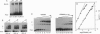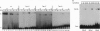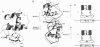Abstract
The 1,25-dihydroxyvitamin D3 receptor, like other members of the nuclear receptor superfamily, forms dimers in solution that are probably stabilized by a dyad symmetrical interface formed by the ligand-binding domain. This receptor, however, recognizes DNA targets that are not dyad symmetric but rather are organized as direct repeats of a hexameric sequence with a characteristic 3-bp spacing. Using molecular modeling and site-directed mutagenesis, we have identified regions within the vitamin D3 receptor zinc finger region that confer selectivity for direct repeats with appropriate spacing. Reflecting the organization of the DNA target, these regions, mapping to the tip of the first zinc finger module and the N and C termini of the second finger module, direct asymmetrical protein-protein contacts. A stereochemical model is proposed for these interactions.
Full text
PDF

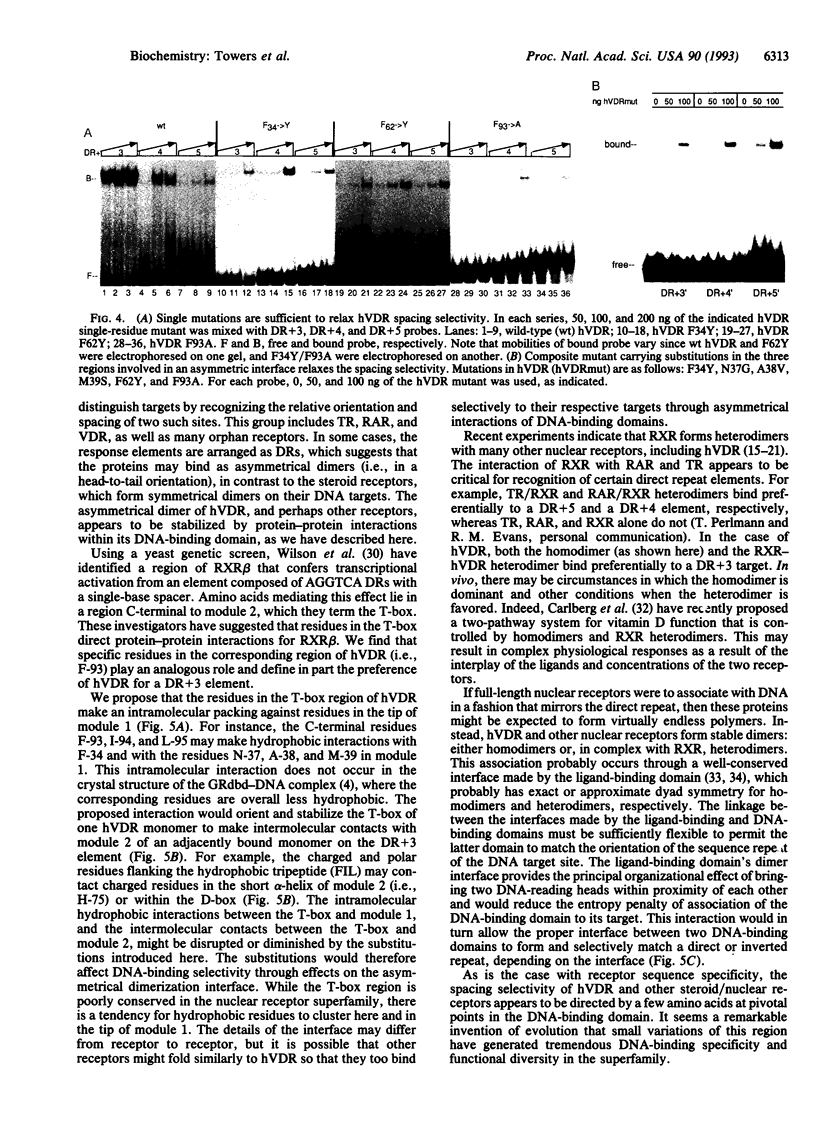

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alroy I., Freedman L. P. DNA binding analysis of glucocorticoid receptor specificity mutants. Nucleic Acids Res. 1992 Mar 11;20(5):1045–1052. doi: 10.1093/nar/20.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A. R., McDonnell D. P., Hughes M., Crisp T. M., Mangelsdorf D. J., Haussler M. R., Pike J. W., Shine J., O'Malley B. W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988 May;85(10):3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlman-Wright K., Wright A., Gustafsson J. A., Carlstedt-Duke J. Interaction of the glucocorticoid receptor DNA-binding domain with DNA as a dimer is mediated by a short segment of five amino acids. J Biol Chem. 1991 Feb 15;266(5):3107–3112. [PubMed] [Google Scholar]
- Freedman L. P., Luisi B. F., Korszun Z. R., Basavappa R., Sigler P. B., Yamamoto K. R. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988 Aug 11;334(6182):543–546. doi: 10.1038/334543a0. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Härd T., Dahlman K., Carlstedt-Duke J., Gustafsson J. A., Rigler R. Cooperativity and specificity in the interactions between DNA and the glucocorticoid receptor DNA-binding domain. Biochemistry. 1990 Jun 5;29(22):5358–5364. doi: 10.1021/bi00474a022. [DOI] [PubMed] [Google Scholar]
- Härd T., Kellenbach E., Boelens R., Maler B. A., Dahlman K., Freedman L. P., Carlstedt-Duke J., Yamamoto K. R., Gustafsson J. A., Kaptein R. Solution structure of the glucocorticoid receptor DNA-binding domain. Science. 1990 Jul 13;249(4965):157–160. doi: 10.1126/science.2115209. [DOI] [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Schwabe J. W., Neuhaus D., Rhodes D. Solution structure of the DNA-binding domain of the oestrogen receptor. Nature. 1990 Nov 29;348(6300):458–461. doi: 10.1038/348458a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]