Abstract
A 29-year-old man was admitted with fevers, cough, left-sided chest pain and lethargy for 1 week. He had a cardiac transplant 10 years prior and was on immunosuppressive drugs. He was found to have a pulmonary lesion and went on to develop a lung abscess. Propionibacterium acnes was identified on matrix-assisted laser desorption ionisation mass spectrometry-time of flight and 16s rRNA gene sequencing after drainage. He was curatively treated with co-trimoxazole and co-amoxiclav. He divulged a longstanding history of seborrhoeic dermatitis with frequent flares leading to large volumes of squames collecting on his bed sheets. We hypothesise this was a possible route of entry: inhalation of the Propionibacterium. This case highlights how a common commensal bacterium, P. acnes, was able to cause pathology in an immunosuppressed patient. This is the only case of a patient with transplantation developing a P. acnes pulmonary infection and the only case of P. acnes causing these clinical features to be reported in the literature.
Background
Propionibacterium acnes is a Gram-positive, anaerobic bacillus; it is part of the normal flora of the skin and mucosa, and is thought to play a role in acne. However, it has been increasingly recognised as a cause of serious infection in immunocompetent as well as in immunosuppressed patients. This case highlights how a common commensal bacterium, P. acnes, was able to cause pathology in a patient whose immune system was ill-functioning due to immunosuppressing drugs post-cardiac transplantation. To the best of our knowledge, this is the only case of a patient with transplantation developing a P. acnes pulmonary infection, and the only case of P. acnes causing these clinical features. We additionally highlight that P. acnes can mimic the presentation of nocardia and the importance of 16s rRNA gene sequencing in organism identification.
Case presentation
A 29-year-old software engineer was admitted with fevers, cough, left-sided chest pain and lethargy of 1-week duration.
The most significant aspect of his medical history was a restrictive cardiomyopathy necessitating a cardiac transplant 10 years prior. He subsequently developed skeletal muscle weakness mainly affecting his distal upper limbs and was put under investigation for causes of myofibrillar myopathy. After an episode of transplant rejection, his therapeutic immunosuppression was increased, with mycophenolate mofetil 1 g twice daily, cyclosporin 125 mg mane and 100 mg nocte, and prednisolone 5 mg daily. He was a non-smoker and very occasionally drank alcohol.
Physical examination was unremarkable, although moderately severe seborrhoeic dermatitis was noted.
Investigations
Blood tests showed neutrophils of 7.25×109/L (range 2–7.5×109/L) and C reactive protein of 42.9 mg/L (range 0–5.0 mg/L). Admission chest X-ray (figure 1) revealed a well-defined rounded lesion in the left mid zone measuring 3.1 cm, which had evolved since his last imaging 2 months previously. A subsequent CT of the chest showed a 3 cm×2.6 cm×3.1 cm necrotic mass with rim enhancement within the left lung, spanning the oblique fissure (figure 2). It involved both the posterior left upper lobe and anterior left lower lobe. He proceeded to a CT-guided biopsy. Two 18G cores were taken consisting mainly of pus, and so the introducer needle was advanced into the centre of the lesion and a small amount of purulent fluid aspirated. The Gram stain revealed Gram-positive rods (figure 3). Owing to the typical radiological findings, Gram stain and significant immunosuppression, the patient was diagnosed with presumed nocardiosis and treatment was started with co-trimoxazole 960 mg three times daily.
Figure 1.

Initial chest X-ray showing a new well-defined rounded lesion in the left mid zone, measuring 3.1 cm in diameter.
Figure 2.
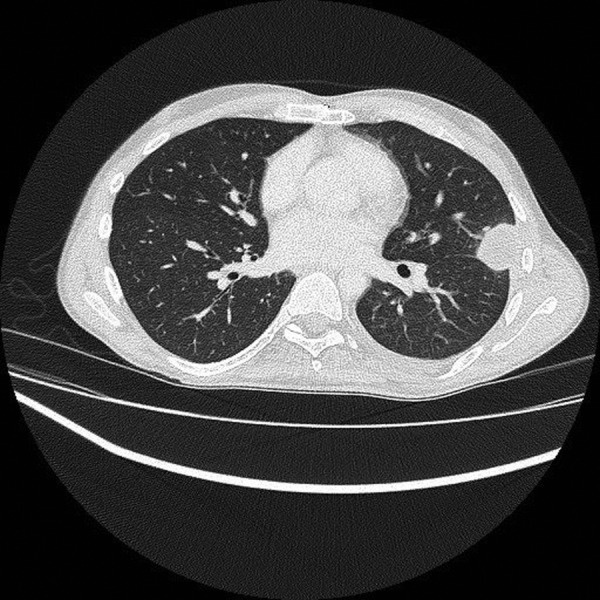
CT scan showing 3 cm×2.6 cm×3.1 cm necrotic mass with rim enhancement within the left lung, spanning the oblique fissure.
Figure 3.
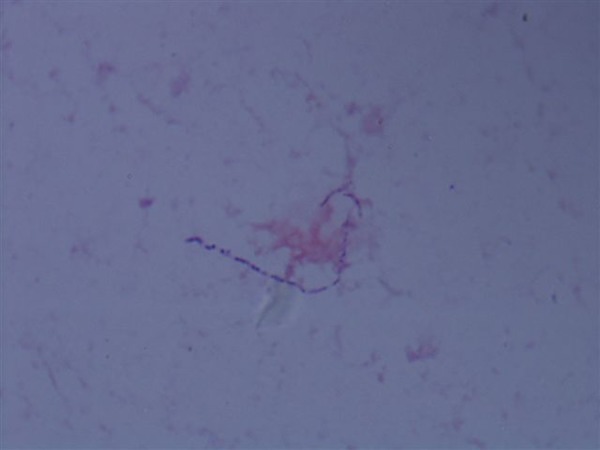
Original Gram stain revealed a Gram-positive rod.
The patient deteriorated with worsening fevers, chest pain and dyspnoea. Blood tests showed a peak neutrophilia of 13.18×109/L and C reactive protein of 422.8 mg/L. He had developed a new large left-sided pleural effusion with the differential diagnoses including lung abscess. He was started on meropenem 1 g three times daily in addition to co-trimoxazole. Ultrasonography revealed a complex left-sided pleural effusion. It contained multiple septations and 400 mL of straw-coloured fluid was obtained on chest drain insertion. No organisms were seen including acid-fast bacilli. pH was 7.26 (range 7.35–7.45), glucose was 3.1 and protein 38 g/L.
Differential diagnosis
The clinical and radiological findings alongside the Gram stain were felt to be compatible with pulmonary nocardiosis, and treatment was started with co-trimoxazole 960 mg three times daily. Despite a good response to therapy, nocardia was not cultured from the multiple aspirates of the lung abscess. Small colonies of a Gram-positive organism were noted on chocolate agar. These were identified as P. acnes, using matrix-assisted laser desorption ionisation mass spectrometry-time of flight Bruker, using their database (level of confidence=2.183. Confirmed by Anaerobe Reference lab, Cardiff, UK) and 16s rRNA gene sequencing (in-house) performed using previously described primers.1 On further questioning, the patient divulged a longstanding history of seborrhoeic dermatitis with frequent flares leading to large volumes of squames collecting on his bed sheets and, after performing his own research, the patient himself suggested that he may have acquired his infection through inhaling dead skin.
Treatment
The patient received 2 weeks of co-trimoxazole to provide appropriate cover for the presumed nocardiosis. P. acnes was identified and found to be sensitive to co-amoxiclav, clindamycin, penicillin G, piperacillin/tazobactam and meropenem. It was resistant to metronidazole. His therapy was changed to co-amoxiclav (625 mg three times daily), on confirmation of the organism, and this was continued for 6 weeks, until radiological resolution of the abscess. Success of treatment was seen with complete resolution of his clinical and radiological abnormalities 6 weeks on.
Outcome and follow-up
At clinic follow-up 7 months after the initial presentation, the patient remained well.
Discussion
P. acnes is a Gram-positive, anaerobic bacillus that is part of the normal flora of the skin and mucosa.2 3 However, it has been increasingly recognised as a cause of pathology. It is believed to colonise the skin immediately prior to puberty when the sebaceous glands are enlarging,3 and may play a role in acne.
P. acnes has been associated with serious infections including of the central nervous system, sepsis, endocarditis and infection of prostheses.2 It has been described as a cause of serious infection in immunocompetent as well as in immunosuppressed patients,2 4 5 however, because of the ubiquity of P. acnes as a commensal, it is often difficult to be certain that it is the pathogen rather than a contaminant.
The case that we present was a pulmonary infection with P. acnes in an adult patient who was immunosuppressed secondary to antirejection drugs for his cardiac transplant. A PubMed literature review revealed three articles reporting on immunosuppressed patients who developed infections in their lungs caused by P. acnes. There were no cases of any transplant recipient with P. acnes pulmonary infection or any case of a lung abscess caused by P. acnes.
Claeys et al4 described a case of a 65-year-old patient with chronic obstructive pulmonary disease who was immunosuppressed due to long-term systemic steroid use. This patient developed a subacute bronchopulmonary pneumonia with a pure P. acnes isolate. Mohsenifar et al5 described the case of a 57-year-old patient with lymphocytic lymphoma who developed multiple micro-abscesses with only P. acnes isolated. This patient was immunosuppressed secondary to hypogammaglobulinaemia and chemotherapy for the lymphoma. Bourdeaut et al3 described two cases of paediatric patients with immunosuppression secondary to chronic granulomatous disease who then developed P. acnes chest infections.
Additionally, Kurz et al6 reported successful treatment of an immunocompetent patient with P. acnes prosthetic valve endocarditis with abscess formation after long-term treatment with antibiotic therapy.
In our case, we can be confident that the lung abscess was caused by P. acnes because no other microorganism was identified from the biopsy and multiple microbiological investigations were performed including molecular testing. Furthermore, the biopsy was performed using an aseptic technique, thus minimising the likelihood of contamination. The likely route of entry was inhalation of the Propionibacterium from the squames on the patient's bed sheets.
Learning points.
This case highlights how a commensal bacterium, in this case Propionibacterium acnes, can cause atypical pathology in individuals with iatrogenic immunosuppression.
To the best of our knowledge, this is the only case of a patient with transplantation developing a P. acnes pulmonary infection and the only case of P. acnes causing these clinical features.
P. acnes can mimic the presentation of nocardia in immunosuppressed patients, and 16s rRNA gene sequencing is important in differentiating and identifying the causative organism.
In immunosuppressed patients, it is important to consider commensal organisms such as P. acnes in the differential of infectious pathogens.
Acknowledgments
The authors thank Dr Colin Brown, infectious diseases registrar, who contributed significantly in the care of this patient.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Harris KA, Hartley JC. Development of broad-range 16s rDNA PCR for use in the routine diagnostic clinical microbiology service. J Med Microbiol 2003;52:685–91. 10.1099/jmm.0.05213-0 [DOI] [PubMed] [Google Scholar]
- 2.Al-Mazrou AM. Propionibacterium acnes: a cause of pneumatocele associated pneumonia. Saudi Med J 2005;26:1127–9. [PubMed] [Google Scholar]
- 3.Bourdeaut F, Quartier P, Alkaer G et al. . Propionibacterium acnes chest infections in patients with chronic granulomatous disease: case reports. Clin Infect Dis 2002;34:853–4. 10.1086/338875 [DOI] [PubMed] [Google Scholar]
- 4.Claeys G, Verschraegen G, Potter CD et al. . Bronchopneumonia caused by Propionibacterium acnes. Eur J Clin Microbiol Infect Dis 1994;13:747–9. 10.1007/BF02276058 [DOI] [PubMed] [Google Scholar]
- 5.Mohsenifar DZ, Klonoff DD, Cassan DS. Propionibacterium acnes pneumonia in a patient with lymphoma. Infection 1979;7:146–8. 10.1007/BF01641315 [DOI] [PubMed] [Google Scholar]
- 6.Kurz M, Kaufmann BA, Baddour LM et al. . Propionibacterium acnes prosthetic valve endocarditis with abscess formation: a case report. BMC Infect Dis 2014;14:105 10.1186/1471-2334-14-105 [DOI] [PMC free article] [PubMed] [Google Scholar]


