Abstract
The water content of the epidermis is a main factor in maintaining skin smoothness and elasticity and preventing skin dryness. Occlusive products can greatly affect skin hydration by forming a barrier on the skin following the topical administration of oil-based formulations. These products repair the skin barrier by restoring the skin lipids as well. Solid lipid nanoparticles (SLNs) have recently been introduced as a novel carrier with several benefits in pharmaceutics and cosmeceutics. It has been suggested that SLNs may have an occlusive effect following topical application. In this study, the occlusion effects of lipidic particles in different size ranges were investigated in vitro, ex vivo, and in vivo, and the results were compared with the positive (vaseline) and negative (blank) controls. Although larger lipidic particles showed better occlusion properties than nanoparticles in vitro, but ex vivo experiments confirmed the benefits of nanoparticles (almost 30% higher occlusion factor for particles in the range of 170 nm than ones in the range of 600 and 1800 nm). The superiority of SLN formulation to Vaseline as a positive reference was confirmed by the in vivo study. SLN formulation resulted in much thicker stratum corneum than Vaseline. It was indicated that in vitro and ex vivo study methods may not be a good reflective of the in vivo method for determining the occlusive properties of nanoparticulate systems. It was concluded that formulations containing SLNs can be used as efficient skin moisturizer products.
Keywords: Solid lipid nanoparticles, Topical occlusive, Cosmeceutics, Skin moisturizer
INTRODUCTION
Dry skin, known as winter xerosis, is characterized by a significant change in the appearance and surface condition of the stratum corneum (SC) (1). Most cases occur in winter when the cold air outside and the hot air inside cause a low relative humidity. Moreover, too frequent bathing or handwashing, vitamins A and D deficiencies, systemic disease, severe sunburn, or some medicines may cause dry skin, which can be treated by administering emollients and moisturizers. Occlusive moisturizers prevent evaporative water loss to the environment by placing an oily substance on the skin surface through which water cannot penetrate, thus replenishing the SC moisture by water moving from the lower viable epidermal and dermal layers (2). It was also proposed that the penetration of energetic compounds to the skin depends on skin hydration which can be influenced by occlusive compounds (3). If the water content of SC (10-20%) decreases, the protective layer of the skin starts to become weakened and cracked. In this case, topical occlusives have to be administered to prevent water loss of the SC. Several suggested topicals with outstanding occlusive properties such as petrolatum, fats, fatty acids, silicon oil, and poly acrylate foils cause an unfavorable cosmetic or visual appearance (4). Vaseline has been proposed as the most effective treatment for dryness among the various occlusives. However, it is a thick waxy material which makes it difficult to handle and inconvenient for general use, especially over large areas of the body (5). Therefore, a remarkable demand for the invention of novel occlusive formulations exists. Solid lipid nanoparticles (SLNs) have recently been introduced as novel carriers in pharmaceutics and cosmeceutics.
SLNs have several benefits, such as the controlled release of incorporated active ingredients and the protection of unstable drugs against chemical degradation (3). It was proposed that SLNs be applied in different ways, including oral (6), ocular (7), buccal (8), pulmonary (9,10), and dermal (11) routes. It has been suggested that SLNs can prevent epidermal water loss by preparing a hydrophobic monolayer film when administered topically for the delivery of pharmaceuticals and active ingredients in cosmetics (4). The film preparation of SLNs on filters was shown in vitro, which caused a reduction in water evaporation through these filters (12).
The film formation properties of SLNs can be attributed to their small size and vast specific surface area, thus making adhesive properties which finally lead to the high penetration of compounds into the skin layers. In spite of various reported investigations regarding the advantages of incorporating active cosmetic and pharmaceutical ingredients into SLNs, there are only a few reports on the occlusion effects of SLNs and their benefits for therapeutic applications. It seems that the size of the lipidic particles plays the key role in the occlusive effect of moisturizers. However, as our literature review showed, there is no report on determining the suitable size for effective occlusion properties. Therefore, the aim of this study was to investigate the occlusion effects of lipidic particles in different sizes in vitro, ex vivo, and in vivo to compare the occlusive properties of SLNs with those of solid lipid microparticles.
MATERIALS AND METHODS
Materials
Glycerylpalmtostearate (Precirol ATO-5®) was obtained from Gattefossé, (Saint-Priest, Cedex, France). Poloxamer 407 was supplied by Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Tween 20® BDH (UK) was used as surfactant. Vaseline (Sepidaj, Iran), Formalin (Merck, Germany), and diethyl ether (KianKaveh Pharmaceutical and Chemical Co., Iran) were used as received.
Preparation of solid lipid particles
SLNs were prepared using the hot melt homogenization method. First, the lipids (Vaseline or Precirol) were melted at about 80 °C, and then Tween 20 as an oil phase surfactant solution was added to the mixture. An aqueous phase was prepared by dissolving Poloxamer 407 in distilled water and heating it to the same temperature as the oil phase. Keeping the temperature at 80 °C, the aqueous phase was dropped into the oil phase by a syringe, and homogenization was carried out (at various stirring speeds for each formulation) using a Silent Crusher M homogenizer (Heidolph, Germany). The final formulation volume was adjusted to 50 ml. Table 1 shows the details of the formulations and production parameters. Poloxamer 407 was used in identical concentrations (2.5% w/v) for all formulations. Formulations F8, F9, and F10 were used for the occlusion effect in subsequent experiments.
Table 1.
Composition and preparation conditions of solid lipid particles and resulted size characteristics (data are shown as mean ± SD, n = 3).
Particle size assessment
The particle size of the SLNs was determined by laser diffraction using a Wing SALD 2101 which provided information about the mean diameter of the bulk population based on volume mean diameter (VMD). The width of the distribution was expressed by span value ratings calculated from the following equation:

where, Dv 10%, Dv 50%, and Dv 90% indicate that 10%, 50%, and 90% of the particles were smaller than the given volume. Prior to being measured, SLNs samples were diluted with double-distilled water.
Zeta potential measurement
Zeta potentials of the IPCs lipidic particles were measured by Zetasizer (Zetasizer-ZS, Malvern Instrument, Malvern, UK). Each sample was measured in duplicate.
Size stability of lipidic particles
Three samples of each formulation (F8, F9, and F10) were stored at room temperature for a period of 90 days. The average size of samples was determined after 45 and 90 days of storage by particle size analyzer.
In vitro occlusion test
The in vitro occlusion test was adapted from the method of Wissing and coworkers (4). Plastic beakers (30 g) were filled with distilled water (25 ml), covered with metal mesh (steel, cut-off size of 50 μm), and sealed carefully with Teflon tape and parafilm. Each selected formulation (350 mg) and Vaseline as a positive control were spread regularly with a spatula on the filter surface in such a way that a visible thin film formation on the top of the metal filter was identifiable throughout the test. The samples were kept at 32 °C (skin temperature) for 72 h in an incubator (Inkubator 1000, Heidoph, Germany). Plastic beakers coated with filter but without an applied sample were provided as blank reference values. The samples were weighed after 6, 12, 24, and 72 h in order to measure water loss as a result of evaporation through the filter. Each experiment was carried out six times (n=6).
Ex vivo occlusion test
Male Wistar rats (170–220 g; Pasteur Institute of Iran, Tehran) housed in the animal facility of the Drug Applied Research Center, Tabriz Medical Science University were used for the ex vivo occlusion experiments. The abdominal hair of the rats was shaved using an electric razor after the animals were sacrificed using excess ether anesthesia. The abdominal skin was surgically removed and kept in saline solution for 12 h before the experiment was begun. The hydrated skin sample was cut to the size of the container. Then the water drops on both sides of the skin were removed with paper towels. The skin was inserted in the fitted plastic container, and the rim of the container was sealed carefully with waterproof silicone grease (GREASIL 4000, CRC Industries, UK). The skin was accurately weighed. Then about 100 mg of each formulation was applied to the skin surface (F8, F9, and F10 formulations and Vaseline as a positive reference). A container coated with skin (without an applied sample) was provided as a blank reference (negative control). The samples were stored at 32 °C (skin temperature) for 24 h and were weighed at 2, 4, 6, 8, and 24 h to determine the amount of water loss resulting from evaporation through the treated skin. The occlusion factor for both in vitro and ex vivo tests was calculated according to the following equation:

where, B is the water loss of the skin treated with the formulations and A is the water loss of the skin without a sample (blank reference). An occlusion factor of zero indicated no occlusive effect compared with the reference, and 100 was the maximum occlusion factor.
In vivo occlusion test
Young male Wistar rats used for the in vivo experiments (200–250 g) were obtained from the Pasteur Institute of Iran, Tehran. The rats’ skin on both lateral sides (flanks) was shaved with electrical straight razors, and then 100 mg of the semisolid prepared formulations was applied. Formulation F8 was applied on the right side, and Vaseline was applied on the left. The upper sides of the skin samples near the sections treated with the formulations were selected as references. The experiment was carried out on six rats. After 24 h, the rats were humanely killed, and the skin (1 cm2 sized) was cut with a surgical blade. Samples were fixed in 10% phosphate buffer formalin for 48 h. Samples were then dehydrated in a descending series of ethyl alcohol, cleared in xylene, and embedded in paraffin. Paraffin sections were cut serially into slices of 5 μm thick on a rotary microtome (Leitz, 1512, Wetzlar, Germany), mounted on slides, stained with hematoxylin-eosin (H&E staining), and examined under a light microscope (Olympus 3H-2). The thickness of SC was measured using the Hund-Wetzlar Image Analyzer software (Helmut Hund Gmbh, Wetzlar, Germany).
Statistical analysis
All results are expressed as mean ± standard deviation (SD). An independent Student's t-test was used to compare the mean differences between two independent groups and a one-way analysis of variance (one-way ANOVA) for multiple comparisons. When the differences between the means were significant, post hoc pair wise comparisons were carried out using Tukey multiple comparison tests (SPSS, version 13.0, Chicago, IL, USA). For all the statistical tests performed, the level of significance (P) was set at 0.05.
RESULTS
Preparation of solid lipid particles
Table 1 shows the VDM and span value data of the investigated formulations. Each sample was measured in triplicate. The small standard deviations (reported in Table 1) indicated the appropriate reproducibility of the preparation. The application of Vaseline in different amounts, even up to 40% (F1-F3), did not result in the preparation of solid lipid particles; it finally led to the liquid formulations. Higher lipid concentrations were not tested, because the aim of this study was to prepare an occlusion product with low lipid amounts. Changing the lipid type to Precirol at a concentration of 15% w/v resulted in a semisolid product (F4) with undesirable particle size and wide size distribution (Table 1). To decrease particle size, lower (Table 1). To decrease particle size, lower amounts of lipid were used (F5 and F6), as the literature suggested that decreasing the lipid concentration in the process of SLN preparation would lead to a reduction in SLN size (13). But lower lipid concentrations (7% and 8%) led to the formation of liquid formulations. Therefore, in the next step, the lipid concentration was increased to 10% (F7), which provided the desired semisolid formulation with a particle size of around 1 μm (Table 1).
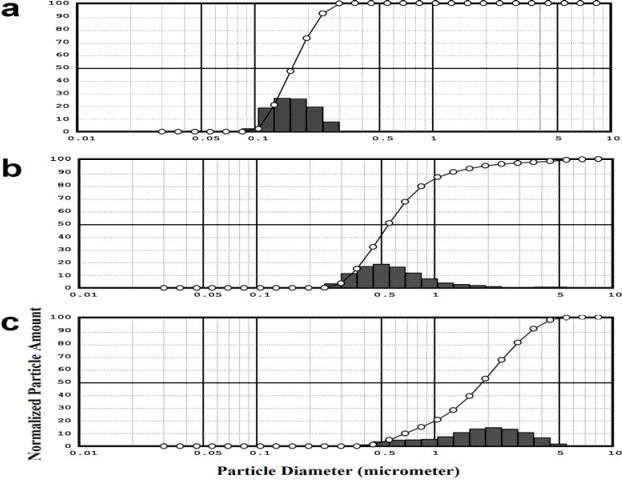
The amount of lipid was fixed at 10 % w/v, the least possible lipid concentration to prepare a semisolid formulation in accordance with the aim of study, which was to prepare an occlusion product containing the least amount of lipids with the aid of SLNs. To decrease the particle size, a higher amount of surfactant was used (F8) as the literature suggested that increasing the lipid concentration while preparing SLN would lead to a reduction in SLN size (13). The particle size of formulations was significantly reduced when the surfactant concentration increased five times from 0.25% to 1% v/v (Table 1). Furthermore, the size distribution pattern of formulation F8 was narrower as shown by the span value comparison of F7 and F8 (Table 1). The size pattern of F8 indicates that 90% of particles were between 100 nm and 200 nm (data was not shown). To prepare lipid particles larger than the particles obtained in F8 with the same composition, the stirring speed of the homogenizer was decreased to 12500 and 9000 rpm. Consequently, larger particle sizes, 0.624 ± 0.081 and 1.813 ± 0.203 μm were obtained for F9 and F10, respectively (Fig. 1).
Fig. 1.
The particle size distributions of a; F8, b; F9 and c; F10.
Stability studies
The stability of the SLNs (F8, F9, and F10) during three months of storage was determined by monitoring changes in particle size and distribution of particles (Table 2). No noticeable color change, agglomeration, or phase separation was detected. Table 2 indicates that the particle size and the size distribution (Span value) did not change significantly as evaluated by the statistical ANOVA method (P>0.05).
Table 2.
Size stability of formulations (data are shown as means ± SD, n=3).
Occlusion tests
In vitro occlusion study
The percentage of the occlusion factor (Fig. 2a) was investigated for each selected formulation (F8-F10), Vaseline as a positive reference, and blank as a negative control during a period of 72 h. The values of the occlusion factor for F8 showed no significant difference in comparison with F9 and F10 in the initial hours of the test, and Vaseline clearly represented the highest occlusion factor. The percentage of water loss of the Vaseline at the end of 72 h was found to be only 5.04%, whereas the percentage of evaporated water for F8, F9, and F10 was increased to 10.2%, 12.7%, and 12.1%, respectively. The negative control showed a fairly high water loss (63.7%). Notably, the occlusion factor of F10 reached to a higher occlusivity after 48 and 72 h, P=0.061 and 0.066, respectively, confirming no significant difference with Vaseline.
Fig. 2.
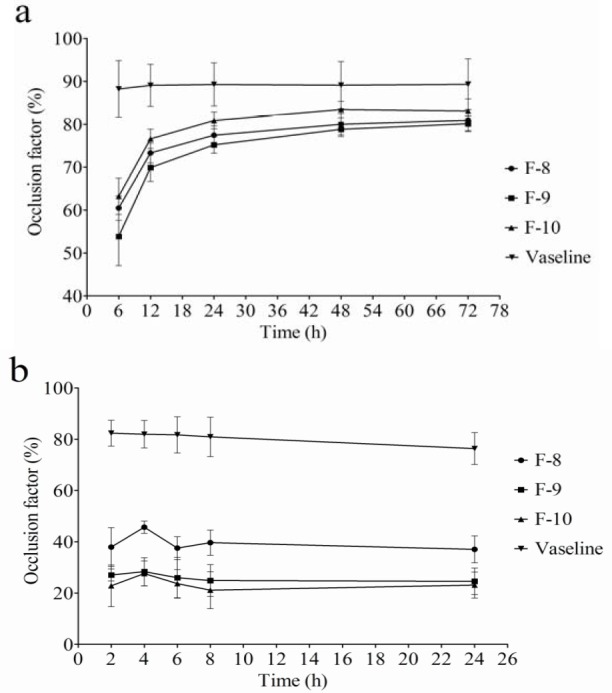
a; In vitro and b; ex vivo occlusion study results of F; occlusion factor vs. time profile following the application of solid lipid particles compared with Vaseline and the untreated sample; data are reported as mean ± SD (n = 6).
Ex vivo occlusion study
Fig. 2b illustrates the percentage of the resulted occlusion factor of the treated skin with the F8, F9, and F10 formulations, Vaseline as a positive reference, and untreated skin as a negative control within 24 h. Vaseline demonstrated the highest occlusion factor (P<0.0001), and its water loss was the least (only 14%) after 24 h. Formulations containing lipid particles showed good and acceptable occlusion properties (water evaporation between 37%-46%) in comparison with the untreated reference skin with 60% water evaporation. However, the amount of water loss for the F8 formulation was significantly lower (37%) than that of F9 and F10 (45% and 46%, respectively) after 24 h. This clearly indicated that smaller particles (F8, 170 nm) represented a higher occlusion effect. There was no statistically significant difference between F9 (particle size around 600 nm) and F10 (particle size around 1.8 μm) in the ex vivo occlusion study (P>0.05 in all time points).
In vivo study
The SLN formulation F8, which showed the highest occlusion factor among the solid lipid particle formulations F9 and F10, was selected for the in vivo study. Vaseline and untreated skin were used as positive and negative references, respectively. Fig. 3 illustrates the microscopic photomicrograph of SLN-treated skin (F8) (Figs. 3a-3d), untreated skin (as a reference for F8) (Fig. 3e), Vaseline treated skin (Figs. 2f and 2g), and untreated skin (as a reference for Vaseline) (Fig. 3h). The upper layer in the photomicrographs represents the top layer of the skin (i.e., SC). The thickness of the SC of the four mentioned groups, which was calculated from Fig. 3, is represented in Fig. 4. As shown in Fig. 4, the application of the SLNs resulted in a significant increase in the thickness of the SC as compared with its control group and Vaseline (P<0.0001). SLN formulation resulted in around three times thicker stratum corneum than Vaseline. Interestingly, although Vaseline showed statistically higher thickness of SC than its control (P=0.042), this difference was not considerable.
Fig. 3.
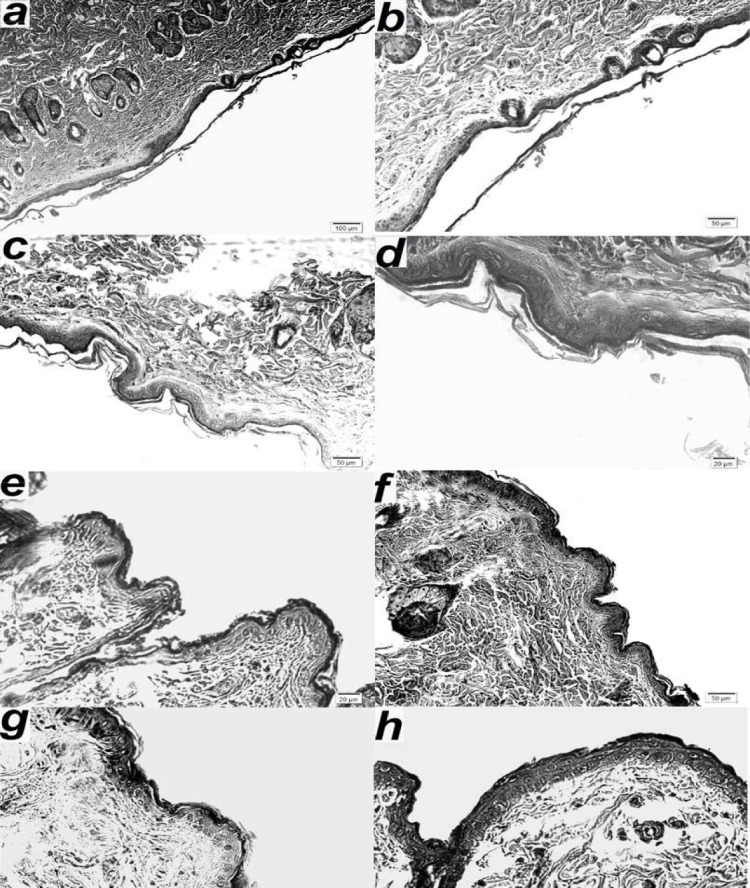
Microscopic photomicrograph of formulation containing solid lipid particles (F8)- a-d; treated skin, e; untreated skin (as a reference for F8), f and g; Vaseline treated skin, and h; untreated skin (as a reference for Vaseline).
Fig. 4.
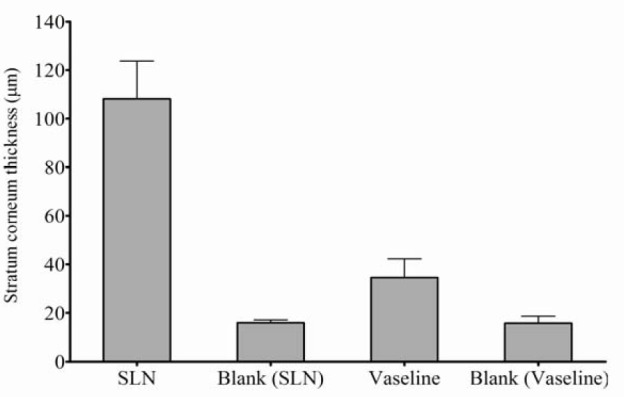
In vivo occlusion study results of formulation containing solid lipid particles (F8), untreated skin as a reference of F8 formulation, skin treated with Vaseline and untreated skin as a reference of Vaseline comparing the thickness of stratum corneum 24 h after application.
DISCUSSION
It was suggested that the occlusion factor depends strongly on particle size (14). Therefore, in this study, SLNs in three different particle sizes (170, 620, and 1800 nm) (Fig. 1) were prepared by developing formulations using different amounts of lipid or surfactant and different homogenizer stirring speeds as processing and experimental variables (Table 1).
In the hot homogenization technique, the lipid is melted at a temperature above its melting point, and preferably a surfactant is dissolved into this melted lipid. In the next step, the lipid phase is emulsified in a hot aqueous surfactant solution (preferably a polymeric surfactant such as PVA and poloxamer) by high-speed stirring. Generally, lower particle sizes are obtained at higher processing temperatures because of the lowered viscosity of the lipid phase (15). However, high temperatures enhance the degradation rate of lipids, which causes particle agglomeration and wide particle size distribution (16). On the other hand, decreasing the temperature of the lipid and aqueous phases during the preparation process results in larger particles by increasing the inner phase viscosity (15). Therefore, the temperature of both phases was adjusted to just above the lipid melting point in this study. Finally, the way the aqueous phase is added to the lipid phase affects particle size.
In the present study, the aqueous phase was dropped into the oil phase by a syringe (27G) which seemed to be effective for increasing homogenization efficiency and decreasing particle size. The prepared SLNs showed at least three months size stability (Table 2). The combination of surfactants (Tween and Poloxamer) at high concentrations (2.5%) may justify the suitable storage stability of SLNs. It was suggested that the high surfactant concentrations on the surface stabilized the SLNs (17). F10 formulation exhibited better occlusion result almost the same as Vaseline in the in vitro occlusion studies. This favorable property of the F10 formulation (which had larger particles) may be attributed to the effective blocking of filter pores. The occlusion factor value of Vaseline followed a stable pattern and remained constant during 72 h. There was, however, a progressive increase in the occlusion factor value for solid lipid particles (all three formulations, F8-10) up to 72 h, and particularly up to 24 h. This could be attributed to the gradual packing of the particles, which led to a continuous increase of barrier formation by lipid particles. In addition, the gradual evaporation of the water composition of solid lipid formulations during the study may lead to a regular increase in lipid concentration and consequently the formation of a thicker barrier and better occlusivity. On the other hand, ex vivo findings were in contrast with the in vitro study results.
The formulation with smaller particles (F8) showed better occlusion in the ex vivo study, while the formulation with larger particles (F10) caused higher occlusion in the in vitro study. In addition, all formulations (F8-F10) showed lower occlusivity than Vaseline in the ex vivo study compared with the in vitro study. Therefore, it can be claimed that the in vitro method which was applied for the occlusion evaluation of various formulations in previous studies (4) may not be accurate enough to predict in vivo outcomes. Such an observation in the ex vivo test may indicate that, except for the occlusive properties of particles, there is a different mechanism for reducing water evaporation through treated skin. For example, the SLNs might demonstrate better penetrability into upper skin layers which could block the pores of skin layers and lead to more effective occlusivity as compared with larger solid lipid particles.
Although Vaseline still was superior to the F8 formulation (an occlusion factor almost two times higher), the SLN formulation (F8) showed suitable occlusion (around 40%) in the ex vivo test. This finding is a brilliant result for the F8 formulation, because it contains just 10% lipids in its formulation in comparison with Vaseline, which is totally greasy. The application of the SLN formulation (F8) showed significantly different results between the in vivo and ex vivo studies. The differences can be attributed to the fact that SLNs probably penetrate into skin pores and block them, which consequently prevents water evaporation from the skin. It was also suggested that small particles like SLN possess a high specific surface area and consequently adhesive properties (12).
This sticky and tight adhesion to the SC may improve occlusion properties as compared with Vaseline. At equal lipid content, reducing the particle size resulted in an increase in the particle number; therefore, the formed film became denser and consequently the occlusion factor increased (18). The zeta potential of prepared nanoparticles were measured and found that those are almost zero (0.0473 and -0.0675 mV in duplicate repetition). Therefore, the role of zeta potential in the prevention of epidermal water loss due to its possible charge interaction by skin compositions might be negligible. As a result, both ex vivo and in vitro study methods were not capable of simulating the in vivo state. Therefore, it can be suggested that in vitro and ex vivo studies are not sufficiently reflective of the in vivo conditions to be used as alternatives for determining the occlusive properties of nanoparticulate dosage forms.
CONCLUSION
In this study, the suitability of formulations containing SLNs as an efficient occlusive product was evaluated in vitro, ex vivo, and in vivo. A comparison of the occlusion factor estimated from different study methods showed that the in vitro and ex vivo studies may not be sufficiently appropriate to determine the occlusive properties of nanoparticulate containing dosage forms. Interestingly, the superiority of the SLN formulation to Vaseline as a positive reference was confirmed by the in vivo studies. This may have been caused by the fact that SLNs probably penetrate into skin pores and by blocking them prevents the SC water evaporation. The findings of this manuscript are assumed to be important and applicable in cosmeceutical industries because the formulation containing just 10% lipid resulted in better occlusion properties than the gold standard, Vaseline, which is a greasy vehicle.
ACKNOWLEDGMENTS
The authors declare that they have no conflict of interest. This paper was extracted from Pharm. D thesis no. 3634 that was submitted to the Faculty of Pharmacy of Tabriz University of Medical Sciences and financially supported by the grant no. 90/75 from the Drug Applied Research Center of the same university.
REFERENCES
- 1.De Rigal J, Losch M, Bazin R, Camus C, Sturelle C, Descamps V, et al. Near infrared spectroscopy: a new approach to the characterization of dry skin. J Soc Cosmet Chem. 1993;44:197. [Google Scholar]
- 2.Wilhelm KP, Cua AB, Maibach HI. Skin aging: effect on transepidermal water loss, stratum corneum hydration, skin surface pH, and casual sebum content. Arch Dermatol. 1991;127:1806–1809. doi: 10.1001/archderm.127.12.1806. [DOI] [PubMed] [Google Scholar]
- 3.Wissing S, Müller R. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int J Pharm. 2002;242:377–379. doi: 10.1016/s0378-5173(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 4.Wissing S, Lippacher A, Müller R. Investigations on the occlusive properties of solid lipid nanoparticles (SLN) J Cosmet Sci. 2001;52:313–324. [PubMed] [Google Scholar]
- 5.Buraczewska I, Berne B, Lindberg M, Törmä H, Lodén M. Changes in skin barrier function following long‐term treatment with moisturizers, a randomized controlled trial. Br J Dermatol. 2007;156:492–498. doi: 10.1111/j.1365-2133.2006.07685.x. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seyfoddin A, Shaw J, Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010;17:467–489. doi: 10.3109/10717544.2010.483257. [DOI] [PubMed] [Google Scholar]
- 8.Reddy PC, Chaitanya K, Rao YM. A review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methods. Daru. 2011;19:385–403. [PMC free article] [PubMed] [Google Scholar]
- 9.Dolatabadi JEN, Hamishehkar H, Eskandani M, Valizadeh H. Formulation, characterization and cytotoxicity studies of alendronate sodium-loaded solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2014;117:21–28. doi: 10.1016/j.colsurfb.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 10.Emami HM, Hamishehkar H, Varshosaz J. Formulation and optimization of solid lipid nanoparticle formulation for pulmonary delivery of budesonide using Taguchi and Box-Behnken design. Res Pharm Sci. 2014;10:17–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Hamishehkar H, Shokri J, Fallahi S, Jahangiri A, Ghanbarzadeh S, Kouhsoltani M. Histopathological evaluation of caffeine-loaded solid lipid nanoparticles in efficient treatment of cellulite. Drug Dev Ind Pharm. 2014 doi: 10.3109/03639045.2014.980426. in press. [DOI] [PubMed] [Google Scholar]
- 12.Jenning V, Gysler A, Schäfer-Korting M, Gohla SH. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 2000;49:211–218. doi: 10.1016/s0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 13.Castro GA, Coelho ALL, Oliveira CA, Mahecha GA, Oréfice RL, Ferreira LA. Formation of ion pairing as an alternative to improve encapsulation and stability and to reduce skin irritation of retinoic acid loaded in solid lipid nanoparticles. Int J Pharm. 2009;381:77–83. doi: 10.1016/j.ijpharm.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Lim S-J, Kim C-K. Formulation parameters determining the physicochemical characteristics of solid lipid nanoparticles loaded with all-trans retinoic acid. Int J Pharm. 2002;243:135–146. doi: 10.1016/s0378-5173(02)00269-7. [DOI] [PubMed] [Google Scholar]
- 15.Kullavadee KO, Uracha R, Smith SM. Effect of surfactant on characteristics of solid lipid nanoparticles (SLN) Adv Mat Res. 2012;364:313–316. [Google Scholar]
- 16.Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007;2:289–300. [PMC free article] [PubMed] [Google Scholar]
- 17.Pople PV, Singh KK. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS PharmSciTech. 2006;7:E63–E9. doi: 10.1208/pt070491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]