Abstract
Prosthetic valve obstruction (PVO) is a rare but feared complication of mechanical valve replacement. Diagnostic evaluation should focus on differentiating prosthetic valve thrombosis (PVT) from pannus formation, as their treatment options differ. History of sub-optimal anti-coagulation and post-op time course to development of PVO are useful clinical characteristics in differentiating thrombus from pannus formation. Treatment of PVT is influenced by the patient’s symptoms, valve location, degree of obstruction and thrombus size and may include thrombolysis or surgical intervention. Alternatively, pannus formation requires surgical intervention. The purpose of this article is to review the pathophysiology, epidemiology, diagnostic approach and treatment options for aortic and mitral valve PVO.
Keywords: Prosthetic valve thrombosis, Pannus overgrowth, Thrombolysis, Prosthetic valve obstruction, Echocardiography
Core tip: Prosthetic valve obstruction (PVO), while rare, is a dreaded complication of mechanical valve replacement. Careful clinical and multiple non-invasive imaging modalities are necessary to assess suspected PVO and evaluate for pannus overgrowth or valve thrombosis. Unlike pannus overgrowth, prosthetic valve thrombosis is more common, occurs earlier in the post-op period, is frequently related to inadequate anti-coagulation, and can often be treated through non-invasive thrombolysis. While the current understanding of pannus overgrowth remains elusive, future clarification of its pathophysiology may allow for the development of non-invasive therapeutic options.
INTRODUCTION
A 60-year-old male underwent 1-vessel coronary artery bypass graft and a 31 mm bileaflet St. Jude’s mechanical mitral valve (MV) replacement for newly diagnosed ischemic cardiomyopathy and functional mitral regurgitation. His post-op course was uneventful and he reported self-compliance with all his medications. Three months after his surgery he was admitted for shortness of breath and was found to be hypotensive with jugular venous distention, warm extremities with pitting edema bilaterally, and a new 3/6 holosystolic murmur with a 2/4 diastolic rumble- both radiating to the axilla. His international normalized ratio (INR) was 1.3.
Transthoracic echocardiography (TTE) revealed an unchanged ejection fraction and a fixed closed mitral leaflet disc with a transmitral Doppler mean gradient of 13 mmHg. His calculated MV area was 0.41 cm2 (via continuity equation), maximum MV E wave velocity of 1.7 m/s and new severe right ventricle dilatation, dysfunction, and tricuspid regurgitation were also present. Transesophageal echocardiography (TEE) confirmed a fixed mitral leaflet (Figure 1), and a soft thrombus in left atrial appendage. A small soft non-mobile mass (5-6 mm) adjacent to the sewing ring on the fixed leaflet was identified. Follow up TTE and cine fluoroscopy (CF) confirmed residual immobility of the posterior occluded prosthetic leaflet.
Figure 1.
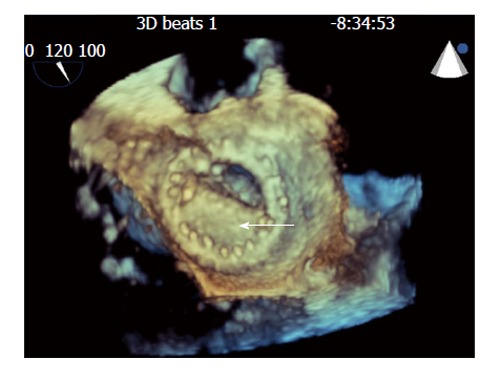
Still frames of 3-dimensional transesophogeal echocardiographic rendering of the mechanical bi-leaflet mitral valve as visualized from the left atrial perspective during diastole showing fixed mitral leaflet (arrow).
He was treated with intravenous furosemide with symptomatic improvement. Tissue plasminogen activator was administered (10 mg bolus centrally through Swan Ganz catheter followed by a 90 mg infusion peripherally over 5 h). Follow up TTE transmitral gradient via Doppler interrogation demonstrated a significant decrease to 4 mmHg. A decision was made to pursue redo-mitral valve replacement with a 31 mm St. Jude’s porcine bioprosthesis since the valve remained in the closed position. Gross sample revealed residual organized thrombus on the mitral valve disc (Figure 2). Three-month follow-up TEE showed no change in transmitral gradient.
Figure 2.
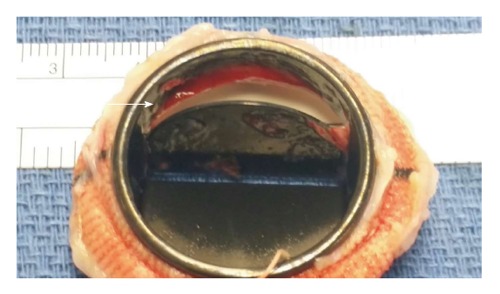
Gross sample of explanted mechanical mitral valve revealing the transesophogeal echocardiography finding residual organized thrombus, apparent on the mitral valve disc (arrow).
Pathophysiology
Prosthetic valve replacement whether mechanical or bioprosthetic carries an inherent risk for serious, sometimes devastating complications. Obstruction of prosthetic valves can result from thrombus, pannus overgrowth, vegitations or combination of thrombus and pannus formation.
Prosthetic valve thrombosis: Prosthetic valve obstruction (PVO) is a rare but dreaded post-surgical complication, with the most common cause being prosthetic valve thrombosis (PVT). PVT occurs more commonly in mechanical, as compared to biologic prostheses, likely related to the underlying pathophysiology of thrombus development[1,2].
Post-surgical endothelization after prosthetic valve surgery occurs over weeks to months. During this time, the exposed and healing endothelium may serve as a nidus for clot formation. Typically, an initial small thrombus may develop and act as a further substrate for additional layering of new thrombus[3]. In addition, the post op course of a newly placed mechanical valve results in the development of turbulent flow and stasis which is an additional contributor to thrombus development. This relative stasis and aberrant flow helps explain why tricuspid valve thrombosis is 20 times as common as left sided thrombosis, and MV thrombosis is more common than aortic valve (AV) thrombosis[3]. Similarly, increased prosthetic surface area has been correlated to a greater formation of both thrombi and pannus[4].
The intrinsic prothrombotic milieu post valve replacement requires strict anticoagulation to avoid complications. Thus, multiple investigators have observed a significantly higher incidence of thrombotic complications among patients with subtherapeutic anticoagulation, which has been validated as the best clinical tool to differentiate pannus from thrombus, as discussed below[1,5,6].
Pannus overgrowth: Although less common than thrombus formation, pannus may develop over prosthetic valves. A biologic reaction to the prosthesis material with unknown mechanism is thought to cause fibroelastic and collagen overgrowth, with subsequent infiltration of endothelial cells, myofibroblasts, and chronic inflammatory cells resulting in fibrinous ingrowth around the prosthetic valve annulus[1,4,7].
The precise trigger for pannus formation remains unclear at this time, further limiting the ability to prevent and treat this phenomenon. Peripheral blood samples of patients with pannus formation have elevated levels of the proliferation and cell differentiation signaling protein transforming growth factor-beta (TGFβ) when compared to a control cohort (87.7 ng/mL vs 73.7 ng/mL, P < 0.05)[7]. A careful immunohistochemical analysis of these patients’ surgical valve specimens revealed endothelial cells, myofibroblasts and macrophages-each with a cell specific expression profile at the left ventricular pre-annular septum. While the profile differed based on cell type, two of the three cells had increased expression of TGFβ, with all three having increased expression of TGFβ receptor 1. Thus, it would appear that aortic valve pannus originates from the healing process occurring at the junction of the neointima, which is mediated by TGFβ.
It should be noted that many investigators have identified mechanical valve obstructions with both elements of pannus and thrombus. It is likely that pannus serves as a nidus for thrombus, with pannus formation being the underlying cause[1]. The prevalence of these concomitant factors has been reported to be between 12%-75% of all PVO[1,3,4].
Epidemiology
The overall incidence of PVO ranges from 0.4%-6.0% annually with the difference in rates depending on the type and location of prosthetic valve replacement[1,8-10]. This may be underestimated as routine post-op screening for PVO is not typically performed, unless patients become symptomatic. For instance, in observing 680 consecutive patients who underwent prosthetic valve surgery, Laplace et al[11] observed 64 patients (9.4%) with evidence of significant valve thrombosis starting as early as 9 d post-op, a significantly higher rate as compared to those who present with symptoms.
An observational study by Deviri et al[1] found thrombus associated obstruction in 78% of cases (both MV and AV), pannus formation in 10.7% cases, and combination of thrombus and pannus for the remaining 11.6% cases. Overall, the time from valve replacement to obstruction ranged from 6 wk to 13 years (median 4 years)[1]. Alternatively, a report by Vitale et al[4] found pannus in 31%, thrombus in 24%, and both pannus and thrombus in 45% of MV PVO. When comparing mitral to aortic valve complications, aortic valves appear to have a higher incidence of pannus, while mitral valves more commonly have PVO from thrombus[1,5,8,12].
The annual incidence of PVT ranges between 0.03%-5.7%[1,3,10,13,14]. PVT can occur in mechanical or bioprosthetic valves and can result in non-obstruction to complete obstruction[9-12]. PVT is more common in mechanical compared to bioprosthetic valves, with the immediate post-op period being the time of highest risk. Although PVT can occur any time after valve replacement, 24% occur within one year postoperatively with subsequent decreases in incidence with each year that follows[1,3]. Compared to pannus formation, thrombotic valvular dysfunction appears to occur at an earlier time with larger masses on imaging[4,5,8,15]. As mentioned above, larger valves, valves exposed to decreased flow (i.e., mitral vs aortic, tricuspid vs left sided valves) and subtherapeutic anticoagulation status have been shown to be significant risk factors for PVT development[3,6,16].
Diagnosis
While a patient’s clinical presentation may suggest a possible prosthetic valve complication, diagnosis of PVO, and differentiating its etiology, requires direct visualization of the valve by various imaging modalities.
Valvular obstruction should be considered when an unexpected rise in trans-valvular gradient is observed on Doppler echocardiography. Non-invasive visualization utilizing modalities such as TTE, TEE, and CF are necessary to accurately diagnose and guide treatment strategies. Since the etiology of obstruction may guide choice of therapy, the differentiation of thrombus from pannus is an essential but often challenging task. Initial diagnostic evaluation should commence with TTE in order to assess valve motion, degree of obstruction, and clot burden but also exclude non-acquired obstruction like patient prosthetic mismatch (PPM).
Echocardiography: TTE with color Doppler is regarded as the initial step for diagnosis of PVO and is required to determine hemodynamic severity and impact on valve function[5,12,17]. Sudden increases in transvalvular gradients from baseline are indicative of valvular obstruction. However, it is important to consider other causes of increased prosthetic valve gradients such as high cardiac output states, pressure recovery [in AV replacement (AVR)], regurgitation, and PPM. Furthermore, TTE may be limited by prosthetic reverberation artifacts. In this scenario, the use of spectral Doppler may detect a stuck valve due to aberration of opening and closing spikes. More importantly, image optimization despite these limitations, can be attempted through the use of 3-dimensional (3D) TEE allowing a more precise and realistic visualization[18]. Girard et al[12] found that TTE correctly identify the pathological mechanism of mechanical AVR obstruction in only 10% of cases but 63% of bioprosthetic AVR. While TTE is usually inadequate for valvular leaflet investigation and often not sensitive enough to identify thrombi as compared to pannus, it is an essential screening modality and may accurately identify obstructive masses in > 80% of cases[5].
MV Doppler echocardiographic evaluation should focus on measuring the mean transmitral gradient and pressure half time (PHT), in addition to the use of continuity equation to calculate valve area[19,20]. PHT ≥ 130 ms has been shown to identify MV PVO in 99% of patients, however, its sensitivity is limited due to its relationship with atrial and ventricular compliance in addition to heart rate[2,21]. While peak early E velocity (PEV) ≥ 1.9 m/s has also been shown to be a useful screening tool for PVO (OR = 3.51; 95%CI: 1.62-7.57; for every 10-unit increments of peak E velocity in cm/s), even with a peak E < 1.9 m/s, the presence of HT ≥ 130 ms still correlates well with PVO. In addition, PHT ≥ 130 ms is especially helpful in differentiating PVO from prosthetic valve dysfunction and regurgitation. Thus, while PEV may suggest PVO, PHT ≥ 130 ms is necessary to indicate PVO, irrespective of PEV (Figure 3)[21].
Figure 3.
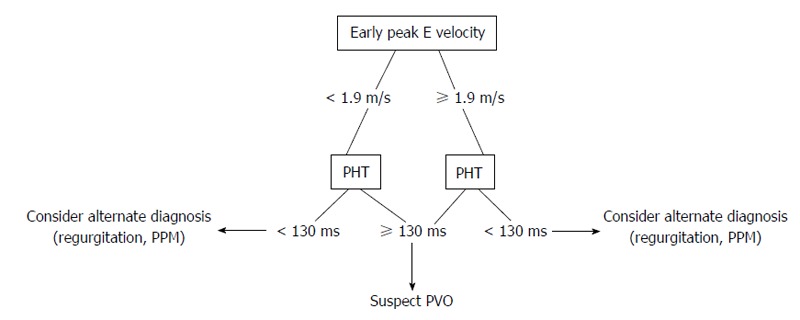
Proposed echocardiographic evaluation for suspected prosthetic mitral valve obstruction. PHT: Pressure half-time; PVO: Prosthetic valve obstruction; PPM: Prosthetic patient mismatch.
Aortic valve PVO investigation by echocardiography should begin with a Doppler peak and mean transvalvular gradients. These acquired values should then be compared to known brand and size specific published values[2]. Severe prosthetic AV stenosis is suggested (assuming a normal stroke volume) with the presence of a rounded symmetric Doppler jet, a peak velocity ≥ 4 m/s, mean gradient ≥ 35 mm/Hg, Doppler velocity index < 0.25, effective orifice area < 0.8 cm2 (or an indexed area to surface body area of < 0.65 cm2), and an acceleration time ≥ 100 ms. A ratio of acceleration time to ejection time of ≥ 0.4 has been demonstrated as a reliable angle-independent variable that is consistent with PVO (Figure 4)[22]. In addition, careful assessment should be made for any abnormal echo densities or valve motions. Other non-valvular parameters should also be closely measured and compared to prior studies, including left ventricular size, function, and hypertrophy[2].
Figure 4.
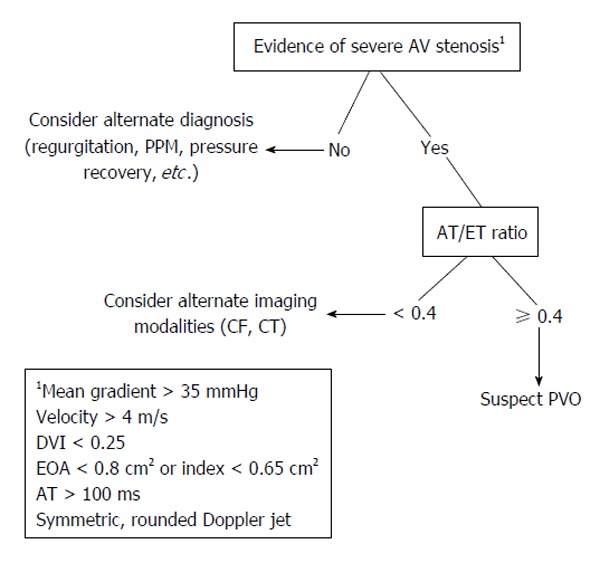
Proposed non-invasive evaluation for suspected prosthetic aortic valve obstruction. AT: Acceleration time; ET: Ejection time; PPM: Prosthetic-patient mismatch; CF: Cine fluoroscopy; CT: Computed tomography; PVO: Prosthetic valve obstruction; EOA: Effective orifice area; DVI: Doppler velocity index; AV: Aortic valve.
If there is a clinical suspicion for PVO but TTE Doppler is equivocal then stress TTE can be considered for further evaluation. While there is limited data regarding strict ranges and diagnostic cutoffs for PVO on stress TTE, a mean transmitral gradient rise of ≥ 15 mmHg (or ≥ 18 mmHg with AV prostheses) with stress has been suggested as a reliable marker to suggest PVO, even if the resting mean gradient is normal[2].
Use of TEE remains the gold standard for diagnosis of PVO and is required to determine the etiology of PVO as well as identifying candidates for thrombolytic therapy vs surgical intervention[12,23,24]. There is an additional benefit in using 3D echocardiography to more precisely visualize and evaluate the anatomy of both aortic and mitral prosthetic valves. As compared to standard 2-dimensional echo, 3D echocardiography allows a more detailed and accurate assessment of valve leaflets, prosthetic rings and struts. However, AV visualization still remains relatively difficult to image, as compared to the MV, given its distance from the transducer and its oblique angle of incidence as related to the ultrasound beam. Additionally, 3D TEE has been shown to have a high correlation with surgical findings, especially in regards to MV pathology[25-27].
Characteristics on TTE and TEE that differentiate pannus from thrombus include a larger size (2.8 cm vs 1.7 cm) and a soft mass-like appearance, as compared to pannus. A quantitative evaluation of mass characteristics can be done by comparison to myocardium using a video intensity ratio (VIR), with a VIR < 0.7 as being similar to myocardium (VIR; video intensity of the mass in relation to the prosthetic material). A VIR < 0.7 has a positive predictive value (PPV) 87% and negative predictive value (NPV) 89% and specificity of 80% with a sensitivity of 93%, slightly better than identification of soft mass alone (NPV 80%, PPV 86%, sensitivity 86%, specificity 80%). Interestingly, a clinical history of inadequate anticoagulation alone had a specificity of 92% and a sensitivity of 79% for thrombus. When it was combined with imaging findings thought to be thrombus-specific on TEE (either soft mass-like or VIR < 0.7), sensitivity and specificity remained the same at 93% and 80% respectively. Furthermore, in the mitral position, unlike pannus formation, thrombi on TEE characteristically extend into the left atrium and appendage (Figure 5)[5].
Figure 5.
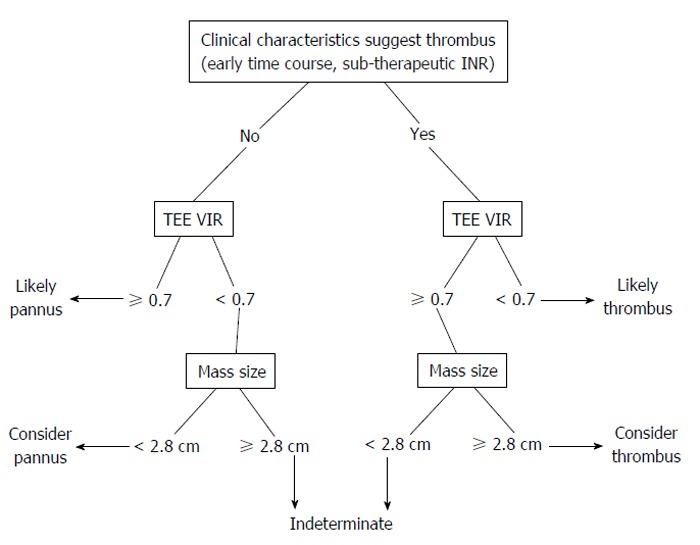
Proposed non-invasive evaluation for differentiating thrombus from pannus as underlying cause of prosthetic valve obstruction. TEE: Transesophogeal echocardiography; VIR: Video intensity ratio; INR: International normalized ratio.
In addition to its diagnostic role, TEE, unlike TTE, has been shown to contribute to risk stratification for embolic phenomenon which in turn may assist to guide therapeutic decision making. A thrombus area of < 0.85 cm2 on TEE has the lowest risk of systemic embolization[17]. As discussed below, fibrinolytic therapy has emerged as a therapeutic option in PVO.
Cine-fluoroscopy: While CF was historically the original imaging technique to evaluate for PVO, it has remained a useful tool to this day. CF allows for direct visualization of the radiopaque valve disks and allows comparison of the opening and closing angles of leaflets to normal or baseline angles, something that has limited usage with tissue valves[2,28]. Abnormal tilting of the ring base may suggest valve dehiscence, which can be confirmed with the injection of contrast dye. Valve obstruction is suggested with incomplete seating of the valves moving parts or impaired excursion[2].
TEE may appear to provide more robust information as compared to CF due to its ability to evaluate valve motion, structure, and hemodynamic parameters. However, CF is an essential complimentary examination to TEE, especially when TTE is insufficient at determining the difference between PVO and PPM[24]. In a comparison study between TTE and CF in the evaluation for PVT, sensitivity and specificity were 75% and 64% for TTE, 87% and 78% for CF[24]. Positive and negative predictive values for TTE and CF were 57%/78% and 80%/91% respectively. When used together, CF and TTE correctly diagnosed PVT in 85% of cases with TEE only required in 15% of cases.
Computed tomography scan: While computed tomography (CT) may appear to have a limited diagnostic role in the evaluation for PVO due to its incomplete evaluation of valve motion and hemodynamics, it may afford superiority over echocardiography when imaging pannus, especially in the atrial position[29]. At this time, there are no comprehensive comparative studies of echocardiography and CT in evaluating PVO. While CT may not be primarily indicated in the evaluation of PVO, it should be considered as an adjunct to TEE and CF, especially if the results are inconclusive[2].
Treatment
Treatment options for PVO include either a medical or surgical approach. In general, medical treatments are favored as an initial therapy, as the mortality of repeat valve surgery can be extremely high, depending on patient specific factors. However, pannus, due to its highly fibrotic makeup does not respond to medical therapy. When indicated, thrombolysis affords a non-invasive approach to clot dissolution and valve restoration. Thrombolysis should be considered based on the level of obstruction, ejection fraction (in aortic obstruction) and symptomatic burden (i.e., NYHA class III-IV)[1,6]. Thrombolysis, when used appropriately, has shown complete resolution of valvular obstruction in 71%-82% of patients with 17% showing a partial hemodynamic resolution. If unsuccessful, a second dose of thrombolysis shows an additive effect and further hemodynamic benefit[30-32]. Thrombolysis may be more effective in aortic valves as compared to mitral valves, however, at this time the data is limited[32]. A multicenter registry has demonstrated complications from PVT thrombolysis treatment in 18% of patients, with death occurring at a rate 6%. Specifically, prior history of stroke and increased thrombus area (for every 1 cm2 ≥ 0.8 cm2, defined by TEE) are independent predictors of complications to thrombolysis for PVT[17].
It remains to be seen if medical therapies can be applied to treatment of pannus obstruction. While a monoclonal antibody targeting TGFβ may seem sensible, it would likely require intervention at the very early stages of pannus formation in order to be fully effective. However, early identification remains difficult and, to date, such an approach has yet to be attempted.
The 2006 ACC/AHA guidelines for management of valvular disease[23] suggest prioritizing surgery over medical therapy in select situations. With left sided valvular obstruction, surgery is considered a first line treatment for valve dysfunction if a patient has significant symptoms (NYHA III-IV), or a large clot burden. The ACCP has suggested a cutoff thrombus area of ≥ 0.8 cm2[10]. Patients at high surgical risk should first be considered for thrombolysis administration. Right sided valvular dysfunction should first be considered for thrombolysis, even if NYHA class is III-IV. Importantly, after treatment, whether medical or surgical, ACC/AHA recommend a new higher chronic INR goal of 3.5 for aortic valve and 4 for mitral valve. Patients that undergo treatment for PVO (especially fibrinolysis, as discussed below) should undergo serial Doppler echocardiography to ensure there is no change in transvalvular gradients that may suggest rethrombosis[2]. The use of novel oral anti-coagulants has yet to be studied in this population and cannot be recommended at this time.
CONCLUSION
PVO, while rare, is a dreaded complication of mechanical valve replacement. Careful clinical and multiple non-invasive imaging modalities are necessary to definitively evaluate a patient with suspected PVO. As compared to pannus formation, PVT is more common, occurs earlier in the post-op period, is commonly related to inadequate anti-coagulation, and in many patients can be treated by thrombolysis. While the pathophysiology of pannus formation remains elusive, a better understanding of pannus may allow for the development of non-invasive therapeutic options.
Footnotes
P- Reviewer: Kosmas P, Said SAM, Ueda H S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: All the authors declare no conflict of interest in relation to this paper.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 4, 2015
First decision: August 8, 2015
Article in press: October 27, 2015
References
- 1.Deviri E, Sareli P, Wisenbaugh T, Cronje SL. Obstruction of mechanical heart valve prostheses: clinical aspects and surgical management. J Am Coll Cardiol. 1991;17:646–650. doi: 10.1016/s0735-1097(10)80178-0. [DOI] [PubMed] [Google Scholar]
- 2.Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, Khandheria BK, Levine RA, Marx GR, Miller FA, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22:975–1014; quiz 1082-1084. doi: 10.1016/j.echo.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Roudaut R, Serri K, Lafitte S. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart. 2007;93:137–142. doi: 10.1136/hrt.2005.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitale N, Renzulli A, Agozzino L, Pollice A, Tedesco N, de Luca Tupputi Schinosa L, Cotrufo M. Obstruction of mechanical mitral prostheses: analysis of pathologic findings. Ann Thorac Surg. 1997;63:1101–1106. doi: 10.1016/s0003-4975(96)01391-4. [DOI] [PubMed] [Google Scholar]
- 5.Barbetseas J, Nagueh SF, Pitsavos C, Toutouzas PK, Quiñones MA, Zoghbi WA. Differentiating thrombus from pannus formation in obstructed mechanical prosthetic valves: an evaluation of clinical, transthoracic and transesophageal echocardiographic parameters. J Am Coll Cardiol. 1998;32:1410–1417. doi: 10.1016/s0735-1097(98)00385-4. [DOI] [PubMed] [Google Scholar]
- 6.Toker ME, Eren E, Balkanay M, Kirali K, Yanartaş M, Calişkan A, Güler M, Yakut C. Multivariate analysis for operative mortality in obstructive prosthetic valve dysfunction due to pannus and thrombus formation. Int Heart J. 2006;47:237–245. doi: 10.1536/ihj.47.237. [DOI] [PubMed] [Google Scholar]
- 7.Teshima H, Hayashida N, Yano H, Nishimi M, Tayama E, Fukunaga S, Akashi H, Kawara T, Aoyagi S. Obstruction of St Jude Medical valves in the aortic position: histology and immunohistochemistry of pannus. J Thorac Cardiovasc Surg. 2003;126:401–407. doi: 10.1016/s0022-5223(03)00702-5. [DOI] [PubMed] [Google Scholar]
- 8.Rizzoli G, Guglielmi C, Toscano G, Pistorio V, Vendramin I, Bottio T, Thiene G, Casarotto D. Reoperations for acute prosthetic thrombosis and pannus: an assessment of rates, relationship and risk. Eur J Cardiothorac Surg. 1999;16:74–80. doi: 10.1016/s1010-7940(99)00124-4. [DOI] [PubMed] [Google Scholar]
- 9.Habets J, Budde RP, Symersky P, van den Brink RB, de Mol BA, Mali WP, van Herwerden LA, Chamuleau SA. Diagnostic evaluation of left-sided prosthetic heart valve dysfunction. Nat Rev Cardiol. 2011;8:466–478. doi: 10.1038/nrcardio.2011.71. [DOI] [PubMed] [Google Scholar]
- 10.Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e576S–e600S. doi: 10.1378/chest.11-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laplace G, Lafitte S, Labèque JN, Perron JM, Baudet E, Deville C, Roques X, Roudaut R. Clinical significance of early thrombosis after prosthetic mitral valve replacement: a postoperative monocentric study of 680 patients. J Am Coll Cardiol. 2004;43:1283–1290. doi: 10.1016/j.jacc.2003.09.064. [DOI] [PubMed] [Google Scholar]
- 12.Girard SE, Miller FA, Orszulak TA, Mullany CJ, Montgomery S, Edwards WD, Tazelaar HD, Malouf JF, Tajik AJ. Reoperation for prosthetic aortic valve obstruction in the era of echocardiography: trends in diagnostic testing and comparison with surgical findings. J Am Coll Cardiol. 2001;37:579–584. doi: 10.1016/s0735-1097(00)01113-x. [DOI] [PubMed] [Google Scholar]
- 13.Puvimanasinghe JP, Steyerberg EW, Takkenberg JJ, Eijkemans MJ, van Herwerden LA, Bogers AJ, Habbema JD. Prognosis after aortic valve replacement with a bioprosthesis: predictions based on meta-analysis and microsimulation. Circulation. 2001;103:1535–1541. doi: 10.1161/01.cir.103.11.1535. [DOI] [PubMed] [Google Scholar]
- 14.Dürrleman N, Pellerin M, Bouchard D, Hébert Y, Cartier R, Perrault LP, Basmadjian A, Carrier M. Prosthetic valve thrombosis: twenty-year experience at the Montreal Heart Institute. J Thorac Cardiovasc Surg. 2004;127:1388–1392. doi: 10.1016/j.jtcvs.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Cleveland JC, Lebenson IM, Dague JR. Early postoperative development of aortic regurgitation related to pannus ingrowth causing incomplete disc seating of a Björk-Shiley prosthesis. Ann Thorac Surg. 1982;33:496–498. doi: 10.1016/s0003-4975(10)60792-8. [DOI] [PubMed] [Google Scholar]
- 16.Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–641. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- 17.Tong AT, Roudaut R, Ozkan M, Sagie A, Shahid MS, Pontes Júnior SC, Carreras F, Girard SE, Arnaout S, Stainback RF, et al. Transesophageal echocardiography improves risk assessment of thrombolysis of prosthetic valve thrombosis: results of the international PRO-TEE registry. J Am Coll Cardiol. 2004;43:77–84. doi: 10.1016/j.jacc.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Faletra FF, Ramamurthi A, Dequarti MC, Leo LA, Moccetti T, Pandian N. Artifacts in three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr. 2014;27:453–462. doi: 10.1016/j.echo.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Barbetseas J, Zoghbi WA. Evaluation of prosthetic valve function and associated complications. Cardiol Clin. 1998;16:505–530. doi: 10.1016/s0733-8651(05)70029-1. [DOI] [PubMed] [Google Scholar]
- 20.Habib G, Benichou M, Bonnet JL, Jau P, Bille J, Djiane P, Luccioni R. Assessment of normal and abnormal prosthetic mitral valves by Doppler echocardiography. Doppler in prosthetic mitral valves. Int J Card Imaging. 1990;6:11–21. doi: 10.1007/BF01798428. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes V, Olmos L, Nagueh SF, Quiñones MA, Zoghbi WA. Peak early diastolic velocity rather than pressure half-time is the best index of mechanical prosthetic mitral valve function. Am J Cardiol. 2002;89:704–710. doi: 10.1016/s0002-9149(01)02343-8. [DOI] [PubMed] [Google Scholar]
- 22.Ben Zekry S, Saad RM, Ozkan M, Al Shahid MS, Pepi M, Muratori M, Xu J, Little SH, Zoghbi WA. Flow acceleration time and ratio of acceleration time to ejection time for prosthetic aortic valve function. JACC Cardiovasc Imaging. 2011;4:1161–1170. doi: 10.1016/j.jcmg.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 24.Montorsi P, De Bernardi F, Muratori M, Cavoretto D, Pepi M. Role of cine-fluoroscopy, transthoracic, and transesophageal echocardiography in patients with suspected prosthetic heart valve thrombosis. Am J Cardiol. 2000;85:58–64. doi: 10.1016/s0002-9149(99)00607-4. [DOI] [PubMed] [Google Scholar]
- 25.Sugeng L, Shernan SK, Weinert L, Shook D, Raman J, Jeevanandam V, DuPont F, Fox J, Mor-Avi V, Lang RM. Real-time three-dimensional transesophageal echocardiography in valve disease: comparison with surgical findings and evaluation of prosthetic valves. J Am Soc Echocardiogr. 2008;21:1347–1354. doi: 10.1016/j.echo.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Tauras JM, Zhang Z, Taub CC. Incremental benefit of 3D transesophageal echocardiography: a case of a mass overlying a prosthetic mitral valve. Echocardiography. 2011;28:E106–E107. doi: 10.1111/j.1540-8175.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- 27.Friedman M, Ahuja K, Christian AJ, Taub CC. A sticky situation. Echocardiography. 2010;27:205. doi: 10.1111/j.1540-8175.2009.01065.x. [DOI] [PubMed] [Google Scholar]
- 28.White AF, Dinsmore RE, Buckley MJ. Cineradiographic evaluation of prosthetic cardiac valves. Circulation. 1973;48:882–889. doi: 10.1161/01.cir.48.4.882. [DOI] [PubMed] [Google Scholar]
- 29.Teshima H, Hayashida N, Enomoto N, Aoyagi S, Okuda K, Uchida M. Detection of pannus by multidetector-row computed tomography. Ann Thorac Surg. 2003;75:1631–1633. doi: 10.1016/s0003-4975(02)04772-0. [DOI] [PubMed] [Google Scholar]
- 30.Roudaut R, Lafitte S, Roudaut MF, Courtault C, Perron JM, Jaïs C, Pillois X, Coste P, DeMaria A. Fibrinolysis of mechanical prosthetic valve thrombosis: a single-center study of 127 cases. J Am Coll Cardiol. 2003;41:653–658. doi: 10.1016/s0735-1097(02)02872-3. [DOI] [PubMed] [Google Scholar]
- 31.Ozkan M, Kaymaz C, Kirma C, Sönmez K, Ozdemir N, Balkanay M, Yakut C, Deligönül U. Intravenous thrombolytic treatment of mechanical prosthetic valve thrombosis: a study using serial transesophageal echocardiography. J Am Coll Cardiol. 2000;35:1881–1889. doi: 10.1016/s0735-1097(00)00654-9. [DOI] [PubMed] [Google Scholar]
- 32.Gupta D, Kothari SS, Bahl VK, Goswami KC, Talwar KK, Manchanda SC, Venugopal P. Thrombolytic therapy for prosthetic valve thrombosis: short- and long-term results. Am Heart J. 2000;140:906–916. doi: 10.1067/mhj.2000.111109. [DOI] [PubMed] [Google Scholar]


