. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature 382: 716–719. [DOI] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. 2003. CD36 mediates the innate host response to β-amyloid. J Exp Med 197: 1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. 2007. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13: 432–438. [DOI] [PubMed] [Google Scholar]
- Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD. 1998. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med 188: 1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. 1998. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol 152: 307–317. [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, Farfara D, Kingery ND, Weiner HL, El Khoury J. 2013. Scara1 deficiency impairs clearance of soluble amyloid-β by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat Commun 4: 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froininann C. 1878. Untersuchungen über die Gewebveränderungen bei der multiplen sklerose des gehirns und ruckenmarks [Studies in multiple sclerosis of the brain]. Gustav Fischer, Jena, Germany. [Google Scholar]
- Garcia JA, Pino PA, Mizutani M, Cardona SM, Charo IF, Ransohoff RM, Forsthuber TG, Cardona AE. 2013. Regulation of adaptive immunity by the fractalkine receptor during autoimmune inflammation. J Immunol 191: 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. 2001. Tumor necrosis factor-α-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J Biol Chem 276: 37993–38001. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. 1986. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci 6: 2163–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Müller PF, Wolf Y, Varol D, Yona S, Brendecke SM, Kierdorf K, Staszewski O, Datta M, et al. 2013. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci 16: 1618–1626. [DOI] [PubMed] [Google Scholar]
- Graeber MB, López-Redondo F, Ikoma E, Ishikawa M, Imai Y, Nakajima K, Kreutzberg GW, Kohsaka S. 1998. The microglia/macrophage response in the neonatal rat facial nucleus following axotomy. Brain Res 813: 241–253. [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kündig TM, Frei K, Ginhoux F, Merad M, et al. 2012. Stroma-derived interleukin-34 controls the development and maintenance of Langerhans cells and the maintenance of microglia. Immunity 37: 1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, et al. 2013. TREM2 variants in Alzheimer’s disease. N Engl J Med 368: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, et al. 1998. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci 95: 10896–10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell CA, Cleary MD, Charo IF. 1999. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem 274: 10053–10058. [DOI] [PubMed] [Google Scholar]
- Haskell CA, Cleary MD, Charo IF. 2000. Unique role of the chemokine domain of fractalkine in cell capture. Kinetics of receptor dissociation correlate with cell adhesion. J Biol Chem 275: 34183–34189. [DOI] [PubMed] [Google Scholar]
- Haskell CA, Hancock WW, Salant DJ, Gao W, Csizmadia V, Peters W, Faia K, Fituri O, Rottman JB, Charo IF. 2001. Targeted deletion of CX3CR1 reveals a role for fractalkine in cardiac allograft rejection. J Clin Invest 108: 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CA, McLaurin J. 2009. Selective targeting of perivascular macrophages for clearance of β-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci 106: 1261–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. 2006. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9: 1512–1519. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, et al. 2013. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. 2008. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci 28: 8354–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. 2013. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 16: 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DM, Mizoue LS, Handel TM, Lubkowski J. 2000. The crystal structure of the chemokine domain of fractalkine shows a novel quaternary arrangement. J Biol Chem 275: 23187–23193. [DOI] [PubMed] [Google Scholar]
- Horuk R. 1994. Molecular properties of the chemokine receptor family. Trends Pharmacol Sci 15: 159–165. [DOI] [PubMed] [Google Scholar]
- Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren HG, Biron CA, et al. 2006. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J 20: 896–905. [DOI] [PubMed] [Google Scholar]
- Hume DA, Perry VH, Gordon S. 1983. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: Phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol 97: 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, et al. 2003. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell–cell adhesion. Blood 102: 1186–1195. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. 1996. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun 224: 855–862. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, et al. 2012. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. 2000. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20: 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, Prinz M. 2013. Factors regulating microglia activation. Front Cell Neurosci 7: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Vallon-Eberhard A, Zigmond E, Farache J, Shezen E, Shakhar G, Ludwig A, Lira SA, Jung S. 2011. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood 118: e156–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, Van Broeckhoven C. 2005. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am J Pathol 167: 527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro C, Di Angelantonio S, Cipriani R, Sobrero F, Antonilli L, Brusadin V, Ragozzino D, Limatola C. 2008. Activity of adenosine receptors type 1 is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol 180: 7590–7596. [DOI] [PubMed] [Google Scholar]
- Lauro C, Cipriani R, Catalano M, Trettel F, Chece G, Brusadin V, Antonilli L, van Rooijen N, Eusebi F, Fredholm BB, et al. 2010. Adenosine A1 receptors and microglial cells mediate CX3CL1-induced protection of hippocampal neurons against Glu-induced death. Neuropsychopharmacology 35: 1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebson L, Nash K, Kamath S, Herber D, Carty N, Lee DC, Li Q, Szekeres K, Jinwal U, Koren J, et al. 2010. Trafficking CD11b-positive blood cells deliver therapeutic genes to the brain of amyloid-depositing transgenic mice. J Neurosci 30: 9651–9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. 2010. CX3CR1 deficiency alters microglial activation and reduces β-amyloid deposition in two Alzheimer’s disease mouse models. Am J Pathol 177: 2549–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Condello C, Schain A, Harb R, Grutzendler J. 2010. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J Neurosci 30: 17091–17101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. 1998. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4 or SDF-1 deficient mice. Proc Natl Acad Sci 95: 9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. 2010. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: A field in its infancy. J Alzheimers Dis 19: 355–361. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. 1987. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett 79: 195–200. [DOI] [PubMed] [Google Scholar]
- McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, et al. 1996. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J 15: 5647–5658. [PMC free article] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L Jr, Baron P, Villalba M, Ferrari D, Rossi F. 1995. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature 374: 647–650. [DOI] [PubMed] [Google Scholar]
- Mizoue LS, Bazan JF, Johnson EC, Handel TM. 1999. Solution structure and dynamics of the CX3C chemokine domain of fractalkine and its interaction with an N-terminal fragment of CX3CR1. Biochemistry 38: 1402–1414. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM, Cardona AE. 2012. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J Immunol 188: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti JM, Nash KR, Grimmig BA, Ranjit S, Small B, Bickford PC, Gemma C. 2012. The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson’s disease. J Neurosci 32: 14592–14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Watanabe Y, Osio N, Higuchi T, Shigeta A, Mizuguchi N, Katou F, Hashimoto K, Kawada A, Yoshie O. 2010. Eotaxin-3/CC chemokine ligand 26 is a functional ligand for CX3CR1. J Immunol 185: 6472–6479. [DOI] [PubMed] [Google Scholar]
- Nash KR, Lee DC, Hunt JB Jr, Morganti JM, Selenica ML, Moran P, Reid P, Brownlow M, Guang-Yu Yang C, Savalia M, et al. 2013. Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy. Neurobiol Aging 34: 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314–1318. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Gross CT. 2011. Microglia in development: Linking brain wiring to brain environment. Neuron Glia Biol 7: 77–83. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. 2011. Synaptic pruning by microglia is necessary for normal brain development. Science 333: 1456–1458. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR III, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. 2013. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155: 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. 1985. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 15: 313–326. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. 2011. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 14: 1227–1235. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. 2005. Selective leukocyte chemoattractants emerge from the primeval sup(ernatants). J Immunol 175: 5567–5568. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. 2010. The myeloid cells of the central nervous system parenchyma. Nature 468: 253–262. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Stevens B. 2011. Neuroscience. How many cell types does it take to wire a brain? Science 333: 1391–1392. [DOI] [PubMed] [Google Scholar]
- Rawji KS, Yong VW. 2013. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin Dev Immunol 2013: 948976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A, von Andrian UH. 2004. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu Rev Immunol 22: 891–928. [DOI] [PubMed] [Google Scholar]
- Rozemuller JM, Eikelenboom P, Stam FC. 1986. Role of microglia in plaque formation in senile dementia of the Alzheimer type. An immunohistochemical study. Virchows Arch B Cell Pathol Incl Mol Pathol 51: 247–254. [DOI] [PubMed] [Google Scholar]
- *.Schafer DP, Stevens B. 2015. Microglia function in central nervous system development and plasticity. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, et al. 2012. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O’Banion MK. 2007. Sustained hippocampal IL-1β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest 117: 1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, Fairchild-Huntress V, Fang Q, Dunmore JH, Huszar D, et al. 2002. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol 125: 59–65. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, et al. 2010. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnemark D, Eltayeb S, Nilsson M, Wallström E, Lassmann H, Olsson T, Berg AL, Ericsson-Dahlstrand A. 2005. CX3CL1 (fractalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: Kinetics and cellular origin. J Neuroinflammation 2: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooyama I, Kimura H, Akiyama H, McGeer PL. 1990. Reactive microglia express class I and class II major histocompatibility complex antigens in Alzheimer’s disease. Brain Res 523: 273–280. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. 2010. Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8: e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2: 732–738. [DOI] [PubMed] [Google Scholar]
- Tsou CL, Haskell CA, Charo IF. 2001. Tumor necrosis factor-α-converting enzyme mediates the inducible cleavage of fractalkine. J Biol Chem 276: 44622–44626. [DOI] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. 2013. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16: 543–551. [DOI] [PubMed] [Google Scholar]
- Walz W, Ilschner S, Ohlemeyer C, Banati R, Kettenmann H. 1993. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. J Neurosci 13: 4403–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. 2012. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13: 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, et al. 2014. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 211: 1533–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, et al. 2014. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17: 400–406. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. 1998. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393: 595–599. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D, Warfel A, Grusky G, Frangione B, Teitel D. 1987. Novel monocyte-like properties of microglial/astroglial cells. Constitutive secretion of lysozyme and cystatin-C. Lab Invest 57: 176–185. [PubMed] [Google Scholar]
- Zujovic V, Schussler N, Jourdain D, Duverger D, Taupin V. 2001. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNF-α and 8-isoprostane production induced by intracerebroventricular injection of LPS. J Neuroimmunol 115: 135–143. [DOI] [PubMed] [Google Scholar]