Abstract
Globally, Brucella melitensis and B. abortus are the most common cause of human brucellosis. The outer membrane protein 31 (Omp31) and L7/L12 are immunodominant and protective antigens conserved in human Brucella pathogens which are considered as potential vaccine candidates. We aimed to design the fusion protein from Brucella L7/L12 and truncated Omp31proteins, in silico, clone the fusion in pET28a vector, and express it in Escherichia coli host. Two possible fusion forms, L7/L12-TOmp31 and TOmp31-L7/L12 were subjected to in silico modeling and analysis. Analysis and validation of the fusion proteins with three dimensional (3D) models showed that both models are in the range of native proteins. However, L7/L12-Tomp31 structure was more valid than the TOmp31-L7/L12 model and subjected to in vitro production. The major histocompatibility complex (MHC II) epitope mapping using IEDB database indicated that the model contained good MHC II binders. The L7/L12-TOmp31 coding sequence was cloned in pET28a vector. The integrity of the construct was confirmed by polymerase chain reaction, restriction enzyme mapping, and sequencing. The fusion was successfully expressed in E. coli BL21 (DE3) by induction with isopropyl β-D-thiogalactopyranoside. The rL7/L12-TOmp31 was purified with Ni-NTA column. The yield of the purified rL7/L12-TOmp31 was estimated by Bradford method and found to be 40 mg/L of the culture. Western blotting with anti-His antibody revealed a specific reactivity with purified rL7/L12-TOmp31 produced in E. coli and showed the functional expression in the prokaryotic system. In this study, a new protein vaccine candidate against brucellosis was constructed with the help of bioinformatics tools and the construct was expressed in the bacterial host. Studies evaluating the immunogenicity and cross-protection of this fusion protein against B. melitensis and B. abortus are underway.
Keywords: Brucella, Omp31, L7/L12, Fusion protein, Cloning, In silico design
INTRODUCTION
Brucellosis is among the most common zoonosis infecting approximately half a million people annually around the world. The disease is especially endemic in countries including Latin America, Middle East, Africa, Asia, and the Mediterranean Basin (1,2). Brucellosis is caused by Breculla, a gram negative, facultative intracellular, partially acid fast coccobacillus lacking capsule or flagellea (2,3). The genus of Brucella consists of more than ten species: B. melitensis, B. abortus, B. suis, B. ovis, B. canis, B.neotomae, B. pinnipedialis, B. microti, B. inopinata, and B. Ceti (1,3). B. abortus, B. melitensis, and B. suis cause most of the animal and human diseases. B. abortus causes abortion in cattle and chronic infection in humans. B. melitensis that mainly infects goats and sheep is considered as the most pathogenic species of Brucella to humans (1,4). Moreover, B. melitensis is the only Brucella species that causes acute infection in both animals and human (2).
At present, there is no commercially available vaccine against human brucellosis and the disease is prevented by immunization of uninfected animals and elimination of the infected ones (5,6). In animals, immunization against Brucella infections is usually performed by administration of the live attenuated smooth Brucellastrains: B. abortus strain S19, B. melitensis strain Rev.1, and non-smooth strain B. abortus RB51 (7). B. abortus S19 is proven to be effective against B. abortus infection in cattle and B. melitensis Rev.1 is effective against B. melitensis and B. ovis infection in sheep and goats. However, live attenuated vaccines have the limitations of causing abortion in immunized pregnant animals, being pathogenic for humans, inducing resistant to antibiotics and interfering with the lipopolysaccharides-based serological tests (7,8). To develop a human Brucella vaccine, those Brucella proteins that exist in Brucella strains pathogenic to humans but absent in Brucella strains and not pathogenic to humans would be ideal for vaccine development. Vaxign identified forty six Brucella potentially immunogenic proteins that are conserved in all B. abortus, B. melitensis, and B. suis genomes not having any similarity with human or mouse protein sequences (9). The 31 kDa outer membrane protein (Omp31) of B. melitensis and 13.5 kDa L7-L12 ribosomal protein of B. abortus are considered as potential candidates for developing human Brucella subunit vaccine.
Omp31 initially cloned from B. melitensis 16M is the only protective antigen and it is the most exposed outer membrane protein identified in B. melitensis strains (10). Omp31 has been identified as an immunodominant antigen in the serological immune response. Moreover, studies have shown that Omp31 from B. suis, B. melitensis, and B. ovis is an hemin-binding protein (HBP), whose expression seems to be induced by iron limitation (11).
The L7/L12 ribosomal protein which also exists in B. melitensis and other Brucella species has been identified as an immunodominant and protective antigen. The recombinant L7/L12 protein and DNA vaccine encoding L7/L12 gene can elicit strong cell-mediated immunity (CD4+T cells) and promote protection against Brucella infection in Balb/c mice (6,12,13,14). Moreover, it has been identified that the L7/L12 from B. melitensis and B.abortusis is a major component in the antigenicity of Brucellin INRA (Brucellergen) for delayed-type hypersensitivity in Brucella-sensitized guinea pigs (12,13,14).
Considering the fact that B. melitensis and B. abortus are the main cause of human brucellosis, design of a new vaccine candidate inducing protectivity against both pathogens would be essential. In the present study, we aimed to design and express a fusion construct from Omp31 and L7/L12 considered as immunodominant, protective, and conserved antigens in human Brucella pathogens. Evaluation of the cross-protective efficacy of the fusion against B. melitensis and B. abortus is is an ongoing project.
MATERIALS AND METHODS
Strains, plasmids and media
The heat killed wild B. abortus strain 544 and B. melitensis strain 16M were purchased from the microbial collection of the Pasteur Institute of Iran (Karaj, Iran). The prokaryotic expression vector pET28a (Novagene, USA) and E. coli, BL21 (DE3) (Stratagene, USA) were used for rL7/L12-TOmp31 protein production. Bacterial strains were cultured in LB broth or agar (Merck, Germany) containing 50 μg/ml of kanamycin, at 37 °C. Unless otherwise stated, all reagents, kits and enzymes used for amplification and cloning were from Fermentas, USA.
In silico design and modeling of the fusion
In silico design, modeling and structure validation of truncated Omp31 were performed based on conserved areas, as previously described (15). Then the L7/L12,L7-L12-TOmp31 and TOmp31-L7/L12 proteins’ modeling was performed using Threading ASSEmbly Refinement (I-TASSER) server.
Validation of the fusion model
Validation and analysis of the models were carried out using protein structure analysis (ProSa) server, Swiss PDB Viewer software (version 4) and Ramachandran Plot Analysis (RAMPAGE) server. The I-TASSER output provides the C-score (a confidence score for estimating the quality of predicted models by I-TASSER), TM score and RMSD (known as standards for measuring structural similarity between two structures which are usually used to measure the accuracy of structure modeling when the native structure is known). Z-score and energy plots were calculated using ProSa server. The Z-score indicates overall model quality in comparison to the Z-scores of all experimentally determined protein chains in current Protein Data Bank. The energy plot shows local model quality by plotting energies as a function of amino acid sequence position and is representative of the error parts. The Ramachanran plot provided by RAMPAGE server shows the number of residues in favoured, allowed, and outer regions.
Major histocompatibility complex II epitope mapping
The human major histocompatibility complex (MHC) II epitope mapping was performed for the selected fusion model using Immune epitope database (IEDB) in order to find fusion MHC II binding capacity. In the IEDB output page, the predicted epitopes of the human MHC II alleles (HLA-DR, HLA-DQ or HLA-DP) within the protein sequence are listed based on their binding affinity. The epitopes’ affinities are presented as percentage (low percentage indicates good binder).
DNA extraction, primer design and PCR amplification
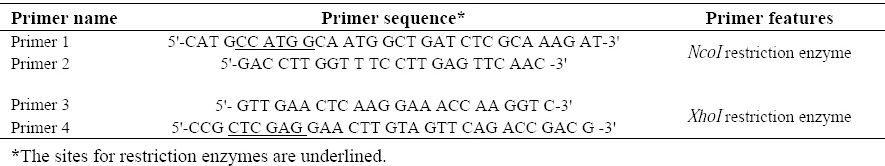
B. melitensis 16M and B. abortus 544 chromosomal DNA was extracted using DNA extraction kit (Roche, Germany) according to the manufacturer recommendations. In order to construct the coding sequence of the L7/L12-TOmp31 fusion protein, the designed TOmp31 (accession number: KJ411877) and the L7/L12 genes (accession number: AQIS01006.1) were separately amplified using overlap primers (TAG Copenhagen, Denmark) and finally fused by overlap polymerase chain reaction (PCR). Briefly, the coding sequence of L7/L12 (375 bp) was amplified using primers 1 and 2 (Table 1). The coding sequence of the TOmp31 (345 bp) was amplified with primers 3 and 4 (Table 1). Then the PCR products were gel purified using high pure PCR product purification Kit (Roche, Germany) according to the manufacturer's recommendations. The purity of the eluted PCR product was assessed by gel electrophoresis. Finally the fusion construct (720 bp) was amplified by primers 1 and 4 (Table 1). All PCRs were performed in 50 μl total volume containing 500 ng of template DNA, 1 μM of each primer, 200 μM dNTP-Mix, 1X pfu buffer containing MgSO4 and 1 unit of pfu DNA polymerase. The amplification conditions were consisted of hot start at 95 °C for 3 min followed by 30 cycles of denaturation step at 94 °C for 45 s, annealing step at 55 °C for 45 s, and extension step at 72 °C for 1 min. The program followed by a final extension at 72 °C for 7 min. The PCR products were analyzed by 1% agarose gel electrophoresis.
Table 1.
PCR primers for amplification of single and fusion genes.
Cloning of L7/L12-TOmp31 in pET28a
The PCR product was gel purified and then digested with fast digest NcoI and XhoI and then ligated to similar digested ends pET28a using T4 DNA ligase at 4 °C overnight. Competent TOP10 and BL21 (DE3) strains of E. coli were prepared by calcium chloride method and were used for transformation of pET28a-L7/L12-TOmp31 plasmid.
The transformed E. coli TOP10 was cultured on LB agar containing 50 μg/ml of kanamycin and was selected by screening the colonies on the media containing antibiotic. Some suspected colonies were further analyzed by PCR and restriction enzyme digestion. The sequencing of the selected plasmid construct was performed with specific T7 promoter and T7 terminator universal primers (GATC Company).
Expression and purification of recombinant L7/L12-TOmp31
E. coli BL21 (DE3) was transformed with the sequenced pET28a-L7/L12-TOmp31 plasmid construct and was cultured in LB broth supplemented with Kanamycin (50 μg/ml) at 37 °C with agitation. Recombinant L7/L12-TOmp31 protein expression was induced with different concentrations of isopropyl-beta-D-thiogalactopyranoside (IPTG) (0.1, 0.2 and 0.4 mM) in a culture of bacteria with an OD 600 of 0.6. Induced bacteria were incubated for 4 h at 37 °C and finally harvested by centrifugation at 4000 rpm for 10 min at 4 °C and stored at -80 °C. The expressed fusion protein was then purified with Ni-NTA column using denaturing method (Genescript, USA) according to manufacturer instructions. The purified protein was dialyzed against 3M urea, 1M urea and phosphate buffered saline (pH 7.2) at 4 °C, overnight. Quality and identity of the purified rL7/L12-TOmp31 protein was analyzed by SDSPAGE (15%) and Western blotting assay, respectively. For western blotting, the SDSPAGE gels were electroblotted onto nitrocellullose (at 100 V for 2 h). The blotted nitrocellulose was then blocked with skim milk for 2 h. After washing the blot three times with TPBS buffer, the blot was incubated with anti-His antibody (Roche, Germany) at a dilution of 1:500 (100 mU/ml) and the rabbit anti-B. abortus RB51 hyperimmune sera (used as the detecting antibody) for 2 h at RT. The blots were washed again three times with TBST and finally reactions were developed by Diaminobenzidine (DAB) solution (Roche, Germany). The second blotted and blocked nitrocellulose was incubated with the rabbit anti-B. abortus RB51/anti-B. melitensis REV-1 hyperimmune sera (used as the detecting antibody) for 2 h at RT and horseradish peroxidase-goat anti-rabbit immunoglobulin G served as the secondary antibody. Following the addition of DAB substrate, the antibody-specific protein band was revealed. Finally, the quantity of the recombinant protein was estimated using bradford assay. The purified rL7/L12-TOmp31 protein was stored at -20 °C for further evaluation of immunogenicity and protective efficacy in mice.
GenBank gene sequence submission
After sequencing of the constructed plasmids with specific T7 promoter and T7 terminator universal primers, the confirmed L7/L12-TOmp31 and TOmp31 sequences were submitted in GenBank.
RESULTS
3D structure modeling and validation of the fusion protein
The fusion gene sequence was designed from the truncated Omp31 and L7/L12 according to the published L7/L12 and TOmp31 gene sequences (15). The 3D structure of two fusion forms were predicted using I-TASSER server. Validation of the 3D models was done using related bioinformatics tools.
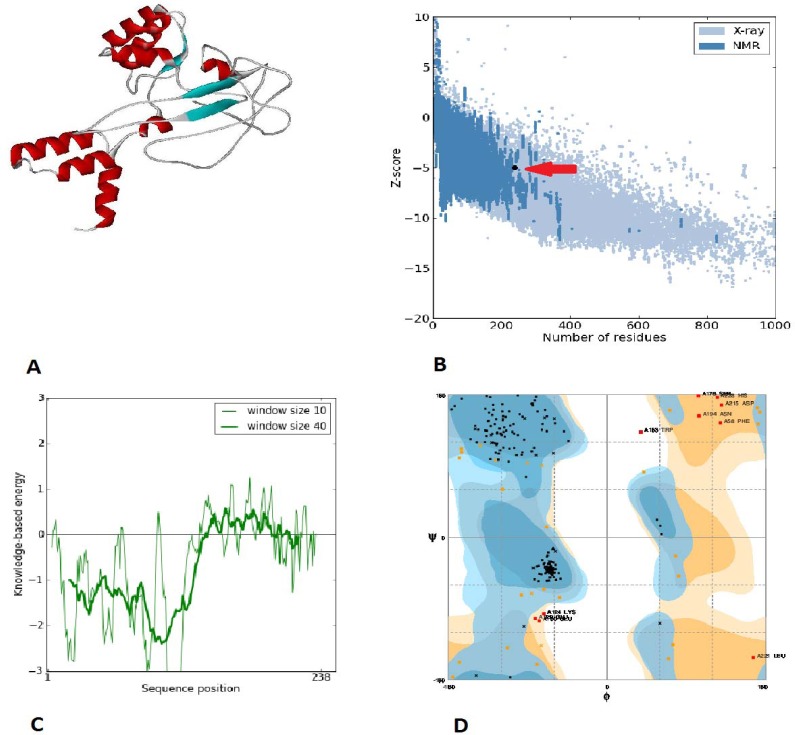
The results are summarized in Table 2. The Z-score for both models showed that they were in the range of native proteins of similar size (in the Nuclear Magnetic Resonance restriction). The residue energies were largely negative for both models with the exception of some peaks in the N-terminal part of the TOmp31-L7/L12 and the C-terminal part of the L7/L12-TOmp31. However, with regard to the fusion protein structures analysis the L7/L12-TOmp31 form was selected for further protein production (Fig. 1).
Table 2.
3D model validation results.

Fig. 1.
L7/L12-TOmp31 protein structure modeling and analyses results: A; L7/L12-TOmp31 fusion protein modeling. B; The Z-score plot: The L7/L12-TOmp31 protein structure is within the range of scores typically found for native proteins of similar size (the arrow shows the location of the fusion protein). C; The plot of residue scores: In this plot, overall residue energies were largely negative for the model. D; the Ramachandran plot of the L7-L12-TOmp31 protein. Number of residues in favored, allowed, and outer region is 84.3%, 11.4% and 4.2%, respectively.
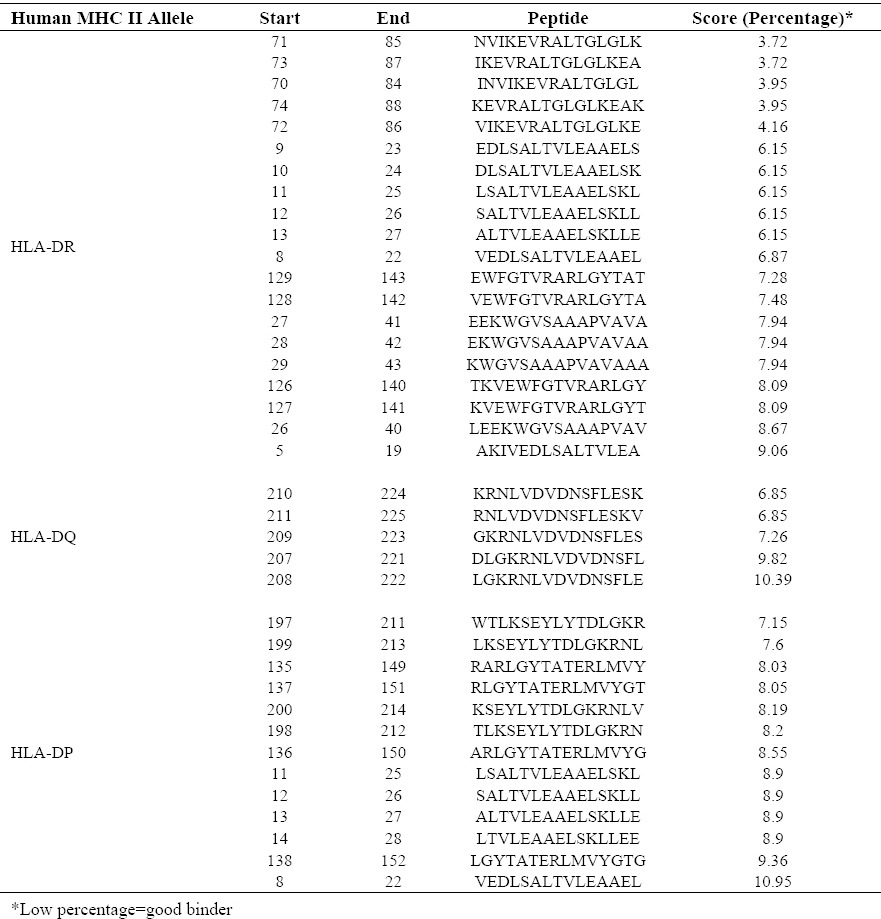
The location of human MHC II good binders was predicted for the fusion protein and top binders are summarized in Table 3.
Table 3.
Human MHC II epitope prediction. Top good binders for three MHC II alleles are summarized.
L7/L12-TOmp31 gene amplification and cloning
Gel electrophoresis of the amplified L7/L12, TOmp31 and L7/L12-TOmp31 sequences showed main fragments of 375 bp, 345 bp and 720 bp, respectively (Fig. 2A). The integrity of the constructed vector pET28-L7/L12-TOmp31 was confirmed by PCR, restriction digestion analysis and sequencing. Fig. 2B shows the digestion results for positive and negative clones. Recombinant plasmids showed two bands; representing 5.3 kb pET28a plasmid and the 720 bp fusion construct. The sequencing result was confirmed by comparing the L7/L12-TOmp31 construct sequence with the in silico designed fusion sequence using Basic Local Alignment Search Tool software.
Fig. 2.
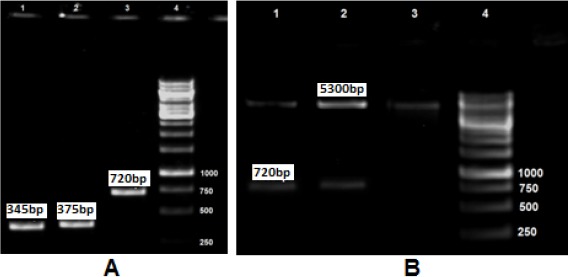
A; L7/L12, Omp31 and Fusion PCR results. Lane 1: TOmp31 (345 bp), Lane 2: L7/L12 (375 bp), Lane 3: Fusion gene (720 bp), Lane 4: 1Kb DNA ladder. B; Digestion results of recombinant and non-recombinant plasmids. Lane 1-2: Recombinant plasmids; the digested PET plasmid (5300 bp) and the fusion sequence (720 bp), Lane 3: Negative clone, Lane 4: 1 Kb DNA ladder.
Expression and purification of the rL7/L12-TOmp31
The L7/L12-TOmp31 recombinant protein was successfully expressed after induction with 0.1, 0.2 and 0.4 mM IPTG. The results of induction with different concentrations of IPTG were the same. Further, rL7/L12-TOmp31 protein was expressed with 0.2 mM IPTG at OD of 600 equal to 0.6 for 4 h at 37 °C. Expected rL7/L12-TOmp31 protein size of 25 kDa was detected after induction of the culture with IPTG and most of it was found to be localized inside the inclusion bodies in the cells, after sonication. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analyses of the induced and un-induced bacterial lysates and the purified rL7/L12-TOmp31 protein indicated the expected molecular mass of 25 kDa (Fig. 3A). The results demonstrated that the L7/L12- TOmp31 fusion protein could be expressed in BL21 (DE3) cells correctly and could be purified specifically and efficiently. The quantity of the purified fusion protein was estimated about 40 mg/L of the culture. Western blotting with antibodies revealed the specific reactivity with purified rL7/L12-TOmp31 produced in E. coli cells and showed the functional expression in the prokaryotic system (Fig. 3B).
Fig. 3.
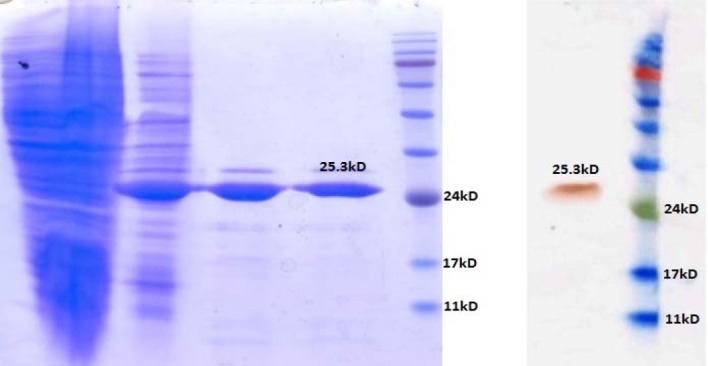
SDS-PAGE and western blotting results of the fusion protein expression and purification. A; Lane 1: Uninduced bacterial lysate, Lane 2: Induction results by 0.2 mM IPTG, Lane 3-4: Purified fusion protein (25.3 kD), Lane 5: Prestained Protein ladder (Vivantis, USA). B; Lane 1: Purified fusion protein (25.3 kD), Lane 2: Pre-stained protein ladder.
GenBank accession numbers
The sequences of the TOmp31 (15) and L7/L12-TOmp31 were submitted in GenBank under accession numbers KJ411877 and KJ396241, respectively.
DISCUSSION
Recently many efforts have been made to indentify new immunogens in Brucella proteome using immunoproteomic approaches. However, not all of these new targets showed in vivo protective efficacy (16,17,18). The B. abortus L7/L12 and B. melitensis Omp31 proteins are considered as potential immunogens to design vaccines against brucellosis and some studies have concentrated on using these antigens as subunit protein and DNA vaccine (19,20,21). During the infection by Brucella, some of the bacterial structural components such as L7/L12 ribosomal protein are involved in stimulating the host immune responses. Recombinant L7/L12 protein, as an antigen, could be detected during acute phase of brucellosis in the sera of the infected human. Additionally, studies have demonstrated that L7/L12 protein and DNA vaccine can induce lymphocyte proliferation and elicit cellular immunity (14,16,17,18,19,22). Antibodies against Omp31 have been detected in serum of infected humans and animals (22). Evaluation of the immunogenicity and protective efficacy of the Omp31 or its 27-amino acid peptide derivative as a subunit vaccine showed that the vaccine could elicit T helper1 (Th1) response mediated by CD4+T cells and conferred protection against B. melitensis and B. ovis infections (8,18,19,23,24,25).
We have previously designed the truncated form of Omp31 based on identifying the conserved area of the Omp31 protein sequence among Brucella species using multiple sequence alignment tools (15). In the current study, we aimed to design a fusion protein from L7/L12 and TOmp31 proteins and to express the fusion in the bacterial expression system. To achieve this purpose, fusion protein modeling was done using I-TASSER database which generates the best 3D structure predictions among all automated servers (26). Protective immunity against Brucella is cell mediated particularly via Th1 immune responses characterized by production of gamma Interferon (IFN-γ) (17,18,19,20). Since Th1 responses mediated by MHC II epitopes are indicated to be the significant key in the defense against Brucella infection, we evaluated the fusion immunogenicity based on predicting epitopes recognized by MHC II (27,28,29). Prediction of the epitopes recognized by MHC II molecules was performed using IEDB MHC epitope predictor and the location of the good binder peptides (High affinity for MHC II) are recognized in the fusion protein sequence (30). The prediction results indicate that the fusion is capable of inducing MHC II molecules. As observed in Table 2, top HLA-DR, HLA-DQ and HLA-DP binders are 71NVIKEVRALTGLGLK85, 210KRNLVDVDNSFLESK224 and 197WTLKSEYLYTDLGKR211, respectively.
Although the main immune responses against Brucella are cell mediated and independent of the protein structure and folding, in order to choose between the two possible fusions (TOmp31-L7/L12 and L7/L12-TOmp31), we decided to perform protein structure prediction. The Z-score for both fusion models showed that they are in the range of native conformations.
Overall the residue energies were largely negative for both models with the exception of some peaks in the N-terminal part of the fusion L7/L12-TOmp31 and the C-terminal part of the fusion L7/L12-TOmp31. These peaks are supposed to correspond to the nature of TOmp31 protein which is an outer membrane protein (high energy) (31). Finally, 3D model prediction and structural analysis of the two possible fusions resulted in L7/L12-TOmp31 selection.
Due to the importance of high level production of recombinant protein in immunological studies, the fusion was cloned in pET28a expression vector.
The pET is the most powerful system has so far been developed for the cloning and expression of the recombinant proteins in E. coli (32). Target genes are cloned in pET plasmids under control of strong bacteriophage T7 transcription and translation signals where expression is induced by providing a source of T7 RNA polymerase in the host cells. T7 RNA polymerase is so active that when fully induced, almost all of the cell's resources are converted to the target gene expression. This vector encodes the N-terminal and C-terminal HisTag sequences that allow easy purification, quantification and detection of target proteins (32). Sequencing of the cloning product confirmed the integrity of the cloning. The plasmid construct pET28a-L7/L12-TOmp31 was transferred into E. coli BL21 DE3 containing T7 RNA polymerase and the expression was induced by the addition of IPTG. Successfully induced expression by different concentration of IPTG and high level production of the fusion demonstrated the high efficiency of our fusion construct.
CONCLUSION
Initial practical steps toward developing new vaccines are prediction and production of the new vaccine targets. In this study, a new candidate protein vaccine, L7/L12-TOmp31, against Brucella infection was designed with the help of bioinformatics tools. Further studies evaluating the immunogenicity and protection conferred by the rL7/L12-TOmp31 against B. melitensis and B. abortus in mice model are underway.
ACKNOWLEDGMENTS
This study was financially supported by Pasteur Institute of Iran (Ph.D grant No. B-8803).
REFERENCES
- 1.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infec Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 2.Avila-Calderón E, Lopez-Merino A, Sriranganathan N, Boyle SM, Contreras-Rodrígue A. A history of the development of Brucella vaccines. BioMed Res Inter 2013. 2013 doi: 10.1155/2013/743509. ID 743509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappas G. The changing Brucella ecology: novel reservoirs, new threats. Int J Antimicrob Agent. 2010;36:1:S8–11. doi: 10.1016/j.ijantimicag.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 4.He Y. Analyses of Brucella pathogenesis, host immunity, and vaccine targets using systems biology and bioinformatics. Front Cell Infect Microbiol. 2012 doi: 10.3389/fcimb.2012.00002. doi: 10.3389/fcimb.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seleem MN, Boyle S. M, Sriranganthan N. Brucellosis: A re-emerging zoonosis. Vet Microbiol. 2010;140:392–398. doi: 10.1016/j.vetmic.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Perkins SD, Smither SJ, Atkins HS. Towards a Brucella vaccine for humans. FEMS Microbiol Rev. 2010;34:379–394. doi: 10.1111/j.1574-6976.2010.00211.x. [DOI] [PubMed] [Google Scholar]
- 7.Corbel MJ. Brucellosis in humans and animals. Geneva, Switzerland: WHO Press, World Health Organization; 2006. [Google Scholar]
- 8.Cassataro J, Estein SM, Pasquevich KA, Velikovsky CA, Barrera S, Bowden R, et al. Vaccination with the recombinant brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+ T helper 1 response that protects against Brucella melitensis infection. Infec Immun. 2005;73:8079–8088. doi: 10.1128/IAI.73.12.8079-8088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, Xiang Z. Bioinformatics analysis of Brucella vaccines andvaccine targets using VIOLIN. Immunome Res. 2010;6:S5. doi: 10.1186/1745-7580-6-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salhi I, Boigegrain RN, Machold J, Weise C, Cloeckaert A, Rouot B. Characterization of new members of the group 3 outer membrane protein family of Brucella spp. Infec Immun. 2003;71:4326–4332. doi: 10.1128/IAI.71.8.4326-4332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delpino AV, Cassataro J, Fossati CA, Goldbaum FA, Baldi PC. Brucella outer membrane protein Omp31 is a haemin-binding protein. Microbes Infect. 2006;8:1203–1208. doi: 10.1016/j.micinf.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Mantur BG, Amarnath SK, Shinde RS. Review of clinical and laboratory features of human Brucellosis. Indian J Med Microbiol. 2007;25:188–202. doi: 10.4103/0255-0857.34758. [DOI] [PubMed] [Google Scholar]
- 13.Bachrach G, Banai M, Fishman Y, Bercovier H. Delayed-Type hypersensitivity activity of the Brucella L7/L12 ribosomal protein depends on posttranslational modification. Infec Immun. 1997;65:267–271. doi: 10.1128/iai.65.1.267-271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazak Esra, Oliveira SC, Goral G, Akalin H, Yilmaz E, Heper Y, et al. Brucella abortus L7/L12 recombinant protein induces strong Th1 response in acute brucellosis patients. Iran J Immunol. 2010;7:132–141. [PubMed] [Google Scholar]
- 15.Golshani M, Zandi P, Bouzari S. In silico design of truncated Omp31 protein of Brucella melitensis: Its cloning and high level expression in Escherichia coli. Vaccine Res. 2014;1:16–20. [Google Scholar]
- 16.ZhaoZ, Li M, Luo D, Xing L, Wu S, Duan Y, et al. Protection of mice from Brucella infection by immunization with attenuated Salmonella enteric serovartyphimurium expressing a L7/L12 and BLS fusion antigen of Brucella. Vaccine. 2009;27:5214–5219. doi: 10.1016/j.vaccine.2009.06.075. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Wang L, Yin J, Wang X, Cheng S, Lang X, et al. Immunoproteomics analysis of Brucella melitensis and identification of a new immunogenic candidate protein for the development of brucellosis subunit vaccine. Mol Immunol. 2011;49:175–184. doi: 10.1016/j.molimm.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Yanng X, Skyberg JA, Cao L, Clapp B, Thornburg T, Pascul DW. Progress in Brucella vaccine development. Front Biol. 2013;8:60–77. doi: 10.1007/s11515-012-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avila-Calderón ED, Lopez-Merino A, Sriranganathan N, Boyle SM, Contreras-Rodríguez A. A history of the development of brucella vaccines. BioMed Res Inter 2013. 2013 doi: 10.1155/2013/743509. ID 743509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira C, Macedo GC, de Almeida LA, de Oliveira FS, Oñate A, Cassataro J, et al. Recent advances in understanding Immunity against brucellosis: application for vaccine development. The Open Vet Sci J. 2010;4:102–108. [Google Scholar]
- 21.Zarrini G, Zargham S, TabatabaeiYazdi M, BehzadiyanNejad Q, Muhammad Hassan Z. Cloning and overexpression of rpll gene of Brucellaabortus in Escherichia coli. Pakistan J BiolSci. 2006;9:1128–1131. [Google Scholar]
- 22.Luo D, Bing N, Li P, Shi W, Zhang SL, Han Y, et al. Protective immunity elicited by a divalent DNA vaccine encoding both the L7/L12 and Omp16 genes of Brucellaabortus in BALB/c mice. Infect Immun. 2006;74:2734–2741. doi: 10.1128/IAI.74.5.2734-2741.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Díaza AG, Clausse M, Paolicchi FA, Fiorentino MA, Ghersi G, Zylberman V, et al. Immune response and serum bactericidal activity against Brucella ovis elicited using a short immunization schedule with the polymeric antigen BLSOmp31 in rams. Vet Immunol Immunopathol. 2013;154:36–41. doi: 10.1016/j.vetimm.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Gupta VK, Radhakrishnan G, Harmsb J, Splitter G. Invasive Escherichia coli vaccines expressing Brucella melitensis outer membrane proteins 31 or 16 or periplasmic protein BP26 confer protection in mice challenged with B. melitensis. Vaccine. 2012;30:4017–4022. doi: 10.1016/j.vaccine.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassataro J, Velikovsky CA, Bruno L, Estein SM, Barrera S, Bowden R, et al. Improved Immunogenicity of a vaccination regimen combining a DNA vaccine encoding Brucella melitensis outer membrane protein 31(Omp31) and recombinant Omp31 boosting. Clinic and Vaccine Immunol. 2007;14:869–874. doi: 10.1128/CVI.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skendros P, Boura P. Immunity to brucellosis. Rev Sci Tech Off Int. 2013;32:137–147. doi: 10.20506/rst.32.1.2190. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira SC, Macedo GC, Almeida LM, Oliveira FS, Oñate A, Cassataro J, et al. Recent advances in understanding immunity against brucellosis: application for vaccine development. The Open Vet Sci J. 2010;4:102–108. [Google Scholar]
- 29.Titball RW. Vaccines against intracellular bacterial pathogens. Drug Discov. 2008;13:596–600. doi: 10.1016/j.drudis.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Sturniolo T, Bono E, Ding J, Raddrizzani L, Tuereci O, Sahin U, et al. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat Biotechnol. 2009;17:555–561. doi: 10.1038/9858. [DOI] [PubMed] [Google Scholar]
- 31.Wiederstein M, Sippl M.J. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.11th ed. Darmstadt, Germany: EMD Chemicals Inc, an affiliate of Merck KGaA; 2011. Novagen pET System Manual. [Google Scholar]