Abstract
Future long-distance space missions will be associated with significant exposures to ionizing radiation, and the health risks of these radiation exposures during manned missions need to be assessed. Recent Earth-based epidemiological studies in survivors of atomic bombs and after occupational and medical low dose radiation exposures have indicated that the cardiovascular system may be more sensitive to ionizing radiation than was previously thought. This has raised the concern of a cardiovascular disease risk from exposure to space radiation during long-distance space travel. Ground-based studies with animal and cell culture models play an important role in estimating health risks from space radiation exposure. Charged particle space radiation has dense ionization characteristics and may induce unique biological responses, appropriate simulation of the space radiation environment and careful consideration of the choice of the experimental model are critical. Recent studies have addressed cardiovascular effects of space radiation using such models and provided first results that aid in estimating cardiovascular disease risk, and several other studies are ongoing. Moreover, astronauts could potentially be administered pharmacological countermeasures against adverse effects of space radiation, and research is focused on the development of such compounds. Because the cardiovascular response to space radiation has not yet been clearly defined, the identification of potential pharmacological countermeasures against cardiovascular effects is still in its infancy.
Keywords: Space radiation, Cardiovascular disease risk, Experimental models, Countermeasures, Ionizing radiation
Core tip: This review article provides an overview of studies in experimental models that have begun to shed light on the potential risks of damage in heart and blood vessels after exposure to space radiation.
INTRODUCTION
Participants of future long-distance space missions will be exposed to significant doses of ionizing radiation in space, and the health risks of these exposures need to be assessed. Because the cardiovascular system has recently been shown to be more sensitive to ionizing radiation than was previously thought, there is current concern that exposure to radiation during long-distance space travel may be associated with a cardiovascular disease risk. This review article provides an overview of studies in experimental models of ionizing radiation exposure relevant to that found in space that have started to shed light on the potential risks for heart and blood vessels.
Characteristics of ionizing radiation
Exposure of living cells and tissues to ionizing radiation, forms of radiation that can remove electrons from the atoms in these cells or tissues, may result in molecular damage, which can eventually lead to early and late injury. Exposure of cells or tissues to ionizing radiation causes DNA damage, which has long been considered as the primary cause of cellular injury and cell death. However, additional mechanisms are now recognized as important in normal tissue radiation injury[1]. Doses of ionizing radiation are indicated in Gray (1 Gy equals 1 Joule of absorbed energy per kilogram of mass, e.g., tissue). Because equal doses of different types of ionizing radiation may not have equal biological effects, one can express radiation exposure as equivalent dose in Sieverts (Sv), which is the absorbed dose multiplied by a unit-less radiation weighting factor and accounts for difference in the biological response[2].
Ionizing radiation can take many forms, including electromagnetic waves and high energy charged particles; the latter deposit their energy along densely ionizing cylindrical tracks. These forms of radiation can be distinguished, among other characteristics, by the amount of energy the radiation transfers to the target material per unit of track length, or linear energy transfer (LET)[2]. Ionizing radiation in the form of electromagnetic waves, such as X-rays or γ-radiation, are considered forms of low-LET radiation and deposit their energy uniformly in target volumes, while high energy charged particles release their energy along dense tracks of ionization and are considered high-LET radiation. Space travel is associated with low-dose-rate exposure to high-LET radiation if the form of galactic cosmic rays (GCR) and occasional high dose rate solar particle events (SPEs)[3].
IONIZING RADIATION AND THE CARDIOVASCULAR SYSTEM
Ionizing radiation has long been known to cause injury in heart and blood vessels. These effects first became apparent from follow-up of patients after radiation therapy, which delivers high doses of low-LET radiation locally to the tumor but in some cases also exposes normal (non-cancer) tissues such as the heart and blood vessels[4-8]. Several previously published review articles[9-11] have provided a comprehensive overview of the effects of low-LET radiation on the cardiovascular system. In short, manifestations of radiation-induced heart disease as a result of exposure to high doses of ionizing radiation include accelerated atherosclerosis, myocardial fibrosis, and cardiac conduction and valve abnormalities. Most deleterious effects in heart and blood vessels are observed years to decades after exposure to ionizing radiation. Therefore, long post-radiation follow-up is required for a full assessment of cardiovascular risk. Mechanisms by which ionizing radiation has its effects in the cardiovascular system are not yet fully known.
Recent reports of health assessments in atomic bomb survivors[12-15] have shown an increased incidence of cardiovascular disease, including ischemic heart disease and stroke, in people several decades after exposure to doses of γ-radiation as low as 2 Gy. Moreover, other epidemiological studies in occupational exposure and low-dose exposure due to medical treatments indicate that cardiovascular disease may occur after lower doses of ionizing radiation than was previously thought[16-20]. The main cardiovascular effects seen in atomic bomb survivors include hypertension and ischemic heart disease, suggesting that after low-dose radiation exposure a vascular component may play a central role in the cardiovascular disease risk.
These recent reports on health effects from exposure to low doses of low-LET radiation have raised the concern about potential risk of cardiovascular disease from exposure to ionizing radiation during space travel[21]. However, care should be taken when the results of terrestrial radiation exposures such as those from atomic bombs are used to support the potential for a cardiovascular disease risk from space radiation, since certain conditions such as dose rate are different between atomic bomb events and radiation exposure in space. The remainder of this review is focused on studies in experimental models that have aimed to shed light on the cardiovascular risk of exposure to space radiation.
SPACE RADIATION
Characteristics of space radiation
While astronauts in the International Space Station are somewhat protected from exposure to space radiation due to the earth’s magnetosphere, future long-distance space travel (beyond low-Earth orbit) will be accompanied by exposure to higher cumulative doses of space radiation, and short-term and long-term health risks need to be assessed[22,23].
GCR and solar emissions are dominated by protons and iron, silicon, oxygen, and carbon that are highly energetic. The greatest particle abundance is found for particles with energies ranging from hundreds of MeV per nucleon (MeV/n) up to about 1 GeV/n[24]. Practical levels of current shielding materials cannot easily protect against these particles[25]. Chronic exposure occurs at a dose rate of 1.3 mGy/d, or the dose equivalent of 4.8 mSv/d, when assuming the radiation weighting factors of the International Commission on Radiological Protection Publication 60 outside the earth’s magnetosphere[26,27]. The exposure is characterized by the traversal of most cells in the body by one or more protons and electrons per day, with infrequent traversals (days to weeks) by ions of higher atomic number (Z).
SPEs consist predominantly of protons, and exposure to the largest SPEs occurs at dose rates up to 0.5 Sv/h over hours to a few days[28]. Energies of SPE protons are less than those for GCR and therefore have shorter ranges in material, which may enable effective shielding inside a spacecraft but not inside a thin spacesuit. These higher dose rate exposures may put an astronaut at risk for acute radiation effects, sometimes collectively called acute radiation sickness[29]. Both SPEs and GCR may also cause long-term degenerative disease in various tissues, including the heart and blood vessels.
Experimental data obtained from animal and cell culture models play an important role in estimating health risks from exposure to space radiation. Appropriate simulation of the space radiation environment, including the long-term low-dose rate exposures to various charged particles and the appropriate energy of these particles, and the choice of the most relevant animal or cell culture model are challenging but key to providing relevant estimates of health risks[30-32]. The concern of adverse cardiovascular effects of exposure to space radiation is relatively new, and studies on the cardiovascular effects in animal models of space radiation exposure are not yet abundant. An overview of existing studies on heart and blood vessels is given below. Since much of this work is ongoing, we have had to occasionally refer to proceeding abstracts, but hope to find the results in peer-reviewed publications in the near future.
Cardiac response in animal models of charged particle exposure
Studies in animal models of charged particle exposure have shown cardiovascular effects at doses lower than those required to cause cardiovascular changes if low-LET radiation is used. This may not be surprising, since high-LET radiation typically causes more damage per unit of absorbed dose. Among studies with charged particles, some previous research has focused on the cardiac response to fission spectrum neutrons in animal models[33-36]. More recently, studies were designed to provide answers about the cardiovascular risk from exposure to high-LET radiation in space. Exposure of male C75Bl/6NT mice at 8-10 mo of age to protons (1 GeV, 0.5 Gy) or iron ions (1 GeV/n, 0.15 Gy) induced cardiac infiltration of CD68-positive cells (monocytes and macrophages), increased DNA oxidation, myocardial fibrosis, and modified cardiac function, both at baseline and in response to myocardial infarction, in a radiation-type specific manner[37-39]. Exposure of male CBA/CaJ mice at 10-12 wk of age to silicone ions (300 MeV/n) at doses between 0.1 and 0.5 Gy caused prolonged apoptosis and increased expression of the common pro-inflammatory cytokines interleukin (IL)-1β, IL-6, or tumor necrosis factor-α in the heart[40]. Low doses of high-LET radiation have been shown to cause long-term alterations in DNA methylation in various organ systems in vivo and cells in culture[41-43]. Similarly, we recently found changes in cardiac DNA methylation in male C57BL/6J mice exposed at 10 wk of age to protons (150 MeV, 0.1 Gy) or iron ions (600 MeV/n, 0.5 Gy) (Figure 1), suggesting that epigenetic alterations may contribute to the cardiac radiation response[44]. Analysis of the response in individual cardiac cell types is also ongoing[45].
Figure 1.
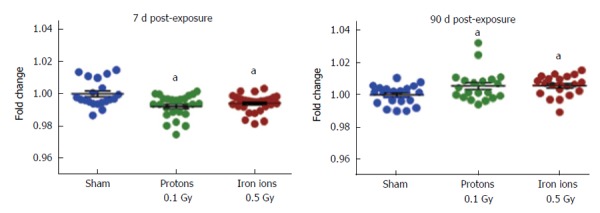
Methylation of genomic DNA isolated from hearts of male C57Bl/6 mice at 7 d and 90 d after exposure to protons (150 MeV, 0.1 Gy) or iron ions (600 MeV/n, 0.5 Gy). DNA methylation of the open reading frame 1 of long interspersed nuclear element-1, a transposable element that comprises about 20% of the mouse genome, as assessed by pyrosequencing and indicated as fold change compared to sham-irradiated controls. Each group contained 4-5 animals. Horizontal lines indicate average ± standard error of the mean. aP < 0.05 vs the sham-irradiated control group.
Vascular response in animal models of charged particle exposure
Whole-body exposure of rats to iron ions at doses of 0.5 and 1 Gy induced long-term indications of endothelial dysfunction and increased aortic stiffness[46]. It is difficult to assess the effects of ionizing radiation on atherosclerosis when using regular rodent models, due to the low prevalence of atherosclerosis in these animals. Targeted exposure of the atherosclerotic-prone apolipoprotein E-deficient (Apo-/-) mouse model to iron ions (600 MeV/n) at doses of 2 and 5 Gy caused accelerated atherosclerosis in the exposed parts of the aorta[47]. Additional studies with lower doses of particle irradiation may provide a more comprehensive estimate of cardiovascular risk in this mouse model. Studies on adhesiveness of endothelium in charged particle-exposed animal models are also underway[48].
The microvasculature also plays an important role in normal organ function, degenerative tissue effects, and tissue injury from ionizing radiation[49,50]. Exposure of 10-wk old male C57BL/6 mice to iron ions (600 MeV/n) at doses between 0.5 and 2 Gy caused a long-term loss of endothelial cells in the hippocampus[51]. More research is required to assess the effects of space radiation on the microvasculature.
Studies on charged particle exposure in cells culture models
Endothelial cells are considered to play a central role in the cardiovascular response to ionizing radiation. Endothelial dysfunction, which is characterized by a proinflammatory and profibrogenic phenotype of endothelial cells, is a critical contributor to the patho-physiological manifestations of radiation injury[52-54]. Experimental models of exposure to low-LET radiation have shown that ionizing radiation can cause prolonged endothelial dysfunction, thereby sustaining a detrimental tissue environment that leads to chronic inflammation and adverse remodeling[55,56].
Because of the central role of endothelial cells in the radiation response, studies are addressing the effects of space radiation on endothelial cells in cultures[57]. Various tissue-relevant cell culture models are being used[58]. For instance, in three-dimensional culture models of human endothelial cells, protons (1 GeV) and iron ions (1 GeV/n) at doses up to 3 Gy caused alterations in vasculogenesis and endothelial cell death in a radiation-type specific manner[59,60]. These results raise the concern of damage of the human vasculature from exposure to charged particles in vivo.
Potential countermeasures against the cardiovascular response to space radiation
Astronauts could potentially be administered pharmacological countermeasures against adverse effects of space radiation, when the countermeasure is safe, stable during long-term space flight, and has a relatively light weight. Therefore, research is focused on the development of countermeasures against various biological effects of space radiation[29]. Interestingly, pharmacological countermeasures are being developed for low-LET radiation in exposure scenarios on earth and may point to potential countermeasures against adverse effects of space radiation. Neupogen [filgrastim, recombinant human granulocyte colony stimulating factor (G-CSF)], for instance, was recently approved by the American Food and Drug Administration as a countermeasure against acute injury from accidental radiation exposure. G-CSF has also been shown to protect animal models against acute injury from exposure to SPE-like protons[61].
Because the cardiovascular response to space radiation has not yet been clearly defined, the identification of potential pharmacological countermeasures against cardiovascular effects is still in its infancy. Nonetheless, similar to the acute response scenario, potential countermeasures against cardiovascular effects of terrestrial radiation exposure, albeit not yet approved for clinical use, may be pursued in space radiation models. For example, the angiotensin converting enzyme (ACE) inhibitor captopril has been shown to reduce cardiac injury in animal models of localized irradiation of the heart[62,63]. In addition, the vitamin E analog γ-tocotrienol is one of the most potent dietary countermeasures to radiation injury currently known. It is safe and nontoxic and has no known drug interactions. It is commercially available, requires no specific storage conditions, and is currently in advanced stages of development for terrestrial applications in radiation protection[64,65]. In addition, γ-tocotrienol has several beneficial effects in the cardiovascular system. It is a potent inhibitor of the cholesterol biosynthesis pathway, thereby reducing the isoprenylation of Rho proteins that modify a wide range of cellular functions, including stress fiber formation, hypertrophy, regulation of NOS, and production of cytokines and growth factors[66]. Indeed, γ-tocotrienol reduces vascular oxidative stress and protects against vascular radiation injury at least in part via HMG-CoA reductase inhibition[67,68]. The protective properties of agents such as ACE inhibitors or γ-tocotrienol against cardiovascular effects of space radiation need to be assessed.
CONCLUSION
The cardiovascular system may be more sensitive to ionizing radiation than was previously thought, which raises the concern of a cardiovascular risk from exposure to ionizing radiation during long-distance space missions. Animal and cell culture models have started to shed light on risk of cardiovascular complications from exposure to charged particle irradiation. Additional studies, including those that employ low radiation doses/dose rates and mixed particle fields to mimic GCR are required to aid in assessing the cardiovascular risk of space radiation.
Footnotes
P- Reviewer: Cosmi E, Natarajan M S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Supported by The National Space Biomedical Research Institute (RE03701) through NCC 9-58; and the National Institutes of Health (CA148679).
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 3, 2015
First decision: August 4, 2015
Article in press: October 8, 2015
References
- 1.Denham JW, Hauer-Jensen M, Peters LJ. Is it time for a new formalism to categorize normal tissue radiation injury? Int J Radiat Oncol Biol Phys. 2001;50:1105–1106. doi: 10.1016/s0360-3016(01)01556-5. [DOI] [PubMed] [Google Scholar]
- 2.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 3.Cucinotta FA, Wu H, Shavers MR, George K. Radiation dosimetry and biophysical models of space radiation effects. Gravit Space Biol Bull. 2003;16:11–18. [PubMed] [Google Scholar]
- 4.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 5.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood RD, Rosenthal A, Cassady R, Jaffe N, Nadas AS. Constrictive pericarditis in childhood due to mediastinal irradiation. Circulation. 1974;50:1033–1039. doi: 10.1161/01.cir.50.5.1033. [DOI] [PubMed] [Google Scholar]
- 7.Russell NS, Hoving S, Heeneman S, Hage JJ, Woerdeman LA, de Bree R, Lohuis PJ, Smeele L, Cleutjens J, Valenkamp A, et al. Novel insights into pathological changes in muscular arteries of radiotherapy patients. Radiother Oncol. 2009;92:477–483. doi: 10.1016/j.radonc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res. 2010;174:865–869. doi: 10.1667/RR1862.1. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol. 2013;10:697–710. doi: 10.1038/nrclinonc.2013.195. [DOI] [PubMed] [Google Scholar]
- 10.Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 11.Fajardo LF, Berthrong M. Vascular lesions following radiation. Pathol Annu. 1988;23 Pt 1:297–330. [PubMed] [Google Scholar]
- 12.Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F, Hall P, et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect. 2012;120:1503–1511. doi: 10.1289/ehp.1204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 14.Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. Noncancer disease incidence in atomic bomb survivors, 1958-1998. Radiat Res. 2004;161:622–632. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 15.Stewart AM, Kneale GW. A-bomb survivors: factors that may lead to a re-assessment of the radiation hazard. Int J Epidemiol. 2000;29:708–714. doi: 10.1093/ije/29.4.708. [DOI] [PubMed] [Google Scholar]
- 16.Adams MJ, Grant EJ, Kodama K, Shimizu Y, Kasagi F, Suyama A, Sakata R, Akahoshi M. Radiation dose associated with renal failure mortality: a potential pathway to partially explain increased cardiovascular disease mortality observed after whole-body irradiation. Radiat Res. 2012;177:220–228. doi: 10.1667/rr2746.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr ZA, Land CE, Kleinerman RA, Weinstock RW, Stovall M, Griem ML, Mabuchi K. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61:842–850. doi: 10.1016/j.ijrobp.2004.07.708. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov VK, Maksioutov MA, Chekin SY, Petrov AV, Biryukov AP, Kruglova ZG, Matyash VA, Tsyb AF, Manton KG, Kravchenko JS. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 2006;90:199–207. doi: 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- 19.Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Paris F, Tapio S, Elliott P. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res. 2008;169:99–109. doi: 10.1667/RR1070.1. [DOI] [PubMed] [Google Scholar]
- 20.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 21.National Council on Radiation Protection & Measurements. NCRP Report No. 153, Information Needed to Make Radiation Protection Recommendations for Space Missions Beyond Low-Earth Orbit. 2006. [Google Scholar]
- 22.Hellweg CE, Baumstark-Khan C. Getting ready for the manned mission to Mars: the astronauts’ risk from space radiation. Naturwissenschaften. 2007;94:517–526. doi: 10.1007/s00114-006-0204-0. [DOI] [PubMed] [Google Scholar]
- 23.Shuchman M. Striving for Mars: what are acceptable risks? CMAJ. 2014;186:E7–E8. doi: 10.1503/cmaj.109-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benton ER, Benton EV. Space radiation dosimetry in low-Earth orbit and beyond. Nucl Instrum Methods Phys Res B. 2001;184:255–294. doi: 10.1016/s0168-583x(01)00748-0. [DOI] [PubMed] [Google Scholar]
- 25.Walker SA, Townsend LW, Norbury JW. Heavy ion contributions to organ dose equivalent for the 1977 galactic cosmic ray spectrum. Adv Space Res. 2013;51:1792–1799. [Google Scholar]
- 26.International Commission on Radiological Protection. ICRP Publication 60. Oxford: Pergamon Press; 1991. [Google Scholar]
- 27.Zeitlin C, Hassler DM, Cucinotta FA, Ehresmann B, Wimmer-Schweingruber RF, Brinza DE, Kang S, Weigle G, Böttcher S, Böhm E, et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science. 2013;340:1080–1084. doi: 10.1126/science.1235989. [DOI] [PubMed] [Google Scholar]
- 28.Parsons JL, Townsend LW. Interplanetary crew dose rates for the August 1972 solar particle event. Radiat Res. 2000;153:729–733. doi: 10.1667/0033-7587(2000)153[0729:icdrft]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy AR. Biological Effects of Space Radiation and Development of Effective Countermeasures. Life Sci Space Res (Amst) 2014;1:10–43. doi: 10.1016/j.lssr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MY, Rusek A, Cucinotta FA. Mixed-field GCR Simulations for Radiobiological Research Using Ground Based Accelerators. Proceedings of the 40th COSPAR Scientific Assembly. Russia: Moscow; 2014. [Google Scholar]
- 31.Chancellor JC, Scott GB, Sutton JP. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life (Basel) 2014;4:491–510. doi: 10.3390/life4030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasi SP, Lee J, Mehrzad R, Morgan JP, Gee H, Song J, Rahimi L, Enderling H, Yan X, Goukassian DA. Different Sequence of Fractionated Proton and Single Low Dose Iron Radiation Induce Divergent Biological Responses in the Heart. Proceedings of the NASA Human Research Program Investigators’ Workshop. USA: Galveston; 2015. [Google Scholar]
- 33.Stearner SP, Yang VV, Devine RL. Cardiac injury in the aged mouse: comparative ultrastructural effects of fission spectrum neutrons and gamma rays. Radiat Res. 1979;78:429–447. [PubMed] [Google Scholar]
- 34.Yang VV, Stearner SP, Ainsworth EJ. Late ultrastructural changes in the mouse coronary arteries and aorta after fission neutron or 60Co gamma irradiation. Radiat Res. 1978;74:436–456. [PubMed] [Google Scholar]
- 35.Yang VV, Stearner SP, Tyler SA. Radiation-induced changes in the fine structure of the heart: comparison of fission neutrons and 60Co gamma rays in the mouse. Radiat Res. 1976;67:344–360. [PubMed] [Google Scholar]
- 36.Yang VV, Stearner SP, Dimitrievich GS, Griem ML. Radiation damage to the microvasculature in the rabbit ear chamber. An electron microscope study. Radiat Res. 1977;70:107–117. [PubMed] [Google Scholar]
- 37.Sasi SP, Yan X, Lee J, Sisakyan H, Carrozza J, Goukassian DA. Radiation-associated degenerative cardiovascular risks during normal aging and after adverse CV event 10 months post-initial exposure. J Radiat Res. 2014;55 Suppl 1:i111–i112. [Google Scholar]
- 38.Yan X, Sasi SP, Gee H, Lee J, Yang Y, Song J, Carrozza J, Goukassian DA. Radiation-associated cardiovascular risks for future deep-space missions. J Radiat Res. 2014;55 Suppl 1:i37–i39. [Google Scholar]
- 39.Yan X, Sasi SP, Gee H, Lee J, Yang Y, Mehrzad R, Onufrak J, Song J, Enderling H, Agarwal A, et al. Cardiovascular risks associated with low dose ionizing particle radiation. PLoS One. 2014;9:e110269. doi: 10.1371/journal.pone.0110269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tungjai M, Whorton EB, Rithidech KN. Persistence of apoptosis and inflammatory responses in the heart and bone marrow of mice following whole-body exposure to 28Silicon (28Si) ions. Radiat Environ Biophys. 2013;52:339–350. doi: 10.1007/s00411-013-0479-4. [DOI] [PubMed] [Google Scholar]
- 41.Aypar U, Morgan WF, Baulch JE. Radiation-induced epigenetic alterations after low and high LET irradiations. Mutat Res. 2011;707:24–33. doi: 10.1016/j.mrfmmm.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Lima F, Ding D, Goetz W, Yang AJ, Baulch JE. High LET (56)Fe ion irradiation induces tissue-specific changes in DNA methylation in the mouse. Environ Mol Mutagen. 2014;55:266–277. doi: 10.1002/em.21832. [DOI] [PubMed] [Google Scholar]
- 43.Nzabarushimana E, Miousse IR, Shao L, Chang J, Allen AR, Turner J, Stewart B, Raber J, Koturbash I. Long-term epigenetic effects of exposure to low doses of 56Fe in the mouse lung. J Radiat Res. 2014;55:823–828. doi: 10.1093/jrr/rru010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boerma M, Koturbash I, Sridharan V, Miousse IR, Hauer-Jensen M, Nelson GA. Cellular and molecular alterations in the heart in response to 56Fe and protons. Proceedings of the 60th Annual Meeting of the Radiation Research Society. USA: Las Vegas; 2014. [Google Scholar]
- 45.Coleman M, Sasi SP, Onufrak J, Natarajan M, Manickam K, Peterson LE, Yan X, Goukassian DA. Delayed Cardiomyocyte Response to Total Body Heavy Ion Particle Radiation Exposure - Identification of Regulatory Gene Networks. Proceedings of the NASA Human Research Program Investigators’ Workshop. USA: Galveston; 2015. [Google Scholar]
- 46.Soucy KG, Lim HK, Kim JH, Oh Y, Attarzadeh DO, Sevinc B, Kuo MM, Shoukas AA, Vazquez ME, Berkowitz DE. HZE 56Fe-ion irradiation induces endothelial dysfunction in rat aorta: role of xanthine oxidase. Radiat Res. 2011;176:474–485. doi: 10.1667/rr2598.1. [DOI] [PubMed] [Google Scholar]
- 47.Yu T, Parks BW, Yu S, Srivastava R, Gupta K, Wu X, Khaled S, Chang PY, Kabarowski JH, Kucik DF. Iron-ion radiation accelerates atherosclerosis in apolipoprotein E-deficient mice. Radiat Res. 2011;175:766–773. doi: 10.1667/RR2482.1. [DOI] [PubMed] [Google Scholar]
- 48.Chanda D, Gupta K, Kabarowski JH, Kucik DF. 56Fe Irradiation of Wild type C57BL/6 Mice Results in Increased Adhesiveness of Aortic Endothelium. Proceedings of the NASA Human Research Program Investigators’ Workshop. USA: Galveston; 2015. [Google Scholar]
- 49.Hopewell JW, Young CM. Changes in the microcirculation of normal tissues after irradiation. Int J Radiat Oncol Biol Phys. 1978;4:53–58. doi: 10.1016/0360-3016(78)90115-3. [DOI] [PubMed] [Google Scholar]
- 50.Hopewell JW, Calvo W, Jaenke R, Reinhold HS, Robbins ME, Whitehouse EM. Microvasculature and radiation damage. Recent Results Cancer Res. 1993;130:1–16. doi: 10.1007/978-3-642-84892-6_1. [DOI] [PubMed] [Google Scholar]
- 51.Mao XW, Favre CJ, Fike JR, Kubinova L, Anderson E, Campbell-Beachler M, Jones T, Smith A, Rightnar S, Nelson GA. High-LET radiation-induced response of microvessels in the Hippocampus. Radiat Res. 2010;173:486–493. doi: 10.1667/RR1728.1. [DOI] [PubMed] [Google Scholar]
- 52.Fajardo LF. The endothelial cell is a unique target of radiation: an overview. In: Rubin DB, editor. Radiation biology of the vascular endothelium. Boca Raton: CRC Press LLC; 1998. pp. 1–12. [Google Scholar]
- 53.Schultz-Hector S, Balz K. Radiation-induced loss of endothelial alkaline phosphatase activity and development of myocardial degeneration. An ultrastructural study. Lab Invest. 1994;71:252–260. [PubMed] [Google Scholar]
- 54.Sharma P, Templin T, Grabham P. Short term effects of gamma radiation on endothelial barrier function: uncoupling of PECAM-1. Microvasc Res. 2013;86:11–20. doi: 10.1016/j.mvr.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Zheng H, Ou X, Fink LM, Hauer-Jensen M. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063–2072. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13:3047–3055. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Natarajan M, Krishnan M, Sureshkumar MA, Zheng H, Mohan S. Inflammatory Response of Vascular Endothelium Exposed to Space Radiation. Proceedings of the NASA Human Research Program Investigators’ Workshop. USA: Galveston; 2015. [Google Scholar]
- 58.Patel ZS, Grande-Allen KJ. Development of a Flow-Perfused and Immunocompetent 3-D Vascular Model for Radiation Risk Assessment of Cardiovascular Disease and Countermeasure Screening. Proceedings of the NASA Human Research Program Investigators’ Workshop. USA: Galveston; 2015. [Google Scholar]
- 59.Grabham P, Sharma P, Bigelow A, Geard C. Two distinct types of the inhibition of vasculogenesis by different species of charged particles. Vasc Cell. 2013;5:16. doi: 10.1186/2045-824X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grabham P, Hu B, Sharma P, Geard C. Effects of ionizing radiation on three-dimensional human vessel models: differential effects according to radiation quality and cellular development. Radiat Res. 2011;175:21–28. doi: 10.1667/RR2289.1. [DOI] [PubMed] [Google Scholar]
- 61.Romero-Weaver AL, Wan XS, Diffenderfer ES, Lin L, Kennedy AR. Kinetics of neutrophils in mice exposed to radiation and/or granulocyte colony-stimulating factor treatment. Radiat Res. 2013;180:177–188. doi: 10.1667/RR3055.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Veen SJ, Ghobadi G, de Boer RA, Faber H, Cannon MV, Nagle PW, Brandenburg S, Langendijk JA, van Luijk P, Coppes RP. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol. 2015;114:96–103. doi: 10.1016/j.radonc.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 63.Yarom R, Harper IS, Wynchank S, van Schalkwyk D, Madhoo J, Williams K, Salie R, Genade S, Lochner A. Effect of captopril on changes in rats’ hearts induced by long-term irradiation. Radiat Res. 1993;133:187–197. [PubMed] [Google Scholar]
- 64.Pathak R, Shao L, Ghosh SP, Zhou D, Boerma M, Weiler H, Hauer-Jensen M. Thrombomodulin contributes to gamma tocotrienol-mediated lethality protection and hematopoietic cell recovery in irradiated mice. PLoS One. 2015;10:e0122511. doi: 10.1371/journal.pone.0122511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suman S, Datta K, Chakraborty K, Kulkarni SS, Doiron K, Fornace AJ, Sree Kumar K, Hauer-Jensen M, Ghosh SP. Gamma tocotrienol, a potent radioprotector, preferentially upregulates expression of anti-apoptotic genes to promote intestinal cell survival. Food Chem Toxicol. 2013;60:488–496. doi: 10.1016/j.fct.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 67.Berbée M, Fu Q, Boerma M, Wang J, Kumar KS, Hauer-Jensen M. gamma-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berbee M, Fu Q, Boerma M, Pathak R, Zhou D, Kumar KS, Hauer-Jensen M. Reduction of radiation-induced vascular nitrosative stress by the vitamin E analog γ-tocotrienol: evidence of a role for tetrahydrobiopterin. Int J Radiat Oncol Biol Phys. 2011;79:884–891. doi: 10.1016/j.ijrobp.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


