Abstract
AIM: To conduct a systematic review relating myocardial strain assessed by different imaging modalities for prognostication following ST-elevation myocardial infarction (STEMI).
METHODS: An online literature search was performed in PubMed and OVID® electronic databases to identify any studies that assessed global myocardial strain parameters using speckle-tracking echocardiography (STE) and/or cardiac magnetic resonance imaging (CMR) techniques [either myocardial tagging or feature tracking (FT) software] in an acute STEMI cohort (days 0-14 post-event) to predict prognosis [either development of major adverse cardiac events (MACE)] or adverse left ventricular (LV) remodelling at follow-up (≥ 6 mo for MACE, ≥ 3 mo for remodelling). Search was restricted to studies within the last 20 years. All studies that matched the pre-defined search criteria were reviewed and their results interpreted. Due to considerable heterogeneity between studies, meta-analysis was not performed.
RESULTS: A total of seven studies (n = 7) were identified that matched the search criteria. All studies used STE to evaluate strain parameters - five (n = 5) assessed global longitudinal strain (GLS) (n = 5), one assessed GLS rate (GLS-R) (n = 1) and one assessed both (n = 1). Three studies showed that GLS independently predicted the development of adverse LV remodelling by multivariate analysis - odds ratio between 1.19 (CI: 1.04-1.37, P < 0.05) and 10 (CI: 6.7-14, P < 0.001) depending on the study. Four studies showed that GLS predicted the development of MACE - hazard ratio (HR) between 1.1 (CI: 1-1.1, P = 0.006) and 2.34 (1.10-4.97, P < 0.05). One paper found that GLS-R could significantly predict MACE - HR 18 (10-35, P < 0.001) - whilst another showed it did not. GLS < -10.85% had sensitivity/specificity of 89.7%/91% respectively for predicting the development of remodelling whilst GLS < -13% could predict the development of MACE with sensitivity/specificity of 100%/89% respectively. No suitable studies were identified that assessed global strain by CMR tagging or FT techniques.
CONCLUSION: GLS measured acutely post-STEMI by STE is a predictor of poor prognosis. Further research is needed to show that this is true for CMR-based techniques.
Keywords: Strain, Speckle tracking, Tagging, Feature tracking, Myocardial infarction, Major adverse cardiac events, Remodelling
Core tip: Global myocardial strain is an objective measure of cardiac function. It can be assessed using post-processing analysis on different imaging modalities such as speckle-tracking echocardiography (STE) and cardiac magnetic resonance imaging (CMR) - tagging and feature tracking. We performed a systematic review that showed global longitudinal strain (GLS) measured acutely by STE following ST-elevation myocardial infarction (STEMI) predicted clinical outcomes and adverse left ventricular remodelling, a surrogate marker of poor prognosis. No relevant studies were found for CMR techniques. GLS may refine risk stratification in the STEMI population but further work is needed to support this.
INTRODUCTION
Ischaemic heart disease (IHD) presents a significant burden to healthcare services and is one of the leading causes of death worldwide[1]. Acute myocardial infarction (MI) results from spontaneous coronary artery occlusion due to thrombus formation as a result of plaque rupture and subsequent platelet aggregation - most commonly seen with the background of IHD[2]. ST-elevation myocardial infarction (STEMI) is an acute emergency that requires prompt reperfusion by either primary percutaneous coronary intervention (PPCI) or thrombolysis, ideally within two hours of symptom onset[3].
Timely reperfusion has led to a reduction in mortality from acute MI[4]. However, despite receiving current best therapy, a significant number of patients still develop complications post-MI that includes new-onset heart failure (HF)[5] - 20.4% of patients develop HF on admission and 8.6% subsequently[6]. The incidence of HF has increased over the past few decades[7] and it is especially prevalent amongst the elderly[8]. Long-term mortality from HF still remains high, even with the best contemporary pharmacological and non-pharmacological interventions[9]. The increase in HF incidence may partly be a result of improved survival post-MI, albeit with greater morbidity in some survivors.
Outcomes after STEMI
Major adverse cardiac events: Major adverse cardiac events (MACE) are often used in cardiovascular studies as a measure of clinical outcomes after STEMI. It is an umbrella term that includes a variety of measures - including all-cause mortality, hospital readmission due to HF, recurrence of MI, need for revascularisation, and occurrence of stroke. Demographic features associated with poor outcomes post-STEMI include age[10], diabetes[11], hypertension[12], infarct location (i.e., anterior MI)[13], large infarct size (IS)[14] and presence of microvascular obstruction[15].
“Hard events” such as mortality are the best markers of outcome. However, these are relatively rare occurrences and so require a considerable sample size to demonstrate statistically significant association with a biomarker, or effects of intervention[16] and some authors believe that studies reporting these need to have a sample size of n > 1000 to be statistically robust[17]. Such large, multi-centre trials are challenging to conduct and need to be carried out over a considerable period of time in order to accrue the required sample sizes and numbers of events. Consequently, surrogate markers of poor outcome such as adverse left ventricular (LV) remodelling can be used in lieu of hard outcomes with much smaller sample sizes to achieve statistically significant results.
Adverse LV remodelling: Adverse LV remodelling post-MI is thought to be the main process underpinning the development of HF and is defined as: “A change in size, shape and function of the heart resulting from cardiac load or injury”[18]. It is a complex process that progresses over a period of weeks to months post-infarct (Figure 1). Adverse LV remodelling post STEMI can be defined as either an increase in end-diastolic volume (EDV) of > 20% or end systolic volume (ESV) of > 15%, at follow-up compared to baseline. However, there is no consensus on which definition is better. Several cellular, extra-cellular, inflammatory, and neuro-hormonal pathways have been implicated to play a role in development of LV remodelling; these include neutrophils[19], macrophages[19], collagen fibres[20], various metallo-proteinases[20] and activation of the sympathetic nervous system along with the renin-angiotensin-aldosterone system (RAAS)[7,18] amongst others. The exact role of these components has not yet been elucidated and there is still some controversy over the initial trigger of remodelling[21]. There is good evidence to suggest that angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, and aldosterone antagonists attenuate the process of adverse remodelling by inhibiting RAAS[18].
Figure 1.
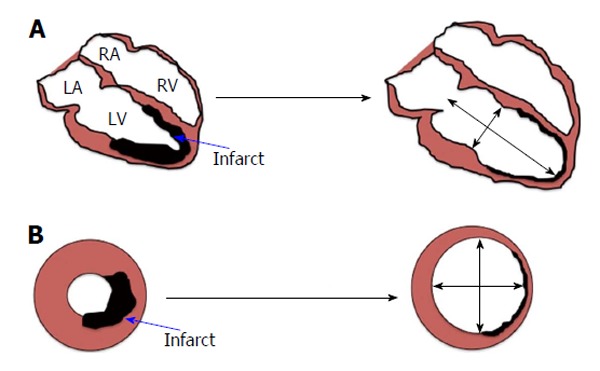
Development of adverse left ventricular remodelling post-myocardial infarction in (A) long axis view and (B) short axis view. LA: Left atrium; LV: Left ventricle; RA: Right atrium; RV: Right ventricle.
Early identification of high-risk patients who are likely to undergo adverse LV remodelling may allow targeted therapeutic intervention in these patients to counteract remodelling processes. Parameters that reflect myocardial dysfunction can potentially be utilised to help identify such patients as cardiac function is often affected post-MI, which usually precedes development of overt HF.
LV dysfunction post-infarction
Traditionally, the systolic phase of the cardiac cycle is often used as a measure of LV function in a clinical setting. A region of myocardium affected by an infarct may have impaired contractility due to death of myocytes in that zone. Ejection fraction (EF) is the most commonly used method to assess systolic function and a reduced EF, commonly measured by echocardiography, is known to be associated with a poor outcome[22]. However, EF is relatively insensitive to regional differences in myocardial function and has been shown to be a poor predictor of late myocardial dysfunction when measured acutely after reperfusion therapy[23]. Wall Motion Score Index (WMSI) has also been used in addition to EF but it has the inherent shortcoming of being a subjective measure based on the experience of the assessor. WMSI is based on either the 16-segment[24] or the 17-segment model[25] of the LV.
An infarct is also thought to affect LV compliance by increasing wall stiffness and hence reducing active relaxation of the myocardium - this can cause diastolic dysfunction[26]. Recent evidence suggests that diastolic dysfunction post-MI measured by echocardiography confers a poor outcome[27,28].
The optimal marker of LV dysfunction would: (1) Be objective and “angle independent”; (2) Be sensitive to myocardial dysfunction early after an MI; (3) Offer an evaluation of both regional and global LV contractility; (4) Provides an assessment of both systolic and diastolic heart function; and (5) Be reproducible and easy to measure.
Myocardial strain
Strain is defined as the change in length of an object relative to its original length[29]. In the heart, myocardial strain is a sensitive measure of contractility. Strain can be calculated at both the segmental and global level and in the three axes of myocardial contraction - circumferential, longitudinal and radial (Figure 2). Strain rate (SR) measures the change in strain for a given vector as a function of time and can also be assessed. Systolic and diastolic strain rates vary throughout the cardiac cycle (Figure 3).
Figure 2.
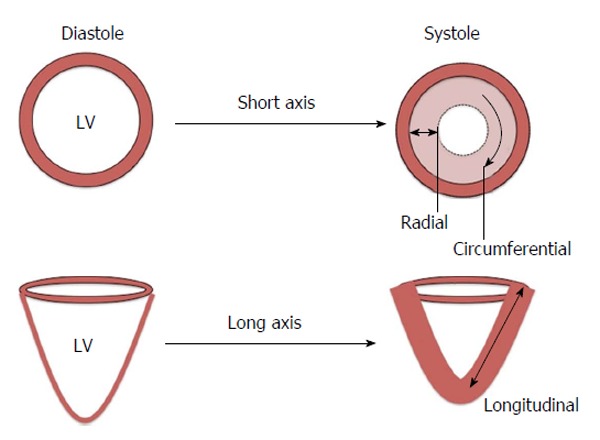
Myocardial contraction in three vectors - circumferential, longitudinal and radial. LV: Left ventricle.
Figure 3.
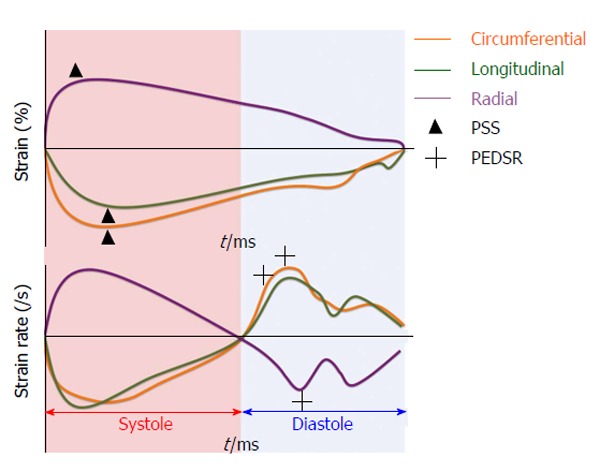
Strain and strain rate values as a function of time - peak systolic strain and peak early diastolic strain rate are annotated. PSS: Peak systolic strain; PEDSR: Peak early diastolic strain rate.
Anatomically, myocardial fibres are orientated longitudinally in the sub-endocardium and circumferentially in the mid-myocardium[30]. This suggests that longitudinal strain (LS) can provide a reflection of sub-endocardial function whilst circumferential strain can inform mid-myocardial function. Radial strain, whilst being potentially informative of myocardial contraction in the short axis, has been shown to have high intra- and inter-observer variability[31] making it unsuitable for routine clinical practice. Peak systolic strain (PSS) is commonly used to assess myocardial contraction whilst peak early diastolic strain rate (PEDSR) is a marker of diastolic function[32]. Consequently, strain/diastolic strain rate assessment provides a comprehensive evaluation of myocardial contractility and compliance.
Myocardial Strain/strain rates can be assessed by a number of different imaging modalities - most frequently by echocardiography, but also by cardiac magnetic resonance imaging (CMR).
Echocardiography
Tissue Doppler imaging can assess myocardial strain but this technique is extremely angle dependent and has been superseded by speckle tracking echocardiography (STE)[33,34]. The ultrasonic images obtained by echocardiography consist of a large number of “speckles” which have individual properties[35]. These “acoustic markers”[34] can be identified and tracked as they move from one frame to the other throughout the cardiac cycle. Endocardial and epicardial borders are pre-defined by the operator and each speckle within this region of interest (ROI) is tracked. The tracking of such movement can be used to derive measures of strain[36] and strain rate[33]. STE is entirely a post-processing analysis. The only minor requirements are a short duration of breath holding by the patient so that respiratory motion does not affect the tracking of cardiac motion and a high frame rate to optimize temporal resolution.
Common echocardiographic imaging protocols include the acquisition of two-, four-chambered and three chamber views from which global LS (GLS) is derived (Figure 4). Short axis views allow circumferential and radial strain to be derived but it is difficult to accurately obtain global measures due to uncertainty of the imaging plane location.
Figure 4.
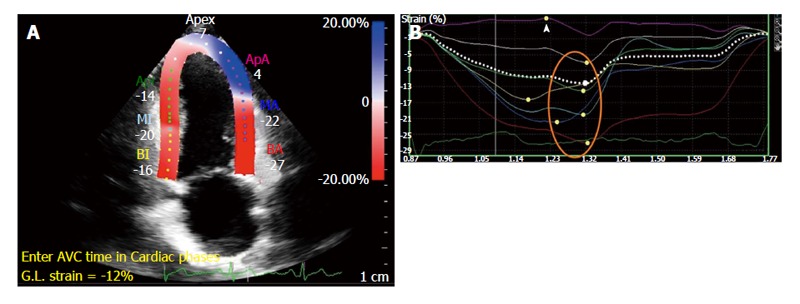
Peak systolic strain calculated by speckle-tracking echocardiography. A: Segmental strain after definition of endocardial and epicardial contours; B: Graphical illustration of segmental peak systolic strain - normal values annotated by orange circle, impaired strain by arrowhead.
STE-derived global strain parameters in the setting of an acute STEMI have shown good reproducibility - intra- and inter-observer variability of 0.92 and 0.85 by Intra-class Correlation Coefficient (ICC) respectively[37]. Repeatability is a measure of the “variation in repeat measurements made on the same subject under identical conditions made within a short period of time over which the underlying value can be considered to be constant”[38]. It is another method of establishing reliability. However, no studies to date have reported the repeatability of global strain measured by STE in acute STEMI.
CMR
CMR is another non-invasive imaging modality and is an alternative method of imaging to echocardiography. CMR can be used in the diagnosis, risk-stratification, and prognosis of a number of cardiac disorders[39,40], including acute MI[41-43]. Typically, strain is assessed on CMR using specialised myocardial tissue tagging sequences that involves the superimposition of horizontal and vertical lines on a cine image that appear in the form of a “grid”[44]. These grids or “tags” are formed onto the tissue by changing the local magnetisation through the use of selective radiofrequency saturation pulses perpendicular to the plane of image acquisition[45]. Tags deform along with the myocardium through the cardiac cycle and this deformation can be used to assess strain. Tagged images are commonly acquired using spatial modulation of magnetisation (SPAMM)[46] and complementary SPAMM sequences[47]. Post-processing analysis of tagged data can be performed using Harmonic phase analysis[48] and local sine wave modelling[49] and they have been shown to have good agreement[50]. Tagging has been validated against other invasive methods of strain assessment such as sonomicrometry[51] and has been used in a variety of animal models[52-54]. Tagging-derived strain parameters have a good intra- and inter-observer variability - ICC of 0.8 for both - along with acceptable test-retest repeatability - ICC of 0.74[55].
Tagging sequences however involve relatively long breath holds that may be difficult in the context of a recent STEMI. In addition, analysis is also labour-intensive and time-consuming[56]. Tagging, particularly with SPAMM sequences, cannot reliably calculate diastolic strain as the tags fade after systole especially at the 1.5 T field strength[45,57]. This can be overcome by using a stronger magnetic field strength (3.0 T) and Steady State Free Precession (SSFP) sequences[45]. However, true reproducibility is poor at 3.0 T CMR[58]. This may in part be due to the fact that by 3.0 T CMR images are also more susceptible to artefacts due to increase in inhomogeneity within the magnetic field[59].
To overcome the issues of tagging, myocardial motion tracking through the cardiac cycle on routinely acquired cine SSFP sequences can be performed by means of the novel feature tracking (FT) software[60]. FT is analogous to STE - endocardial and epicardial borders are defined and then subsequently propagated through the cardiac cycle. The software tracks the motion of the defined ROI from one frame to the next - PSS and PEDSR can be derived from this motion[60]. FT has shown excellent reproducibility - intra- and inter-observer variability of 0.988 and 0.971 in terms of ICC[61] - and acceptable test-retest repeatability - ICC of 0.77[56] - for PSS. Additionally, PSS by FT can predict global recovery of LV function in terms of EF[62].
Figure 5 illustrates a comparison of global circumferential strain (GCS) evaluation by tagging and FT.
Figure 5.
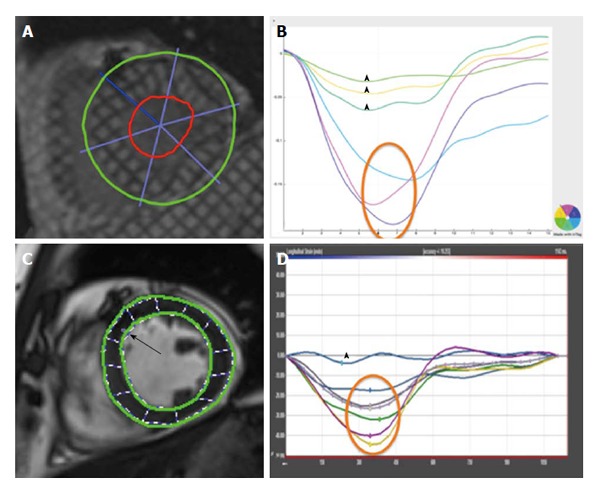
Comparison of tagging (A and B) and feature tracking (C and D) for evaluation of global circumferential strain - normal peak systolic strain annotated by orange circle, impaired peak systolic strain by arrowhead.
STE vs CMR to assess strain
STE has several advantages over CMR in the assessment of strain (Table 1). There is good agreement between STE-derived and CMR derived global values of strain - this is true both for tagging[63,64] and FT[65]. This suggests that these methods could be used interchangeably in the assessment of global strain. A detailed comparison of different imaging modalities to be used in the setting of an acute MI can be found elsewhere[36].
Table 1.
Advantages and disadvantages of speckle-tracking echocardiography vs cardiac magnetic resonance imaging
| Advantages | Disadvantages |
| Cheaper than CMR scan | Cannot acquire SAX views easily - needed to calculate circumferential strain |
| Can be performed at the bedside | Cannot routinely obtain stress imaging as part of acquisition protocol |
| Short duration: 10-20 min for STE vs 45-60 min for CMR | Not possible to ascertain infarct size, oedema, microvascular obstruction |
| Significant contraindications for CMR - for example, pacemaker/ICD, brain aneurysmal clip, claustrophobia, eGFR < 30 mL/min per 1.73 m2 - vs almost none for STE | CMR has much higher spatial resolution than STE. Consequently, a greater percentage of images are analysable by CMR than STE |
CMR: Cardiac magnetic resonance imaging; eGFR: Estimated glomerular filtration rate; ICD: Insertable cardioverter defibrillator; SAX: Short axis; STE: Speckle-tracking echocardiography.
Aims of systematic review
Global myocardial strain can objectively evaluate LV dysfunction post-STEMI and can be measured by STE and CMR techniques with good reproducibility and repeatability. We looked to review the literature for studies that evaluated the ability of global strain measured acutely post-STEMI by either STE or CMR to predict either MACE or development of adverse LV remodelling.
MATERIALS AND METHODS
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol[66].
Eligibility criteria
Table 2 highlights the eligibility criteria for the review. Studies were limited to acute STEMI patients to represent the setting of an acute MI - NSTEMI patients were excluded since the diagnosis is more complex, heterogeneous presentations and that their subsequent management is based on risk-stratification[67]. There was no limitation placed on the management of the STEMI - both in terms of method of revascularisation (PPCI or thrombolysis) and success/failure. Strain parameters were restricted to peak systolic GCS and GLS and PEDSR in the same two vectors. Both segmental strain values and radial strain parameters were excluded since they both have been shown to have poor intra- and inter-observer variability[31,58]. We limited the timeframe for the baseline scan to be 0-14 d post- to limit the effects of subsequent remodelling. The timeframe for outcome measures were ≥ 3 mo for adverse LV remodelling (since it is a dynamic process that takes months to fully develop[68]). Minimum follow-up time for development of MACE was six months. We included studies that quoted either changes in EDV or ESV.
Table 2.
Eligibility criteria for systematic review
| Type of characteristic | |
| Population type | Acute STEMI |
| Measured parameters | Global longitudinal and/or circumferential strain and/or strain rate - PSS or strain rate (PSS-R) or PEDSR |
| Imaging modalities | STE or cardiac MRI tagging or cardiac MRI FT |
| Timeframe for baseline scan | Days 0-14 post-STEMI |
| Outcomes reported | MACE or adverse LV remodelling |
| Timeframe for follow-up | MACE - ≥ 6 mo |
| Adverse LV remodelling - s ≥ 3 mo | |
| Year published | Within the last 20 yr |
STEMI: ST-elevation myocardial infarction; PSS: Peak systolic strain; PEDSR: Peak early diastolic strain rate; STE: Speckle-tracking echocardiography; FT: Feature tracking; MACE: Major adverse cardiac events; LV: Left ventricular; MRI: Magnetic resonance imaging.
Search protocol
The literature search was performed in PubMed and OVID® electronic databases. The final date on which the online search was performed was January 27th, 2015 (Table 3) for list of keywords used.
Table 3.
Keywords used for search of electronic databases
| "Cardiac MRI" OR "CMR" OR "magnetic resonance imaging [MeSH Term]" OR "cardiac magnetic resonance" OR "feature tracking" OR "tissue tracking" OR "tagging" OR "tag" OR "tagged" OR "SPAMM" OR "CPSAMM" OR "HARP" OR "SinMOD" OR "Echocardiography [MeSH Term]" OR "Speckle tracking", "2D speckle" OR "3D speckle" OR "two dimensional speckle" OR "three dimensional speckle". MIs were searched using "myocardial infarction [MeSH Term]" OR "acute MI" OR "STEMI" OR "ST elevation". Strain was searched using "strain" OR "myocardial strain" OR "strain rate" OR "deformation" OR "myocardial deformation" OR "systolic" OR "diastolic" OR "PSS" OR "PEDSR" OR "longitudinal" OR "circumferential". Outcomes were searched using "Predict" OR "Outcome" OR "Risk" OR "Prognosis" OR "Logistic Models [MeSH Term]" OR "risk" OR "multivariable" OR "multivariate" OR "odds" OR "MACE" OR "mortality [MeSH Term]" OR "remodelling" OR "remodelling" OR "adverse" OR "cardiac" OR "left ventricular" |
| Note: MeSH terms were only available on PubMed |
MRI: Magnetic resonance imaging; CMR: Cardiac magnetic resonance imaging; STEMI: ST-elevation myocardial infarction; PSS: Peak systolic strain; PEDSR: Peak early diastolic strain rate.
Study selection
Figure 6 highlights the process of study selection. Initial electronic search yielded 1920 studies; 1330 remained after addition of relevant filters. The titles and abstracts of these studies were then screened to assess for eligibility for inclusion in the systematic review (Table 2). A majority of the studies were deemed inappropriate for inclusion based on the aforementioned criteria (n = 1310). The remaining 20 papers were further scrutinised by searching for and evaluating the full-text article. A further 13 studies were excluded - some did not actually assess strain at all (n = 4), some assessed torsion (n = 3), three had included NSTEMI patients and the rest did not have full-text articles available as they were presented as posters (n = 3). Consequently, there were seven studies that matched our inclusion criteria for the review.
Figure 6.
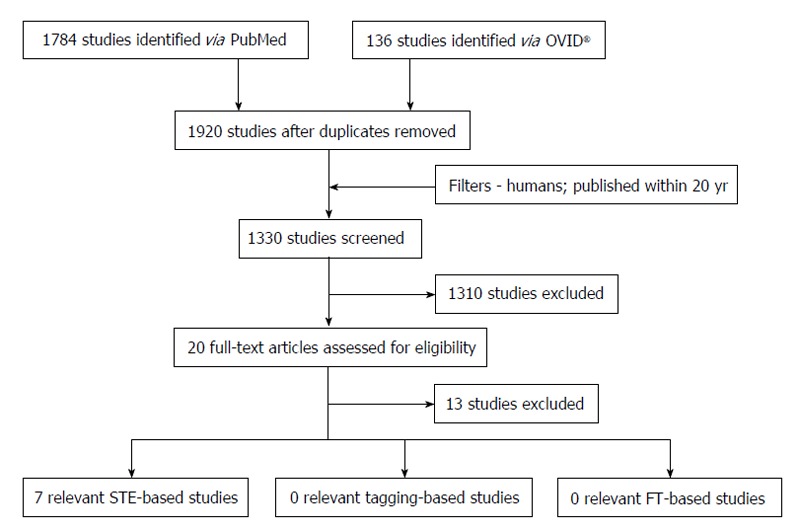
Flowchart illustrating the search for relevant studies. FT: Feature tracking; STE: Speckle-tracking echocardiography.
RESULTS
Strain measured by STE
Seven STE-based studies that matched our inclusion criteria were found (Table 4) highlights studies that assessed global strain to predict adverse LV remodelling and Table 5 highlights studies that used global strain to predict MACE. Six studies reported peak systolic longitudinal strain parameters to predict outcomes - only one study used diastolic strain. All the patients were treated with PPCI.
Table 4.
All studies that have used speckle-tracking echocardiography-based strain to predict adverse left ventricular remodelling
| Ref. | Age (yr) | Sample size (male) | Baseline ejection fraction (%) | Timeframe baseline scan | Timeframe follow-up scan(s) | Definition of adverse remodelling | Other parameters in multivariate model | Results | Limitations |
| Bochenek et al[70] | 59.6 ± 10.3 | 66 (53) | 49.7 ± 9.2 | 4-6 d post-infarct | 3 mo | EDV > 20% | Diabetes | 22 patients remodelled; GLS can predict LV remodelling - OR = 1.19 (1.04-1.37), P < 0.05 - shown by multivariate analysis GLS > -12.5% can predict remodelling - AUC = 0.77 for ROC, sensitivity/specificity of 69%/79% respectively | Only longitudinal strain measured. Too many variables in multivariate analysis |
| Anterior MI | |||||||||
| Leuk. Count | |||||||||
| Time to reperfusion | |||||||||
| WMSI | |||||||||
| Max. Trop | |||||||||
| ST-elevation max pre-PCI | |||||||||
| Joyce et al[74] | 60 ± 12 | 1041 (792) | 47.0 ± 9.0 | 2 d post-PPCI | 3 and 6 mo | EDV ≥ 20% | Male sex | GLS > -15% can predict remodelling at 3 and 6 mo vs GLS < -15% (both P < 0.001): OR = 6.7 (2.8-11) for 3 mo; OR = 10 (6.7-14) for 6 mo | Only longitudinal strain measured; Prognostic data divided categorically - i.e., GLS > -15% or < -15%; Excluded patients with re-infarction before follow-up and cardiogenic shock - could potentially have been used as another endpoint |
| LAD infarct | |||||||||
| Max. Trop | |||||||||
| Discharge heart rate | |||||||||
| LA volume index | |||||||||
| WMSI | |||||||||
| Cong et al[71] | 59.9 ± 11.6 | 127 (103) | 51.8 ± 5.1 | 1 d post-PPCI | 6-9 mo | ESV ≥ 15% | Anterior MI | 41 patients developed remodelling; GLS predicted remodelling - OR = 0.39 (0.26-0.57), P < 0.01; GLS = -10.85% had sensitivity/specificity of 89.7%/91.7% respectively by ROC to predict remodelling | Only longitudinal strain measured; Too many variables in the multivariate analysis |
| Time to reperfusion | |||||||||
| ∑ST before PPCI | |||||||||
| ∑ST post-PPCI | |||||||||
| Raised CK-MB/Trops | |||||||||
| Baseline ESV/EF | |||||||||
| WMSI |
AUC: Area under curve; CK: Creatine kinase; EDV: End diastolic volume; ESV: End systolic volume; GLS: Global longitudinal strain; LA: Left atrium; LAD: Left anterior descending; LV: Left ventricle; OR: Odds ratio; PPCI: Primary percutaneous coronary intervention; ROC: Receiver operator characteristic; WMSI: Wall motion score index; ∑ST: Sum of ST-elevation.
Table 5.
All studies that have used speckle-tracking echocardiography-based strain to predict major adverse cardiac events
| Ref. | Age (yr) | Sample size (male) | Baseline ejection fraction (%) | Timeframe baseline scan | Follow-up period | Outcome measures | Other parameters in multivariate model | Results | Limitations |
| Antoni et al[69] | 60 ± 12 | 759 (517) | 46.0 ± 8.0 | 2 d post-PPCI | 21 ± 13 mo | GLS and/or GL-strain rate to predict: A: Mortality; B: Composite of revascularisation/readmission for HF/re-infarction | Age (A) | 179 patients reached one or more endpoints; GLS independent predictor of all-cause mortality - HR = 1.2 (1.1-1.3), P = 0.002; GLS-R independent predictor of B endpoints - HR = 22 (11-48), P < 0.001; Both GLS and GLS-R independent predictors of combined A and B endpoints - HR = 1.1 (1 -1.1, P = 0.006) and 18 (10-35, P < 0.001) respectively | Sample size n < 1000 - potentially not large enough to predict "hard" events like mortality; Only longitudinal strain measured; SR analysis feasible in only 89% of segments |
| HTN (A) | |||||||||
| Multi-vessel disease (A/B) | |||||||||
| Peak Trop (A) | |||||||||
| QRS duration (A/B) | |||||||||
| EF (A/B) | |||||||||
| Severe MR (A) | |||||||||
| Smoking (B) | |||||||||
| Diabetes (B) | |||||||||
| Shanks et al[73] | 59.7 ± 11.6 | 371 (288) | 45.2 ± 8.0 | 2 d post-PPCI | 17.3 ± 12.2 mo | GL-PEDSR to predict: Mortality; Readmission for HF; Re-infarction; Revascularisation | EF | Combined clinical endpoints occurred in 84 patients; GL-PEDSR does not predict clinical outcomes | Sample size potentially too small to assess "hard" endpoint such as mortality; No measure of GLS; Only longitudinal parameters obtained |
| TIMI 0-1 | |||||||||
| ESV-index | |||||||||
| Iso-volumetric relaxation SR | |||||||||
| Woo et al[72] | 64.4 | 98 (65) | 52.6 ± 12.0 | Pre-PPCI and 3 d post-PPCI | 13.1 ± 3.8 mo | GLS to predict: Mortality; Readmission for HF | Initial Trop | 7 patients developed endpoints; Pre-PPCI GLS predictor of outcomes - HR = 1.41 (1.01-1.98), P < 0.05; Post-PPCI GLS more likely to predict outcomes - HR = 2.34 (1.10-4.97), P < 0.05; Pre-PPCI GLS < 14% had sensitivity/specificity of 85%/75% respectively - post-PPCI GLS < 13% of 100%/89% | Very small sample size; Only longitudinal strain measured; Too many variables in multivariate analysis |
| Initial NT-pro BNP | |||||||||
| EF (baseline) | |||||||||
| WMSI (follow-up) | |||||||||
| E/e’sr | |||||||||
| EF (follow-up) | |||||||||
| WSMI (follow-up) | |||||||||
| Munk et al[78] | 63.1 | 576 (446) | 50.0 ± 10.0 (without composite endpoint), 47.0 ± 12.0 (with composite endpoint) | 1 d post-PPCI | 24 (IQ range 13-61) mo | GLS to predict: Mortality/re-infarction/stroke/hospitalisation for HF; Crude mortality | EF | 162 patients experienced composite endpoints; GLS alone predicted outcomes within 1 yr post-MI - HR = 1.2 (1.12-1.29), P < 0.01; GLS alone could not predict outcomes later than 1yr post-MI | GLS could only be obtained in 74% of 576 patients - 26% excluded due to poor image quality (no difference in event rates, however); Only longitudinal strain measured |
| WMSI | |||||||||
| ESV-index (Separately and in combination with each other) | |||||||||
| Cong et al[71] | 59.9 ± 11.6 | 127 (103) | 51.8 ± 5.1 | 1 d post-PPCI | 16.9 ± 1.6 mo | GLS to predict: Mortality; Development of HF | Anterior MI | GLS predicted outcomes - OR = 0.56 (0.34-0.91), P = 0.02; GLS > -9.55% had sensitivity/specificity of 83.3%/83.5% respectively | Sample size could potentially be too small to significantly predict "hard" events such as mortality |
| Time to reperfusion | |||||||||
| ∑ST before PPCI | |||||||||
| ∑ST post-PPCI | |||||||||
| Raised CK-MB/Trops | |||||||||
| Baseline ESV/EF | |||||||||
| WMSI |
CK: Creatine kinase; ESV: End systolic volume; GLS: Global longitudinal strain; HF: Heart failure; OR: Odds ratio; HR: Hazard ratio; HTN: Hypertension; IQ: Inter-quartile range; MR: Mitral regurgitation; PEDSR: Peak early diastolic strain rate; PPCI: Primary percutaneous coronary intervention; TIMI: Thrombolysis in myocardial infarction; WMSI: Wall motion score index; ∑ST: Sum of ST-elevation; MI: Myocardial infarction.
Multivariate analyses in all the studies have shown that peak systolic GLS can independently predict both adverse LV remodelling and MACE. Such analyses have shown that this is independent of factors such as age, diabetes, location of infarct, EF and WMSI. One study showed that global longitudinal SR (GLS-R) also had significant impact on prognosis[69] - patients with impaired GLS-R, and GLS, were 18-times more likely to suffer from composite endpoint of mortality, readmission due to HF, revascularisation, or re-infarction. One study showed that a cut-off GLS > -12.5% (i.e., LV unable to contract more than 12.5% of its original length in the longitudinal vector) could predict development of remodelling - OR 1.19 (1.04-1.37), P < 0.05, sensitivity/specificity of 69%/79%[70]. Another showed a cut-off of GLS = 10.85% - OR 0.39 (0.26-0.57), P < 0.01, sensitivity/specificity of 89.7%/91.7%[71]. A cut-off for prediction of MACE ranged from GLS > -13% [HR = 2.34 (1.10-4.97), P < 0.05, sensitivity/specificity of 100%/89%][72] to GLS > -9.55% [OR = 0.56 (0.34-0.91), P = 0.02, sensitivity/specificity of 83.3/83.5%][71].
PEDSR was only measured in one study[73]. There was no significant difference in PEDSR in between patients that reached clinical endpoints and those that did not.
Strain measured by CMR
There were no studies that used CMR-based strain measurement techniques - either tagging or FT - to predict outcomes post-STEMI that matched our eligibility criteria.
DISCUSSION
This systematic review has shown that certain strain parameters measured by STE - namely, GLS[70-72,74,75] and GLS-R[69] - are independent predictors of adverse outcomes post-STEMI. Impaired GLS can predict both clinical endpoints and adverse LV remodelling, a surrogate marker of poor prognosis. When combined with routine clinical functional parameters such as EF and WMSI, strain provides incremental value in the prognostication of STEMI patients.
However, studies that monitored “hard” events such as mortality could not match the large sample size of n > 1000 that some authors believe is important for the evidence to be considered statistically robust[17]. Only one of the studies we assessed had such a large sample size - but the authors monitored remodelling and not “hard” events[74].
Some of the studies that monitored MACE had only a small number of patients that had reached their defined endpoints. Despite this, they constructed models for multivariate analysis that included a large number of independent variables (in addition to GLS). It is believed that one variable should be added for every 10 events to ensure that the regression estimates have reasonable precision[76]. Therefore, all of these studies may have included an inappropriately high number of variables to assess independent predictors of clinical endpoints and the models are likely to suffer from over-fitting.
PEDSR does not seem to provide any benefit at predicting these outcomes although has only been assessed in one study. Consequently, further studies are surely needed to determine if diastolic dysfunction has any role to play in prognostication after a STEMI[27].
Data in this review is limited to GLS measured by STE. We cannot comment on whether GCS is of any added value or has similar predictive properties as GLS since no studies assessed these two parameters together.
Evidence suggests that GLS measured by STE is related to IS[37,77]. The question remains as to whether GLS provides additional information to IS in post-STEMI prognostication and it can only be adequately answered using CMR. However, no studies were found that showed global strain measured by CMR could predict development of remodelling or MACE.
Limitations
We could rule out publication bias - unpublished data were not included as part of our review and could possibly affect our results, especially if it contradicted the seven studies that were assessed. We did not include three search results that were presented as posters since we could not access either the poster itself or the full-text articles associated with it. Regardless, we do not feel this exclusion would significant affect the results of the review since the titles of all three posters stated that GLS could predict post-STEMI outcomes. There is outcome data available in strain measured by TDI but we decided to exclude it from our review since its major limitation of “angle dependence” has been superseded by STE.
Conclusion
Global longitudinal strain when measured by STE is an independent predictor of both adverse LV remodelling and MACE after STEMI and provides incremental prognostic value when combined with traditional LV functional parameters such as EF and WMSI. No such data exist for CMR, but this modality could inform us as to whether strain provides prognostic data in addition to IS.
ACKNOWLEDGMENTS
This systematic review was performed as part of a student Intercalated Bachelor of Science degree, funded by the University of Leicester and the National Institute for Health Research (NIHR) in the United Kingdom. The author declares no competing interests and no relationship with industry. The author would also like to thank the following individuals for providing support and supervision of the project: Dr. Gerry McCann - NIHR Career Development Fellow and Reader in Cardiovascular Imaging, Department of Cardiovascular Sciences, University of Leicester and the NIHR Leicester Cardiovascular Biomedical Research Unit, Glenfield Hospital, Leicester, United Kingdom; Professor Iain B Squire - Professor of Cardiovascular Medicine/Honorary Consultant Physician, Department of Cardiovascular Sciences, University of Leicester; Dr. Sheraz Nazir - Clinical Research Fellow - Glenfield Hospital, United Kingdom; Dr. Jamal Khan - Clinical Research Fellow - Glenfield Hospital, United Kingdom; The author would also like to thank Dr. Anna-Marie Marsh for providing sample images of STE.
COMMENTS
Background
Left ventricular (LV) dysfunction is an important determinant of prognosis following ST-elevation myocardial infarction (STEMI). Routinely used measures of LV dysfunction such as ejection fraction (EF) may not be able to detect subtle changes in cardiac function. Myocardial strain describes the relative change in length of myocardium through the cardiac cycle and is an objective measure of LV function. It can be measured during both systole and diastole and hence provides a reflection of both systolic and diastolic LV contractility. Acutely measured strain post-STEMI may help in predicting markers of poor prognosis [such as development of adverse LV remodelling or major adverse cardiac events (MACE)] at follow-up.
Research frontiers
Strain can be assessed using post-processing speckle-tracking echocardiography (STE) or cardiac magnetic resonance imaging (CMR)-based techniques [such as tagging or novel feature tracking (FT) software]. Such techniques can quantify strain at a segmental and global level and may provide additional information to LV volumes and EF.
Innovations and breakthroughs
This is the first paper to review the literature and present all the studies that have assessed acutely measured global strain parameters to predict markers of outcome post-STEMI. Three studies have shown that global longitudinal strain (GLS) measured by STE is a predictor of adverse remodelling following STEMI whilst four studies have shown that it can predict MACE at follow-up. Therefore, GLS may be a useful clinical measure of identifying patients at a “high risk” of developing poor outcomes. There were also no CMR-based studies assessing strain and its relation to prognosis following STEMI.
Applications
GLS may help improve risk stratification following STEMI but further studies are required to show that this improves outcome.
Terminology
Myocardial strain describes the relative change in length of myocardium through the cardiac cycle -GLS is a measure of LV contractility in the longitudinal vector; STE is an echocardiography-based post-processing software that analyses myocardial deformation parameters (such as global strain) by tracking the motion of “speckles” from one frame to another through the cardiac cycle; Tagging is a post-processing CMR-based software that evaluates strain on tagged sequences - examples of such sequences include spatial modulation of magnetisation (SPAMM) and complementary SPAMM; FT is a post-processing software that assesses strain on cine steady-state free precession images, a type of sequence that is routinely acquired during a clinical CMR scan.
Peer-review
The article is interesting, well-written and supported by updated references.
Footnotes
P- Reviewer: Biondi-Zoccai G, Tadic M S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: The authors have no conflicts of interest relevant to the manuscript.
Data sharing statement: Not applicable.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 30, 2015
First decision: August 25, 2015
Article in press: October 13, 2015
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar P, Clark M. Acute Coronary Syndromes - Cardiovascular Disease. Kumar and Clark’s Clinical Medicine. 8th ed. Elsevier: 2012. pp. 733–740. [Google Scholar]
- 3.Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 4.Volmink JA, Newton JN, Hicks NR, Sleight P, Fowler GH, Neil HA. Coronary event and case fatality rates in an English population: results of the Oxford myocardial infarction incidence study. The Oxford Myocardial Infarction Incidence Study Group. Heart. 1998;80:40–44. doi: 10.1136/hrt.80.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilansky S, Moreno CA, Lester SJ. Complications of myocardial infarction. Crit Care Med. 2007;35:S348–S354. doi: 10.1097/01.CCM.0000270244.90395.67. [DOI] [PubMed] [Google Scholar]
- 6.Jhund PS, McMurray JJ. Heart failure after acute myocardial infarction: a lost battle in the war on heart failure? Circulation. 2008;118:2019–2021. doi: 10.1161/CIRCULATIONAHA.108.813493. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart. 2000;83:596–602. doi: 10.1136/heart.83.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jelani A, Jugdutt BI. STEMI and heart failure in the elderly: role of adverse remodeling. Heart Fail Rev. 2010;15:513–521. doi: 10.1007/s10741-010-9177-3. [DOI] [PubMed] [Google Scholar]
- 9.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, Luengo-Fernandez R, Rayner M. Coronary Heart Disease Statistics. In: Foundation BH, editor. London: British Heart Foundation; 2012. [Google Scholar]
- 11.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 12.Picariello C, Lazzeri C, Attanà P, Chiostri M, Gensini GF, Valente S. The impact of hypertension on patients with acute coronary syndromes. Int J Hypertens. 2011;2011:563657. doi: 10.4061/2011/563657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman JD, Shimbo D, Baggett C, Liu X, Crow R, Abraham JM, Loehr LR, Wruck LM, Folsom AR, Rosamond WD. Trends in myocardial infarction rates and case fatality by anatomical location in four United States communities, 1987 to 2008 (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2013;112:1714–1719. doi: 10.1016/j.amjcard.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller TD, Christian TF, Hodge DO, Hopfenspirger MR, Gersh BJ, Gibbons RJ. Comparison of acute myocardial infarct size to two-year mortality in patients < 65 to those > or =65 years of age. Am J Cardiol. 1999;84:1170–1175. doi: 10.1016/s0002-9149(99)00529-9. [DOI] [PubMed] [Google Scholar]
- 15.Wu KC. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J Cardiovasc Magn Reson. 2012;14:68. doi: 10.1186/1532-429X-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitcher A, Ashby D, Elliott P, Petersen SE. Cardiovascular MRI in clinical trials: expanded applications through novel surrogate endpoints. Heart. 2011;97:1286–1292. doi: 10.1136/hrt.2011.225904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Aidi H, Adams A, Moons KG, Den Ruijter HM, Mali WP, Doevendans PA, Nagel E, Schalla S, Bots ML, Leiner T. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: a systematic review of prognostic studies. J Am Coll Cardiol. 2014;63:1031–1045. doi: 10.1016/j.jacc.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 18.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 19.Anzai T. Post-infarction inflammation and left ventricular remodeling: a double-edged sword. Circ J. 2013;77:580–587. doi: 10.1253/circj.cj-13-0013. [DOI] [PubMed] [Google Scholar]
- 20.Müller AL, Dhalla NS. Role of various proteases in cardiac remodeling and progression of heart failure. Heart Fail Rev. 2012;17:395–409. doi: 10.1007/s10741-011-9269-8. [DOI] [PubMed] [Google Scholar]
- 21.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 22.de Waha S, Eitel I, Desch S, Fuernau G, Lurz P, Stiermaier T, Blazek S, Schuler G, Thiele H. Prognosis after ST-elevation myocardial infarction: a study on cardiac magnetic resonance imaging versus clinical routine. Trials. 2014;15:249. doi: 10.1186/1745-6215-15-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christian TF, Behrenbeck T, Gersh BJ, Gibbons RJ. Relation of left ventricular volume and function over one year after acute myocardial infarction to infarct size determined by technetium-99m sestamibi. Am J Cardiol. 1991;68:21–26. doi: 10.1016/0002-9149(91)90703-n. [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 26.Møller JE, Pellikka PA, Hillis GS, Oh JK. Prognostic importance of diastolic function and filling pressure in patients with acute myocardial infarction. Circulation. 2006;114:438–444. doi: 10.1161/CIRCULATIONAHA.105.601005. [DOI] [PubMed] [Google Scholar]
- 27.Ersbøll M, Andersen MJ, Valeur N, Mogensen UM, Fahkri Y, Thune JJ, Møller JE, Hassager C, Søgaard P, Køber L. Early diastolic strain rate in relation to systolic and diastolic function and prognosis in acute myocardial infarction: a two-dimensional speckle-tracking study. Eur Heart J. 2014;35:648–656. doi: 10.1093/eurheartj/eht179. [DOI] [PubMed] [Google Scholar]
- 28.Møller JE, Egstrup K, Køber L, Poulsen SH, Nyvad O, Torp-Pedersen C. Prognostic importance of systolic and diastolic function after acute myocardial infarction. Am Heart J. 2003;145:147–153. doi: 10.1067/mhj.2003.46. [DOI] [PubMed] [Google Scholar]
- 29.Zwanenburg JJM. Mapping Asynchrony of Circumferential Shortening in the Human Heart with High Temporal Resolution MRI Tagging. Amsterdam: Vrije University; 2005. [Google Scholar]
- 30.Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH. Left ventricular fibre architecture in man. Br Heart J. 1981;45:248–263. doi: 10.1136/hrt.45.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leischik R, Dworrak B, Hensel K. Intraobserver and interobserver reproducibility for radial, circumferential and longitudinal strain echocardiography. Open Cardiovasc Med J. 2014;8:102–109. doi: 10.2174/1874192401408010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan JN, Wilmot EG, Leggate M, Singh A, Yates T, Nimmo M, Khunti K, Horsfield MA, Biglands J, Clarysse P, et al. Subclinical diastolic dysfunction in young adults with Type 2 diabetes mellitus: a multiparametric contrast-enhanced cardiovascular magnetic resonance pilot study assessing potential mechanisms. Eur Heart J Cardiovasc Imaging. 2014;15:1263–1269. doi: 10.1093/ehjci/jeu121. [DOI] [PubMed] [Google Scholar]
- 33.Feigenbaum H, Mastouri R, Sawada S. A practical approach to using strain echocardiography to evaluate the left ventricle. Circ J. 2012;76:1550–1555. doi: 10.1253/circj.cj-12-0665. [DOI] [PubMed] [Google Scholar]
- 34.Gorcsan J, Tanaka H. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol. 2011;58:1401–1413. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 35.Feigenbaum H, Armstrong W, Ryan T. Physics of Echocardiography. Feigenbaum’s Echocardiography. London: Lippincott Williams and Wilkins; 2005. p. 12. [Google Scholar]
- 36.Flachskampf FA, Schmid M, Rost C, Achenbach S, DeMaria AN, Daniel WG. Cardiac imaging after myocardial infarction. Eur Heart J. 2011;32:272–283. doi: 10.1093/eurheartj/ehq446. [DOI] [PubMed] [Google Scholar]
- 37.Sjøli B, Ørn S, Grenne B, Ihlen H, Edvardsen T, Brunvand H. Diagnostic capability and reproducibility of strain by Doppler and by speckle tracking in patients with acute myocardial infarction. JACC Cardiovasc Imaging. 2009;2:24–33. doi: 10.1016/j.jcmg.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol. 2008;31:466–475. doi: 10.1002/uog.5256. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Mattei JC, Shah DJ. The role of cardiac magnetic resonance in valvular heart disease. Methodist Debakey Cardiovasc J. 2013;9:142–148. doi: 10.14797/mdcj-9-3-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, Wansapura J, Klimeczek P, Al-Khalidi HR, Chung ES, et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 2010;3:144–151. doi: 10.1016/j.jcmg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Chen MY, Tsai JW, Chang MS, Yu BC. Assessment of heart wall motion: modified spatial modulation of magnetization for MR imaging. Proc Natl Sci Counc Repub China B. 1995;19:47–53. [PubMed] [Google Scholar]
- 42.Neizel M, Lossnitzer D, Korosoglou G, Schäufele T, Peykarjou H, Steen H, Ocklenburg C, Giannitsis E, Katus HA, Osman NF. Strain-encoded MRI for evaluation of left ventricular function and transmurality in acute myocardial infarction. Circ Cardiovasc Imaging. 2009;2:116–122. doi: 10.1161/CIRCIMAGING.108.789032. [DOI] [PubMed] [Google Scholar]
- 43.Inoue Y, Yang X, Nagao M, Higashino H, Hosokawa K, Kido T, Kurata A, Okayama H, Higaki J, Mochizuki T, et al. Peri-infarct dysfunction in post-myocardial infarction: assessment of 3-T tagged and late enhancement MRI. Eur Radiol. 2010;20:1139–1148. doi: 10.1007/s00330-009-1657-2. [DOI] [PubMed] [Google Scholar]
- 44.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59–63. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- 45.Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:55. doi: 10.1186/1532-429X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171:841–845. doi: 10.1148/radiology.171.3.2717762. [DOI] [PubMed] [Google Scholar]
- 47.Fischer SE, McKinnon GC, Maier SE, Boesiger P. Improved myocardial tagging contrast. Magn Reson Med. 1993;30:191–200. doi: 10.1002/mrm.1910300207. [DOI] [PubMed] [Google Scholar]
- 48.Osman NF, Kerwin WS, McVeigh ER, Prince JL. Cardiac motion tracking using CINE harmonic phase (HARP) magnetic resonance imaging. Magn Reson Med. 1999;42:1048–1060. doi: 10.1002/(sici)1522-2594(199912)42:6<1048::aid-mrm9>3.0.co;2-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arts T, Prinzen FW, Delhaas T, Milles JR, Rossi AC, Clarysse P. Mapping displacement and deformation of the heart with local sine-wave modeling. IEEE Trans Med Imaging. 2010;29:1114–1123. doi: 10.1109/TMI.2009.2037955. [DOI] [PubMed] [Google Scholar]
- 50.Miller CA, Borg A, Clark D, Steadman CD, McCann GP, Clarysse P, Croisille P, Schmitt M. Comparison of local sine wave modeling with harmonic phase analysis for the assessment of myocardial strain. J Magn Reson Imaging. 2013;38:320–328. doi: 10.1002/jmri.23973. [DOI] [PubMed] [Google Scholar]
- 51.Yeon SB, Reichek N, Tallant BA, Lima JA, Calhoun LP, Clark NR, Hoffman EA, Ho KK, Axel L. Validation of in vivo myocardial strain measurement by magnetic resonance tagging with sonomicrometry. J Am Coll Cardiol. 2001;38:555–561. doi: 10.1016/s0735-1097(01)01397-3. [DOI] [PubMed] [Google Scholar]
- 52.Heijman E, Strijkers GJ, Habets J, Janssen B, Nicolay K. Magnetic resonance imaging of regional cardiac function in the mouse. MAGMA. 2004;17:170–178. doi: 10.1007/s10334-004-0082-4. [DOI] [PubMed] [Google Scholar]
- 53.Ivancevic MK, Daire JL, Hyacinthe JN, Crelier G, Kozerke S, Montet-Abou K, Gunes-Tatar I, Morel DR, Vallée JP. High-resolution complementary spatial modulation of magnetization (CSPAMM) rat heart tagging on a 1.5 Tesla Clinical Magnetic Resonance System: a preliminary feasibility study. Invest Radiol. 2007;42:204–210. doi: 10.1097/01.rli.0000255646.58831.4b. [DOI] [PubMed] [Google Scholar]
- 54.Croisille P, Moore CC, Judd RM, Lima JA, Arai M, McVeigh ER, Becker LC, Zerhouni EA. Differentiation of viable and nonviable myocardium by the use of three-dimensional tagged MRI in 2-day-old reperfused canine infarcts. Circulation. 1999;99:284–291. doi: 10.1161/01.cir.99.2.284. [DOI] [PubMed] [Google Scholar]
- 55.Donekal S, Ambale-Venkatesh B, Berkowitz S, Wu CO, Choi EY, Fernandes V, Yan R, Harouni AA, Bluemke DA, Lima JA. Inter-study reproducibility of cardiovascular magnetic resonance tagging. J Cardiovasc Magn Reson. 2013;15:37. doi: 10.1186/1532-429X-15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson. 2012;14:43. doi: 10.1186/1532-429X-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeung MY, Germain P, Croisille P, El ghannudi S, Roy C, Gangi A. Myocardial tagging with MR imaging: overview of normal and pathologic findings. Radiographics. 2012;32:1381–1398. doi: 10.1148/rg.325115098. [DOI] [PubMed] [Google Scholar]
- 58.Singh A, Steadman CD, Khan JN, Horsfield MA, Bekele S, Nazir SA, Kanagala P, Masca NG, Clarysse P, McCann GP. Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 Tesla: a comparison of feature-tracking and tagging in patients with aortic stenosis. J Magn Reson Imaging. 2015;41:1129–1137. doi: 10.1002/jmri.24625. [DOI] [PubMed] [Google Scholar]
- 59.Oshinski JN, Delfino JG, Sharma P, Gharib AM, Pettigrew RI. Cardiovascular magnetic resonance at 3.0 T: current state of the art. J Cardiovasc Magn Reson. 2010;12:55. doi: 10.1186/1532-429X-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hor KN, Baumann R, Pedrizzetti G, Tonti G, Gottliebson WM, Taylor M, Benson DW, Mazur W. Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp. 2011;(48):pii: 2356. doi: 10.3791/2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann GP. Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol. 2015;84:840–848. doi: 10.1016/j.ejrad.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Buss SJ, Krautz B, Hofmann N, Sander Y, Rust L, Giusca S, Galuschky C, Seitz S, Giannitsis E, Pleger S, et al. Prediction of functional recovery by cardiac magnetic resonance feature tracking imaging in first time ST-elevation myocardial infarction. Comparison to infarct size and transmurality by late gadolinium enhancement. Int J Cardiol. 2015;183:162–170. doi: 10.1016/j.ijcard.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 63.Amundsen BH, Crosby J, Steen PA, Torp H, Slørdahl SA, Støylen A. Regional myocardial long-axis strain and strain rate measured by different tissue Doppler and speckle tracking echocardiography methods: a comparison with tagged magnetic resonance imaging. Eur J Echocardiogr. 2009;10:229–237. doi: 10.1093/ejechocard/jen201. [DOI] [PubMed] [Google Scholar]
- 64.Singh GK, Cupps B, Pasque M, Woodard PK, Holland MR, Ludomirsky A. Accuracy and reproducibility of strain by speckle tracking in pediatric subjects with normal heart and single ventricular physiology: a two-dimensional speckle-tracking echocardiography and magnetic resonance imaging correlative study. J Am Soc Echocardiogr. 2010;23:1143–1152. doi: 10.1016/j.echo.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Padiyath A, Gribben P, Abraham JR, Li L, Rangamani S, Schuster A, Danford DA, Pedrizzetti G, Kutty S. Echocardiography and cardiac magnetic resonance-based feature tracking in the assessment of myocardial mechanics in tetralogy of Fallot: an intermodality comparison. Echocardiography. 2013;30:203–210. doi: 10.1111/echo.12016. [DOI] [PubMed] [Google Scholar]
- 66.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–29, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 67.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina) Circulation. 2000;102:1193–1209. doi: 10.1161/01.cir.102.10.1193. [DOI] [PubMed] [Google Scholar]
- 68.Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, Santoro GM, Antoniucci D. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–2357. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 69.Antoni ML, Mollema SA, Delgado V, Atary JZ, Borleffs CJ, Boersma E, Holman ER, van der Wall EE, Schalij MJ, Bax JJ. Prognostic importance of strain and strain rate after acute myocardial infarction. Eur Heart J. 2010;31:1640–1647. doi: 10.1093/eurheartj/ehq105. [DOI] [PubMed] [Google Scholar]
- 70.Bochenek T, Wita K, Tabor Z, Grabka M, Krzych Ł, Wróbel W, Berger-Kucza A, Elżbieciak M, Doruchowska A, Gluza MT. Value of speckle-tracking echocardiography for prediction of left ventricular remodeling in patients with ST-elevation myocardial infarction treated by primary percutaneous intervention. J Am Soc Echocardiogr. 2011;24:1342–1348. doi: 10.1016/j.echo.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Cong T, Sun Y, Shang Z, Wang K, Su D, Zhong L, Zhang S, Yang Y. Prognostic Value of Speckle Tracking Echocardiography in Patients with ST-Elevation Myocardial Infarction Treated with Late Percutaneous Intervention. Echocardiography. 2015;32:1384–1391. doi: 10.1111/echo.12864. [DOI] [PubMed] [Google Scholar]
- 72.Woo JS, Kim WS, Yu TK, Ha SJ, Kim SY, Bae JH, Kim KS. Prognostic value of serial global longitudinal strain measured by two-dimensional speckle tracking echocardiography in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2011;108:340–347. doi: 10.1016/j.amjcard.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 73.Shanks M, Ng AC, van de Veire NR, Antoni ML, Bertini M, Delgado V, Nucifora G, Holman ER, Choy JB, Leung DY, et al. Incremental prognostic value of novel left ventricular diastolic indexes for prediction of clinical outcome in patients with ST-elevation myocardial infarction. Am J Cardiol. 2010;105:592–597. doi: 10.1016/j.amjcard.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 74.Joyce E, Hoogslag GE, Leong DP, Debonnaire P, Katsanos S, Boden H, Schalij MJ, Marsan NA, Bax JJ, Delgado V. Association between left ventricular global longitudinal strain and adverse left ventricular dilatation after ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. 2014;7:74–81. doi: 10.1161/CIRCIMAGING.113.000982. [DOI] [PubMed] [Google Scholar]
- 75.Munk K, Andersen NH, Nielsen SS, Bibby BM, Bøtker HE, Nielsen TT, Poulsen SH. Global longitudinal strain by speckle tracking for infarct size estimation. Eur J Echocardiogr. 2011;12:156–165. doi: 10.1093/ejechocard/jeq168. [DOI] [PubMed] [Google Scholar]
- 76.Campbell MJ, Machin D, Walters SJ. Medical Statistics - A Textbook for The Health Sciences. 4th ed. John Wiley and Sons Ltd: 2007. p. 331. [Google Scholar]
- 77.Gjesdal O, Helle-Valle T, Hopp E, Lunde K, Vartdal T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. Noninvasive separation of large, medium, and small myocardial infarcts in survivors of reperfused ST-elevation myocardial infarction: a comprehensive tissue Doppler and speckle-tracking echocardiography study. Circ Cardiovasc Imaging. 2008;1:189–196, 2 p following 196. doi: 10.1161/CIRCIMAGING.108.784900. [DOI] [PubMed] [Google Scholar]
- 78.Munk K, Andersen NH, Terkelsen CJ, Bibby BM, Johnsen SP, Bøtker HE, Nielsen TT, Poulsen SH. Global left ventricular longitudinal systolic strain for early risk assessment in patients with acute myocardial infarction treated with primary percutaneous intervention. J Am Soc Echocardiogr. 2012;25:644–651. doi: 10.1016/j.echo.2012.02.003. [DOI] [PubMed] [Google Scholar]


