Abstract
Pulmonary fibrosis is a progressive disease of the lungs, which leads to death in human. It has been suggested that transforming growth factor beta 1 (TGF-β1) together with oxidative stress play a central role in the pathogenesis of the ailment. The objective of this study was to evaluate the possible curative effects of black radish, Raphanus sativus L. var niger (RSN) on bleomycine (BLM) -induced pulmonary fibrosis in a rat model. In this study, thirty-six male Wistar rats were divided into six groups, including: (I) positive (BLM) control group, (II) negative (normal saline) control group, (III) sham group (R. sativus extract 150 mg/kg), and (IV-VI) treatment groups. In order to induce pulmonary fibrosis, four groups were treated with a single dose of BLM sulfate (7.5 U/kg) through intratracheal instillation. Treatment groups (IV-VI) received RSN extract (75, 150, and 300 mg/kg) orally a week before and two weeks after the administration of BLM. At the end of the treatment course, blood and lung tissue samples were taken and the measurement of TGF-β1 and histopathological examination of the lung tissues performed. The results showed that RSN, at 300 mg/kg dose, could significantly decrease the serum level of TGF-β1 and severity of the histological lesions as compared to the positive control group. The results of the current study indicate that the components present in the extract can remarkably prevent the aggravation of pulmonary fibrosis via decreasing TGF-β1 level.
Keywords: Pulmonary fibrosis, Bleomycin, Raphanus sativus L. var niger, TGF-β1
INTRODUCTION
Pulmonary fibrosis is an irreversible, chronic, and progressive disease of the lungs interstitium characterized by inflammation and deposition of collagen in the alveolar septa. The etiology and pathogenesis of the disease have not been completely elucidated (1). Recent studies have focused on the role of inflammatory cytokines and superficial molecules of cells involving in pro-fibrotic/anti-fibrotic mechanisms (2,3,4). It is believed that poorly balanced immune response, including imbalance of cytokines with positive (e.g. TGFβ1) and negative (e.g. interferon-γ) fibrogenic effects, is a characteristic feature of human progressive lung fibrosis such as idiopathic pulmonary fibrosis (4). Epithelial damage, aggregation of inflammatory cells and generation of free radicals trigger the release of cytokines. Consequently, these events result in collagen deposition and changes in the lung structure (5,6). Human and animal model studies have shown that transforming growth factor beta 1 (TGF-β1) is one of the most important cytokines, playing a key role in the pathophysiology of the disease (7). Herpes virus infection and undesired effects of some chemical drugs such as bleomycin (BLM) and methotrexate are two important etiologic factors (8). Long-term inhalation of toxic fumes might be a further reason (9). BLM is an anti-neoplastic agent, which is used for chemotherapy of a number of human cancers particularly skin tumors (10). It is not clear, how BLM can induce pulmonary fibrosis; however it seems that, generation of free radicals and its consequent epithelial cells injury is the underlying mechanism (11). Unfortunately, based on the available evidences there is not a satisfactory remedy for the disease (12) and the existing therapies are usually futile and associated with considerable adverse side effects (13). Currently, the only definitive treatment is lung transplantation (14). Therefore, the investigation of novel and cost-effective cures is an attractive position. In the recent years, synthetic drugs have been widely replaced with herbal medicines and plant extracts, because of their little undesirable properties and extensive beneficial effects (15).
Raphanus sativus L. var niger (black radish) (RSN) is an annual, edible plant belonging to the Brassicaceae family (16) which has been used in the folk medicine of China and Turkey for the treatment of asthma, bronchitis and other chest complaints (17,18). In Iranian traditional medicine seeds of the plant ethno-pharmacologically have been used as a diuretic, carminative, anti-fever, antitussive and stomach tonic (19). In addition, it has been prescribed as an antidote and a remedy for urinary system disorders since antiquity (20). Black radish extract contains high concentration of vitamin C, tocopherol and polyphenols. Experimental studies demonstrated that, these constituents possess pharmacological properties mostly antioxidant (21,22,23). Another active component is quercetin, which has potential anti-proliferative effects (21,24,25).
Unique pharmacological properties of black radish extract motivated us to suppose that, it may be effective for the treatment of pulmonary fibrosis. Thus, the current study conducted to evaluate possible curative effects of hydro-alcoholic extract of RSN on the rat model of pulmonary fibrosis induced by BLM.
MATERIALS AND METHODS
Reagents and chemicals
BLM was purchased from Kakaya Ltd, China. Black radish (RSN), genuine plant materials were purchased from a local market and identified at the Faculty of Medicine, Urmia University, Iran. Voucher specimens of the plant under the herbarium reference number of 77207 were deposited in this institute.
The enzyme-linked immunosorbent assay (ELISA) kit for the measurement of TGF-β1 was obtained from BENDER MED, Austria (Rat TGF- β1 Platinum Elisa, Lot NO# 57659004).
Hydro-alcoholic extract of underground parts of black radish
After pharmacognostic verification of black radish, aerial parts of the plant were removed and underground parts (root) air-dried. For preparation of hydro-alcoholic extract, dried and finely powdered root of the plant (350 g) was wetted by 800 ml of ethanol:water (70:30) and perculated using extra volume of solvent for 48 h, then extracted at room temperature by maceration method (26). The solvent (ethanol/water) was removed using a rotary evaporator under vacuum at 50 °C. Semisolid extract with gelling nature was obtained and kept in the refrigerator. Adequate amounts of the dried extract was suspended in sterilized normal saline (vehicle) and administered to animals through gavages.
Animals
Pathogen-free, 7–8 weeks old mature male Wistar rats were obtained from Urmia University Laboratory Animal Center, Faculty of Veterinary Medicine, Urmia, Iran. Animals were maintained in a standard specific pathogen-free environment at a temperature of 23 ± 2 °C and controlled humidity (60 ± 10%), under a 12:12-h light–dark cycle. Rats were administered distilled water and a standard diet ad libitum. All rats were acclimatized to their new surroundings for 2 weeks prior to the experimental procedures. All the experiments involving laboratory animals were approved by the Urmia Animal Care and Use Committee and performed at the Animal Housing Department, Faculty of Veterinary Medicine.
Experimental protocols
Thirty-six rats weighing 190-220 g were randomly divided into the following six groups: (I) positive control group: received a single intratracheal dose of BLM solution at 7.5 U/kg BW; (II) negative control group: was given a single intratracheal dose of normal saline; (III) sham group was administered 150 mg/kg of R. sativus extract; (IV, V, and VI) treatment group: received 75, 150, and 300 mg/kg BW of plant extracts orally once a day a week before and for two weeks after administration of a single intratracheal dose of BLM (7.5 U/kg BW).
Negative control and sham groups were instilled intratracheally with normal saline at a volume of 2 ml/kg BW and orally treated with sterilized distilled water and extract 150 mg/kg BW, respectively. Rats in the positive control (BLM) group received sterilized distilled water, after intratracheal instillation of BLM solution.
The previously described procedure was utilized to induce pulmonary fibrosis (27). At the end of the treatment course, the animals were humanly euthanized and their blood and lung tissue specimens were obtained according to the previous method (3,4).
Histological examination
Lung tissues were fixed in 10% neutral buffered formalin. After processing, samples were embedded in paraffin and sections were cut in 5 μm thickness and then stained with Hematoxylin and Eosin for microscopic examination. Histopathological evaluation is a current gold standard for diagnosis and staging of pulmonary fibrosis. Therefore, fibroblasts (as the fibrosis marker) were counted in at least ten microscopic fields using a Graticule lens (OLYMPUS, America) in 400 × magnifications.
Measurement of serum TGFβ-1 level
Serum samples were prepared by centrifugation of the blood samples at 2500 rpm for 5 min at room temperature and preserved in a -70 °C refrigerator. Measurement of TGFβ-1 was performed according to the kit manual (Rat TGF-β1 Platinum Elisa, Lot NO# 57659004).
Statistical analysis
The data were entered into a database and analyzed by SigmaPlot software (version 12). One-way analysis of variance (ANOVA) followed by the Holm-Sidak was used. P values less than 0.05 was considered significant. The results were expressed as means ± standard error of means (SEM) for six rats per each experimental group.
RESULTS
TGF-β1 levels
Fourteen days after BLM instillation in the positive control group, TGF-β1 level was significantly (P<0.001) increased as compared with negative (11500 ± 274, pg/ml) or sham group (11000 ± 186, pg/ml). RSN could reduce TGF-β1 serum concentrations dose-dependently and differed significantly from the positive control group. However, 300 mg/kg of RSN had a more noticeable effect. No significant differences observed in the TGF-β1 serum concentrations between negative control and sham groups indicating that oral administration of RSN is safe (Fig. 1).
Fig. 1.

TGF-β1 content in serum specimens of the rats (n=6). Each value represents Mean ± SEM. Significant difference versus bleomycin group has been shown by ***P<0.001. Treat1; 75 mg/kg, Treat2; 150 mg/kg, Treat3; 300 mg/kg.
Histopathologic findings
Sections from the negative control and sham groups revealed a normal structure with no pathological changes under a light microscope. No thickening of the inter-alveolar septa was found in the alveolar septa of these groups (Fig. 2A).
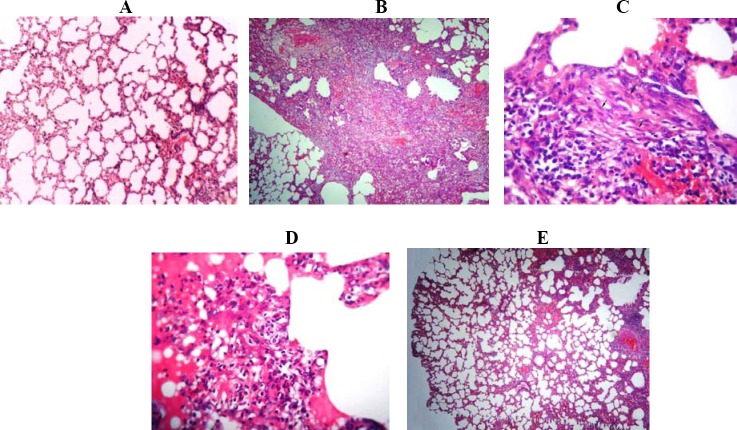
Fig. 2.
Photomicrographs of lung tissue sections of the rats in the study groups; the staining method is Hematoxylin and Eosin for all images: A; saline–water-treated rat with normal structure of lung tissue (× 40). Bleomycin treated rats: B; a vast area is affected (× 40), C; infiltration of inflammatory cells and proliferation of fibroblasts (arrows) (× 400) and D; leakage of proteinaceous fluid in the alveolar space (× 400). E; treatment group (300 mg/kg) (× 40).
Lungs of the positive control group were widely affected. Rats treated with BLM showed lymphocytic infiltration and proliferation of fibroblasts in the alveolar septa. Increased thickness of the inter-alveolar septa, accumulation of collagen and fibrosis were the other microscopic findings (Fig. 2B and 2C). In addition, there was a severe edema in the alveolar and interstitial space (Fig. 2D).
Treatment with the extract could help the recuperation of some pathologic changes induced by BLM. The numbers of fibroblasts and the severity of fibrosis were alleviated in RSN treated rats, in a dose-dependent manner (P<0.001, Fig. 3). In different treatment groups, the severity of changes varied from slight to moderate. However, 300 mg/kg of RSN had the best curative effect; the extensity of fibrosis and thickening of the alveolar septa were minor in comparison with the BLM group (Fig. 2E).
Fig. 3.
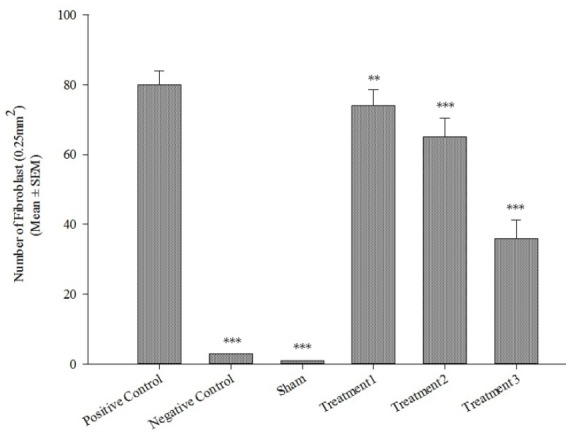
The effect of black radish extract on the quantity of fibroblasts (n=6). Each value represents Mean ± SEM. A significant difference versus the bleomycin group has been shown by ***P<0.001) and **P<0.01). Treat1; 75 mg/kg, Treat 2; 150 mg/kg, Treat3; 300 mg/kg.
DISCUSSION
The results of this study demonstrate that the constituents present in RSN hydro-alcoholic extract could relatively prevent lung pathological changes in the rat model of pulmonary fibrosis. Curative effects of the extract were verified by the decrease of fibroblasts number and TGF-β1 level (as an important fibrogenic factor) at the end of the treatment course.
The initial stage of pulmonary fibrosis is the migration and activation of inflammatory cells including macrophages, neutrophils, and lymphocytes. Inflammatory and epithelial cells release cytokines/growth factors such as (IL)-1, (IL)-6 and (IL)-8, TNF-α, TGF-β, insulin-like growth factor and platelet-derived growth factor, which lead to the proliferation of fibroblasts and final accumulation of collagen and fibrin, the end stage of the disease (28,29). In addition, accumulation of activated inflammatory cells in the site of lung injury leads to the generation of reactive oxygen species (ROS) and severe tissue damage (30).
The injury increases permeability of the pulmonary epithelium and endothelium, resulting in extravasation of plasma proteins and alveolar edema (31,32). This lesion was observed in the positive control group (Fig. 2D).
The prevention of pro-inflammatory cytokines release and ROS generation are attractive targets for the development of therapeutics for the treatment of lung fibrosis. Potential antioxidant properties of black radish extract have been shown previously (21). Moreover, the extract contains a large amount of quercitin, possessing potential anti-proliferative effect on several cell lines (33,34,35).
According to the critical role of the reactive oxygen species in the pathophysiology of pulmonary fibrosis, it has been suggested that black radish, a strong antioxidant, can effectively inhibit the progress of tissue damage through its antioxidant and free radical scavenging properties. The results of this study are in agreement with a previous report that black radish (due to the presence of polyphenols such as catechin, proto-catechuic acid, vitamin C and tocopherol) can neutralize oxidative stress and scavenge free radicals (21,36). Another possible mechanism refers to quercetin which can inhibit the proliferation of fibroblasts and induce apoptosis (37). It is likely that dysregulations in the balance of some growth factors play a major role in determining the differences between normal and pathologic tissue repair (3). Among these, TGF-β1 is one of the key cytokines involved in the pathogenesis of pulmonary fibrosis (38). It is well established that TGF-β1 promotes differentiation of fibroblasts into activated myofibroblasts which is an essential factor for the production of extra-cellular matrix (38,39). The BLM-induced pulmonary fibrosis in rodents is one of the most commonly used animal models to illustrate pathobiology and to identify new targets for medication (40). This model is also a good tool to assess efficacy of potential compounds in general as proof of principle, although there are some limitations (41).
In the current study, delivery of BLM via the intratracheal route resulted in notable histopathological changes in the lungs including thickening of alveolar wall, collapsing of alveolar spaces, infiltration of inflammatory cells and excessive collagen deposition. These findings were consistent with previous reports (3,40).
Our results showed that in positive control group, the concentrations of TGF-β1 were significantly increased in comparison with the negative control and sham groups. In fact, RSN particularly at the dose of 300 mg/kg could remarkably decrease concentrations of this key cytokine.
One of the most relevant findings in our study was the effect of RSN on the quantity of fibroblasts and collagen deposition. As mentioned before, pulmonary fibrosis is characterized by the infiltration of inflammatory cells, fibroblast proliferation and collagen deposition. The increased collagen in the lung has been associated with increased numbers of fibroblasts in the interstitium and increased collagen synthesis by these cells. Relative reduction in lung fibrosis and the numbers of fibroblasts were observed in the group treated with a low dose of 75 mg/kg RSN. The best curative effect was obtained with the highest dose of RSN (300 mg/kg), which significantly reduced collagen deposition and lung fibrosis. The histological findings of the current research certainly point out that RSN noticeably alleviates the extent and severity of the pathological changes in pulmonary fibrosis. These observations are confirmed by the reduction of the numbers of fibroblasts.
The results of this study showed that the beneficial effects of the hydro-alcoholic extract of RSN in rat pulmonary fibrosis is dose-dependent and the best curative effect was found to attain at the highest dose of the extract.
CONCLUSION
Taking together, the present results demonstrate that the administration of black radish hydro-alcoholic extract by oral route can ameliorate the BLM-induced pulmonary fibrosis in a dose dependent manner. This improvement is associated with the decrease of collagen deposition and the number of fibroblasts. It is concluded that black radish exerts its beneficial effects via modulation of TGF-β1, an important pro-inflammatory cytokine, which has the crucial role in the proliferation of fibroblasts and the stimulation of collagen synthesis. Therefore, RSN could be considered as a valuable novel agent for prophylaxis or treatment of the pulmonary fibrosis.
Investigation of the precise underlying mechanisms of such protective roles of black radish and the comparison of the extract efficacy with other chemical medicines of this model, as well as on other animal models of lung fibrosis, should be covered in further studies.
ACKNOWLEDGMENTS
The content of this paper is part of the data of DVM thesis of Mr. Mohammad Hossein Asghari (NO. 1229). We also would like to thank Mr. H. Jafari-Namin for his tremendous support.
REFERENCES
- 1.Vij R, Noth I. Peripheral blood biomarkers in idiopathic pulmonary fibrosis. Transl Res. 2012;159:218–227. doi: 10.1016/j.trsl.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larki-Harchegani A, Hemmati AA, Arzi A, Ghafurian-Boroojerdnia M, Shabib S, Zadkarami MR, et al. Evaluation of the effects of caffeic acid phenethyl ester (CAPE) on prostaglandin E2 and two key cytokines involve in bleomycin-induced pulmonary fibrosis. Iran J Basic Med Sci. 2013;16:850–857. [PMC free article] [PubMed] [Google Scholar]
- 4.Larki A, Hemmati AA, Arzi A, Borujerdnia MG, Esmaeilzadeh S, Zad Karami MR. Regulatory effect of caffeic acid phenethyl ester on type I collagen and interferon-gamma in bleomycin-induced pulmonary fibrosis in rat. Res Pharm Sci. 2013;8:243–252. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen F, Gong L, Zhang L, Wang H, Qi X, Wu X, et al. Short courses of low dose dexamethasone delay bleomycin induced lung fibrosis in rats. Eur J Pharmacol. 2006;536:287–295. doi: 10.1016/j.ejphar.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorax Soc. 2006;3:285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Vos C, Mitchev K, Pinelli ME, Derde MP, Boev R. Non-interventional study comparing treatment satisfaction in patients treated with antihistamines. Clin Drug Investig. 2008;28:221–230. doi: 10.2165/00044011-200828040-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kuwano K, Kunitake R, Maeyama T, Hagimoto N, Kawasaki M, Matsuba T, et al. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am J Physiol Lung Cell Molecular Physiol. 2001;280:L316–L325. doi: 10.1152/ajplung.2001.280.2.L316. [DOI] [PubMed] [Google Scholar]
- 9.Arab M, Mirzaei R, Karimi M, Mashhadi R. The study of histological effects of solder fumes in rat lungs. Iran J Basic Med Sci. 2010;13:63–68. [Google Scholar]
- 10.Adamson IYR, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974;77:185–198. [PMC free article] [PubMed] [Google Scholar]
- 11.Steinsvoll S, Halstensen TS, Schenck K. Extensive expression of TGF-beta1 in chronically-inflamed periodontal tissue. J Clin Periodontol. 1999;26:366–373. doi: 10.1034/j.1600-051x.1999.260606.x. [DOI] [PubMed] [Google Scholar]
- 12.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gogali A, Wells A. New pharmacologicalbstrategies for the treatment of pulmonary fibrosis. Ther Adv Respir Dis. 2010;4:353–366. doi: 10.1177/1753465810379454. [DOI] [PubMed] [Google Scholar]
- 14.Klingsberg RC, Mutsaers SE, Lasky JA. Curren tclinical trials for the treatment of idiopathic pulmonary fibrosis. Respirology. 2010;15:19–31. doi: 10.1111/j.1440-1843.2009.01672.x. [DOI] [PubMed] [Google Scholar]
- 15.Nazarizadeh A, Mikaili P, Moloudizargari M, Aghajanshakeri S, Javaherypour S. Therapeutic uses and pharmacological properties of plantago major l. and its active constituents. J Basic Appl Sci Res. 2013;3:212–221. [Google Scholar]
- 16.Castro-Torres I, Naranjo-Rodrıguez E, Domınguez-Ortız M, Gallegos-Estudillo J, Saavedra-Velez M. Antilithiasic and hypolipidaemic effects of Raphanus sativus L. var niger on mice fed with a lithogenic diet. J Biomed Biotechnol 2012. 2012:1–8. doi: 10.1155/2012/161205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duke JA, Ayensu ES. Medicinal plants of China. 2nd ed. Michigan: Algonac; 1985. [Google Scholar]
- 18.Sargın SA, Akçicek E, Selvi S. An ethnobotanical study of medicinal plant sused by the local people of Alaşehir (Manisa) inTurkey. J Ethnopharmacol. 2013;150:860–874. doi: 10.1016/j.jep.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 19.Afsharypuor S, Hoseiny-Balam M. Volatile constituents of Raphanus sativus L. var. niger seeds. J Essent Oil Res. 2005;17:440–441. [Google Scholar]
- 20.Salah-Abbès JB, Abbès S, Ouanes Z, Houas Z, Abdel-Wahhab MA, Bacha H, et al. Tunisian radish extract (Raphanus sativus) enhances the antioxidant status and protects against oxidative stress induced by zearalenone in Balb/c mice. J Appl Toxicol. 2008;28:6–14. doi: 10.1002/jat.1240. [DOI] [PubMed] [Google Scholar]
- 21.Lugasi A, Blázovics A, Hagymási K, Kocsis I, Kéry A. Antioxidant effect of squeezed juice from black radish (Raphanus sativus L. var niger) in alimentary hyperlipidaemia in rats. Phytother Res. 2005;19:587–591. doi: 10.1002/ptr.1655. [DOI] [PubMed] [Google Scholar]
- 22.Deger Y, Yur F, Ertekin A, Mert N, Dede S, Mert H. Protective effect of α-tocopherol on oxidative stress in experimental pulmonary fibrosis in rats. Cell Biochem Funct. 2007;25:633–637. doi: 10.1002/cbf.1362. [DOI] [PubMed] [Google Scholar]
- 23.Taş S, Dirican M, Sarandöl E, Serdar Z. The effect of taurine supplementation on oxidative stress in experimental hypothyroidism. Cell Biochem Funct. 2004;24:153–158. doi: 10.1002/cbf.1198. [DOI] [PubMed] [Google Scholar]
- 24.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, Thannickal VJ. NOX enzymes and pulmonary disease. Antioxid Redox Signal. 2009;11:2505–2516. doi: 10.1089/ars.2009.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Paola R, Mazzon E, Muia C, Genovese T, Menegazzi M, Zaffini R, et al. Green tea polyphenol extract attenuates lung injury in experimental model of carrageenan-induced pleurisy in mice. Respir Res. 2005;6:66. doi: 10.1186/1465-9921-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minaiyan M, Ghannadi A, Mahzouni P, Nabi-Meibodi M. Anti-ulcerogenic effect of ginger (rhizome of Zingiber officinale Roscoe) hydroalcoholic extract on acetic acid-induced acute colitis in rats. Res Pharm Sci. 2008;3:15–22. [Google Scholar]
- 27.Schraufnagel DE, Mehta D, Harsbarger R, Trevianus K, Wang NS. Capillary remodelling in bleomycin induced pulmonary fibrosis. Am J Pathol. 1986;125:97–106. [PMC free article] [PubMed] [Google Scholar]
- 28.Snider GL. Interstitial pulmonary fibrosis: which cell is the culprit? Am Rev Respir Dis. 1983;127:535–539. doi: 10.1164/arrd.1983.127.5.535. [DOI] [PubMed] [Google Scholar]
- 29.Entzian P, Schlaak M, Seitzer U, Bufe A, Acil Y, Zabel P. Anti-inflammatory properties of colchicines: implications for idiopathic pulmonary fibrosis. Lung. 1997;175:41–51. doi: 10.1007/pl00007555. [DOI] [PubMed] [Google Scholar]
- 30.Weissler JC. Idiopathic pulmonary fibrosis: cellular and molecular pathogenesis. Am J Med Sci. 1989;297:91–104. doi: 10.1097/00000441-198902000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Winkler MK, Fowlkes JL. Metalloproteinase and growth factor interactions: do they play a role in pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol. 2002;283:L1–L11. doi: 10.1152/ajplung.00489.2001. [DOI] [PubMed] [Google Scholar]
- 32.Cortijo J, Cerdá-Nicolás M, Serrano A, Bioque G, Estrela JM, Santangelo F, et al. Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J. 2001;17:1228–1235. doi: 10.1183/09031936.01.00049701. [DOI] [PubMed] [Google Scholar]
- 33.Choi EJ, Bae SM, Ahn WS. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch Pharm Res. 2008;31:1281–1285. doi: 10.1007/s12272-001-2107-0. [DOI] [PubMed] [Google Scholar]
- 34.Gulati N, Laudet B, Zohrabian VM, Murali R, Jhanwar-Uniyal M. The antiproliferative effect of quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006;26:1177–1182. [PubMed] [Google Scholar]
- 35.Orsolić N, Knezević AH, Sver L, Terzić S, Basić I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J Ethnopharmacol. 2004;94:307–315. doi: 10.1016/j.jep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Beevi SS, Narasu ML, Gowda BB. Polyphenolics profile, antioxidant and radical scavenging activity of leaves and stem of Raphanus sativus L. Plant Foods Hum Nutr. 2010;65:8–17. doi: 10.1007/s11130-009-0148-6. [DOI] [PubMed] [Google Scholar]
- 37.Brisdelli F, Coccia C, Cinque B, Cifone MG, Bozzi A. Induction of apoptosis by quercetin: different response of human chronic myeloid (K562) and acute lymphoblastic (HSB-2) leukemia cells. Mol Cell Biochem. 2007;296:137–149. doi: 10.1007/s11010-006-9307-3. [DOI] [PubMed] [Google Scholar]
- 38.Kelly M, Kolb M, Bonniaud P, Gauldie J. Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm Des. 2003;9:39–49. doi: 10.2174/1381612033392341. [DOI] [PubMed] [Google Scholar]
- 39.Todd NW, Luzina IG, Atamas SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012;5:11. doi: 10.1186/1755-1536-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou XM, Wen GY, Zhao Y, Liu YM, Li JX. Inhibitory effects of alkaline extract of Citrus reticulataon pulmonary fibrosis. J Ethnopharmacol. 2013;146:372–378. doi: 10.1016/j.jep.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]