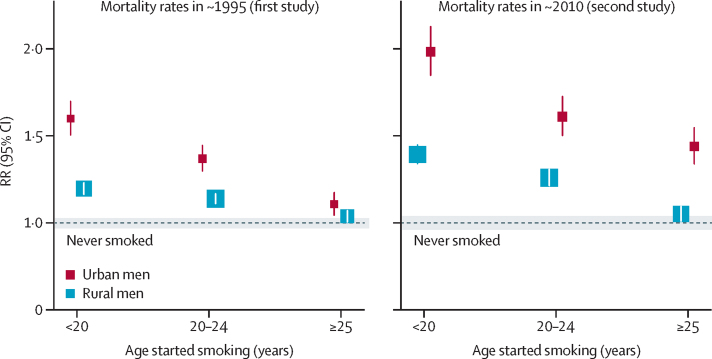Summary
Background
Chinese men now smoke more than a third of the world's cigarettes, following a large increase in urban then rural usage. Conversely, Chinese women now smoke far less than in previous generations. We assess the oppositely changing effects of tobacco on male and female mortality.
Methods
Two nationwide prospective studies 15 years apart recruited 220 000 men in about 1991 at ages 40–79 years (first study) and 210 000 men and 300 000 women in about 2006 at ages 35–74 years (second study), with follow-up during 1991–99 (mid-year 1995) and 2006–14 (mid-year 2010), respectively. Cox regression yielded sex-specific adjusted mortality rate ratios (RRs) comparing smokers (including any who had stopped because of illness, but not the other ex-smokers, who are described as having stopped by choice) versus never-smokers.
Findings
Two-thirds of the men smoked; there was little dependence of male smoking prevalence on age, but many smokers had not smoked cigarettes throughout adult life. Comparing men born before and since 1950, in the older generation, the age at which smoking had started was later and, particularly in rural areas, lifelong exclusive cigarette use was less common than in the younger generation. Comparing male mortality RRs in the first study (mid-year 1995) versus those in the second study (mid-year 2010), the proportional excess risk among smokers (RR-1) approximately doubled over this 15-year period (urban: RR 1·32 [95% CI 1·24–1·41] vs 1·65 [1·53–1·79]; rural: RR 1·13 [1·09–1·17] vs 1·22 [1·16–1·29]), as did the smoking-attributed fraction of deaths at ages 40–79 years (urban: 17% vs 26%; rural: 9% vs 14%). In the second study, urban male smokers who had started before age 20 years (which is now typical among both urban and rural young men) had twice the never-smoker mortality rate (RR 1·98, 1·79–2·19, approaching Western RRs), with substantial excess mortality from chronic obstructive pulmonary disease (COPD RR 9·09, 5·11–16·15), lung cancer (RR 3·78, 2·78–5·14), and ischaemic stroke or ischaemic heart disease (combined RR 2·03, 1·66–2·47). Ex-smokers who had stopped by choice (only 3% of ever-smokers in 1991, but 9% in 2006) had little smoking-attributed risk more than 10 years after stopping. Among Chinese women, however, there has been a tenfold intergenerational reduction in smoking uptake rates. In the second study, among women born in the 1930s, 1940s, 1950s, and since 1960 the proportions who had smoked were, respectively, 10%, 5%, 2%, and 1% (3097/30 943, 3265/62 246, 2339/97 344, and 1068/111 933). The smoker versus non-smoker RR of 1·51 (1·40–1·63) for all female mortality at ages 40–79 years accounted for 5%, 3%, 1%, and <1%, respectively, of all the female deaths in these four successive birth cohorts. In 2010, smoking caused about 1 million (840 000 male, 130 000 female) deaths in China.
Interpretation
Smoking will cause about 20% of all adult male deaths in China during the 2010s. The tobacco-attributed proportion is increasing in men, but low, and decreasing, in women. Although overall adult mortality rates are falling, as the adult population of China grows and the proportion of male deaths due to smoking increases, the annual number of deaths in China that are caused by tobacco will rise from about 1 million in 2010 to 2 million in 2030 and 3 million in 2050, unless there is widespread cessation.
Funding
Wellcome Trust, MRC, BHF, CR-UK, Kadoorie Charitable Foundation, Chinese MoST and NSFC
Introduction
For national disease control strategies, and for individual decisions about smoking, what matters is not just current but also future tobacco-attributed mortality rates. China now consumes over a third of the world's cigarettes,1 but the increase is too recent for the full effect of current cigarette consumption levels on the hazard per continuing smoker to have emerged.2, 3 There has been a large intergenerational increase in cigarette smoking by young men, first in urban and then in rural areas. In contrast, there has been a large intergenerational decrease in smoking among women, at least until women born in the 1970s.4, 5, 6, 7 So, assessment of future tobacco hazards must allow for urban versus rural and, particularly, male versus female differences in lifelong smoking patterns.
Research in context.
Systematic review
Existing reviews of smoking and death in China include mainly studies established during the 1980s or 1990s.23, 24 We searched PubMed for further such studies using the terms “smoking” AND “mortality” AND “China”, but found no large studies reported in English. The previous studies mainly involved people born before 1950 who, unlike those born more recently, had not smoked cigarettes persistently since early adulthood, or had smoked forms of tobacco (eg, pipes) that carry a lower risk than manufactured cigarettes. These previous studies could not assess directly the growing risks of tobacco in the present century. For, the hazards among smokers depend importantly not only on recent smoking patterns, but also on patterns in early adult life,2, 13, 14 and consumption of substantial numbers of cigarettes from early adulthood used to be uncommon, particularly in rural areas. Future hazards can be assessed only by studies of large numbers who have smoked cigarettes ever since early adulthood. Our two nationally representative studies can help to assess how the epidemic of smoking in mainland China has developed, and how it will evolve.
Interpretation
There are contrasting male and female trends. Among women, tobacco-attributed mortality is currently about 5%, 3%, 1%, and <1% of all mortality in those born in the 1930s, 1940s, 1950s, and since 1960. Female tobacco-attributed mortality is likely to fall as the pre-1950 generation, in which some women smoke, is replaced by the next generation, in which far fewer do so. Among men, the situation is opposite; the proportion of male deaths from smoking has been increasing, first in urban and then in rural areas, and already by 2010 tobacco accounted for a quarter of urban male deaths at ages 40–79 years. Among urban male smokers who started to smoke cigarettes (rather than other forms of tobacco) before age 20 years, which is the uptake pattern that is now typical among young men throughout China, overall mortality is already twice as great as among otherwise similar men who never smoked. As the epidemic matures, first in urban and then in rural areas, and the population grows, Chinese tobacco deaths will rise from about 1 million in 2010, to 2 million in 2030, and 3 million in 2050, unless there is widespread cessation.
Assessment of current and future tobacco hazards must also allow for other changes in mortality. Communicable disease mortality is decreasing steeply and non-communicable disease mortality is also decreasing, albeit more slowly.8, 9 This health transition is continuing, so all-cause mortality is falling in children and in adults, despite the net effects of changes in smoking and in adiposity. At 1970 Chinese rates, half would die before age 70 years (including 10% before age 5 plus another 10% before age 50). At 2010 rates, however, only a quarter would die before age 70 years (including only 1% before age 5 plus another 4% before age 50).9 These improvements are likely to continue (except perhaps in male smokers), due to better treatment and reductions in many other causes of disease.
The Chinese population has stabilised below age 50, but at ages 60–79 years it will double from 2010 to 2030, then stabilise.10 The rising over-50 population and falling age-specific death rates affect projections of tobacco-attributed mortality, and of the eventual risks for those smokers who, as has become usual for men in urban and then rural China, started substantial cigarette usage before age 20 years. The hazards currently seen in urban men who started cigarette smoking before age 20 years are particularly important, as they foreshadow what nationwide smoker versus non-smoker mortality rate ratios (RRs) will eventually become.2, 3
Successive nationally representative epidemiological studies, conducted some time apart, can help to monitor and predict trends in tobacco-attributed mortality.11, 12, 13, 14, 15 We report two large studies in China, one monitoring deaths from 1991–99 (mid-year 1995),16 and the second monitoring deaths 15 years later, from 2006–14 (mid-year 2010).17, 18 Changing smoker versus non-smoker RRs between the two studies show how the epidemic is evolving. The baseline survey of the second study (which was more detailed than that of the first) yields data on intergenerational changes in smoking patterns among urban and rural men and women, and prospective follow-up of mortality yields the RRs associated with each pattern, including the particularly informative hazards among urban men who have smoked cigarettes since before age 20 years.
Methods
Study populations
After a pilot phase in 1990–91, the first nationwide prospective study (Chinese Prospective Smoking Study [CPSS]) had its main recruitment phase from April 9, to Dec 31, 1991, with follow-up to Dec 31, 1999 (mid-year 1995). The second (China Kadoorie Biobank [CKB] study) recruited from June 25, 2004, to July 15, 2008, with follow-up to Jan 1, 2014 (mid-year 2010). The designs and methods of both studies have been described previously.16, 17, 18
In the first, 225 721 men were recruited from 45 study areas, chosen at random from 145 nationally representative Disease Surveillance Points (DSPs). A typical DSP monitors cause-specific deaths in 50 000–100 000 residents in five to ten nearby residential units (groups of rural villages or urban street committees). In each study area (22 rural, 23 urban), two or three such units were randomly selected and all men aged 40 years or older were identified through local residential records and invited to take part; about four-fifths participated (including 219 893 at ages 40–79 years). In local study clinics, trained health workers measured blood pressure, height, weight, and peak expiratory flow rate and administered a standardised questionnaire on demographics, education, occupation, smoking, drinking, diet, and self-reported medical history. Survey records were handwritten on paper, with subsequent data entry double-punched.
In the second study, 210 222 men and 302 669 women were recruited in 2004–08 from ten diverse locations across China, four urban and six rural (or semi-rural in the case of Suzhou). These study areas chosen from the DSPs to span a range of socioeconomic levels, risk factor patterns, and disease patterns. The set of all DSPs is nationally representative, and after excluding a few with organisational difficulties, the choice of ten study sites from the remaining DSPs was made centrally, knowing local characteristics and cause-specific death rates. The exact choice of areas was decided carefully, aiming successfully to retain geographic and social balance and balance across deciles of mortality from major diseases, so the set of ten study sites should still be approximately nationally representative. All 1 801 167 registered residents of age 35–74 years were identified through local records and invited to survey clinics, and 500 223 (28%) participated; another 12 668 just outside this age range also participated. As a substantial minority of registered residents would actually have been living elsewhere, we estimate that about a third of the invitees actually living in the study areas participated. Trained health workers took blood for long-term storage; measured height, weight, waist, hips, bioimpedance, blood pressure, heart rate, and lung function; and administered laptop-based questionnaires on tobacco, alcohol, diet, indoor air pollution, physical activity, education, socio-demographic status, medical history, and female reproductive history.17
Assessment of smoking
In both studies, questions about smoking included frequency, type, amount (current and past), age first began, age stopped, and main reason for cessation (already ill or stopped by choice), with additional information in the second study on inhalation and exhaled CO (MicroCO meter, Carefusion, San Diego, CA, USA).19 Regular smokers used one or more cigarettes (or ≥1 g tobacco) daily for at least 6 months. Of smokers who had stopped (≥6 months), about half had done so because they were ill, and (to avoid bias) were still counted with smokers in the main analyses; the remainder, who are described as having stopped by choice, helped assess the effects of cessation.
Mortality follow-up
Cause-specific mortality in both studies was monitored through DSP death registries,20 and checked annually against local residential records, with active confirmation of survival through street committees or village administrators.18 In the second study, deaths were also monitored through the new nationwide health insurance system (yielding few additional cases). Causes were checked against any available medical records. For the few deaths (about 5% in both studies) without recent medical attention, standardised procedures determined probable causes from symptoms or signs described by informants (usually family). Deaths were coded by trained staff, blind to baseline data, using International Classification of Diseases (ICD)-9 in the first study and ICD-10 in the second (appendix p 3). At ages 40–79 years, each study had about 25 000 deaths.
Statistical analysis
Cox regression yielded multi-covariate-adjusted smoker versus never-smoker RRs at ages 40–79 years. Pack-years were not used, as ten cigarettes per day for 40 years may have effects very different from those of 20 cigarettes per day for 20 years. Analyses were stratified for location (first study 45 areas, second study ten areas) and 5-year age-at-risk groups, and adjusted for education (tertiary, secondary, primary, or none completed) and alcohol consumption (never, occasional, or ever-regular).
The RR for each smoking category is accompanied by a CI derived only from the variance of the log risk in that one category. Hence, each RR, including that for the reference group, is associated with a group-specific CI that can be thought of as reflecting the amount of data only in that one category.21 The 95% group-specific CI for RR is (RR/T, RR×T), where T=exp (1·96√v) and v is the variance of the log risk.
If the reference group with RR=1 and another group with RR=R have, respectively, group-specific CIs (a, b) and (x, y), then the CI for R that allows for the variation in both of the groups is (√ [xy/k], √ [xyk]), where log (k) is given by √(log2 [y/x] + log2 [b/a]); since k>y/x, this CI is wider than (x, y).
If RR is causal, the fraction of all deaths in the population that is attributed to smoking (ie, the population-attributed fraction; PAF) is P(RR-1) divided by RR, P being the prevalence of smoking among those dying of the relevant cause.22 Setting P=1 yields (RR–1) divided by RR, the fraction of the mortality among smokers that is attributed to smoking. Analyses used SAS version 9.3.
Role of the funding sources
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. ZC, RP, and LL had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
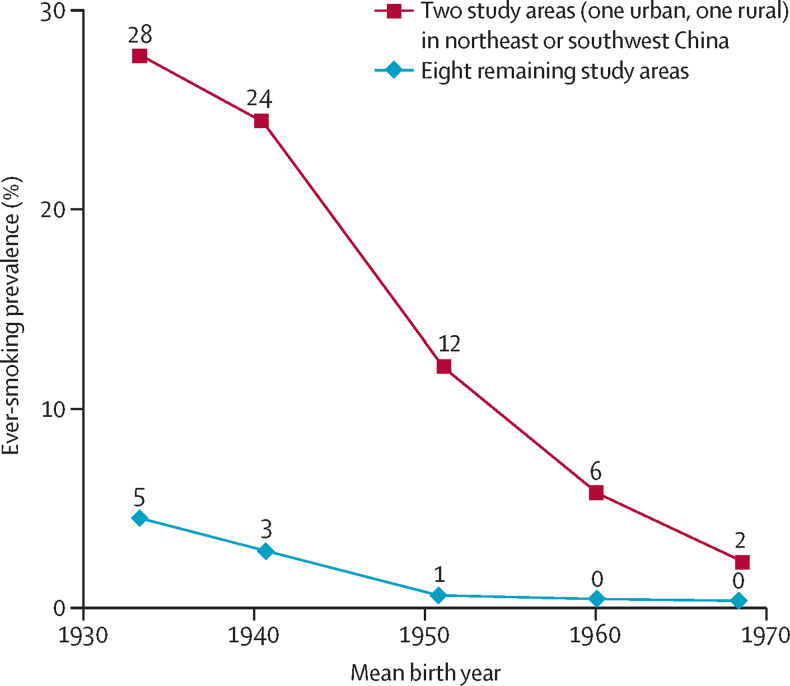
Women were included only in the second study. Of 302 669 women enrolled in 2004–08, only 3·2% were ever regular smokers, including 0·5% who stopped by choice. Smoking uptake decreased steeply across successive generations of women. Hence, there were much lower prevalences among those born in more recent decades (figure 1). Taking all study areas together, the prevalences of smoking among women born in the 1930s, 1940s, 1950s, and since the 1960s were 10%, 5%, 2%, and 1% (see figure 1 legend). In most locations, few women smoked, although in urban Harbin (northeast China) and rural Sichuan (southwest China), appreciable numbers of older women did so. Both in these two locations and elsewhere, the prevalence was about tenfold lower in women born in the 1960s (age about 40 years in 2006) than in women born in the 1930s (age about 70 years in 2006). On average, female smokers had started at age 27 years and currently smoked about 10 g/day (ten cigarette equivalents/day, though not necessarily as cigarettes).
Figure 1.
Chinese female smoking uptake rate by year of birth and locality
300 000 women seen in ten study areas in about 2006, with birth years grouped as: before 1935, 1935–44, 1945–54, 1955–64, and 1965 or later. The two areas where many older women smoked are in Harbin (urban northeast China) and Sichuan (rural southwest China). Taking all ten areas together, the prevalences of ever-smoking among women born in the 1930s, 1940s, 1950s, 1960s, and 1970s were, respectively, 10%, 5%, 2%, 1%, and <1% (3097/30 943, 3265/62 246, 2339/97 344, 926/94 772, and 142/17 161).
During 2·0 million woman-years of follow-up (mid-year 2010), 9934 women died at ages 40–79 years. Female mortality rates were significantly related to smoking for all causes (RR 1·51, 1·40–1·63) and for lung cancer, ischaemic heart disease, and chronic obstructive pulmonary disease (COPD) (appendix p 4). The proportional increase in overall mortality among smokers was greater in urban (RR 1·72, 1·52–1·96) than in rural (RR 1·40, 1·27–1·54) women, but numbers were too small for subdivision by smoking patterns. If these associations are causal, then about a third (0·51/1·51) of all deaths of female smokers were due to tobacco. As, however, few women smoked, the fraction of female mortality attributed to smoking was only 3% for deaths at ages 40–79 years (and 1·1%, 2·7%, and 5·2% for deaths at ages 40–59, 60–69, and 70–79 years). This dependence on age can also be expressed in terms of decade of birth: smoking caused about 5%, 3%, 1%, and less than 1% of all deaths among Chinese women born in the 1930s, 1940s, 1950s, and since 1960. The prevalences of smoking in these four birth cohorts were 10%, 5%, 2%, and 1%, respectively.
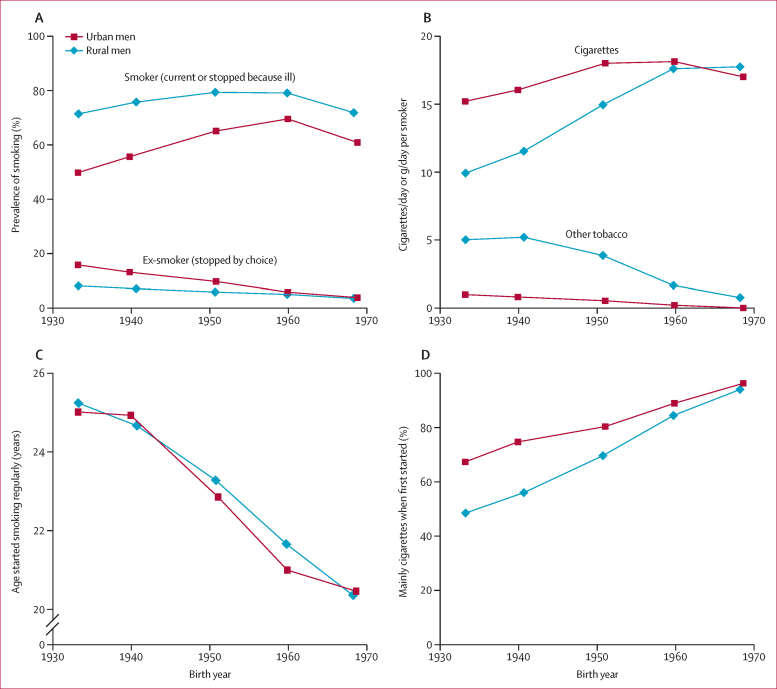
Among men, the smoking patterns were very different. The first of these two prospective studies began about 15 years before the second (1991 vs 2006), so those in it had, on average, an earlier year of birth (1938 vs 1954) and were less likely to have smoked only cigarettes when they started smoking, or last smoked (appendix p 5). The prevalences of ever-smoking among men in the two studies were similar, and did not depend strongly on age. In the second study, 68% were smokers (including those who had stopped because they were ill) and another 7% were ex-smokers (described as stopping by choice). Figure 2, from the baseline survey for the second study, describes smoking patterns by birth year. Male smokers born in 1970 had started around age 20 years and used only cigarettes, but male smokers born in the 1930s (an earlier generation) had started around age 25 years, smoked other tobacco types, and (not shown) were slightly less likely to inhale deeply. The proportion of smokers who had used cigarettes throughout adulthood was higher in urban than in rural men.
Figure 2.
Urban and rural Chinese male smoking patterns, by year of birth—prevalence, consumption, age started, and tobacco type smoked initially
210 000 men seen in about 2006 (at the 2004–08 baseline survey for the second prospective study). Prevalence of smoking (A); amount smoked per day when last smoked (B); Mean age started smoking regularly (C); and percentage of all smokers who used cigarettes when first started (D). To avoid reverse causality biasing the apparent effects of smoking and of cessation, in panel (A) and in the main analyses, the few men who had stopped smoking because they were ill are combined with the continuing smokers, leaving the ex-smokers who had stopped by choice. The overall proportions of men who had stopped because they were ill were 2·16%, 2·47%, 2·19%, 1·08% and 0·12% for those born during the 1930s, 1940s, 1950s, 1960s, and 1970s respectively.
Male deaths at ages 40–79 years numbered 25 548 in the first study and 14 241 in the second. In both, smokers' mortality rates were significantly elevated (first study: RR 1·17, 1·14–1·21; second study: RR 1·33, 1·28–1·39; table 1). So, although the studies were only 15 years apart (mid-years of follow-up 1995 and 2010), the proportional excess mortality among smokers (RR-1) had approximately doubled. Within each study, the RRs for all-cause mortality were not as extreme in rural as in urban men (first study: rural RR 1·13, 1·09–1·17 and urban RR 1·32, 1·24–1·41; second study: rural RR 1·22, 1·16–1·29 and urban RR 1·65, 1·53–1·79). Apart from the rural versus urban difference, the RRs in the second study differed little across study sites (appendix p 6). If the associations of smoking with death are largely causal, then the proportion of all male deaths at ages 40–79 years attributed to smoking rose between 1995 and 2000 from about 11% in the first study (PAF 9% rural, 17% urban) to about 18% in the second study (PAF 14% rural, 26% urban). If the PAF rose from 11% in 1995 to 18% in 2010, it will be at least 20% by the mid-2010s. Hence, smoking will cause about 20% of all male deaths at ages 40–79 years during the 2010s (ie, 2010–19).
Table 1.
Age started smoking regularly versus cause-specific mortality rate ratio (RR) among urban and rural men in about 2010 (second study) and about 1995 (first study)
| Breath CO, ppm |
All causes |
Lung cancer |
Ischaemic stroke or ischaemic heart disease |
COPD |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of deaths | RR* (95% group-specific CI) | Number of deaths | RR* (95% group-specific CI) | Number of deaths | RR* (95% group-specific CI) | Number of deaths | RR* (95% group-specific CI) | |||
| Urban men, about 2010 (2006–14) | ||||||||||
| Age started smoking (mean), years | ||||||||||
| Age <20 (16·9) | 16·5 | 843 | 1·98 (1·85–2·13) | 127 | 3·78 (3·15–4·54) | 200 | 2·03 (1·75–2·35) | 43 | 9·09 (6·60–12·50) | |
| Age 20–24 (21·0) | 15·2 | 797 | 1·61 (1·50–1·73) | 127 | 3·17 (2·67–3·77) | 182 | 1·50 (1·30–1·74) | 24 | 3·89 (2·62–5·79) | |
| Age ≥25 (30·4) | 12·6 | 754 | 1·44 (1·34–1·55) | 96 | 2·23 (1·82–2·73) | 196 | 1·49 (1·29–1·72) | 24 | 2·89 (1·93–4·32) | |
| All smokers | 14·9 | 2394 | 1·65 (1·59–1·73) | 350 | 2·98 (2·66–3·33) | 578 | 1·63 (1·49–1·77) | 91 | 4·61 (3·71–5·71) | |
| Non-smokers | 4·5 | 924 | 1·00 (0·94–1·07) | 69 | 1·00 (0·79–1·27) | 242 | 1·00 (0·88–1·14) | 18 | 1·00 (0·62–1·60) | |
| Trend p value† | <0·0001 | 0·0002 | 0·0032 | <0·0001 | ||||||
| Rural men, about 2010 (2006–14) | ||||||||||
| Age started smoking (mean), years | ||||||||||
| <20 (16·8) | 15·1 | 2710 | 1·39 (1·34–1·45) | 271 | 2·91 (2·58–3·29) | 334 | 1·37 (1·23–1·53) | 315 | 1·88 (1·68–2·10) | |
| 20–24 (21·2) | 13·6 | 2788 | 1·26 (1·21–1·31) | 275 | 2·45 (2·17–2·75) | 397 | 1·35 (1·23–1·49) | 261 | 1·41 (1·24–1·59) | |
| ≥25 (31·3) | 11·8 | 2434 | 1·05 (1·01–1·10) | 177 | 1·63 (1·41–1·90) | 347 | 1·04 (0·93–1·16) | 233 | 1·07 (0·94–1·22) | |
| All smokers | 13·6 | 7932 | 1·22 (1·20–1·25) | 723 | 2·30 (2·13–2·48) | 1078 | 1·24 (1·17–1·32) | 809 | 1·41 (1·31–1·51) | |
| Non-smokers | 6·0 | 2031 | 1·00 (0·96–1·05) | 92 | 1·00 (0·81–1·23) | 316 | 1·00 (0·89–1·12) | 172 | 1·00 (0·86–1·16) | |
| Trend p value† | <0·0001 | <0·0001 | 0·0005 | <0·0001 | ||||||
| Urban men, about 1995 (1991–99) | ||||||||||
| Age started smoking (mean), years | ||||||||||
| <20 (16·7) | .. | 1158 | 1·60 (1·51–1·70) | 142 | 3·20 (2·69–3·80) | 178 | 1·58 (1·36–1·84) | 194 | 1·82 (1·57–2·21) | |
| 20–24 (21·2) | .. | 1358 | 1·37 (1·30–1·44) | 130 | 2·31 (1·95–2·75) | 208 | 1·37 (1·19–1·57) | 209 | 1·43 (1·25–1·64) | |
| ≥25 (30·6) | .. | 1136 | 1·11 (1·04–1·17) | 97 | 1·73 (1·42–2·12) | 178 | 1·17 (1·01–1·36) | 186 | 1·16 (1·00–1·34) | |
| All smokers | .. | 3652 | 1·32 (1·28–1·37) | 369 | 2·32 (2·08–2·59) | 564 | 1·35 (1·23–1·47) | 589 | 1·42 (1·30–1·55) | |
| Non-smokers | .. | 1381 | 1·00 (0·95–1·06) | 82 | 1·00 (0·80–1·25) | 240 | 1·00 (0·87–1·14) | 197 | 1·00 (0·87–1·15) | |
| Trend p value† | <0·0001 | <0·0001 | 0·0052 | <0·0001 | ||||||
| Rural men, about 1995 (1991–99) | ||||||||||
| Age started smoking (mean), years | ||||||||||
| <20 (16·8) | .. | 5107 | 1·20 (1·16–1·23) | 236 | 1·98 (1·73–2·26) | 435 | 1·12 (1·02–1·23) | 1373 | 1·40 (1·33–1·48) | |
| 20–24 (20·9) | .. | 6215 | 1·14 (1·11–1·17) | 280 | 1·82 (1·62–2·05) | 605 | 1·17 (1·08–1·27) | 1573 | 1·28 (1·22–1·34) | |
| ≥25 (29·6) | .. | 3901 | 1·04 (1·01–1·07) | 139 | 1·37 (1·16–1·62) | 385 | 1·08 (0·98–1·18) | 986 | 1·06 (0·99–1·13) | |
| All smokers | .. | 15 223 | 1·13 (1·11–1·15) | 655 | 1·76 (1·62–1·91) | 1425 | 1·13 (1·07–1·20) | 3932 | 1·25 (1·21–1·30) | |
| Non-smokers | .. | 4855 | 1·00 (0·97–1·03) | 150 | 1·00 (0·85–1·18) | 499 | 1·00 (0·91–1·09) | 1114 | 1·00 (0·94–1·06) | |
| Trend p value† | <0·0001 | <0·0001 | 0·0620 | <0·0001 | ||||||
Group-specific CI for the non-smoker RR of 1·00 reflects the variance of the log risk in non-smokers; for each of the other RRs the CI is correspondingly wider than the group-specific CI. COPD=chronic obstructive pulmonary disease.
RRs (smokers vs non-smokers) were adjusted for 5-year age group and region, alcohol, and education; additional adjustment for occupation made no material difference to the RRs.
Trend test in smokers, ignoring non-smokers; if trend tests in this table had included non-smokers, each would have yielded p<0·0001.
Similarly, the proportional excess mortality among smokers (ie, RR-1) increased substantially between 1995 and 2010 for lung cancer (first study: RR 1·95, 1·68–2·26; second study: RR 2·58, 2·17–3·05), all vascular disease (first study: RR 1·12, 1·07–1·18; second study: RR 1·24, 1·16–1·33), and COPD (first study: RR 1·28, 1·20–1·36; second study: RR 1·62, 1·38–1·90).
Figure 3 shows, for both studies, the urban and rural RRs for overall mortality by age started smoking regularly. In each case, men who had started before age 20 years (mean age 17 years) were at substantially greater risk than were those who had started at ages 20–24 years (mean age 21 years). Differences in the reported ages at which men had started smoking regularly did not, however, suffice to explain the more extreme RRs in the second study, as even within each category of age started, the RR was substantially more extreme in the second study (table 1).
Figure 3.
All-cause smoker versus never-smoker mortality rate ratio (RR) among urban and rural Chinese men in two prospective studies, by time period (about 1995 or about 2010) and by age started smoking regularly
Each study followed about 200 000 men. Each group-specific CI (including that for never-smokers, given by the width of the shaded strip) reflects the variance of the log risk in that one group, so comparisons of RRs use variances from more than one group.
Among urban male smokers in the second study who had started before age 20 years (as is now typical throughout urban and rural China), most had always used manufactured cigarettes, and their all-cause mortality rate was double that of non-smokers (RR 1·98, 1·79–2·19). This suggests that about half (0·98/1·98) of their deaths were due to tobacco. This proportion could well rise still further. The few urban men who had started before age 15 years were at even greater risk (RR 2·64, 2·19–3·19).
The remaining analyses are given separately for urban and rural men in the two studies. The discussion particularly emphasises findings in the second study for urban smokers, among whom the RR was 1·65 (1·53–1·79) for all causes, 2·98 (2·28–3·89) for lung cancer, 1·63 (1·39–1·90) for ischaemic stroke or ischaemic heart disease, and 4·61 (2·75–7·73) for COPD; the RRs were even more extreme for men who had started before age 20 years (table 1). Smokers also had elevated mortality from some other diseases (appendix p 4), including stomach and oesophagus cancer, which are still major causes of death in some parts of China. In rural men, the RRs were generally less extreme, especially for vascular disease and COPD.
For the same disease groupings, table 2 shows urban and rural RRs in both studies by amount last smoked (<15, 15–24 and ≥25 g/day, adding together cigarettes per day and g per day of tobacco). It is only the RRs (not the absolute mortality rates) that are compared between areas and studies, as in table 1. For smokers in the second study, among urban men there were highly significant dose-response relationships for mortality from all causes, lung cancer, ischaemic stroke and ischaemic heart disease (each trend p<0·0001), and COPD (trend p=0·0021), but in rural men, the dose-response relationships among smokers (ignoring the non-smokers) were significant only for lung cancer, a disease with little time between symptom onset and death. The lack of a dose-response relationship for COPD (a major cause of death in rural areas) may reflect biases from reverse causality, whereby COPD symptoms could reduce the amount smoked. For all four disease groupings, tests for trend that included the never-smokers as having zero dose were highly significant in urban and rural areas in both studies (16 trend tests each p<0·0001; table 2).
Table 2.
Amount last smoked versus cause-specific mortality rate ratio (RR) among urban and rural men in about 2010 (second study) and about 1995 (first study)
| Breath CO, ppm |
All causes |
Lung cancer |
Ischaemic stroke or ischaemic heart disease |
COPD |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of deaths | RR* (95% group-specific CI) | Number of deaths | RR* (95% group-specific CI) | Number of deaths | RR* (95% group-specific CI) | Number of deaths | RR* (95% group-specific CI) | |||
| Urban men, about 2010 (2006–14) | ||||||||||
| Daily amount smoked (mean) | ||||||||||
| <15 (8·3) | 12·0 | 890 | 1·48 (1·39–1·59) | 115 | 2·28 (1·90–2·74) | 209 | 1·36 (1·19–1·56) | 26 | 2·94 (1·99–4·35) | |
| 15–24 (19·2) | 16·2 | 1091 | 1·70 (1·60–1·80) | 167 | 3·28 (2·82–3·83) | 261 | 1·73 (1·53–1·96) | 45 | 5·40 (4·04–7·23) | |
| ≥25 (34·9) | 17·6 | 413 | 1·93 (1·75–2·12) | 68 | 4·12 (3·24–5·24) | 108 | 2·24 (1·85–2·71) | 20 | 7·26 (4·66–11·32) | |
| All smokers | 14·9 | 2394 | 1·65 (1·59–1·73) | 350 | 2·98 (2·66–3·33) | 578 | 1·63 (1·49–1·77) | 91 | 4·61 (3·71–5·71) | |
| Non-smokers | 4·5 | 924 | 1·00 (0·94–1·07) | 69 | 1·00 (0·79–1·27) | 242 | 1·00 (0·88–1·14) | 18 | 1·00 (0·62–1·60) | |
| Trend p value† | <0·0001 | <0·0001 | <0·0001 | 0·0021 | ||||||
| Rural men, about 2010 (2006–14) | ||||||||||
| Daily amount smoked (mean) | ||||||||||
| <15 (7·7) | 12·1 | 3298 | 1·25 (1·20–1·29) | 202 | 1·81 (1·57–2·09) | 537 | 1·27 (1·16–1·39) | 347 | 1·52 (1·36–1·70) | |
| 15–24 (19·3) | 14·5 | 3113 | 1·17 (1·13–1·22) | 333 | 2·38 (2·14–2·65) | 380 | 1·14 (1·03–1·27) | 306 | 1·32 (1·18–1·47) | |
| ≥25 (35·3) | 14·0 | 1521 | 1·27 (1·20–1·34) | 188 | 3·20 (2·75–3·72) | 161 | 1·37 (1·16–1·61) | 156 | 1·34 (1·13–1·59) | |
| All smokers | 13·6 | 7932 | 1·22 (1·20–1·25) | 723 | 2·30 (2·13–2·48) | 1078 | 1·24 (1·17–1·32) | 809 | 1·41 (1·31–1·51) | |
| Non-smokers | 6·0 | 2031 | 1·00 (0·96–1·05) | 92 | 1·00 (0·81–1·23) | 316 | 1·00 (0·89–1·12) | 172 | 1·00 (0·86–1·16) | |
| Trend p value† | 0·7875 | <0·0001 | 0·9951 | 0·1126 | ||||||
| Urban men, about 1995 (1991–99) | ||||||||||
| Daily amount smoked (mean) | ||||||||||
| <15 (8·2) | .. | 1332 | 1·23 (1·16–1·30) | 104 | 1·77 (1·46–2·15) | 202 | 1·22 (1·06–1·40) | 223 | 1·28 (1·12–1·46) | |
| 15–24 (19·2) | .. | 1660 | 1·35 (1·29–1·42) | 190 | 2·62 (2·27–3·03) | 261 | 1·40 (1·24–1·59) | 256 | 1·45 (1·28–1·64) | |
| ≥25 (36·1) | .. | 660 | 1·51 (1·39–1·63) | 75 | 2·80 (2·21–3·53) | 101 | 1·55 (1·27–1·89) | 110 | 1·78 (1·46–2·15) | |
| All smokers | .. | 3652 | 1·32 (1·28–1·37) | 369 | 2·32 (2·08–2·59) | 564 | 1·35 (1·23–1·47) | 589 | 1·42 (1·30–1·55) | |
| Non-smokers | .. | 1381 | 1·00 (0·95–1·06) | 82 | 1·00 (0·80–1·25) | 240 | 1·00 (0·87–1·14) | 197 | 1·00 (0·87–1·15) | |
| Trend p value† | <0·0001 | 0·0015 | 0·0389 | 0·0071 | ||||||
| Rural men, about 1995 (1991–99) | ||||||||||
| Daily amount smoked (mean) | ||||||||||
| <15 (8·9) | .. | 3959 | 1·16 (1·09–1·16) | 132 | 1·37 (1·15–1·63) | 434 | 1·10 (1·00–1·21) | 1005 | 1·25 (1·17–1·33) | |
| 15–24 (18·8) | .. | 6886 | 1·13 (1·10–1·15) | 327 | 1·90 (1·70–2·12) | 659 | 1·18 (1·09–1·28) | 1800 | 1·23 (1·18–1·29) | |
| ≥25 (36·5) | .. | 4378 | 1·13 (1·10–1·17) | 196 | 1·84 (1·58–2·13) | 332 | 1·08 (0·97–1·21) | 1127 | 1·29 (1·22–1·38) | |
| All smokers | .. | 15 223 | 1·13 (1·11–1·15) | 655 | 1·76 (1·62–1·91) | 1425 | 1·13 (1·07–1·20) | 3932 | 1·25 (1·21–1·30) | |
| Non-smokers | .. | 4855 | 1·00 (0·97–1·03) | 150 | 1·00 (0·85–1·18) | 499 | 1·00 (0·91–1·09) | 1114 | 1·00 (0·94–1·06) | |
| Trend p value† | 0·7163 | 0·0233 | 0·8946 | 0·4014 | ||||||
Daily amount smoked adds together cigarettes plus g of other tobacco. Group-specific CI for the non-smoker RR of 1·00 reflects the variance of the log risk in non-smokers. COPD=chronic obstructive pulmonary disease.
RRs (smokers vs non-smokers) were adjusted for 5-year age group and region, alcohol, and education; additional adjustment for occupation made no material difference to the RRs.
Trend test in smokers, ignoring non-smokers; if trend tests in this table had included non-smokers, each would have yielded p<0·0001.
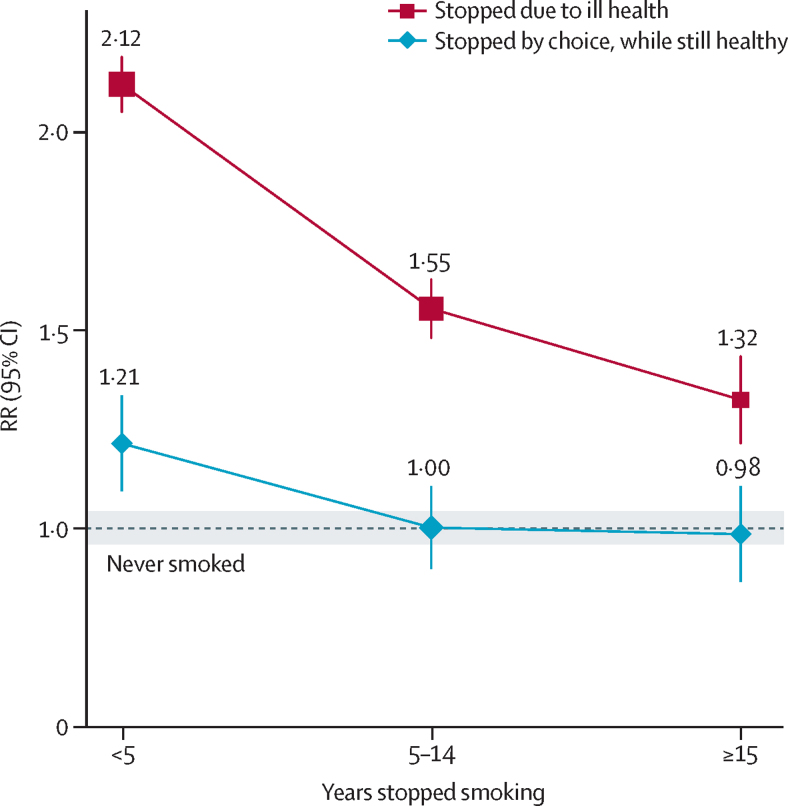
The proportion of male smokers who had stopped by choice rose appreciably over the 15 years from 1991 to 2006, from only 3% (4306/160 971) in the first baseline survey to 9% (14 080/156 313) in the second, and stopping by choice avoided nearly all the excess risk that would have been seen at the mortality rates of continuing smokers (figure 4). Among ex-smokers who had stopped by choice, the RR for all-cause mortality was 1·02 (0·95–1·10), and it attenuated with quitting duration. For men who had stopped by choice less than 5 years, 5–14 years, and 15 or more years before baseline, the RRs were 1·21 (1·07–1·37), 1·00 (0·90–1·11), and 0·98 (0·87–1·11) (trend p=0·01). These findings were not materially changed by inclusion of the first-study results (appendix p 7). Numbers who stopped by choice and died from specific conditions were too small for separate analysis. Among men who had stopped because they were already ill, the protective effects of quitting cannot be assessed straightforwardly. For, even if cessation is substantially protective, the illness that had made them stop could still cause them to be at misleadingly elevated risk (figure 4).
Figure 4.
Ex-smoker versus never-smoker all-cause mortality rate ratio (RR), by years stopped smoking and reason stopped, for men in the second study
Each group-specific CI (including that for never-smokers, given by the width of the shaded strip) reflects the variance of the log risk in that 1 group, so comparisons use variances from more than one group.
The smoker versus non-smoker RRs for lung cancer in these studies are much less extreme than in recent US prospective studies (appendix p 8–10), but the absolute excess lung cancer mortality in Chinese smokers is still substantial, as Chinese people have much higher non-smoker lung cancer rates than Americans do, especially in later middle age.3 Within China, never-smoker lung cancer rates fell slightly between the two prospective studies, whereas the smokers' lung cancer rates rose, resulting in greater RRs in the second study.
Discussion
Among Chinese men, tobacco-attributed mortality has grown considerably since the 1990s, and during the 2010s, smoking will cause about 20% of all male deaths at ages 40–79 years, up from only about 10% in the early 1990s. Moreover, the mortality rate ratio of 2 already seen among urban male smokers who started before age 20 years (the uptake pattern now typical in both urban and rural China) suggests that about half of these men's deaths were caused by smoking. This mortality rate ratio of 2 is still increasing, foreshadowing substantially greater future hazards for Chinese men (panel). Also, although this was not assessed in the present report, tobacco causes many non-fatal disease episodes, and much disability.
Among Chinese women, however, tobacco-attributed mortality is much smaller, and is currently still falling. Fewer women than men are smokers, and there was a large intergenerational decrease in the female prevalence of smoking, from about 10% among women born in the 1930s down to only about 1% among women born around 1970, which has been confirmed in an independent nationwide survey.6 Hence, although the present study suggests that the hazard per smoker is comparable for men and women, as did previous studies,13, 14, 23 tobacco-attributed mortality in the entire female population is low, and will fall further by 2030 as the present generation of women in which few smoke is succeeded by a generation in which even fewer smoke. (A recent WHO report mistakenly suggested, using methods3 inappropriate for China, that male and female tobacco-attributed proportions of deaths are similar;24 this is impossible, given the 20-fold difference in smoking prevalence.) But, this favourable trend may cease (with women born around 1980 and now in their thirties being the least exposed generation), as tobacco use by adolescent females has recently begun to increase in some parts of China.7 This underlines the danger that, as in many Western countries, social changes may well lead young Chinese women to start smoking. It will, however, take decades, probably until after 2050, for an increase in female smoking to cause a substantial increase in mortality, and over the next 20 years, while tobacco-attributed deaths increase among Chinese men, they should decrease among Chinese women, perhaps from about 3% of all female deaths now to less than 1% by the 2030s.2
A few large studies have provided reasonably robust evidence about the hazards of smoking in specific Chinese male populations, but most of the people who died in them were born before 1950,16, 25, 26, 27, 28, 29 so the smokers were at limited risk, as in the USA 60 years ago.11, 14 Some studies29, 30 have attempted to assess the extent of the Chinese epidemic in the early 2000s, but they involved cohorts established decades ago of men born before 1950, or used data from atypical regions (eg, Shanghai) where manufactured cigarettes have long been available.31
In China, cigarette consumption became widespread earlier in urban than in rural areas, mainly because of limited rural availability (and, until recent decades, affordability) of cigarettes.32 Hence, the hazard associated with a given current smoking pattern is more extreme in urban than in rural areas. However, this urban versus rural difference is likely to diminish, or even be reversed, over the next few decades, because rural men born after the 1960s not only tended to start at the same age as urban men and to smoke only cigarettes, but also had a somewhat higher smoking prevalence (figure 2). As earlier generations of urban and rural men get replaced by generations who have smoked cigarettes persistently since early adulthood, tobacco-attributable risks in middle age may soon reach those seen in many Western populations, as has almost happened in the subgroup of urban men who started smoking before age 20 years in the present study (and in Hong Kong, where cigarette use peaked about 20 years earlier than in mainland China33).
For the chief diseases by which tobacco causes death, there are large quantitative differences between China and elsewhere, between urban and rural China, and between past and future decades.2 In many Western populations, tobacco used to cause far more deaths from vascular than from respiratory disease,11, 12, 13, 14, 15 whereas in China the opposite is true, especially in rural areas. Although the RRs for COPD, lung cancer, and stroke may at present be smaller than in many Western populations, the absolute risks associated with smoking are not, as Chinese non-smoker death rates are high. Indeed, 1980s lung cancer mortality among Chinese never-smokers was more than three times that in US never-smokers, perhaps due partly to indoor air pollution from heating and cooking.2 While US never-smoker lung cancer rates have remained roughly constant over the past half-century,3 those in Chinese never-smokers seem to be decreasing while those in smokers are increasing, causing increasing smoker versus non-smoker lung cancer mortality rate ratios.
Absolute mortality rates are likely to be lower in prospective studies than in the general population. Hence, to estimate absolute numbers of tobacco-attributed deaths in China in 2010, the smoking-attributed fractions of all deaths in our second prospective study have been applied to independent estimates of male and female cause-specific numbers of deaths in mainland China34 at ages 35–69, 70–79, and 80 years or older (appendix p 4). This shows that there were about 1 million smoking-attributed deaths in 2010 (840 000 male, 130 000 female; table 3), mainly from diseases already known to be affected by smoking (lung cancer, ischaemic heart disease, ischaemic stroke, COPD, and other neoplastic, vascular, and respiratory conditions).3, 11 Counterbalancing the increasing RRs for all-cause mortality, age-specific under-70 mortality rates in China are decreasing due to many social, occupational and health-care changes, falling by about 15% during 2000–10,9 so the absolute death rate from smoking is not increasing as fast as would be suggested just by the increasing RRs.
Table 3.
Deaths attributed to tobacco in China, 2010
| Male | Female | Both | |
|---|---|---|---|
| 30–69 years | 375/2030 | 20/968 | 395/2998 |
| 70–79 years | 250/1370 | 45/907 | 295/2277 |
| ≥80 years* | 215/1178 | 65/1269 | 280/2447 |
| All ages | 840/4578 | 130/3144 | 970/7722 |
Data are number of deaths caused by tobacco (thousands)/total number of deaths (thousands). Below age 30 years, the total number of deaths (thousands) was 398 in men and 200 in women.
Estimated indirectly by applying mortality rate ratios at age 70–79 years.
About two-thirds of young Chinese men become cigarette smokers in early adult life. Unless they stop, the present study suggests that at least half of them will eventually be killed by their habit, and future studies may well show that a somewhat greater proportion will be killed by it. The first generation of men to experience the full hazards will probably be those born during the 1970s or 1980s, who reached adulthood when nationwide cigarette consumption was high. Conversely, this may well be the least exposed female generation.
China's 2030 sustainable development goals include reducing non-communicable disease mortality by a third, and monitoring the changes. If current smoking patterns persist, then as the smoker versus non-smoker RRs increases, mortality from other causes decreases, and the over-60 population doubles, Chinese tobacco deaths are likely to rise from 1 million in 2010 to about 2 million in 2030. Nowadays, in China more than 6 million young men a year begin smoking. If most persist, and (as in the USA14 and UK12, 13) smokers eventually have more than double the non-smoker mortality rates, there will in 2050 be about 3 million Chinese tobacco deaths, when those born in 1970 reach age 80 years. Although continuation of our second prospective study will monitor how the epidemic develops over the next decade or two, large new prospective studies of people born after 1970 will be needed to continue monitoring it thereafter. Fortunately, China's nationally representative household surveys regularly record smoking and the reasons for smoking cessation, and electronic linkage of this information with routine mortality records should allow reliable monitoring of the evolution of the epidemic for many decades to come.
Avoiding uptake of smoking by young people will greatly reduce tobacco deaths in the second half of the century. Moreover, stopping before the onset of life-threatening illness is remarkably protective, and an increasing proportion of smokers have stopped by choice (9% in 2006 vs only 3% in 1991). With effective measures to accelerate cessation, the growing epidemic of premature death from tobacco can be halted and then reversed, as in other countries. Widespread smoking cessation offers China one of the most effective, and cost-effective,35 strategies to avoid disability and premature death over the next few decades.
Acknowledgments
Acknowledgments
The chief acknowledgment is to the participants, the project staff, the China National Centre for Disease Control and Prevention, and its regional offices. The two prospective studies already span 25 years, throughout which Cancer Research UK, the UK Medical Research Council, and the British Heart Foundation core-funded the Oxford CTSU. The first study's baseline survey was funded by the World Bank and Canadian International Development Research Centre, with long-term continuation funded by the CTSU and organised by Gonghuang Yang; fuller acknowledgments have been published.16 The second study's baseline survey was funded by the Kadoorie Charitable Foundation, with blood storage through the core-funded CTSU Wolfson Laboratories (director Mike Hill) and Fu Wai China National Cardiovascular Centre, with long-term continuation funded by the CTSU, Wellcome Trust (088158/Z/09/Z, 104085/Z/14/Z), MRC (Newton Fund MCPC13049), Chinese Ministry of Science and Technology (2011BAI09B01), and National Natural Science Foundation of China (81390541); the Chinese National Health Insurance scheme provides linkage to all hospital admission data.
China Kadoorie Biobank collaborative group
International Steering Committee—Junshi Chen (co-chair), Zhengming Chen (co-principal investigator), Rory Collins, Liming Li (co-principal investigator), and Richard Peto (co-chair). International Co-ordinating Centre, Oxford—Daniel Avery, Derrick Bennett, Yumei Chang, Yiping Chen, Zhengming Chen, Robert Clarke, Huaidong Du, Xuejuan Fan, Haiyan Gao, Simon Gilbert, Michael Holmes, Andri Iona, Rene Kerosi, Ling Kong, Om Kurmi, Garry Lancaster, Sarah Lewington, John McDonnell, Winnie Mei, Iona Millwood, Qunhua Nie, Jayakrishnan Radhakrishnan, Paul Ryder, Sam Sansome, Dan Schmidt, Paul Sherliker, Margaret Smith, Rajani Sohoni, Robin Walters, Jenny Wang, Lin Wang, Alex Williams, Ling Yang, and Xiaoming Yang. National Co-ordinating Centre, Beijing—Zheng Bian, Ge Chen, Lei Guo, Yu Guo, Bingyang Han, Can Hou, Peng Liu, Jun Lv, Pei Pei, Shuzhen Qu, Yunlong Tan, Canqing Yu, and Huiyan Zhou. Qingdao Regional Co-ordinating Centre—Zengchang Pang, Shaojie Wang, Yun Zhang, and Kui Zhang (Qingdao CDC); Silu Liu and Wei Hou (Licang CDC). Heilongjiang Regional Co-ordinating Centre—Zhonghou Zhao, Shumei Liu, and Zhigang Pang (Provincial CDC); Weijia Feng, Shuling Wu, Liqiu Yang, Huili Han, Hui He, and Bo Yu (Nangang CDC). Hainan Regional Co-ordinating Centre—Xianhai Pan, Shanqing Wang, and Hongmei Wang (Provincial CDC); Xinhua Hao, Chunxing Chen, Shuxiong Lin, and Xiangyang Zheng (Meilan CDC); Jiangsu Regional Co-ordinating Centre—Xiaoshu Hu, Minghao Zhou, Ming Wu, and Ran Tao (Provincial CDC); Yeyuan Wang, Yihe Hu, Liangcai Ma, Renxian Zhou, Guanqun Xu, and Yan Lu (Suzhou CDC). Guangxi Regional Co-ordinating Centre—Baiqing Dong, Naying Chen, and Ying Huang (Provincial CDC); Mingqiang Li, Jinhuai Meng, Zhigao Gan, Jiujiu Xu, Yun Liu, and Jingxin Qing (Liuzhou CDC). Sichuan Regional Co-ordinating Centre—Xianping Wu, Yali Gao, and Ningmei Zhang (Provincial CDC); Guojin Luo, Xiangsan Que, Xiaofang Chen (Pengzhou CDC). Gansu Regional Co-ordinating Centre—Pengfei Ge, Jian He, and Xiaolan Ren (Provincial CDC); Hui Zhang, Enke Mao, Guanzhong Li, Zhongxiao Li, Jun He, Yulong Lei, and Xiaoping Wang (Maiji CDC). Henan Regional Co-ordinating Centre—Guohua Liu, Baoyu Zhu, Gang Zhou, and Shixian Feng (Provincial CDC); Yulian Gao, Tianyou He, Li Jiang, Jianhua Qin, and Huarong Sun (Huixian CDC). Zhejiang Regional Co-ordinating Centre—Liqun Liu, Min Yu, Yaping Chen, and Ruying Hu (Provincial CDC); Zhixiang Hu, Jianjin Hu, Yijian Qian, Zhiying Wu, Chunmei Wang, and Lingli Chen (Tongxiang CDC). Hunan Regional Co-ordinating Centre—Wen Liu, Guangchun Li, and Huilin Liu (Provincial CDC); Xiangquan Long, Xin Xu, Youping Xiong, Zhongwen Tan, Xuqiu Xie, Yunfang Peng, and Weifang Jia (Liuyang CDC).
Contributors
ZC, RP, and LL had full access to the data. All authors were involved in study design, conduct, long-term follow-up, analysis of data, interpretation, or writing the report.
Declaration of interests
We declare no competing interests.
Contributor Information
Zhengming Chen, Email: zhengming.chen@ctsu.ox.ac.uk.
Liming Li, Email: lmlee@vip.163.com.
Supplementary Material
References
- 1.Yang G. Marketing ‘less harmful, low-tar’ cigarettes is a key strategy of the industry to counter tobacco control in China. Tob Control. 2014;23:167–172. doi: 10.1136/tobaccocontrol-2012-050691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peto R, Chen ZM, Boreham J. Tobacco—the growing epidemic. Nat Med. 1999;5:15–17. doi: 10.1038/4691. [DOI] [PubMed] [Google Scholar]
- 3.Peto R, Lopez AD, Boreham J, Thun M, Heath C., Jr Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet. 1992;339:1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]
- 4.Weng XZ, Hong ZG, Chen DY. Smoking prevalence in Chinese aged 15 and above. Chin Med J (Engl) 1987;100:886–892. [PubMed] [Google Scholar]
- 5.Yang G, Fan L, Tan J. Smoking in China: findings of the 1996 National Prevalence Survey. JAMA. 1999;282:1247–1253. doi: 10.1001/jama.282.13.1247. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Wang L, Li Y. Cross-sectional survey on smoking and smoking cessation behaviours among Chinese adults in 2010. Chin Prev Med. 2012;46:404–408. (in Chinese). [PubMed] [Google Scholar]
- 7.Han J, Chen X. A Meta-analysis of cigarette smoking prevalence among adolescents in China: 1981–2010. Int J Environ Res Public Health. 2015;12:4617–4630. doi: 10.3390/ijerph120504617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang G, Wang Y, Zeng Y. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norheim OF, Jha P, Admasu K. Avoiding 40% of the premature deaths in each country, 2010–30: review of national mortality trends to help quantify the UN Sustainable Development Goal for health. Lancet. 2015;385:239–252. doi: 10.1016/S0140-6736(14)61591-9. [DOI] [PubMed] [Google Scholar]
- 10.UN Population Devision . World population prospects: the 2012 Revision. United Nations; New York: 2012. [Google Scholar]
- 11.US Department of Health and Human Services . The health consequences of smoking: a report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, Office on Smoking and Health; Atlanta: 2004. [Google Scholar]
- 12.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirie K, Peto R, Reeves GK, Green J, Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133–141. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thun MJ, Carter BD, Feskanich D. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 16.Niu SR, Yang GH, Chen ZM. Emerging tobacco hazards in China: 2. Early mortality results from a prospective study. BMJ. 1998;317:1423–1424. doi: 10.1136/bmj.317.7170.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen ZM, Lee LM, Chen JS. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC) Int J Epidemiol. 2005;34:1243–1249. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 18.Chen ZM, Chen J, Collins R. China Kadoorie Biobank of 0·5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Li L, Smith M. Exhaled carbon monoxide and its associations with smoking, indoor household air pollution and chronic respiratory diseases among 512 000 Chinese adults. Int J Epidemiol. 2013;42:1464–1475. doi: 10.1093/ije/dyt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang GH, Stroup DF, Thacker SB. National public health surveillance in China: implications for public health in China and the United States. Biomed Environ Sci. 1997;10:1–13. [PubMed] [Google Scholar]
- 21.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 22.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 24.WHO . WHO Global Report: Mortality attributable to tobacco. World Health Organization; Geneva: 2012. [Google Scholar]
- 25.Yuan JM, Ross RK, Wang XL, Gao YT, Henderson BE, Yu MC. Morbidity and mortality in relation to cigarette smoking in Shanghai, China. A prospective male cohort study. JAMA. 1996;275:1646–1650. [PubMed] [Google Scholar]
- 26.Chen ZM, Xu Z, Collins R, Li WX, Peto R. Early health effects of the emerging tobacco epidemic in China. A 16-year prospective study. JAMA. 1997;278:1500–1504. doi: 10.1001/jama.278.18.1500. [DOI] [PubMed] [Google Scholar]
- 27.Lam TH, He Y, Li LS, He SF, Liang BQ. Mortality attributable to cigarette smoking in China. JAMA. 1997;278:1505–1508. [PubMed] [Google Scholar]
- 28.Liu BQ, Peto R, Chen ZM. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317:1411–1422. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu D, Kelly TN, Wu X. Mortality attributable to smoking in China. N Engl J Med. 2009;360:150–159. doi: 10.1056/NEJMsa0802902. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W, McLerran DF, Rolland BA. Burden of total and cause-specific mortality related to tobacco smoking among adults aged ≥45 years in Asia: a pooled analysis of 21 cohorts. PLoS Med. 2014;11:e1001631. doi: 10.1371/journal.pmed.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JB, Jiang Y, Wei WQ, Yang GH, Qiao YL, Boffetta P. Estimation of cancer incidence and mortality attributable to smoking in China. Cancer Causes Control. 2010;21:959–965. doi: 10.1007/s10552-010-9523-8. [DOI] [PubMed] [Google Scholar]
- 32.Huang XL. The China tobacco history. China Press; Beijing: 2006. [Google Scholar]
- 33.Lam TH, Ho SY, Hedley AJ, Mak KH, Peto R. Mortality and smoking in Hong Kong: case-control study of all adult deaths in 1998. BMJ. 2001;323:361. doi: 10.1136/bmj.323.7309.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinese Ministry of Health . China statistical yearbook of health. Publishing house of Peking Union Medical College; Beijing: 2011. [Google Scholar]
- 35.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med. 2014;370:60–68. doi: 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.