Abstract
Osseous metastasis from a primary breast cancer is common, and the skeletal related morbidity is high. However, solitary acral bony metastasis is rare and the diagnosis of these lesions poses a challenge to the physician. We describe a case of a patient treated with primary breast cancer who later presented with metastatic osteolytic bone lesions confined to a forefinger and toe.
Background
The majority of destructive lesions in the bone are metastatic.1 Almost 50% of primary breast malignancies develop bone metastases.2 Even though cutaneous acral metastases have been reported in the past,3 bony metastases to the hand and foot are rare, and account for <0.5% of cases.4 There are isolated reports of single metastasis and of multiple synchronous lesions.2 4 The unusual pattern and site of presentation lead to a diagnostic dilemma for the clinician. Often, the delay in diagnosis results in poor outcome of the disease.
This is a report of a patient treated with primary breast cancer who later presented with metastatic permeative osteolytic bone lesions confined to a forefinger and toe. Our objective is to highlight the existence of uncommon patterns of metastasis in a treated case of breast malignancy and to document a probable change in the pattern of metastasis following tyrosine kinase inhibitor (TKI) (lapatinib) treatment.
Case presentation
A 40-year-old south Indian premenopausal, multiparous woman with no medical comorbidities was diagnosed as having locally advanced right-sided breast cancer in June 2009. Her biopsy was reported as intraductal carcinoma and she was staged as cT4bN1M0, group stage IIIB (American Joint Committee on Cancer, AJCC 7th edition). She received four cycles of neoadjuvant chemotherapy using adriamycin and cyclophosphamide, followed by modified radical mastectomy. The postoperative histopathology was infiltrating duct carcinoma—modified Bloom-Richardson (MBR) grade II, (3+2+2=7 score) and was restaged as ypT2N0Mx (AJCC 7th edition). The patient's immunohistochemistry study proved to be luminal B subtype (oestrogen receptor: diffuse strong nuclear positive; progesterone receptor: negative, Her2Neu: score 3, and Ki-67 index: 40–45%). She then received four cycles of adjuvant chemotherapy using paclitaxel, and adjuvant external beam radiotherapy to chest wall and supraclavicular fossa with a dose of 50 Gy in 20 fractions. Her entire treatment extended over 7 months and she was maintained on a hormonal agent (tamoxifen) during her subsequent follow-up.
Seventeen months post-treatment, the patient developed multiple bilateral pulmonary metastases, which were diagnosed radiologically, and a biopsy proved metastases from the primary. She was started on monoclonal antibodies using trastuzumab and also received six cycles of docetaxel, which she completed in 6 months. Complete radiological resolution of lung metastases was noticed and she was kept on follow-up with a change in her hormonal agent to letrozole (second line). She was also started on lapatinib, a dual TKI.
Six months later, the patient fractured her left forefinger (proximal phalanx) secondary to a local trauma. She reported severe pain during movement of the affected area, with associated swelling and redness of the forefinger (figure 1). A radiological examination of her left hand revealed a lytic expansile lesion with features of bone destruction in the proximal phalanx (figure 2). Considering a differential diagnosis of osteomyelitis, a fine needle aspiration cytology (FNAC) from the lesion was carried out, which showed features suggestive of metastasis from breast primary (figure 3). A bone scan performed at that point showed lytic lesions in the left index finger (figure 4). As the bone was destroyed beyond salvage, a ray amputation was suggested by our orthopaedics team. The patient’s unwillingness prompted us to offer her a non-invasive alternative treatment, in the form of palliative external beam radiotherapy. A palliative dose of 30 Gy in 10 fractions (5 fractions a week) to the painful lesion was delivered. Following this treatment, she had adequate pain relief. Considerable shrinkage in the soft tissue swelling of her finger was observed (figure 5) and the range of movement of the affected finger also improved, which reflected on her quality of life.
Figure 1.

Clinical photograph of metastasis to the proximal phalanx of the left hand.
Figure 2.
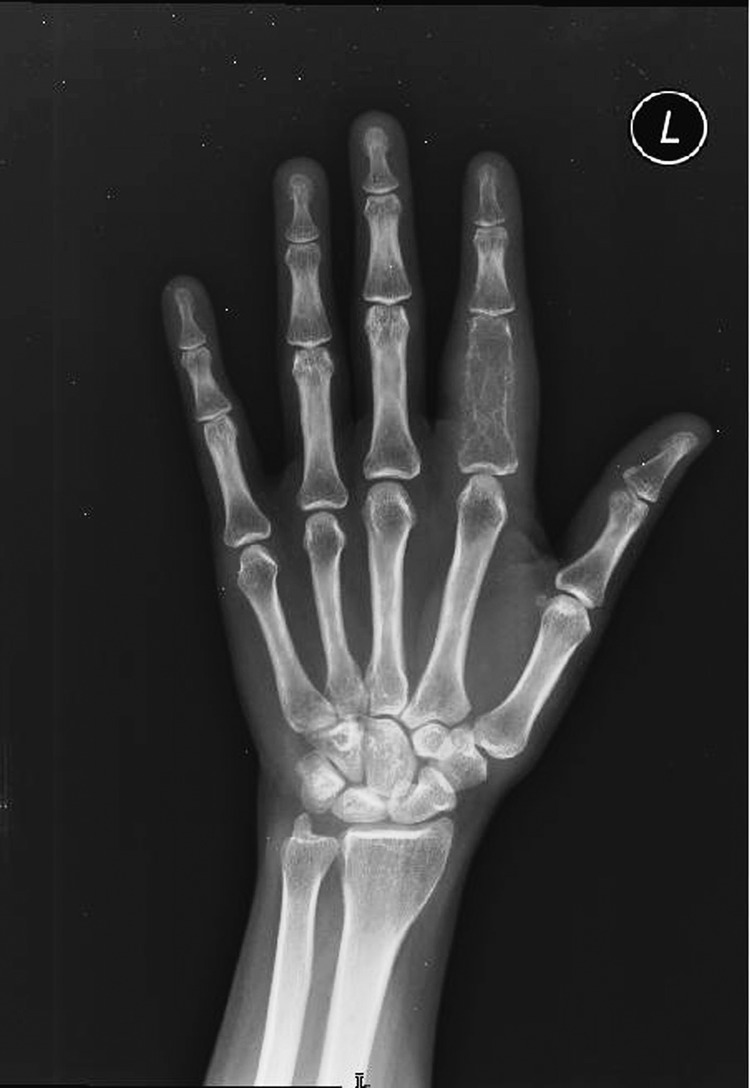
Radiographic image of the proximal phalanx of the left hand: destructive lesion confined to one joint space.
Figure 3.
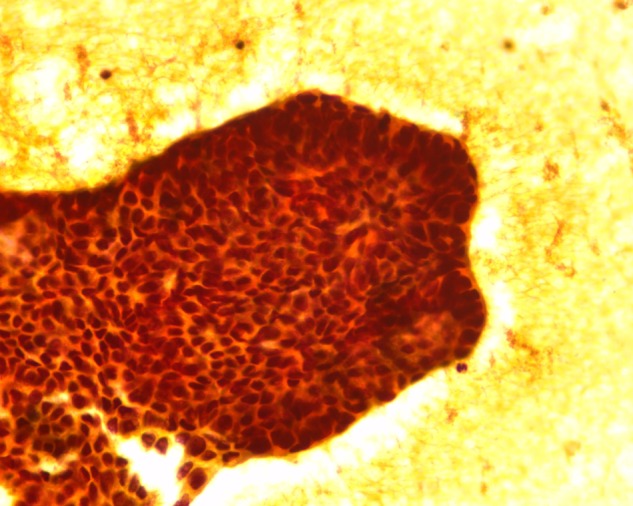
Fine needle aspiration cytology from the proximal phalanx of the left hand, showing features suggestive of metastasis (×40 view).
Figure 4.
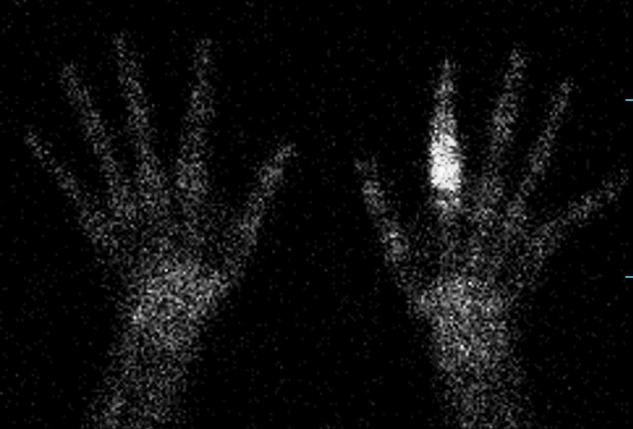
Whole body skeletal scintigraphy showing uptake in the lytic lesion of the proximal left phalanx.
Figure 5.
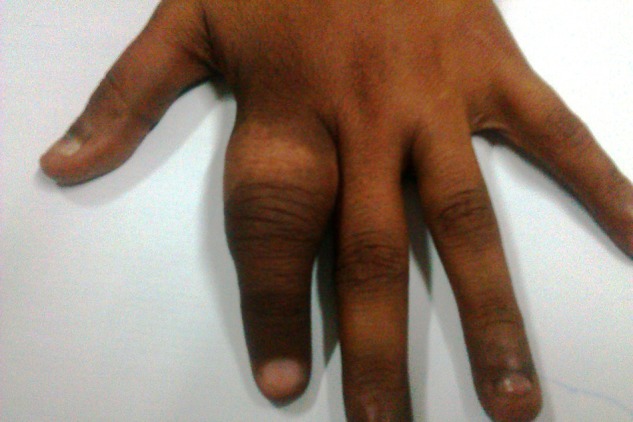
Clinical photograph of metastasis to the proximal phalanx of the left hand—post external beam radiotherapy.
Four months later, the patient had a trauma to her right toe, which later progressed to a non-healing ulcer. The progressive worsening of symptoms resulted in removal of the toenail of the affected toe, performed in a local clinic. This further resulted in a non-healing ulcer with frequent bleeding (figure 6). Retrospective evaluation revealed that the patient had been experiencing mild pain in the right big toe since her previous treatment, and also that there was a doubtful lytic lesion of the proximal phalanx, with no tracer uptake in the bone scan carried out at that time (figure 7). Finding no solution to her ailment, she was offered external beam radiation with a dose of 2500 cGy in five fractions, as a palliative measure. Good haemostasis and pain relief were achieved with this intervention. On subsequent visits, the wound showed resistance to healing, so the patient underwent ray amputation at the metacarpophalangeal level. The postoperative histopathology was suggestive of metastatic carcinoma from breast primary (figure 8). She was again started on hormonal agents and is on regular institutional follow-up.
Figure 6.
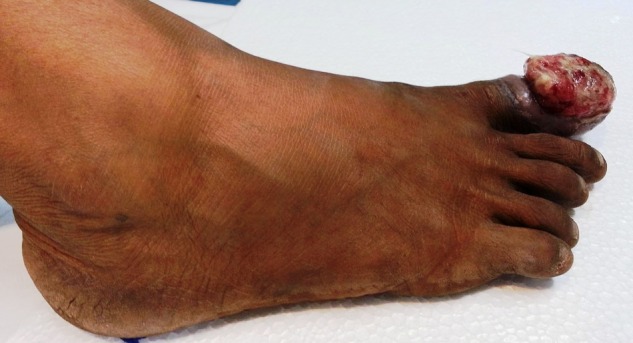
Clinical photograph of metastasis to the distal phalanx of the right toe with non-healing ulcer.
Figure 7.
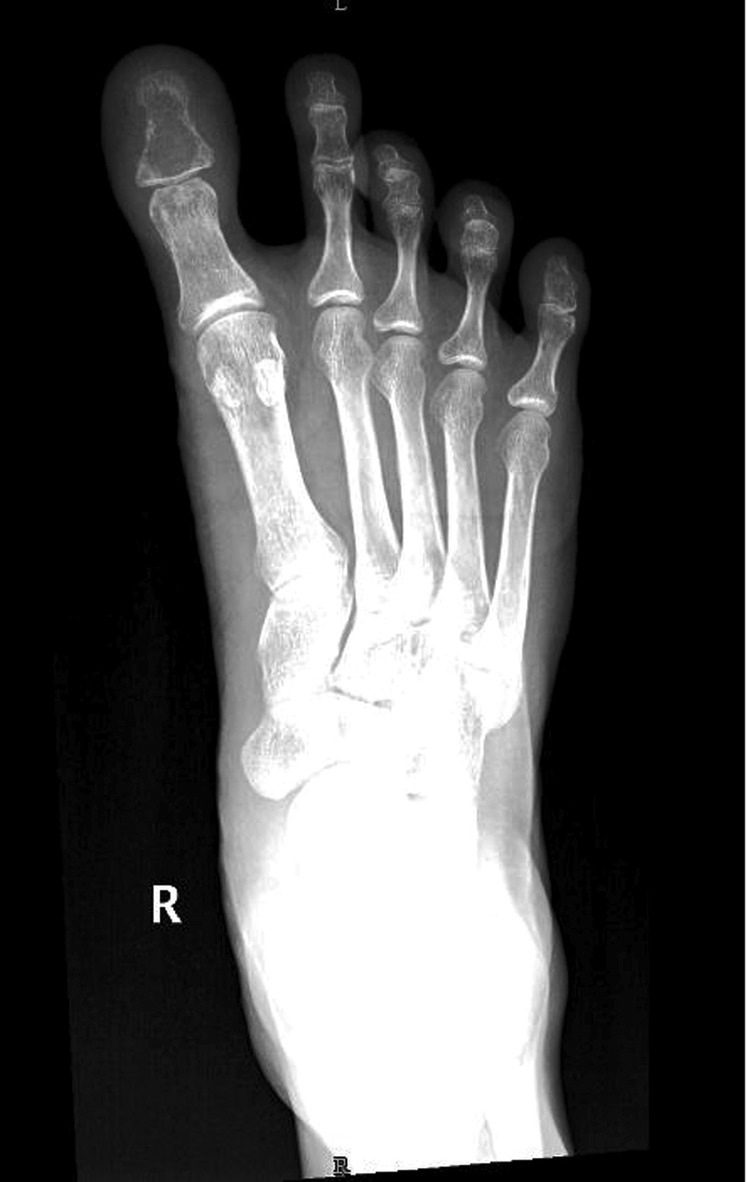
Radiographic image of metastasis to the distal phalanx of the right toe, lytic lesion being noted.
Figure 8.
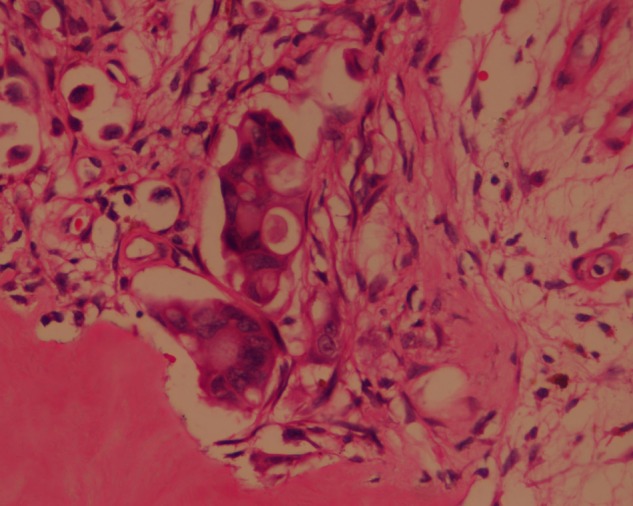
Postoperative histopathology of the right toe, showing high nuclear:cytoplasmic (N:C) ratio and hyperchromatic nuclei, suggestive of metastasis.
Investigations
Differential diagnosis
Osteomyelitis
Infection of nail bed
Benign bone tumours
Outcome and follow-up
The patient is alive on follow-up. At the time of writing this manuscript, she was reported to have symptomatic multiple brain metastasis, for which she received palliative whole brain radiotherapy; she is on best supportive care.
Discussion
Bony metastases stand third (after lung and liver) among common sites of metastases and the incidence range from 6% to 85%.1 Malignancies arising from the lungs, breast, prostate, thyroid and kidneys, account for about 80% of this osseous metastasis.1 5 6 Vertebrae are the commonest, followed by bones of the pelvis, ribs, skull and humerus.1 7 Metastases below the elbow and knee are rare with an incidence of 7%, and mainly originate from primaries of breast and lung carcinoma.1 However, metastatic tumours to the hand and wrist are rare, accounting for approximately 0.1% of all metastatic lesions to the skeleton, and metastasis to the foot and ankle is roughly 1%.1 Metastases to distal phalanges of the toes are extremely rare, accounting for <0.05% of all bone metastases.5 Isolated metastases confined only to the finger and toe synchronously at presentation are very rare.8 Libson et al5 revealed that hand involvement is double that of metastasis to the foot.9
Acral metastases are nearly twice as common in men as compared with women. Median age of presentation is 58 years. Most metastases to the hand originated from supradiaphragmatic structures such as primaries from the lungs, breast, head and neck, while those in the foot were from subdiaphragmatic regions such as the gastrointestinal and genitourinary organs.2 5 9 10 Previous studies have shown that the lesions were more in the dominant hand, accounting for the increased lesions in the right hand. The commonly affected digit is the third finger followed by lesions in the thumb. Of the digits, the distal phalanx is the most affected.2 Usually, these are solitary lesions.9 In lesions of the foot, the tarsal bones are mostly affected, followed by the calcaneus and cuneiforms.2 5 9 11
Acral metastases often present as non-arthritic, painful, red, swollen joints and lesions not crossing the joint space.12 History of trauma mostly heralds these lesions. Commonly reported symptoms include joint pain, progressive swelling of the affected joint, non-healing ulcer, and erythema with local tenderness and warmth.2 6 12 However, for a subset of patients, pain precedes the lesion, which might be evident in routine radiographic images.12 13 Diagnosis of metastasis from known or occult primary malignancy is often overlooked, as these lesions mimic several non-metastatic conditions such as local infection of the nail bed, felon, gout, thromboembolic phenomena, thrombi, osteomyelitis, rheumatoid arthritis and, rarely, tuberculosis, and also benign bone tumours such as enchondroma.2 4 6 9 10 12
Radiographic imaging, supported by radionuclide skeletal scintigraphy and a biochemical assay of serum alkaline phosphatase complement the diagnosis. However, a tissue diagnosis is mandatory and confirmatory.12 14 15 Radiological characteristics of metastatic acral bony lesions include demineralisation of bone, which may progress to partial or complete destruction of the involved bone. Absence of inflammatory changes, for instance, periosteal reaction and reactive bone changes, and the fact that lesions are confined to one bony joint space, with uninvolved adjacent cartilages in spite of extensive destruction of bones, are also characteristic.12 14
Metastatic bone lesions can be lytic, blastic or mixed. Purely lytic lesions point to primaries in the lungs, uterus, thyroid, kidneys and adrenals, and to melanoma or gastrointestinal cancers. Blastic lesions are secondary to prostate and bladder cancers, medulloblastoma and bronchial carcinoid tumours. Mixed lesions are typical of secondaries from breast, ovary, testis, cervix or lymphatic tissues.1 14 Seventy per cent of skeletal metastases are detected radiographically and lytic lesions are seen in more than 80% of the scans.1 12 14
Usually, bone involvement is secondary to haematogenous spread. For a metastatic nidus to develop, it requires red marrow. Abundant red marrow in cancellous bones, such as vertebrae, accounts for increased vertebral involvement in bony metastases.1 Less red bone marrow in the bones of the fingers and toes makes them less conducive to skeletal metastases.1 6 12 16 The pathophysiology leading to acral metastases is less clear, though several hypotheses have been put forth.2
The first hypothesis is that physical trauma leads to increased blood flow to the area, which may result in a metastatic nidus at this site, explaining the reason for more lesions in the dominant hand.2 According to Joll’s concept of trauma-induced acrometastases, repetitive trauma can degrade the resistance of surrounding tissues, and the presence of chemotactic factors such as prostaglandins, released after a trauma, may be responsible for cell migration and help tumour emboli to settle in skeletal tissues.2 6 17
A second theory highlights the ‘osteotrophic’ property of certain tumours to metastasise to areas in the bone marrow with low blood flow (0.4 ml/min/g).1 2 It is also noted that visceral tumours other than primary lung lesions are less likely to have acrometastases as their primary emboli, and usually only reach the systemic arterial circulation after they have passed through the hepatic or pulmonary capillary bed.2 A third possibility is that the tumour cells first drain through the lymphatics and then into the venous system, from which they may enter systemic circulation and spread distally.12 18
Studies have also shown that TKIs might initiate stromal and microenvironmental defence mechanisms, which contribute to eventual loss of drug activity with an increased ability to metastasise.19 20 In the index case, the change in the pattern of the metastasis could be attributed to TKI, in this case lapatinib.
Acrometastases, sadly, portend a poor prognosis and shorter survival.2 4 10 12 The priorities hence are mainly palliative. The performance status of the patient, and the site of the lesion and site of primary malignancy, determine the treatment approach. Surgical interventions such as ray amputation of the affected digit, conservative modes, such as immobilisation and usage of braces, and systemic chemotherapy, have all been tried.2 4 6 9 12 14 A limb-saving approach, as in our case, using palliative external beam radiotherapy with doses of 20–30 Gy divided in 5–10 fractions (5 fractions a week), is a viable option to alleviate pain and preserve mobility of the affected limb. It would, in addition, aid in sclerosis, new bone formation and, at times, complete resolution of lytic lesions, in a small percentage of patients.2 6 14 Earlier studies have opined that patients treated with surgery have better survival compared to those treated with radiotherapy.
Recent advances in cancer treatment bolstered by newer targeted agents are likely to change the traditional concepts of tumour behaviour, and lead to weird patterns of metastases, including acrometastases. The index case may be one such rare manifestation after TKI treatment. Yet, larger clinical data are needed to obtain a vivid picture. Acrometastasis, though a rare presentation, needs to be considered as a differential diagnosis. A high quotient of suspicion would go a long way, and prompt accurate diagnosis and management in this age of advances in cancer therapy would help in improving survival and palliation of symptoms to achieve a better quality of life
Learning points.
In a patient with a known history of breast cancer, if clinically suspicious, osseous metastasis that is confined only to the acral bones should be considered in the differential diagnosis.
The wide use of biological targeted therapy in the present era could lead to a changing pattern of unusual metastases such as that highlighted in this case.
Acknowledgments
The authors would like to acknowledge Dr Arun Lal, Assistant Professor, Department of Radiation Oncology, Amrita Institute of Medical Sciences, whose assistance was instrumental in compiling this manuscript.
Footnotes
Contributors: CGP helped in compiling and reviewing the manuscript. BCG assisted in data recovery and image editing. MD helped in proof reading this article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kundu S, Shankar S, Mitra S et al. Below-elbow and below-knee metastases in breast cancer—a case report. Indian J Med Paediatr Oncol 2007;28. [Google Scholar]
- 2.Flynn CJ, Danjoux C, Wong J et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol 2008;15:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho EY, Kim TH, Park SD et al. Acral metastasis in a patient with ampullary carcinoma. Korean J Intern Med 2007;22:55–8. 10.3904/kjim.2007.22.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janne PA, Datta MW, Johnson BE. Lung cancer presenting with solitary bone metastases. Case 2: acrometastasis as an initial presentation of non-small-cell lung carcinoma. J Clin Oncol 1999;17:2998–3001. [DOI] [PubMed] [Google Scholar]
- 5.Libson E, Bloom RA, Husband JE et al. Metastatic tumors of bones of the hand and foot: a comparative review and report of 43 additional cases. Skeletal Radiol 1987;16:387–92. 10.1007/BF00350965 [DOI] [PubMed] [Google Scholar]
- 6.Weidmann CE, Ganz PA. Multiple synchronous lesions of acral metastasis. West J Med 1984;140:451–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Letanche G, Dumontet C, Envrard P et al. Distal metastases of bronchial cancers; bone and soft tissue metastases. Pubmed 1990;77:1025–30. [PubMed] [Google Scholar]
- 8.Biyi A, Oufroukhi Y, Doudouh A [Hand and foot acrometastasis secondary to breast carcinoma]. Chir Main 2010;29:40–3. 10.1016/j.main.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 9.Robert M. Solitary lateral cuneiform bladder carcinoma metastasis. Ch5. http://www.podiatryinstitute.com/pdfs/Update_2003/2003_05.pdf.
- 10.Perdonà S, Autorino R, Gallo L et al. Renal cell carcinoma with solitary toe metastasis. Int J Urol 2005;12:401–4. 10.1111/j.1442-2042.2005.01060.x [DOI] [PubMed] [Google Scholar]
- 11.Zindrick MR, Young MP, Daley RJ et al. Metastatic tumors of the foot: case report and literature review. Clin Orthop Relat Res 1982;(170):219–25. 10.1097/00003086-198210000-00029 [DOI] [PubMed] [Google Scholar]
- 12.Mir NA, Baba AN, Ahmad SM et al. Big toe metastases as first clinical sign of occult bronchogenic carcinoma. J Coll Physicians Surg Pak 2010;20:699–700. 10.2010/JCPSP.699700 [DOI] [PubMed] [Google Scholar]
- 13.Kaplansky DB, Kademian ME, VanCourt RB. Metastatic squamous cell carcinoma resembling cellulitis and osteomyelitis of the fifth toe. J Foot Ankle Surg 2006;45:182–4. 10.1053/j.jfas.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 14.Jacofsky DJ, Frassica DA, Frassica FJ. Metastatic disease to bone. Hosp Physician 2004;40:21–8. [Google Scholar]
- 15.Amiot RA, Wilson SE, Reznicek MJ et al. Endometrial carcinoma metastasis to the distal phalanx of the hallux: a case report. J Foot Ankle Surg 2005;44:462–5. 10.1053/j.jfas.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 16.Wu KK. Bronchgenic carcinoma with metastasis to the foot: a report of two cases. J Foot Ankle Surg 1995;34:322–6. 10.1016/S1067-2516(09)80068-9 [DOI] [PubMed] [Google Scholar]
- 17.Joll CA. Metastatic tumors of bone. Br J Surg 1923;11:38–72. 10.1002/bjs.1800114105 [DOI] [Google Scholar]
- 18.Ozdemir HM, Yildiz Y, Yilmaz C et al. Tumors of the foot and ankle: analysis of 196 cases. J Foot Ankle Surg 1997;36:403–8. 10.1016/S1067-2516(97)80089-0 [DOI] [PubMed] [Google Scholar]
- 19.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008;8:592–603. 10.1038/nrc2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sendur MA, Aksoy S, Ozdemir NY et al. Tyrosine kinase inhibitors may change the metastatic pattern. J BUON 2013;18:544–50. [PubMed] [Google Scholar]


