Abstract
Purpose of the review
This review highlights recent evidence describing the outcomes associated with fluid overload in critically ill patients and provides an overview of fluid management strategies aimed at preventing fluid overload during the resuscitation of patients with shock.
Recent findings
Fluid overload is a common complication of fluid resuscitation and is associated with increased hospital costs, morbidity and mortality.
Summary
Fluid management goals differ during the resuscitation, optimization, stabilization and evacuation phases of fluid resuscitation. To prevent fluid overload, strategies that reduce excessive fluid infusions and emphasize the removal of accumulated fluids should be implemented.
Keywords: Fluid overload, fluid resuscitation, shock
INTRODUCTION
Fluid overload is a relatively frequent occurrence in critically ill patients and is often a consequence of critical care intervention. Despite a common perception that it is benign, fluid overload in the critically ill is independently associated with increased morbidity and mortality. Thus intravenous fluids need to be dosed appropriately to reduce the risk of harm associated with this potentially life-saving therapy. This review presents recent evidence relevant to fluid overload in critically ill patients and provides an overview of fluid management strategies aimed at preventing fluid overload during the resuscitation of patients presenting with shock.
OUTCOMES ASSOCIATED WITH FLUID OVERLOAD
Fluid extravasation into the interstitial space can adversely affect multiple organ systems (Table 1). Although characterizing fluid overload in every organ system is difficult (i.e. some systems lend themselves to fluid measurement and correlation with function more than others), increasing evidence has been noted in the pulmonary, cardiac, renal, and gastrointestinal systems. Graded increases in extravascular lung water impair oxygenation and are independently associated with mortality [1]. Increased renal interstitial fluid may reduce capillary blood flow and lead to renal ischemia, which can cause or worsen acute kidney injury (AKI). Fluid overload can also worsen myocardial and liver function, impair coagulation, delay wound healing and is a risk factor for intra-abdominal hypertension [2]. In the gastrointestinal system, diffuse intestinal wall edema can cause malabsorption and ileus. Other organ systems, such as the neurologic system, are more difficult to assess for fluid overload and associated complications.
Table 1.
The pathophysiologic effects of fluid overload on organ systems.
| Body System | Effect of Fluid Overload | Clinical Manifestation |
|---|---|---|
| Central Nervous System | Cerebral edema | Impaired cognition Delirium |
| Respiratory System | Pulmonary edema Pleural effusions |
Increased work of breathing Impaired gas exchange Decreased lung compliance Increased extravascular lung water |
| Cardiovascular System | Myocardial edema Pericardial effusions |
Impaired contractility Diastolic dysfunction Conduction abnormalities |
| Gastrointestinal System | Gut wall edema Ascites |
Malabsorption Ileus Bacterial translocation Intra-abdominal hypertension |
| Hepatobiliary System | Hepatic congestion | Cholestasis Impaired synthetic function |
| Renal System | Renal interstitial edema Elevated renal venous pressure |
Acute Kidney Injury Uremia Salt and water retention |
| Skin and Musculoskeletal System | Tissue edema Impaired lymphatic drainage Deranged microcirculation |
Poor wound healing Pressure ulcers Wound infection |
The effect of tissue edema on organ function likely contributes to the association between fluid overload and increased morbidity and mortality. This association has been demonstrated in multiple studies involving patients with severe sepsis and the acute respiratory distress syndrome (ARDS) [1, 3*–6]. In addition, Silversides and colleagues analyzed data from a prospectively collected registry of 492 ICU patients with AKI who received more than 2 days of renal replacement therapy [7*]. The study identified that a higher mean daily fluid balance independently increased the odds of hospital mortality (odds ratio (OR [95% confidence interval (CI)]: 1.36 per 1L positive [1.18 to 1.57]).
Similar results have been shown among surgical and trauma patients. In a prospective observational study of 144 acute care surgery patients admitted to a surgical ICU, those patients who had a negative fluid-balance on the fifth ICU day had an independently lower odds of hospital mortality (OR [CI], 0.31 [0.13 to 0.76]; p=.010) [8*]. A recent meta-analysis of 3 randomized controlled trials and 7 observational studies comparing liberal to restrictive fluid management strategies in trauma patients concluded that liberal fluid resuscitation may be associated with higher mortality [9*].
Fluid overload is also associated with increased hospital costs and greater hospital resource utilization. A single center study by Kelm and colleagues found that 77% of patients with sepsis had signs of persistent fluid overload on the third day after receiving a standardized early goal directed therapy protocol [10*]. Fluid overload independently increased subsequent diuretic use (OR [CI]: 1.66 [1.01 – 2.74]), thoracenteses (OR [CI]: 3.83 [1.74 – 9.15]), and hospital death (OR [CI]: 1.92 [1.16 – 3.22]). A retrospective matched cohort study of 63,974 adult patients from a multicenter ICU database found that fluid overload statistically significant increases in the average total hospital costs per visit (56.7%) and the average total ICU cost per visit (92.6%) compared to patients without fluid overload (p<0.001) [11*]. Patients with fluid overload also had a two-fold longer ICU stay, 4% higher hospital mortality, and 0.5% higher risk for 30-day readmissions.
HOW TO PREVENT FLUID OVERLOAD
Fluid management strategies including two elements appear to be helpful in preventing fluid overload in patients with shock. First, during resuscitation excessive fluid administration should be avoided. Aggressive fluid resuscitation is associated with a high incidence of fluid overload. This was recently demonstrated in a retrospective cohort study of 405 patients with severe sepsis or septic shock who received early goal directed therapy [10]. There was clinical evidence of fluid overload 24 hours after admission in 67% of patients and this persisted until day three of admission in 48% of patients. Patients with fluid overload on day 1 and 3 had a higher risk of hospital mortality compared to patients without fluid overload, highlighting the possible dangers of over-resuscitation with intravenous fluids. Second, removal of excess fluids should be promoted in patients whose shock has resolved. In patients with septic shock who have been adequately resuscitated, conservative fluid management leading to negative fluid balances is associated with a decrease in hospital mortality [4].
These principles can be applied during the resuscitation of patients with shock which can be viewed as occurring in four phases corresponding to the acronym “ROSE”: resuscitation, optimization, stabilization and evacuation [12*]. The goals of fluid administration as well as the associated risk and benefits will vary depending on phase of resuscitation (Figure 1). The primary goal of fluid administration during the resuscitation phase is to rapidly correct systemic hypotension. During the optimization phase, the goal of fluid administration is to improve oxygen delivery to the tissues. In the stabilization phase patients are hemodynamically stable and fluid administration should be restricted. In the evacuation phase interventions are targeted at fluid removal.
Figure 1.
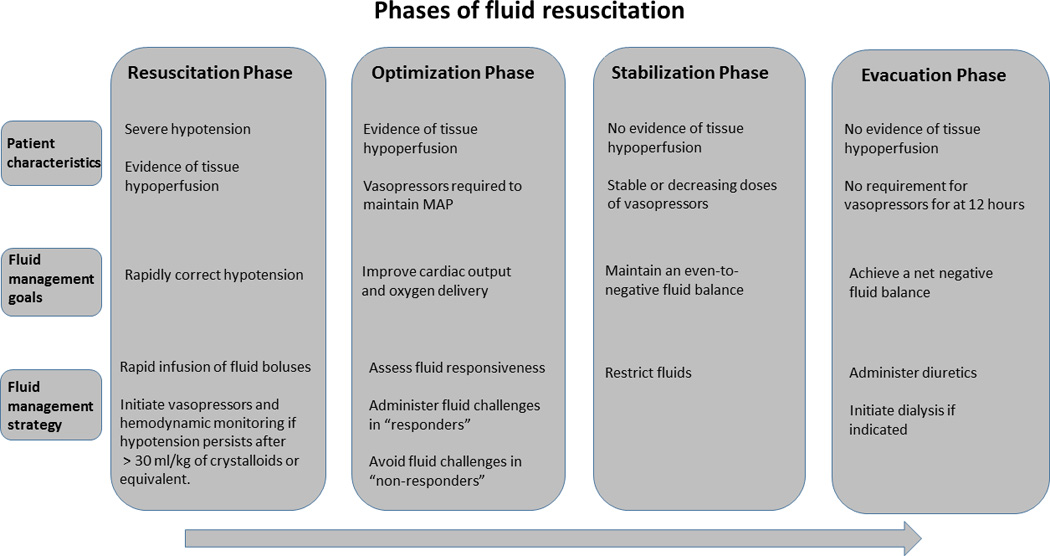
The phases of fluid resuscitation in patients with shock. MAP, mean arterial blood pressure.
Resuscitation Phase
This is the initial phase of resuscitation in patients with severe systemic hypotension and evidence of tissue hypoperfusion, such as an elevated serum lactate. In this phase the immediate goal is to correct the hypotension and intravenous fluids are typically first-line therapy [13]. The volume of fluid required during this phase must be individualized and will necessarily vary between patients and according to fluid type. In sepsis-induced hypotension, the Surviving Sepsis Campaign guidelines recommend an initial minimum crystalloid fluid bolus of 30 mL/kg of body weight [13]. Multiple fluid boluses are often given to achieve a predefined blood pressure target and frequently with limited hemodynamic monitoring such as routine bedside vital signs. Fluids should be given quickly as earlier resuscitation is associated with better outcomes. This was recently demonstrated in a study of 594 with severe sepsis and septic shock that examined the proportion of total fluids received during the first 3 hours compared to the later 3 hours and found that a higher proportion of total fluids received within the first 3 hours of resuscitation was associated with improved survival [14*].
The blood pressure targets will depend on the patient’s underlying diagnosis. The recommended mean arterial pressure in the majority of patients with septic shock is 65 mm Hg. This recommendation is supported by a recent randomized controlled trial that compared the effect of a high mean arterial pressure target (80 to 85 mm Hg) to a low mean arterial pressure target (65 to 70 mm Hg) in patients with septic shock [15*]. There were no significant differences in 28-day or 90-day mortality between both groups. However, patients with chronic hypertension in the group targeting a higher mean arterial pressure required less renal-replacement therapy than those in the group targeting a mean arterial pressure between 65 and 70 mm Hg. This suggests that blood pressure targets must be individualized, as a higher mean arterial pressure may confer benefit to specific populations of critically ill patients with shock.
While the initial focus of resuscitation should be on fluid administration, vasopressor support should not be delayed in those with persistent hypotension. There is no consensus on the optimal timing of vasopressor use during this phase. However, the administration of vasopressors prior to adequate fluid resuscitation in hypovolemic patients can worsen organ perfusion and function. A retrospective study of 2849 patients with septic shock that examined the influence of the timing of fluid and vasopressor therapy as well as the total volume of fluids given on hospital mortality suggested that starting vasopressors within the first hour of resuscitation may be harmful [16*]. Conversely, delayed initiation of vasopressors in patients with persistent hypoperfusion despite fluid administration can also worsen end-organ damage and is associated with increased mortality [17*, 18*]. Ongoing or unmonitored volume resuscitation in patients with persistent hypotension increases the likelihood of excessive fluid administration. A reasonable approach for patients whose hypotension has not resolved after receiving a volume of crystalloid fluid equivalent to 30 ml/kg of body weight is to start a vasopressor while continuing fluid therapy guided with the aid of hemodynamic monitoring.
Optimization Phase
The primary problem during the optimization phase of resuscitation is ongoing or occult tissue hypoperfusion. In this phase the goal of fluid administration is to increase oxygen delivery to the tissues in order to meet cellular oxygen demands. Oxygen delivery (DO2) is primarily a function of cardiac output (heart rate × stroke volume), hemoglobin concentration and arterial oxygen saturation. The purpose of administering a fluid challenge is to increase oxygen delivery by increasing stroke volume and thus cardiac output. Not all critically ill patients will respond in this manner to fluid bolus therapy (i.e. an increase in cardiac output, known as a fluid “responder”). It is estimated, however, that 50% of patients are not fluid responders (“non-responders”) and will not increase stroke volume in response to a fluid challenge [19]. Further fluid challenges in such patients potentially exposes them only to the harmful effects of fluid accumulation without providing any of the potential benefits. Consequently, fluid therapy aimed at improving oxygen delivery should be avoided in patients who are fluid non-responders and a greater emphasis put on the use of vasoactive agents.
There is no gold standard for determining fluid responsiveness but there is growing consensus favoring the use of dynamic measures of fluid responsiveness over static measures such as central venous pressure (CVP) or central venous oxygen saturation (ScvO2) [20*]. Multiple studies have shown that CVP is a poor measure of volume status and does not reliably predict fluid responsiveness, with the possible exception of tracking changes in CVP induced by passive leg raising [21, 22]. Using CVP to guide fluid administration may easily lead to excess fluid administration and fluid overload. There are several available methods to accurately assess fluid responsiveness in both spontaneously breathing and mechanically ventilated patients [20]. Pulse pressure variation, stroke volume variation as well respiratory variations in the inferior vena cava, superior vena cava and internal jugular vein as measured by ultrasound have all been shown to accurately predict fluid responsiveness in mechanically ventilated patients [23*–28*]. The passive leg raise technique in combination with measurement of cardiac output, stroke volume or aortic blood flow is effective in spontaneously breathing patients [29, 30]. In practice the use of these techniques varies widely. A prospective multicenter study of adult patients with shock in 19 French ICUs showed significant between-center differences in the use of hemodynamic monitoring to guide fluid administration along with differences in both the total volume of fluids given during the first 4 days and the average size of individual fluid boluses [31*].
During the resuscitative phase fluids must be administered in a timely and targeted fashion with appropriate limits to fluid administration. Surrogate marker of tissue perfusion such as serum lactate and central venous oxygen saturation (ScvO2) are often used to determine the end-point of resuscitation. Three recent large multicenter randomized controlled studies in sepsis showed no benefit in using protocols that required continuous ScvO2 monitoring over usual care. The American Protocolized Care for Early Septic Shock (ProCESS) trial, the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial and the UK Protocolized Management in Sepsis trial (ProMISe) trial all showed no difference in 90-day mortality when comparing early goal directed therapy to usual care in the management of severe sepsis and septic shock [32*–34*]. These findings argue against routinely targeting absolute values of measures such as ScvO2 during the resuscitation of patients with septic shock. The adequacy of resuscitation should be monitored using clinical judgment as well as physiologic and biochemical parameters and should be individualized to the patient. Clear criteria, such as the absence of fluid responsiveness or the presence of clinical evidence of fluid overload, should be used to determine when to stop giving fluid challenges in patients who fail to achieve their resuscitation goals.
Stabilization Phase
Patients in the stabilization phase of resuscitation have adequate tissue perfusion and may still require hemodynamic support with vasopressors, although the doses of these medications will be stable or decreasing. In this group, careful weighing of the potential risks and benefits of further fluid administration is required and further fluid administration should be restricted in patients without ongoing losses. In patients who are fluid responsive, a fluid challenge may be reasonable with the goal of decreasing vasopressor requirements. This must be balanced against the evidence that late goal-directed therapy may be associated with worse outcomes [35].
Fluids should be restricted in patients not requiring hemodynamic support. In the absence of evidence suggesting hypoperfusion, fluid challenges should not be given, even in those who are fluid responsive. Careful attention must be paid to assess hypoperfusion, whether physiologically or with clinical laboratory tests. Maintenance fluids are usually not required and should be discontinued when possible as critically ill patients frequently receive sufficient fluids with medications and nutrition to account for insensible losses.
Evacuation Phase
In this phase the goal is to remove excess fluids in patients who are hemodynamically stable and do not have evidence of tissue hypoperfusion. Recent studies have demonstrated that protocols aimed at achieving a negative fluid balance are feasible and are associated with decreased morbidity. The Fluid and Catheter Treatment Trial (FACTT trial) enrolled 1000 patients with ARDS who were managed with either a conservative or liberal fluid strategy [36]. Patients with a mean arterial pressure > 60 mm Hg, no vasopressor support for > 12 hours, adequate urine output and circulation were either given furosemide targeted to achieve a CVP < 4 mm Hg or pulmonary artery occlusion pressure (PAOP) < 8 mm Hg (fluid conservative group) or CVP (< 10 mm Hg) and PAOP (< 14 mm Hg) (fluid liberal group). In this study, there was no difference in mortality but the conservative fluid strategy led to a more negative fluid balance at 7 days, 2.5 more days alive and free of mechanical ventilation and a shorter ICU length of stay.
In order to more easily operationalize a fluid restrictive strategy, a simplified version of the FACTT fluid management protocol was evaluated in a recent study [37*]. Using the “FACTT lite” protocol, patients with MAP > 60 mm Hg and who were off vasopressors for > 12 hours were given furosemide to target a CVP < 4 mm Hg or PAOP < 8 mm Hg if they had adequate urine output [37]. A total of 1124 patients who were managed with the FACTT lite protocol were retrospectively compared to the 497 patients managed with FACTT liberal fluid strategy and the 503 patients who were managed with the FACTT conservative fluid strategy. The investigators found that management with the FACTT lite protocol resulted in a significantly lower fluid balance than the FACTT liberal protocol and no difference in the number of ventilator-free days compared to the FACTT conservative protocol. It appears this simplified version of a fluid conservative approach achieves similar fluid balance, and we believe it would result in similar improved outcomes if compared to a historical liberal approach to fluid management.
Biomarkers may also be used to guide fluid removal in critically ill patients. In the B-type Natriuretic Peptide for the Fluid Management of Weaning (BMW) study, 304 mechanically ventilated patients were randomized to a B-type Natriuretic Peptide (BNP)-driven fluid management strategy or usual care [38, 39]. Patients in the BNP-driven group received diuretics and had fluid restricted on days when their BNP was ≥ 200 pg/ml. Duration of weaning was significantly shorter in the BNP-driven group and there was a decreased incidence of ventilator-associated pneumonia and ventilator-associated events.
There is no consensus about the optimal timing of fluid removal and there is limited data to guide a specific recommendation. In both the FACCT trial and the BMW trial, patients were required to have been off vasopressor therapy for at least 12 hours before diuretics were administered. A reasonable approach is to begin restricting fluids when patients achieve hemodynamic stability and/or normal tissue perfusion, and to consider initiating diuresis in patients who are hemodynamically stable with clinical evidence of fluid overload and unable to achieve a negative fluid balance spontaneously. An expert discussion of the pharmacologic management of fluid overload and the indications and management of mechanical fluid removal in critically ill patients was recently published [40*, 41*].
There are understandable concerns about the risk of organ hypoperfusion associated with fluid removal. The data in this regard are inconclusive. The development of shock in patients managed with the FACTT lite protocol was not significantly different compared with patients managed with the FACTT liberal protocol [37]. There are conflicting results regarding organ dysfunction with conservative fluid management in ARDS: greater cardiovascular dysfunction, and less acute neurological dysfunction [36], although a subsequent report found a higher incidence of long term cognitive dysfunction with conservative fluid therapy [42]. In the BMW trial there was no difference in the incidence of renal failure between the intervention and control groups [38]. The impact of fluid conservative strategies on organ function requires additional study, particularly as it relates to the ebb and flow of fluid therapy in sepsis. Given these concerns, fluid removal should be performed cautiously with clear limits and appropriate safety monitoring to avoid inducing hypovolemia.
CONCLUSION
Fluid overload in the critically ill is a potentially preventable complication that is associated with increased morbidity and mortality. Specific fluid management strategies can be implemented during each phase of resuscitation to help mitigate the effects of fluid overload and thus improve outcomes. Ultimately, the most effective fluid management strategy is one that is individualized and emphasizes adequate early resuscitation and a more restrictive approach to fluid administration in the latter phases. Further research on how best to personalize fluid therapy in the critically ill is needed, particularly in the dynamic critically ill patient.
Key points.
Fluid overload is associated with increased morbidity and mortality in critically ill patients
Aggressive fluid resuscitation increases the risk of excessive fluid administration and fluid overload
Fluid responsiveness should be assessed prior to fluid administration to reduce the risk of excessive fluid administration
Fluid resuscitation strategies should include clear limits and individualized end-points to reduce the risk of excessive fluid administration
Interventions aimed at achieving a negative fluid balance should be considered after adequately resuscitating patients with shock.
Acknowledgements
None
Financial support and sponsorship
This manuscript was supported, in part, by funding from the Emory Critical Care Center, the Grady Health System, the National Institutes of Health and the Food and Drug Administration, including grant numbers T32 GM095442 (OO), KL2 TR000455 (OO), R01 FD003440 (GSM) and UL1 TR000454 (GSM).
Dr. Martin conducts research on fluid management in critically ill patients and his institution (Emory University) has received funding from Abbott Laboratories and Baxter Healthcare for research, and Dr. Martin has served as a medical advisor to CSL Behring and Grifols.
Footnotes
Conflicts of interest
Dr. Ogbu and Dr. Murphy have no conflicts to disclose.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review have been highlighted as:
* of special interest
** of outstanding interest
- 1.Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Huber W, et al. Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Annals of intensive care. 2012;2:S1. doi: 10.1186/2110-5820-2-S1-S1. (Suppl 1 Diagnosis and management of intra-abdominal hyperten) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nature reviews Nephrology. 2010;6(2):107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 3. Sirvent JM, Ferri C, Baro A, Murcia C, Lorencio C. Fluid balance in sepsis and septic shock as a determining factor of mortality. The American journal of emergency medicine. 2014 doi: 10.1016/j.ajem.2014.11.016. A prospective observational study of 42 patients with severe sepsis or septic shock demostrating an association between a positive fluid balance at 24, 48, 72 and 96 hours and mortality.
- 4.Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 5.Sakr Y, Vincent JL, Reinhart K, Groeneveld J, Michalopoulos A, Sprung CL, et al. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128(5):3098–3108. doi: 10.1378/chest.128.5.3098. [DOI] [PubMed] [Google Scholar]
- 6.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Critical care medicine. 2011;39(2):259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 7. Silversides JA, Pinto R, Kuint R, Wald R, Hladunewich MA, Lapinsky SE, et al. Fluid balance, intradialytic hypotension, and outcomes in critically ill patients undergoing renal replacement therapy: a cohort study. Critical care. 2014;18(6):624. doi: 10.1186/s13054-014-0624-8. A secondary analysis of a prospective registry of ICU patients with acute kidney injury who received renal replacement therapy that showed an association between a higher daily fluid balance and mortality.
- 8. Barmparas G, Liou D, Lee D, Fierro N, Bloom M, Ley E, et al. Impact of positive fluid balance on critically ill surgical patients: a prospective observational study. J Crit Care. 2014;29(6):936–941. doi: 10.1016/j.jcrc.2014.06.023. A prospective observational study of critically ill acute care surgery patients that showed an association between an early negative fluid balance and a reduced risk for hospital mortality.
- 9. Wang CH, Hsieh WH, Chou HC, Huang YS, Shen JH, Yeo YH, et al. Liberal versus restricted fluid resuscitation strategies in trauma patients: a systematic review and meta-analysis of randomized controlled trials and observational studies*. Critical care medicine. 2014;42(4):954–961. doi: 10.1097/CCM.0000000000000050. A systematic review and meta-analysis of studies comparing restrictive versus a liberal fluid resuscitative strategies in trauma patients.
- 10. Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock (Augusta, Ga) 2015;43(1):68–73. doi: 10.1097/SHK.0000000000000268. A retrospective cohort study of 405 patients with severe sepsis or septic shock that demonstrated a high incidence of fluid overload after EGDT and an association betwee persistent fluid overload and increased mortality.
- 11. Child DL, Cao Z, Seiberlich LE, Brown H, Greenberg J, Swanson A, et al. The costs of fluid overload in the adult intensive care unit: is a small-volume infusion model a proactive solution? ClinicoEconomics and outcomes research : CEOR. 2015;7:1–8. doi: 10.2147/CEOR.S72776. A secondary analysis of a large multicenter ICU database that compared ICU and hospital costs in patients with and without evidence of fluid overload.
- 12. Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiology intensive therapy. 2014;46(5):361–380. doi: 10.5603/AIT.2014.0060. A systematic review of studies examining the association between fluid overload and outcomes as well as studies comparing interventions aimed at achieving a negative fluid balance to usual care.
- 13.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical care medicine. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 14. Lee SJ, Ramar K, Park JG, Gajic O, Li G, Kashyap R. Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: a retrospective cohort study. Chest. 2014;146(4):908–915. doi: 10.1378/chest.13-2702. A retrospective cohort study of 651 patients who received fluid resuscitation for severe sepsis or septic shock that showed improved survival in patients who received a greater proportion of fluids within the first 3 hours of resuscitation.
- 15. Asfar P, Teboul JL, Radermacher P. High versus low blood-pressure target in septic shock. The New England journal of medicine. 2014;371(3):283–284. doi: 10.1056/NEJMc1406276. A randomized controlled trial of 388 patients with septic shock that showed no difference in 90-day mortality when comparing targeting a mean arterial pressure of 80 to 85 mm Hg compared to a mean arterial presssure of 60 to 65 mm Hg.
- 16. Waechter J, Kumar A, Lapinsky SE, Marshall J, Dodek P, Arabi Y, et al. Interaction between fluids and vasoactive agents on mortality in septic shock: a multicenter, observational study. Critical care medicine. 2014;42(10):2158–2168. doi: 10.1097/CCM.0000000000000520. A retrospective cohort study of 2849 patients with septic shock that examined the association of hospital mortality with the timing and volume of intravenous fluid resuscitation and with the timing of initiating vasoactive medications.
- 17. Bai X, Yu W, Ji W, Lin Z, Tan S, Duan K, et al. Early versus delayed administration of norepinephrine in patients with septic shock. Critical care. 2014;18(5):532. doi: 10.1186/s13054-014-0532-y. A retrospective cohort study of 213 adult patients with septic shock tha examined the association between the timing of norepinephrine initiation and 28-day mortality.
- 18. Beck V, Chateau D, Bryson GL, Pisipati A, Zanotti S, Parrillo JE, et al. Timing of vasopressor initiation and mortality in septic shock: a cohort study. Critical care. 2014;18(3):R97. doi: 10.1186/cc13868. A secondary analysis of a large multicenter database of adult patients with septic shock that examined the association between the timing of vasopressor or inotropic initiation and survival.
- 19.Cecconi M, Parsons AK, Rhodes A. What is a fluid challenge? Current opinion in critical care. 2011;17(3):290–295. doi: 10.1097/MCC.0b013e32834699cd. [DOI] [PubMed] [Google Scholar]
- 20. Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive care medicine. 2014;40(12):1795–1815. doi: 10.1007/s00134-014-3525-z. An updated report from 12 experts containing 44 consensus statements regarding the diagnosis, management and monitoring of shock.
- 21.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Critical care medicine. 2013;41(7):1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 22.Lakhal K, Ehrmann S, Runge I, Benzekri-Lefevre D, Legras A, Dequin PF, et al. Central venous pressure measurements improve the accuracy of leg raising-induced change in pulse pressure to predict fluid responsiveness. Intensive care medicine. 2010;36(6):940–948. doi: 10.1007/s00134-010-1755-2. [DOI] [PubMed] [Google Scholar]
- 23. Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Critical care. 2014;18(6):650. doi: 10.1186/s13054-014-0650-6. A systematic review and meta-analysis of 22 clinical trials that demonstrated that pulse pressure variation accurately predicts fluid responsiveness in critically ill patients receiving controllled mechanical ventilation and who do not have cardiac arrhythmias.
- 24.Mohsenin V. Assessment of preload and fluid responsiveness in intensive care unit. How good are we? J Crit Care. 2015 doi: 10.1016/j.jcrc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Critical care medicine. 2009;37(9):2642–2647. doi: 10.1097/CCM.0b013e3181a590da. [DOI] [PubMed] [Google Scholar]
- 26.Barbier C, Loubieres Y, Schmit C, Hayon J, Ricome JL, Jardin F, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive care medicine. 2004;30(9):1740–1746. doi: 10.1007/s00134-004-2259-8. [DOI] [PubMed] [Google Scholar]
- 27.Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, et al. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive care medicine. 2004;30(9):1734–1739. doi: 10.1007/s00134-004-2361-y. [DOI] [PubMed] [Google Scholar]
- 28. Guarracino F, Ferro B, Forfori F, Bertini P, Magliacane L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Critical care. 2014;18(6):647. doi: 10.1186/s13054-014-0647-1. A prospective study of 50 mechanically ventilated patients with sepsis that examined the efficacy of using internal jugular vein size and distensibility to assess fluid responsiveness.
- 29.Duus N, Shogilev DJ, Skibsted S, Zijlstra HW, Fish E, Oren-Grinberg A, et al. The reliability and validity of passive leg raise and fluid bolus to assess fluid responsiveness in spontaneously breathing emergency department patients. J Crit Care. 2015;30(1):217.e1–217.e5. doi: 10.1016/j.jcrc.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Cavallaro F, Sandroni C, Marano C, La Torre G, Mannocci A, De Waure C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive care medicine. 2010;36(9):1475–1483. doi: 10.1007/s00134-010-1929-y. [DOI] [PubMed] [Google Scholar]
- 31. Boulain T, Boisrame-Helms J, Ehrmann S, Lascarrou JB, Bougle A, Chiche A, et al. Volume expansion in the first 4 days of shock: a prospective multicentre study in 19 French intensive care units. Intensive care medicine. 2015;41(2):248–256. doi: 10.1007/s00134-014-3576-1. A prospective multicenter evaluation of fluid resuscitation practices in 19 French ICUs.
- 32. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. The New England journal of medicine. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. A randomized controlled trial that compared early goal-directed therapy, protocol-based standard therapy and usual care in 1341 patients with septic shock.
- 33. Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock. The New England journal of medicine. 2015 doi: 10.1056/NEJMoa1500896. A randomized controlled trial that compared early goal-directed therapy to usual care in 1260 patients with septic shock.
- 34. Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, et al. Goal-directed resuscitation for patients with early septic shock. The New England journal of medicine. 2014;371(16):1496–1506. doi: 10.1056/NEJMoa1404380. A randomized controlled trial that compared early goal-directed therapy to usual care in 1600 patients with septic shock.
- 35.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. The New England journal of medicine. 1994;330(24):1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 36.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. The New England journal of medicine. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 37. Grissom CK, Hirshberg EL, Dickerson JB, Brown SM, Lanspa MJ, Liu KD, et al. Fluid Management With a Simplified Conservative Protocol for the Acute Respiratory Distress Syndrome*. Critical care medicine. 2015;43(2):288–295. doi: 10.1097/CCM.0000000000000715. A retrospective comparison of three fluid management strategies in a population of patients with the acute respiratory distress syndrome who were enrolled in ARDS network studies.
- 38.Mekontso Dessap A, Katsahian S, Roche-Campo F, Varet H, Kouatchet A, Tomicic V, et al. Ventilator-associated pneumonia during weaning from mechanical ventilation: role of fluid management. Chest. 2014;146(1):58–65. doi: 10.1378/chest.13-2564. [DOI] [PubMed] [Google Scholar]
- 39.Mekontso Dessap A, Roche-Campo F, Kouatchet A, Tomicic V, Beduneau G, Sonneville R, et al. Natriuretic peptide-driven fluid management during ventilator weaning: a randomized controlled trial. American journal of respiratory and critical care medicine. 2012;186(12):1256–1263. doi: 10.1164/rccm.201205-0939OC. [DOI] [PubMed] [Google Scholar]
- 40. Rosner MH, Ostermann M, Murugan R, Prowle JR, Ronco C, Kellum JA, et al. Indications and management of mechanical fluid removal in critical illness. British journal of anaesthesia. 2014;113(5):764–771. doi: 10.1093/bja/aeu297. A review of the indications and management of mechanical fluid removal in critically ill patients.
- 41. Goldstein S, Bagshaw S, Cecconi M, Okusa M, Wang H, Kellum J, et al. Pharmacological management of fluid overload. British journal of anaesthesia. 2014;113(5):756–763. doi: 10.1093/bja/aeu299. A review of the pharmacologic management of fluid overload in critically ill patients.
- 42.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The Adult Respiratory Distress Syndrome Cognitive Outcomes Study. American journal of respiratory and critical care medicine. 2012;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


