Abstract
Black-pigmented bacteria (BPB) are Gram-negative anaerobic, non-motile, proteolytic rods strongly implicated in the pathogenesis of periodontal disease. Although pigments are produced in vitro, black pigmentation is rarely found clinically. However, it may compromise aesthetics and contribute to gingival inflammation. The aim of this report is to describe a clinical case of a 10-year-old boy showing black pigmentation covering all teeth and to propose an alternative therapy for removal of black pigmentation, based on photodynamic therapy (PDT). In order to perform microbiological analysis, plaque samples were collected before and after PDT, and analysed by real-time-PCR (RT-PCR). The results showed a significant reduction in BPB levels after therapy, along with clinical evidence of absence of black pigmentation and reduction in gingival bleeding, although the plaque index remained unaltered. This case showed that PDT is effective for eliminating black pigmentation caused by BPB, without recurrence during a follow-up period of 7 months.
Background
Black pigmentation is a dental extrinsic staining defined as a thin, dark pigmented line usually localised at the enamel, following the contour of the gingival margin.1–3 It may also manifest as incomplete coalescent small dark dots (≤0.5 mm diameter)—parallel to the gingival margin at dental smooth surfaces—that rarely extend beyond the cervical third of the crown of at least two different teeth.2
Enamel stains can be classified as intrinsic or extrinsic dark discolorations of the enamel.4 Intrinsic stains are located within the tooth structure and occur during tooth development as a result of metabolic disorder or systemic factors, such as porphyria and tetracycline administration.4 Extrinsic enamel stains are superficial tooth discolorations adsorbed within the acquired pellicle or dental plaque and may also be retained on the tooth surface via ion exchange.4 Black-pigmented bacteria (BPB) are the cause of extrinsic black stains.3
BPB are predominantly proteolytic, strictly anaerobic Gram-negative rods that play an essential role in the pathogenesis of periodontal disease, since many well recognised periodontal pathogens are BPB, including P. gingivalis, P. intermedia, P. melaninogenica and P. nigrescens.5 BPB are found at 47.1% of healthy sites in healthy periodontal patients and at 87.8% of active sites in patients with periodontal disease.6 BPB need iron to produce the dark pigment and to establish colonies.7 8 These species depend largely on external haem as an iron source for their growth9 and accumulate a cell surface black pigment that mainly consists of oxo bis-haem of iron protoporphyrin IX (PpIX) in P. gingivalis and monomeric iron PpIX (haematin) in P. intermedia and P. nigrescens.9 Specialised mechanisms to acquire iron from the microenvironment are developed by BPB using lysis of haemoglobin, since small concentrations of free iron ions are available.8 9 Black pigments are considered as virulence factors, and can vary among different species.8–10 Prevotella and Porphyromonas species are related to increased gingival bleeding,11 12 coaggregating with Aggregatibacter actinomycetemcomitans in the periodontal infection.13 Some authors found that 70% of children aged 6–12 years old with black stains were positive for A. actinomycetemcomitans, while only 20% of children free from black stains were positive for this species.11
Current estimates of black stain prevalence vary from 1% to 20%,2 14 and are more frequent in children than in adults, affecting both deciduous and permanent dentitions.11 The stains are usually difficult to remove because there is more calcium and phosphate content in the microbial biofilm of black pigments produced by BPB.3 Polishing with a rubber cup removes these stains, however, relapse is common using this method.1
Photodynamic therapy (PDT) has been suggested as an alternative therapy for elimination of pathogenic bacteria for the treatment of periodontitis, peri-implantitis and other infections.15–21 PDT is based on the concept that non-toxic photosensitisers can be preferentially located in certain tissues and subsequently activated by light of an appropriate wavelength to generate singlet oxygen and free radicals that are cytotoxic to cells of the target tissue.22 23 Oral bacteria seem to be susceptible to PDT in planktonic cultures23 and plaque scrapings.24 PDT-induced bacterial killing is able to reduce the microbial count 10-fold when toluidine blue O or erythrosine is used as a photosensitiser.25 26
This paper describes a case of recurrent black teeth pigmentations treated by PDT after a number of unsuccessful attempts to remove the pigmentations by professional prophylaxis. This is the first successful case of oral BPB treated by PDT, which could give a valuable contribution to the current state-of-the-art.
Case presentation
A 10-year-old boy presented with recurrent generalised black stains affecting the permanent and deciduous teeth, along with gingival bleeding. These black stains were characterised by continuous pigmented lines extending beyond half of the cervical third of the tooth surface14 (figure 1). The pigmentations appeared for the first time when the boy was 3 years of age. Since then, he was routinely submitted to professional prophylaxis with carbonate, and recurrence generally occurred within 15–30 days. His mother apprised us regarding the patient's dietary habits and medication intake—there were no relevant findings. He was under psychiatric treatment due to bullying by his classmates, related to the black teeth stains.
Figure 1.
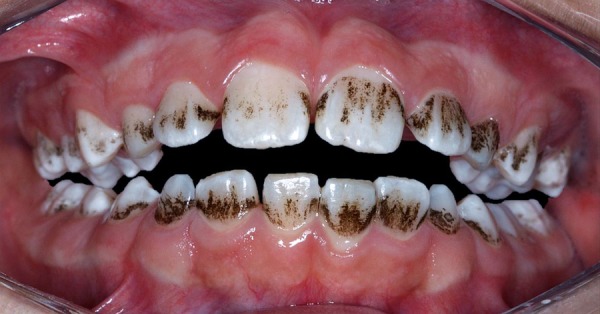
Clinical aspect of generalised darkened pigmentation in all teeth.
Investigations
Blood testing performed from 2006 to 2010 revealed an increase in plasma iron levels (highest value: 128 µg/dL in 2008; reference values for the age range from 35 to 90 µg/dL). The aetiology for such increase was unknown, since the medical staff responsible for physical examination of the patient did not detect any systemic disorder. All examinations were performed at the Brasília University Hospital (Brasília, DF, Brazil).
A specialist performed complete periodontal examinations at baseline and follow-up periods, which included dental radiographs, probing depth (PD), clinical attachment level (CAL), plaque index (PI)27 and gingival bleeding index (GBI).28
Plaque samples were collected by scraping the area with a sterilised paper strip n° 80 (Tanari Cell Pack, Manaus, Amazonas, Brazil) after isolation of the area with sterilised gauze. Samples were collected from 10 sites showing the highest amount of black stains. Immediately after collection, the samples were stored in Eppendorfs containing sterile saline solution (0.9% NaCl) frozen with dry ice (DryIce, Núcleo Bandeirante-DF, Brazil) until microbiological analysis by real-time PCR (RT-PCR). Sequences of genetic and microbiological primers are shown in table 1.
Table 1.
Sequence of genetic and microbiological primers
| Bacteria | Primer sequence |
|---|---|
| A. actinomycetemcomitans | ATGCCAACTTGACGTTAAAT AAACCCATCTCTGAGTTCTTCT |
| P. gingivalis | TACCCATCGTCGCCTTGGT CGGACTAAAACCGCATACACTT |
| P. intermedia | CCACATATGGCATCTGACGTG TCAATCTGCACGCTACTTGG |
| P. melaninogenica | GTGGGATAACCTGCCGAAAG CCCATCCATTACCGATAAATCTTTA |
| P. nigrescens | ATGAAACAAAGGTTTTCCGG CCCACGTCTCTGTGGGCTGGCGA |
Treatment
Removal of the black stains was performed according to the principles of PDT.19 26 Briefly, after isolation of the area with sterilised gauze and air-drying of tooth surfaces, a toluidine blue solution photosensitiser (Farmacotécnica, Brasília, DF, Brazil) was applied to tooth surfaces29 with a micro brush (KG Sorensen, São Paulo, SP, Brazil). The toluidine blue solution consisted of: 1% toluidine blue (10 mg/mL);30 0.18% methylparaben and purified water 30 mL. Irradiation was performed with a diode Indium Gallium Aluminium Phosphorus (InGaAlP) laser (FlashlaseIII—DMC São Carlos, SP, Brazil) at a 660 nm wavelength, continuously, using 100 mW and a focal spot of 600 µm. PDT was performed in five sessions—once a week for 5 weeks—according to the area of laser irradiation: (1) anterior mandibular teeth; (2) posterior mandibular teeth; (3) anterior maxillary teeth; (4) posterior maxillary teeth; (5) all tooth surfaces. After the application of a photosensitiser, irradiation was applied at 70 J/cm2 in non-contact mode, for 23 s, near the dental surface (figure 2). Note: the laser was activated and the optical fibre assembly moved with sweeping strokes in an apico-coronal direction, parallel to the vestibular and lingual surfaces. Proximal areas were treated with prophylaxis.
Figure 2.
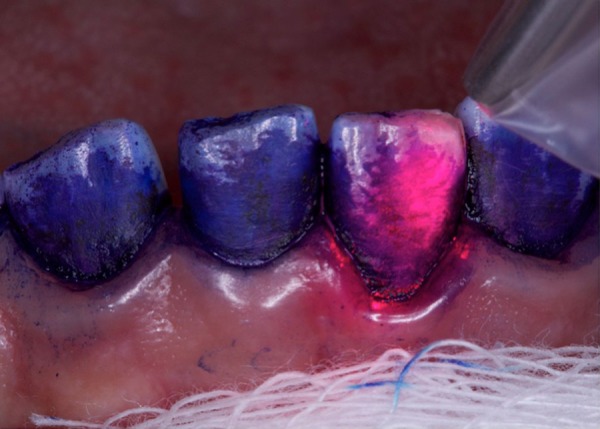
Application of photodynamic therapy with a red InGaAlP laser, 660 nm, 100 mW, 70 J/cm2, 23 s/tooth surfaces.
After performing the PDT, removal of the remaining black-pigmented stains was accomplished using Gracey curettes. The blue solution was removed by prophylactic paste and rubber cap. During the PDT, clinical signs of enamel demineralisation were observed (figure 3), which was accompanied by hypersensitivity being felt by the patient. Considering this, topical neutral fluoride was applied to the treated teeth for 1 min at postoperative follow-up.
Figure 3.
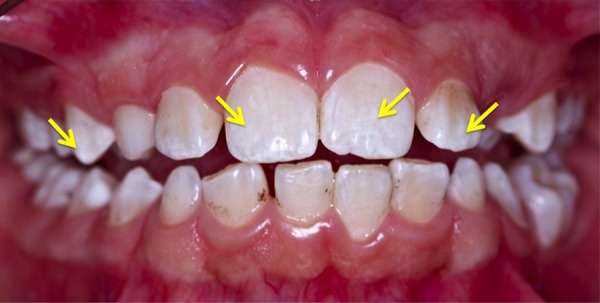
Clinical aspect after photodynamic therapy: notice the white aspect of tooth demineralisation after intervention. Persistent stains have been removed by scaling with Gracey curettes in the same session.
Outcome and follow-up
The black-pigmented stains were completely removed from the teeth after PDT, with no recurrence of the stains at 7 months of follow-up (figure 4).
Figure 4.
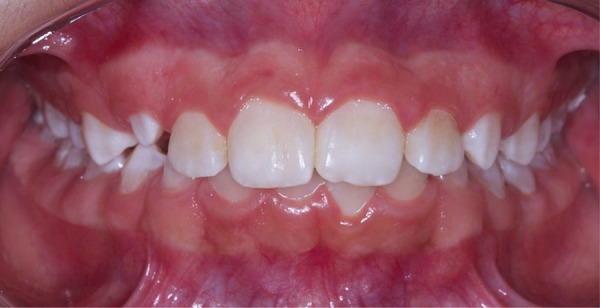
Clinical aspect after photodynamic therapy, seen without recurrence after a 7-month period of follow-up.
After removal of the stains by PDT, plaque samples were obtained from the 10 sites previously tested, as described earlier. Qualitative analysis of the microbial composition of the board samples obtained before and after the PDT is presented in table 2.
Table 2.
Qualitative analysis by RT-PCR of bacterial samples obtained before and after PDT
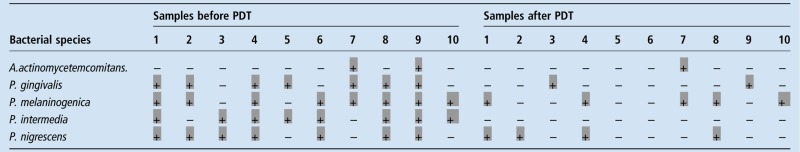 |
(−)=bacteria absence; (+)=bacteria presence
PDT, photodynamic therapy; RT-PCR, real-time PCR.
Bacterial species identified by RT-PCR were A. actinomycetemcomitans, P. gingivalis, P. intermedia, P. melaninogenica and P. nigrescens. Microbiological analysis of the samples obtained before PDT demonstrated an overall 64% prevalence of the bacteria investigated, with higher prevalence of the species P. melaninogenica and P. intermedia, present in 80% of the samples, while A. actinomyctemcomitans was found in 20% of the samples. After treatment, a marked reduction in overall bacteria prevalence was observed (22%), especially for P. intermedia, which was not identified in any of the samples after treatment.
Periodontal analyses of CAL and PD were within normal range as was the radiographic examination. The initial PI and GBI were 96.87% and 46.52%, respectively. The second clinical analysis was carried out 7 months after the PDT. There was a considerable reduction of GBI (13.58%). In relation to PI, there was a reduction, however, it was not a significant improvement (93.51%).
Discussion
Black-pigmented lesions are rarely observed in the literature; they are usually characterised by the presence of small spots concentrated mainly at the cervical areas.1–3 In this case, the lesions were overspread, affecting all deciduous and permanent teeth, as well as middle and incisal thirds, besides affecting the cervical area. Previous attempts at stain removal by professional prophylaxis were unsuccessful, with recurrence of the lesions within 30 days, which had a negative impact in the patient's self-confidence and quality of life. As far as we know, this is the first time that removal of black-pigmented stains has been successfully treated by PDT followed by professional prophylaxis.
The pigment produced by BPB is an important virulence factor,3 and BPB such as P. gingivalis and P. intermedia may contribute to the development of early periodontal disease.5 In this clinical case, although overspread, the stains were more concentrated at gingival margin at the dental smooth surfaces,1–3 possibility related to the facility of BPB colonies obtaining nutrients from gingival fluid.1–3 7 8 Gingival inflammation was characterised by redness of the gingival margin and bleeding on probing the affected teeth.
Studies reporting the association of black stains and periodontal disease are scarce in the literature. Most have reported the association of pigment with lower indices of caries lesions.2 3 The existence of a higher calcium and phosphate content in gingival debris of children with darkened pigmentation may contribute to caries immunity by reducing the dissolution of tooth enamel or by possibly maintaining a more stable pH, which is detrimental to colonisation of caries-related bacteria.3 In this case report, the patient did not present caries lesions.
Microbiological analysis by RT-PCR showed the presence of five BPB species (A. actinomycetemcomitans, P. gingivalis, P. intermedia, P. melaninogenica and P. nigrescens) in the samples obtained before PDT treatment, with higher prevalence of P. melaninogenica and P. intermedia, and lower prevalence of A. actinomycetemcomitans. This result differs from previous reports, which showed a higher prevalence of A. actinomycetemcomitans in plaque samples collected from black-pigmented stains on children's teeth.11
No consensus in treatment protocol to achieve pigment removal was found in the literature.15–24 In this case report, PDT was performed in order to suppress BPB species, commonly found in patients with persistent infection and lower response after conventional periodontal treatment.15 24 The stains in our patient were much easier to remove after PDT. The results obtained highlighted the reduction in overall BPB counting after PDT treatment, especially for P. intermedia, which was not detected in any of the plaque samples obtained after treatment. This species has been associated with increases in gingival bleeding,5 6 which can explain the reduction of gingival bleeding after the intervention.
For the elimination of bacteria in supragingival and subgingival plaque, antimicrobial PDT has been applied with various combinations of lasers and photosensitising agents.19 In antimicrobial PDT, the phenothiazine dyes (toluidine blue O and methylene blue) are the major photosensitisers applied clinically in the medical field. Both have similar chemical and physicochemical characteristics.16 Besides, it has been shown in vitro that toluidine blue O interacts with lipopolysaccharide more effectively than does methylene blue.17
There is not a definitive protocol for PDT application.15–19 Some clinical studies have had adequate antimicrobial activity with red light irradiation between 60 and 100 J/cm2.20 21 We used the laser at 660 nm to irradiate toluidine blue. It is important to clarify that, though it is possible to use this wavelength to irradiate toluidine, it is less effective than using 630 nm.
PDT as an adjunctive therapy is associated with reduction in some key periodontal pathogens, but some studies failed to show statistically significant benefits in clinical outcome.21 In the present case, a positive result was obtained after five sessions with 1-week intervals. PDT resulted in lower prevalence of BPB 3 months after intervention, along with reduction of clinical gingival inflammation and elimination of black stains. A negative collateral effect reported by the patient was the development of postoperative hypersensitivity, probably related to the effect of enamel demineralisation by the laser, with blue toluidine application. Management of this condition included topical application of a neutral fluoride solution, resulting in complete remission of signs and symptoms of enamel demineralisation and hypersensitivity.
Laboratory examinations revealed an increase in iron plasma levels, of unknown aetiology, which might explain the burden of widespread black-pigmented staining of deciduous and permanent teeth, since BPB use iron as a nutrient for growth, producing the black-pigmented stains.5 7–10 Although the clinical periodontal parameters improved after treatment, no significant differences in plaque index were observed before and after treatment. This could be explained by the depressive state of the patient, who suffered from constant bullying by his classmates because of the black-pigmented stains on his teeth, leading to psychiatric treatment.
This case report is the first to show that PDT contributes to elimination of black-pigmented stains, reduction in BPB prevalence and remission of clinical parameters of the gingiva in a 10-year-old child, without recurrence of the disease after a 7-month period of follow-up.
Learning points.
Black-pigmented stains are produced by black-pigment producing bacterial species including P. melaninogenica, P. intermedia, P. nigrescens and P. gingivalis, which are implicated in the aetiology of periodontal diseases.
Toluidine blue solution used as a photosensitiser resulted in enamel demineralisation and postoperative hypersensitivity, which were successfully reversed by the application of neutral fluoride.
Photodynamic therapy was able to reduce the levels of black-pigmented bacteria and gingival inflammation.
Acknowledgments
The authors acknowledge the assistance of Dryice (Núcleo Bandeirante-DF), Instituto Sabin, for blood analysis and Farmacotécnica (Brasília-DF) for their contribution of toluidine blue solution. The authors also thank the Department of Biological Sciences—Bauru School of Dentistry, for microbiological analysis.
Footnotes
Contributors: LP and VG were responsible for clinical analysis and treatment of the patient. ACPSA and CD were responsible for bacterial analysis and for editing the manuscript. All the authors reviewed the article and contributed to the text.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ronay V, Attin T. Black stain—a review. Oral Health Prev Dent 2011;9:37–45. [PubMed] [Google Scholar]
- 2.Koch JM, Bove M, Schroff J et al. Black stain and dental caries in schoolchildren in Potenza, Italy. J Dent Child 2001;68:353–5. [PubMed] [Google Scholar]
- 3.Theilade J, Slots J, Fejerskov O. The ultrastructure of black stain on human primary teeth. Scand J Dent Res 1973;81:528–32. [DOI] [PubMed] [Google Scholar]
- 4.Schoenly JE, Seka W, Featherstone JD et al. Near-UV laser treatment of extrinsic dental enamel stains. Lasers Surg Med 2012;44:339–45. 10.1002/lsm.22017 [DOI] [PubMed] [Google Scholar]
- 5.Albandar JM, DeNardin AM, Adesanya MR et al. Associations between serum antibody levels to periodontal pathogens and early-onset periodontitis. J Periodontol 2001;72:1463–9. 10.1902/jop.2001.72.11.1463 [DOI] [PubMed] [Google Scholar]
- 6.Maeda N, Okamoto M, Kondo K et al. Incidence of Prevotela intermedia and Prevotella nigrescens in periodontal health and disease. Microbiol Immunol 1998;42:583–9. 10.1111/j.1348-0421.1998.tb02328.x [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee S. The role of crevicular fluid iron in periodontal disease. J Periodontol 1985;56:22–7. 10.1902/jop.1985.56.11s.22 [DOI] [PubMed] [Google Scholar]
- 8.Lewis JP. Metal uptake in host-pathogen interactions: role of iron in Porphyromonas gingivalis interactions with host organisms. Periodontol 2000 2010;52:94–116. 10.1111/j.1600-0757.2009.00329.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smalley JW, Silver J, Birss AJ et al. The haem pigment of the oral anaerobes Prevotella nigrescens and Prevotella intermedia is composed of iron (III) protoporphyrin IX in the monomeric form. Microbiology 2003;149:1711–18. 10.1099/mic.0.26258-0 [DOI] [PubMed] [Google Scholar]
- 10.Smalley JW, Birss AJ, Silver J. The periodontal pathogen Porphyromonas gingivalis harnesses the chemistry of the u-oxo bishaem of iron protoporphyrin IX to protect against hydrogen peroxide. FEMS Microbiol Lett 2000;183:159–64. [DOI] [PubMed] [Google Scholar]
- 11.Saba C, Solidani M, Berlutti F et al. Black stains in the mixed dentition: a PCR microbiological study of the etiopathogenic bacteria. J Clin Pediatr Dent 2006;30:219–24. 10.17796/jcpd.30.3.q1561155x22u0774 [DOI] [PubMed] [Google Scholar]
- 12.Lie MA, van der Weijden GA, Timmerman MF et al. Occurrence of Prevotella intermedia and Prevotella nigrescens in relation to gingivitis and gingival health. J Clin Periodontol 2001;28:189–93. 10.1034/j.1600-051x.2001.028002189.x [DOI] [PubMed] [Google Scholar]
- 13.Rhodes ER, Menke S, Shoemaker C et al. Iron acquisition in the dental pathogen Actinobacillus actinomycetemcomitans: what does it use as a source and how does it get this essential metal? Biometals 2007;20:365–77. 10.1007/s10534-006-9058-3 [DOI] [PubMed] [Google Scholar]
- 14.Gasparetto A, Conrado CA, Maciel SM et al. Prevalence of black tooth stains and dental caries in Brazilian schoolchildren. Braz Dent J 2003;14:157–61. 10.1590/S0103-64402003000300003 [DOI] [PubMed] [Google Scholar]
- 15.Hayek RR, Araújo NS, Gioso MA et al. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J Periodontol 2005;76:1275–81. 10.1902/jop.2005.76.8.1275 [DOI] [PubMed] [Google Scholar]
- 16.Takasaki AA, Aoki A, Mizutani K et al. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 2000 2009;51:109–40. 10.1111/j.1600-0757.2009.00302.x [DOI] [PubMed] [Google Scholar]
- 17.Usacheva MN, Teichert MC, Biel MA. The interaction of lipopolysaccharides with phenothiazine dyes. Lasers Surg Med 2003;33:311–19. 10.1002/lsm.10226 [DOI] [PubMed] [Google Scholar]
- 18.Wilson M, Dobson J, Sarkar S. Sensitization of periodontopathogenic bacteria to killing by light from a low-power laser. Oral Microbiol Immunol 1993;8:182–7. 10.1111/j.1399-302X.1993.tb00663.x [DOI] [PubMed] [Google Scholar]
- 19.Usacheva MN, Teichert MC, Biel MA. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg Med 2001;29:165–73. 10.1002/lsm.1105 [DOI] [PubMed] [Google Scholar]
- 20.Kharkwal GB, Sharma SK, Huang IY et al. Photodynamic therapy for infections: clinical applications. Lasers Surg Med 2011;43:755–67. 10.1002/lsm.21080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theodoro LH, Silva SP, Pires JR et al. Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med Sci 2012;27:687–93. 10.1007/s10103-011-0942-x [DOI] [PubMed] [Google Scholar]
- 22.Dougherty TJ, Gomer CJ, Henderson BW et al. Photodynamic therapy. J Nat Cancer Inst 1998;75:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontana CR, Abernethy AD, Som S et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res 2009;44:751–9. 10.1111/j.1600-0765.2008.01187.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkar S, Wilson M. Lethal photosensitization of bacteria in subgingival plaque samples from patients in chronic periodontitis. J Periodontal Res 1993;28:204–20. 10.1111/j.1600-0765.1993.tb01070.x [DOI] [PubMed] [Google Scholar]
- 25.Wood S, Metcalf D, Devine D et al. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother 2006;57:680–4. 10.1093/jac/dkl021 [DOI] [PubMed] [Google Scholar]
- 26.Zanin IC, Lobo MM, Rodrigues LK et al. Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. Eur J Oral Sci 2006;114:64–9. 10.1111/j.1600-0722.2006.00263.x [DOI] [PubMed] [Google Scholar]
- 27.O'Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol 1972;43:38 10.1902/jop.1972.43.1.38 [DOI] [PubMed] [Google Scholar]
- 28.Ainamo J; Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J 1975;25:229–35. [PubMed] [Google Scholar]
- 29.Chondros P, Nikolidakis D, Christodoulides N et al. Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. Lasers Med Sci 2009;24: 681–8. 10.1007/s10103-008-0565-z [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro SL, Donegá JM, Seabra LM et al. Capacity of photodynamic therapy for microbial reduction in periodontal pockets. Lasers Med Sci 2010;25:87–91. 10.1007/s10103-009-0671-6 [DOI] [PubMed] [Google Scholar]


