Abstract
Stress has a profound impact on the nervous system and causes cognitive problems that are partly related to the inflammatory effects. Besides influencing the content of neurotransmitters, antidepressants such as doxepin are likely to have anti-inflammatory, anti-oxidative, and anti-apoptotic effects. Therefore, the present study investigated the effects of doxepin on passive avoidance learning and the levels of tumor necrosis factor-alpha (TNF-α) in the rat hippocampus following repeated restraint stress. Male Wistar rats were divided into five groups. Chronic stress was induced by keeping animals within an adjustable restraint chamber for 6 h every day for 21 successive days. In stress-doxepin group, stressed rats were given 1, 5 and 10 mg/kg of doxepin intraperitoneally (i.p) for 21 days and before placing them in restraint chamber. Healthy animals who served as control group and stressed rats received normal saline i.p. For evaluation of learning and memory, initial latency and step-through latency were determined using passive avoidance learning test. TNF-α levels were measured in hippocampus by enzyme-linked immunosorbant assay (ELISA) at the end of experiment. Induced stress considerably decreased the step through latencies in the rats (P<0.05) but doxepin administration prevented these changes. Stress-doxepin groups did not reveal any differences compared to control group at any given doses. TNF-α level was increased significantly (P<0.05) in stress group. Only the low dose of doxepin (1 mg/kg) decreased TNF-α level. The present findings indicated that learning and memory are impaired in stressful conditions and doxepin prevented memory deficit. It seems that inflammation may involve in induced stress memory deficits, and that doxepin is helpful in alleviating the neural complications due to stress.
Keywords: Doxepin, Stress, Learning and memory, TNF-α
INTRODUCTION
Chronic stress produces destructive changes in the central nervous system. One of the regions that roles in the forming of the brain responses to stress is hippocampus. Hippocampus has direct and indirect anatomical connections with hypothalamus-pituitary-adrenal (HPA) axis and bears significant structural changes following stress (1). Chronic stress through HPA axis performs hypertrophy and hyperplasia in the renal gland and increases glucocorticoids production (2,3,4,5,6). Studies have shown that excessive glucocorticoids increases apoptosis of neurons in the hippocampus (1). Also, it is reported that stress increased and reduced pro-apoptotic (e.g. BAX) and anti-apoptotic (e.g. Bcl-2) gene expression, respectively (7). Stress reduces the expression of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor 1 (IGF1), nerve growth factor (NGF), epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF). Therefore, stress can impair neurogenesis and development of the hippocampus. Also, it has been demonstrated that the production of proinflammatory cytokines and oxidative stress are stimulated in the patients who are experiencing stress or anxiety (8,9). Besides influencing the content of neurotransmitters, antidepressants are revealed that have anti-inflammatory, anti-oxidative, and anti-apoptotic effects (10). Antidepressants, like fluoxetine, clomipramine, maprotiline, amitriptyline and desipramine have been identified that have anti-inflammatory effects (11,12,13). Also, in some previous studies the neuroprotective effects of anti-depressants have been shown (10,14,15,16,17,18).
Doxepin is a tricyclic antidepressant that inhibits reuptake of norepinephrine and serotonin (19). Like other antidepressants, doxepin has anti-inflammatory effects; however, its anti-inflammatory role in the central nervous system has not been studied. Local application of doxepin has therapeutic effects in atopic dermatitis (20) and it is used as second-line therapy for chronic urticarial (21). Doxepin has also been proposed as a protective factor against oxidative stress. Doxepin by reducing calcium signaling, which is a key factor in the oxidative stress-induced damages, results in protection against oxidative stress (22). Moreover, doxepin increased antioxidants such as superoxide dismutase and reduced lipid peroxidation (23).
In our previous study, we observed that doxepin could improve cognitive processes (24). Therefore, this study was designed based on probable neuroprotective effects of doxepin. Because, one of the major regions for evaluating the effects of antidepressants on brain physiology is the hippocampus (25), the purpose of this study was to investigate the effect of doxepin on learning and memory and tumor necrosis factor alpha (TNF-α) levels in the hippocampus of the stressed rats.
MATERIAL AND METHODS
Male Albino Wistar rats (the Pasteur institute of Iran, Tehran) about 10 weeks old weighing 200 to -250 g were housed in an air-conditioned colony room on a light/dark cycle (23 ± 1 °C and 30- 40% humidity) and supplied with standard pelleted diet and tap water ad libitum. The Ethic Committee for Animal Experiments at Isfahan University approved the study and all experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Protocols of stress induction and treatment
Male Wistar rats were randomly divided into five groups of 10 each. Chronic stress was induced by keeping animals within an adjustable restraint chamber for 6 h every day for 21 successive days (26). It was not possible for animals to move or turn around. The stress procedure was carried out in the institutional animal facility throughout the experimental period at 8:00 am -14:00 pm each day. The animals from each group were randomly assigned to one apparatus. In stress-doxepin group, stressed rats were given 1, 5 and 10 mg/kg of doxepin dissolved in normal saline (Ray Chemicals Pvt. Ltd.) intraperitoneally (i.p) for 21 days and before placing them in restraint chamber (24). Healthy animals who served as the control group and stressed rats received the same volume of saline in the same manner.
Passive avoidance learning
Learning and memory performance in the rats were evaluated using passive avoidance learning test.
The apparatus (BPT Co., Tehran) consisted of two separate chambers connected through a guillotine door. One chamber was illuminated, while the other was dark. The floor of both chambers consisted of steel grids for delivering electric shocks by an isolated stimulator. In the acquisition trail, on the nineteenth day and before receiving the treatment and stress, each rat was individually placed in the illuminated chamber while its back was faced to the guillotine door. After 30 s of habituation, the guillotine door separating the illuminated and dark chambers was opened and the initial latency to enter the dark chamber (acquisition latency time) was recorded. The guillotine door was closed immediately after the rat entered the dark chamber and an electric foot shock (75 V, 0.2 mA, 50 Hz) was delivered to the floor grids for 3 s. Rats with initial latency greater than 60 s were excluded from the study. Then the rat was removed from the dark chamber and returned to its home cage. Seventy-two hours later, on the twenty-second day of the experiment, retention latency time (step-through latency) to enter the dark chamber was determined in the same way as in the acquisition trail, but the foot shock was not delivered and the latency time was recorded up to a maximum of 600 s as the cut-off.
Enzyme-linked immunosorbant assay for cytokine detection
After behavioral study, the left hemisphere of the rat brain was dissected and hippocampus was immediately removed on a cold artificial CSF and stored at −80 °C. Tissues were homogenized with ice-cold 10 mM Tris-HCl buffer (pH 7.4) (27). After centrifugation at 9,000 × g for 30 min at 4 °C, the supernatant was collected for the measurement of total protein and TNF-α. Protein concentration was determined by a commercial protein assay, Bio-Rad RC-DC Protein assay (Bio-Rad, UK), based on the traditional method of Lowry (28). The levels of TNF-α were measured by enzyme-linked immunosorbant assay (ELISA) using Rat TNF-α Standard ELISA Development Kit (Peprotech, USA; Cat. No. 900-k73).
Statistical analysis
Behavioral results were analyzed statistically using Kruskal-Wallis Test (non-parametric ANOVA) and Dunn's Multiple Comparisons for post-test. The hippocampal levels of TNF-α were analyzed using one-way ANOVA and Tukey for post-test. The significant level was set at P<0.05. Results are expressed as mean ± S.E.M.
RESULTS
Passive avoidance learning
Figs. 1 and 2 respectively shows the initial latency (acquisition latency time) and step-through latency (retention latency time) of all groups in a single trial passive avoidance test, respectively. As shown in Fig. 1 induction of stress in the rats significantly decreased the mean initial latency time (P<0.05) as compared to that of the control group. This shows that the stressed rats did not spend enough time to assess the light compartment to refuse to enter the dark compartment. Therefore, stress obviously decreased acquisition and recall of passive avoidance response in this group. The reduction in initial latency time may be due to excessive anxiety. Doxepin at 5 mg/kg significantly (P<0.05) reversed this effect to the point that the initial latency time did not differ as compared to the control group which might be due to the reduction of anxiety.
Fig. 1.
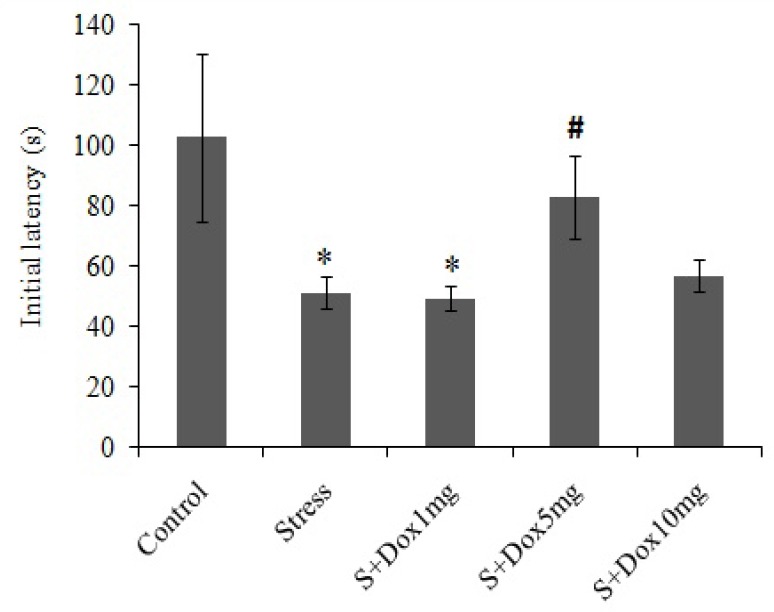
Effects of doxepin and stress on initial latency in passive avoidance test. Data are expressed as mean ± SEM. (n=10). S + Dox stands for stress-doxepin group; *P<0.05 with respect to the control group, #P<0.05 with respect to the stress group.
Fig. 2.
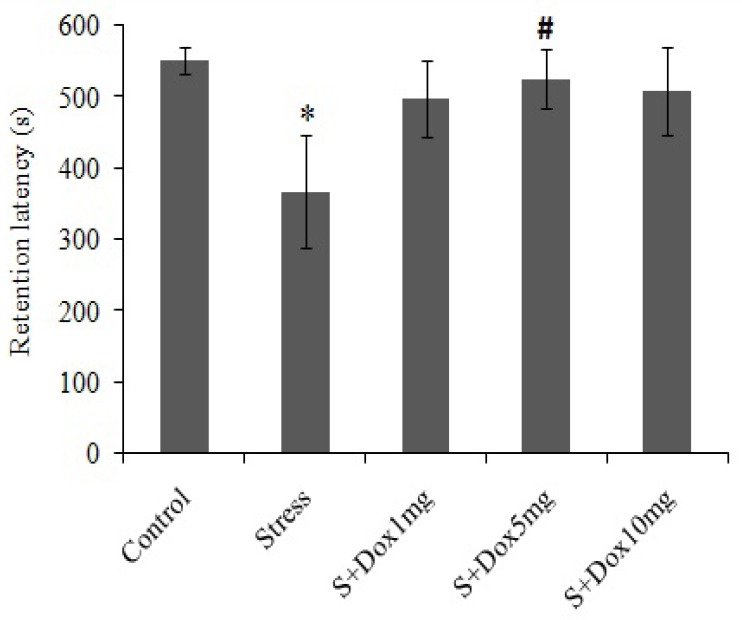
Effects of doxepin and stress on retention latency in passive avoidance test. Data are expressed as mean ± SEM. (n=10). S + Dox stands for stress-doxepin group; *P<0.05 with respect to the control group, #P<0.05 with respect to the stress group.
In the stress group, the mean retention latency was significantly lower than that of the control group (367.19 ± 79.51 s vs 551.09 ± 19.65 s, respectively; P<0.05) indicating poor performance in the stressed rats. Retention latencies showed improvement at all doses of doxepin administered, but it was more significant at 5 mg/kg of doxepin compared to the control group.
Tumor necrosis factor alpha level
As shown in Fig. 3, stress significantly (P<0.05) increased the ratio of TNF-α level to the total protein content in the rat hippocampus compared to the control group. Doxepin significantly (P<0.05) decreased this ratio only in the stress-doxepin group at 1 mg/kg when compared to the stress group. However, there is no difference between stress group received 1 mg/kg doxepin and the control group.
Fig. 3.
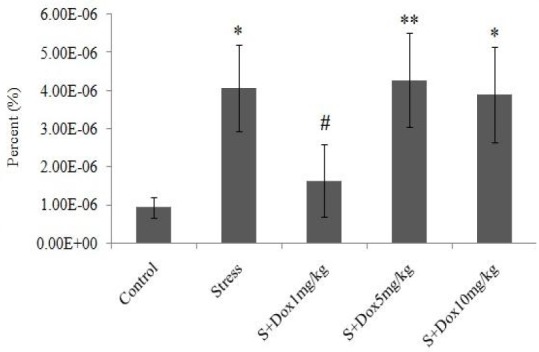
Effects of doxepin and stress on ratio of TNF-α to total protein (%) in the hippocampus. Data are expressed as mean ± SEM. (n=7). S + Dox stands for stress-doxepin group; *P<0.05 and **P<0.01 with respect to the control group, #P<0.05 with respect to the stress group.
Weight of adrenal gland
The weight of the adrenal glands of the stressed animals increased significantly (P<0.01) as compared to that of the control group. Doxepin significantly (P<0.05) prevented enhancment of adrenal gland weight only in the stress-doxepin 1 mg/kg compared to the stress group (Fig. 4).
Fig. 4.
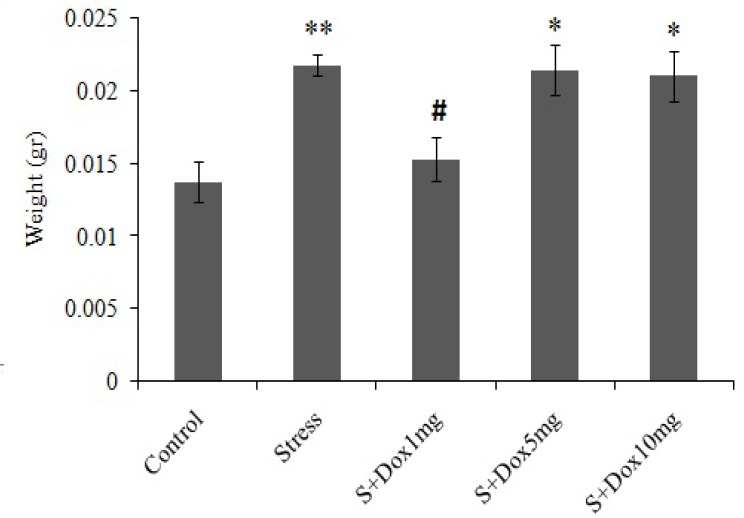
Effects of doxepin and stress on adrenal gland weight. Data are expressed as mean ± SEM. (n=8). S + Dox stands for stress-doxepin group, *P<0.05 and **P<0.01 with respect to the control group, #; P<0.05 with respect to the stress group.
DISCUSSION
The results of the current study showed that stress could impair learning and memory performance in the animal model of stress which can be reversed by giving doxepin. Doxepin at the dose of 5 mg/kg improved initial latency in the stressed animals. Doxepin also improved retention latency at all three doses administered, but the improvement was more significant at the dose of 5 mg/kg. This indicates that different doses of doxepin can have different effects, for example, doxepin at low doses but not at high doses has hypnagogic effects (29). Rats in the stress group spent less time in the light chamber than the control group which is a sign of great anxiety in the rats in this group.
Studies have shown that stress could damage the central nervous system (30) and memory processing. In this regard, different mechanisms such as death of neurons, loss of dendritic branches, and reduction of neurogenesis have been proposed (26). It has been reported that stress through activation of the Hypothalamo-Pituitary-Adrenal (HPA) system and resulted adrenal hypertrophy and high levels of glucocorticoids secretion, can impair hippocampal neurons (6). In the present study, we observed that stress induced adrenal hypertrophy and increased the weight of adrenal glands. It has been reported that glucocorticoids probably damage the neural circuits in the areas that are involved in learning and memory, particularly hippocampus. These impairments are due to production of free radicals, enhancment of serum pro-inflammatory cytokines and gene expression of pro-apoptotic factors (such as BAX), and the reduction of gene expression of anti-apoptotic factors (eg, Bcl-2) and neurotrophic factors (1,31).
Our results showed that stress increased hippocampal content of TNF-α, therefore inflammation may be a probable reason for neuronal impairments in stress. Stress can cause inflammation by various factors. For example, during the restraint stress, releasing of glutamate causes glutamate toxicity, inflammation and degenerative changes in nervous system. Inflammation stimulates the HPA axis (32), which has deleterious effects on the hippocampus (1).
Also pesent results revealed that low doses of doxepin decreased TNF-α level in the hippocampus. This suggests that doxepin is likely to have anti-inflammatory effects in the central nervous system. Correlation between inflammation and depression has been proven and studies have shown that tricyclic antidepressants have anti-inflammatory effects (33). Anti-inflammatory effects of doxepin in skin inflammation has been shown (20) and in this study, central effects of doxepin in the reduction of pro-inflammatory cytokines was observed. However, high doses of doxepin did not reduce the levels of TNF-α. This shows that high doses of doxepin may improve learning and memory through other mechanisms.
These results are consistent with the weight of the adrenal glands in the stress group that received doxepin 1 mg/kg. These results shows that the rats in this group have experienced less stress and HPA axis has been less affected.
It is possible that antidepressants improve balance of oxidative stress in hippocampus (10) and hereby perform some part of their neuroprotective effects (34,35,36). Doxepin has been shown that protects cultured neurons against oxidative stress-induced injury (23). Recent studies demonstrated the effect of sleep on memory consolidation (19). It seems that hippocampal activities through specific coordinated neuropsychological processes facilitates entry of new information into pre-existing network of cortex and thereby contribute to memory consolidation during sleep (37). Awakening during biological sleeping time, can induce cognitive, emotional and learning disorders (38). In this study, stress was induced at the morning. Since rats are nocturnal, the stress was induced at the times that coincide with the early hours of their sleeping time. Doxepin, especially in its low doses, has a strong antagonistic effect on H1 receptors (29). This effect induces sleep and may be effective in improving sleep disorders (34,35).
CONCLUSION
In conclusion, stress increased adrenal weight and hippocampal TNF-α levels. These reflect a probable relationship between the levels of glucocorticoids and inflammatory factors in the brain (6). Low doses of doxepin reduced both adrenal weight and hippocampal TNF-α levels. It can be concluded that low doses of doxepin affect the hypothalamus-pituitary-adrenal axis and can prevent the deleterious effects of stress. High doses of doxepin probably reduces the effects of stress on memory; however, further studies are needed to understand the exact mechanisms.
ACKNOWLEDGMENTS
The content of this paper is extracted from the Master thesis NO. 393288 submitted by A.A. Azadbakht which was financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran
REFERENCES
- 1.Drzyzga LR, Marcinowska A, Obuchowicz E. Antiapoptotic and neurotrophic effects of antidepressants: a review of clinical and experimental studies. Brain Res Bull. 2009;79:248–257. doi: 10.1016/j.brainresbull.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Alario P, Gamallo A, Beato M, Trancho G. Body weight gain, food intake and adrenal development in chronic noise stressed rats. Physiol Behav. 1987;40:29–32. doi: 10.1016/0031-9384(87)90181-8. [DOI] [PubMed] [Google Scholar]
- 3.Ganong W, Hume D. Absence of stress-induced and compensatory adrenal hypertrophy in dogs with hypothalamic lesions. Endocrinology. 1954;55:474–483. doi: 10.1210/endo-55-4-474. [DOI] [PubMed] [Google Scholar]
- 4.Ingle DJ. The time for the occurrence of cortico-adrenal hypertrophy in rats during continued work. Am J Physiol. 1938;124:627–630. [Google Scholar]
- 5.Schapiro S. Pituitary ACTH and compensatory adrenal hypertrophy in stress-non-responsive infant rats. Endocrinology. 1962;71:986–989. doi: 10.1210/endo-71-6-986. [DOI] [PubMed] [Google Scholar]
- 6.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 7.Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21:871–877. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Franco R, Sánchez-Olea R, Reyes-Reyes EM, Panayiotidis MI. Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mutat Res. 2009;674:3–22. doi: 10.1016/j.mrgentox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Lucassen P, Meerlo P, Naylor A, Van Dam A, Dayer A, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, et al. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Salam OM, Baiuomy AR, Arbid MS. Studies on the anti-inflammatory effect of fluoxetine in the rat. Pharmacol Res. 2004;49:119–131. doi: 10.1016/j.phrs.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi M, Sacerdote P, Panerai AE. Chlomipramine differently affects inflammatory edema and pain in the rat. Pharmacol Biochem Behav. 1994;48:1037–1040. doi: 10.1016/0091-3057(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 13.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim CM, Kim SW, Park JY, Kim C, Yoon SH, Lee JK. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. J Neurosci Res. 2009;87:1037–1045. doi: 10.1002/jnr.21899. [DOI] [PubMed] [Google Scholar]
- 16.Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Steven RJ, Li X-M. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology. 2003;28:53–62. doi: 10.1038/sj.npp.1300009. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- 19.Vermeeren A, Coenen AM. Effects of the use of hypnotics on cognition. Prog Brain Res. 2011;190:89–103. doi: 10.1016/B978-0-444-53817-8.00005-0. [DOI] [PubMed] [Google Scholar]
- 20.Drake LA, Fallon JD, Sober A. Relief of pruritus in patients with atopic dermatitis after treatment with topical doxepin cream. J Am Acad Dermatol. 1994;31:613–616. doi: 10.1016/s0190-9622(94)70225-x. [DOI] [PubMed] [Google Scholar]
- 21.Hajak G, Rodenbeck A, Voderholzer U, Riemann D, Cohrs S, Hohagen F, et al. Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psychiatry. 2001;62:453–463. doi: 10.4088/jcp.v62n0609. [DOI] [PubMed] [Google Scholar]
- 22.Ray SK, Fidan M, Nowak MW, Wilford GG, Hogan EL, Banik NL. Oxidative stress and Ca2+ influx upregulate calpain and induce apoptosis in PC12 cells. Brain Res. 2000;852:326–334. doi: 10.1016/s0006-8993(99)02148-4. [DOI] [PubMed] [Google Scholar]
- 23.Ji B-S, Ji H, Liu G-Q. Doxepin protects cultured neurons against oxidative stress-induced injury. Acta Pharmacol Sin. 2004;25:297–300. [PubMed] [Google Scholar]
- 24.Gharzi M, Dolatabadi HR, Reisi P, Javanmard SH. Effects of different doses of doxepin on passive avoidance learning in rats. Adv Biomed Res. 2013;2:66. doi: 10.4103/2277-9175.115823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levkovitz Y, Grisaru N, Segal M. Transcranial magnetic stimulation and antidepressive drugs share similar cellular effects in rat hippocampus. Neuropsychopharmacology. 2001;24:608–616. doi: 10.1016/S0893-133X(00)00244-X. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res. 2005;1040:55–63. doi: 10.1016/j.brainres.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 27.Lin CY, Huang CS, Huang CY, Yin MC. Anticoagulatory, antiinflammatory, and antioxidative effects of protocatechuic acid in diabetic mice. J Agric Food Chem. 2009;57:6661–6667. doi: 10.1021/jf9015202. [DOI] [PubMed] [Google Scholar]
- 28.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Krystal AD, Durrence HH, Scharf M, Jochelson P, Rogowski R, Ludington E, et al. Efficacy and safety of doxepin 1 mg and 3 mg in a 12-week sleep laboratory and outpatient trial of elderly subjects with chronic primary insomnia. Sleep. 2010;33:1553–1561. doi: 10.1093/sleep/33.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs E, Flügge G. Cellular consequences of stress and depression. Dialogues Clin Neurosci. 2004;6:171–183. doi: 10.31887/DCNS.2004.6.2/efuchs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haynes LE, Barber D, Mitchell IJ. Chronic antidepressant medication attenuates dexamethasone-induced neuronal death and sublethal neuronal damage in the hippocampus and striatum. Brain Res. 2004;1026:157–167. doi: 10.1016/j.brainres.2004.05.117. [DOI] [PubMed] [Google Scholar]
- 32.Christian KM, Miracle AD, Wellman CL, Nakazawa K. Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience. 2011;174:26–36. doi: 10.1016/j.neuroscience.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung YC, Kim SR, Jin BK. Paroxetine prevents loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in an experimental model of Parkinson's disease. J Immunol. 2010;185:1230–1237. doi: 10.4049/jimmunol.1000208. [DOI] [PubMed] [Google Scholar]
- 34.Merry D, Korsmeyer S. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y, Lim CM, Kim SW, Park JY, Seo JS, Han PL, et al. Fluoxetine attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Brain Res. 2009;1281:108–116. doi: 10.1016/j.brainres.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson MD, Raff MC. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995;374:814–816. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara M, Moroni F, De Gennaro L, Nobili L. Hippocampal sleep features: relations to human memory function. Front Neurol. 2012;3:57. doi: 10.3389/fneur.2012.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright KP, Lowry CA, LeBourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50. doi: 10.3389/fnmol.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]


