Abstract
AIM:
To compare clomiphene citrate (CC), metformin or the combination of CC and metformin as the first line ovulation induction drug in Asian Indian women with polycystic ovary syndrome (PCOS).
METHODS:
One hundred and five newly diagnosed, treatment naive PCOS women were recruited. They were randomized into any of the three groups: Group I (CC 50–150 mg/day), Group II (metformin 1700 mg/day), and Group III (CC + metformin in similar dosage to Groups I and II). Patients underwent follicular monitoring and advice on timed intercourse. The study period was 6 months, or till pregnant, or till CC resistant. Primary outcome studied was live birth rate (LBR). Secondary outcomes were ovulation rate, pregnancy rate, and early pregnancy loss rate.
RESULTS:
There was no significant difference among the groups in baseline characteristics and biochemical parameters. LBR was 41.6%, 37.5%, and 28.1%, respectively in Groups III, II, and I. Group III (CC + metformin) had the highest ovulation (83.3%), pregnancy (50%), and LBRs (41.6%). Group II (metformin) was as good as Group I (CC) in all the outcomes. CC + metformin (Group III) had statistically significantly higher ovulation rate as compared to CC alone (Group I) (P = 0.03; odds ratio: 95% confidence interval: 3.888 [1.08–13.997]).
CONCLUSION:
Thus, our study shows that metformin was as good as CC in terms of “LBR” and the combination of CC and metformin gave the highest ovulation and LBR.
KEY WORDS: Clomiphene citrate, metformin, ovulation induction, polycystic ovary syndrome, pregnancy rate
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy to affect women in the reproductive age.[1] Although prevalence varies in different socio-ethnic groups, usually 5–20% women suffer from PCOS.[2] PCOS is a syndrome with a varied clinical presentation and long term metabolic risks.[3] In the reproductive age group, PCOS women have low fecundity, anovulation, increased early pregnancy loss, and many obstetric complications.[4,5,6] Induction of ovulation in PCOS women is a challenge and the best ovulation induction drug is still debatable. Clomiphene citrate (CC) is a standard treatment for ovulation induction in these women and is still considered the first line drug.[7] CC has many disadvantages; 25–30% women fail to ovulate and there is a significant discrepancy between ovulation and pregnancy rates. Peripheral antiestrogenic effects of CC at the level of endometrium and cervical mucous, long half-life, multi follicular ovulation are some are other disadvantages. The main factors that predict the outcome of treatment are obesity, hyperandrogenemia, hyperinsulinemia, insulin resistance, and age.[8] PCOS women who are CC resistant or CC failure, have to go through laparoscopic ovarian drilling or gonadotropin therapy as the second line treatment. These are expensive and are associated with many side effects.[9] Thus, many adjuvants have been tried, with the aim to increase the efficacy of CC or as independent drugs aiding in ovulation. The basic etiology of anovulation associated with PCOS is thought to be insulin resistance and hyperinsulinemia.[10,11] Thus, insulin sensitizing drugs are a rational therapeutic option.[12]
Metformin is the most extensively studied insulin lowering agent in PCOS.[10] Current recommendations, limit metformin use in PCOS women who are glucose intolerant.[7] However, since the response to treatment varies so much and PCOS is a heterogenous entity, researchers continue to explore metformin, trying to find the subgroup of PCOS ideal for this drug.
Thus, CC and metformin are the two most commonly used pharmacological agents and a large amount of data is available. However, there exists marked heterogeniety in outcomes.[13] Comparative efficacy between these two agents and also response following combination of the two are important issues that need to be addressed. There are not many published studies of Indian PCOS women and metformin efficacy. The present study was undertaken to compare CC, metformin, and a combination of the two as the first line ovulation induction drug, in Indian PCOS women.
MATERIALS AND METHODS
This was a prospective randomized controlled trial at a private hospital in Bhubaneswar, India from November 2011 to December 2013. Women attending gynecology outpatient with the primary complaints of infertility and oligomenorrhea were evaluated for PCOS. Diagnosis was based on Rotterdam criteria,[14] which included at least two of the three following criteria: (1) Chronic anovulation; (2) the clinical or biochemical hyperandrogenemia; (3) polycystic ovarian morphology.
Chronic anovulation was defined as menses <21 days and >35 days. Mostly, patients were diagnosed as hyperandrogenic by a modified Ferriman–Gallwey (F-G) score of ≥8. Polycystic morphology was defined as the presence of 12 or more follicles in a single sonographic plane (with one ovary being sufficient for diagnosis) measuring 2–9 mm, or ovarian volume more the 10 cm3. All scans were performed by a single sonologist (author), using voluson E6 (GE healthcare). Women with PCOS, normal male factor and at least one patent tube by hysterosalpingography were included in the study. Only treatment naive patients with the 1st time diagnosis and the evaluation of infertility were included. Women with any major systemic illness such as diabetes, liver, heart, or kidney disease were excluded from the study. All patients gave written consent. The study was approved by Institutional Ethics Committee.
Eligible women were randomized by picking up envelopes to either one of the three groups, consisting of CC (Group I), metformin (Group II), or a combination of metformin and CC (Group III). Equal numbers of envelopes for the three groups labeled I, II, and III were prepared by a nurse, naive to this study. The patients picked up the envelope and returned to the investigator for further advice.
Patients randomized to CC only (Group I) were given CC at an initial dose of 50 mg on days 2–6 of menses, increasing to a maximum of 150 mg/day. Transvaginal sonography for follicular monitoring was done starting day 8. If ovulatory, timed intercourse advice was given, and the same dose of CC was repeated. In case of anovulation, the cycle was canceled, progesterone withdrawal given, and CC dose increased in the next cycle. Women who failed to ovulate with 150 mg CC were termed CC resistant.
Patients randomized to metformin group (Group II) received sustained release 850 mg/day initial dose, increased to 1700 mg/day, over 2 weeks’ time. Patients were asked to report spontaneous menses and come for follicular tracking from day 8.
In the combination group (Group III), metformin was given in the same manner as metformin group. CC was given at a dose of 50 mg on days 2–6. Increased to a maximum of 150 mg. Transvaginal sonography and follicular monitoring were done in a manner similar to CC group. All women were enrolled for 6 months. The study was continued till pregnant or CC resistant or until 6 months as applicable individually. Once pregnant, fetal cardiac activity documented at 6 weeks of pregnancy. In metformin group, metformin was continued up to 14 weeks irrespective of glucose status. All pregnant women were followed up till delivery.
Statistical analysis
Primary outcome measure was live birth rate (LBR); secondary outcomes were ovulation rate, pregnancy rate, and early pregnancy loss. Quantitative variables were expressed as a mean ± standard deviation and were compared among groups using one-way ANOVA, ANOVA and “Tamhane” post-hoc statistical tests. Qualitative variables were expressed as frequencies in percent and were analyzed by Chi-square tests. Statistical analysis was done using SPSS version 18 (IBM corporation Developers). A P < 0.05 was considered statistically significant.
A total of 105 patients diagnosed as PCOS and found eligible for this study were randomized into thirty-five patients in each group. However, a total of eighty-one women completed the study, that is, 6 months of follow-up, or till pregnant, or CC resistant. Group I 32 patients (104 cycles), Group II 24 (70 cycles), and Group III 24 patients (84 cycles). Patient's data with the partially complete study was not included.
RESULTS
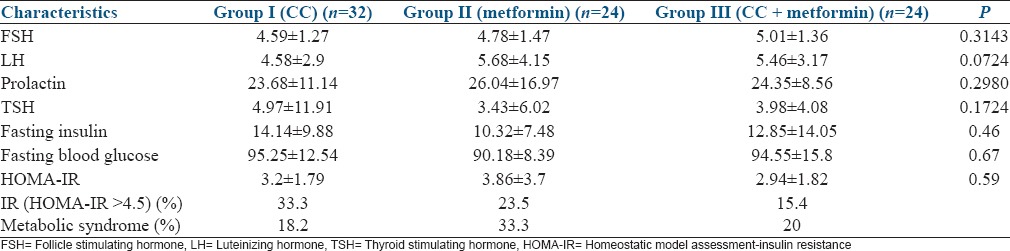
There was no significant difference among the three groups in the baseline variables [Table 1]. Age, body mass index (BMI), duration of infertility, waist, hip circumference, and F-G scores were comparable among the three groups. The biochemical parameters, such as follicle stimulating hormone, luteinizing hormone, thyroid stimulating hormone, prolactin, insulin, fasting blood glucose, homeostatic model assessment-insulin resistance, prevalence of insulin resistance, and metabolic syndrome were also comparable among the groups [Table 2].
Table 1.
Baseline characteristics of women in the three groups
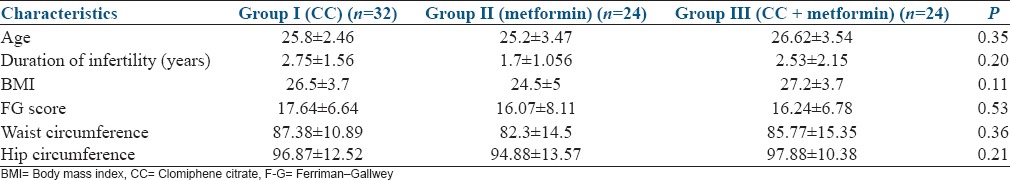
Table 2.
Biochemical parameters in the three groups
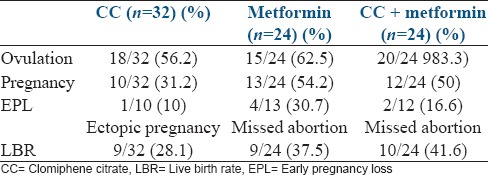
The ovulation rate per patient was 56.2%, 62.5%, and 83.3% in CC, metformin, and CC + metformin groups, respectively. There was no statistically significant difference in ovulation rate between CC and metformin (P = 0.63), metformin and CC + metformin (P = 0.11). However, CC + metformin group was statistically significantly higher than the CC group (P = 0.03). Monofollicular ovulation was the highest in the metformin group (94.4%), against 71.8% in CC + metformin and 60% in CC group. Group II also had the longest days to ovulation (19.2 ± 5.25) as compared to the other two groups (15.2 ± 3.2 in Group I, 16.26 ± 3.41 in Group III).
LBR was the highest in the CC + metformin Group III (41.6%), followed by metformin group 37.5%, and CC group (28.1%). Treatment outcome data per patient for the three groups has been shown in Table 3.
Table 3.
Outcomes (rates per patient)
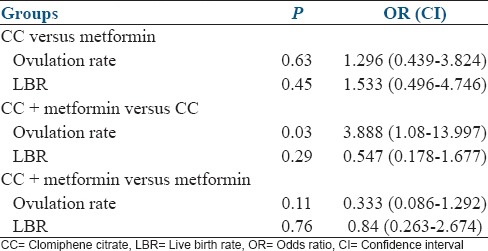
The results of ovulation and LBR, when compared between two groups, have been shown in Table 4. The combination group had a significantly higher ovulation rate as compared to CC only group (P = 0.03) odds ratio: 3.888 (1.08–13.997). There was no significant difference in LBR among the three groups.
Table 4.
Comparing the groups
DISCUSSION
CC has been a time tested drug for ovulation induction for the last 30–40 years. Ovulation occurs in 60–80% of women and pregnancy is achieved in about 35–40%.[15] This discrepancy between ovulation and pregnancy is also well documented. The probable causes are the antiestrogenic effect of CC on the cervix and endometrium, which results in thick cervical mucous, impending sperm transport and thinning of the endometrium which could affect implantation.[16] In PCOS women, CC has no effects on hyperinsulinemia and hyperandrogenism. This study shows an ovulation rate of 56% in CC group which appears low. We noted CC resistance in 47.5% women (14/32) who failed to ovulate with 150 mg of CC for 5 days. This could be related to ethnic variations in the population of the Indian subcontinent, who are known to have a high prevalence of visceral obesity and hyperinsulinemia.[17,18]
Metformin has also been extensively used as an oral antihyperglycemic agent, which inhibits hepatic glucose uptaken and increases peripheral glucose uptake.[19] Thus, metformin reduces peripheral insulin levels and improves glucose tolerance. Furthermore, metformin may directly decrease ovarian androgen production.[20] The role of metformin as the first line ovulation induction drug is still debatable. Some studies demonstrate efficacy[10,21,22] others disagree.[23,24,25] Major problems of these studies are small sample size, variation in populations, treatment, and outcomes reported. A recent meta-analysis[26] of four high quality randomized controlled trial (RCTs) concluded that owing to conflicting findings and heterogeniety across the RCTs, there is insufficient evidence to establish a difference between metformin and CC in all outcomes. There are no RCTs in Indian PCOS women, as seen in PubMed search.
In our study, metformin (Group II) showed an ovulation rate per patient of 62.5%, the pregnancy rate of 54.2% and LBR of 37.5% which appears to be very high. In the metformin group patients were expected to come for ovulation study only after spontaneous cycles. Four patients came back pregnant within 1–3 months of therapy, without ever going through follicular monitoring. Therefore, a number of cycles studied in metformin group was smaller. Furthermore, all patients in our study were very young, with short duration of infertility, BMI < 30, and treatment naive [Table 1] which explains the high pregnancy and LBR across all groups. Early pregnancy loss was highest in Group II (30.7%). In our study pregnancy rate and LBR was comparable between metformin and CC groups. This is in contradiction to a large multicentric trial by Legro et al.[12] which reported no advantage of metformin, either alone or in combination with CC.
Palomba et al.[27] reported much higher LBR with metformin as compared to CC (52% vs. 18%) in naive young nonobese PCOS women and a comparable ovulation rate. In this study, metformin alone has shown a high LBR, comparable to the other two groups. The small sample size is definitely a big disadvantage to project the success of metformin in our PCOS population. The study results have prompted us to undertake an RCT comparing metformin versus placebo to show efficacy of metformin in our ethnic population. Neveu et al.[28] showed metformin was better than CC for ovulation induction and equivalent for pregnancy achievement. However, they showed no advantage of adding the two together. This study had the disadvantage of lack of randomization and standardization. Our study shows a clear advantage of CC + metformin as compared to CC, or metformin alone both in the ovulation rate and LBR [Table 3], which is also statistically significant. This result is comparable to many studies while comparing the two.[25,29,30]
It is well documented that race and ethnicity influence the phenotypic manifestations and response to treatment in PCOS women. Published research articles mostly represent European and North American population. Studies on Asian women are few. A good RCT[25] involving 115 PCOS women of Asian origin was from Malaysia having mostly Malay ethnic group. The Indian subcontinent is a large population with exploding issues of obesity, trunkal obesity, diabetes mellitus, hypertension, metabolic syndrome, and PCOS. It is likely the Asian Indian ethnic group is genetically predisposed to abdominal obesity, insulin resistance.[17] We have shown a high prevalence of metabolic syndrome, insulin resistance, and abdominal obesity in Indian PCOS women.[18] Further randomized well-designed studies are required to see benefits of metformin therapy in our population. Our study highlights that metformin may be an effective alternative or adjuvant to CC for ovulation induction in PCOS women.
CONCLUSION
Thus, this study shows that metformin was as good as CC in terms of “LBR” and the combination of CC and metformin gave the highest ovulation and LBR. Larger multicentric trials should be taken up comparing metformin, with CC and placebo to consolidate the role of metformin in young infertile PCOS women.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Frank S. Polycystic ovary syndrome. N Engl J Med. 1995;333:833–61. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 2.March W, Moore V, Willson K, Phillips D, Norman R, Davies M. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;2:544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 3.Wild RA. Long-term health consequences of PCOS. Hum Reprod Update. 2002;8:231–41. doi: 10.1093/humupd/8.3.231. [DOI] [PubMed] [Google Scholar]
- 4.Hull MG. Epidemiology of infertility and polycystic ovarian disease: Endocrinological and demographic studies. Gynecol Endocrinol. 1987;1:235–45. doi: 10.3109/09513598709023610. [DOI] [PubMed] [Google Scholar]
- 5.Homburg R, Armar NA, Eshel A, Adams J, Jacobs HS. Influence of serum luteinising hormone concentrations on ovulation, conception, and early pregnancy loss in polycystic ovary syndrome. BMJ. 1988;297:1024–6. doi: 10.1136/bmj.297.6655.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–83. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 7.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM Sponsored 3 rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.e5. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil Steril. 2002;77:91–7. doi: 10.1016/s0015-0282(01)02929-6. [DOI] [PubMed] [Google Scholar]
- 9.Kim LH, Taylor AE, Barbieri RL. Insulin sensitizers and polycystic ovary syndrome: Can a diabetes medication treat infertility? Fertil Steril. 2000;73:1097–8. doi: 10.1016/s0015-0282(00)00540-9. [DOI] [PubMed] [Google Scholar]
- 10.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: A randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139–46. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 11.Fleming R, Hopkinson ZE, Wallace AM, Greer IA, Sattar N. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo-controlled trial. J Clin Endocrinol Metab. 2002;87:569–74. doi: 10.1210/jcem.87.2.8261. [DOI] [PubMed] [Google Scholar]
- 12.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–66. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 13.Costello MF, Eden JA. A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertil Steril. 2003;79:1–13. doi: 10.1016/s0015-0282(02)04554-5. [DOI] [PubMed] [Google Scholar]
- 14.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 15.Hughes E, Collins J, Vandekerckhove P. Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea. Cochrane Database Syst Rev. 2000;2:CD000056. doi: 10.1002/14651858.CD000056. [DOI] [PubMed] [Google Scholar]
- 16.Gonen Y, Casper RF. Sonographic determination of a possible adverse effect of clomiphene citrate on endometrial growth. Hum Reprod. 1990;5:670–4. doi: 10.1093/oxfordjournals.humrep.a137165. [DOI] [PubMed] [Google Scholar]
- 17.Prasad DS, Kabir Z, Dash AK, Das BC. Abdominal obesity, an independent cardiovascular risk factor in Indian subcontinent: A clinico epidemiological evidence summary. J Cardiovasc Dis Res. 2011;2:199–205. doi: 10.4103/0975-3583.89803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kar S. Anthropometric, clinical, and metabolic comparisons of the four Rotterdam PCOS phenotypes: A prospective study of PCOS women. J Hum Reprod Sci. 2013;6:194–200. doi: 10.4103/0974-1208.121422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1991;73:1294–301. doi: 10.1210/jcem-73-6-1294. [DOI] [PubMed] [Google Scholar]
- 20.Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril. 2001;76:517–24. doi: 10.1016/s0015-0282(01)01975-6. [DOI] [PubMed] [Google Scholar]
- 21.Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med. 1998;338:1876–80. doi: 10.1056/NEJM199806253382603. [DOI] [PubMed] [Google Scholar]
- 22.Velázquez E, Acosta A, Mendoza SG. Menstrual cyclicity after metformin therapy in polycystic ovary syndrome. Obstet Gynecol. 1997;90:392–5. doi: 10.1016/s0029-7844(97)00296-2. [DOI] [PubMed] [Google Scholar]
- 23.Ehrmann DA, Cavaghan MK, Imperial J, Sturis J, Rosenfield RL, Polonsky KS. Effects of metformin on insulin secretion, insulin action, and ovarian steroidogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:524–30. doi: 10.1210/jcem.82.2.3722. [DOI] [PubMed] [Google Scholar]
- 24.Sahin Y, Yirmibes U, Kelestimur F, Aygen E. The effects of metformin on insulin resistance, clomiphene-induced ovulation and pregnancy rates in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2004;113:214–20. doi: 10.1016/j.ejogrb.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Zain MM, Jamaluddin R, Ibrahim A, Norman RJ. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: A randomized controlled trial. Fertil Steril. 2009;91:514–21. doi: 10.1016/j.fertnstert.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Misso ML, Costello MF, Garrubba M, Wong J, Hart R, Rombauts L, et al. Metformin versus clomiphene citrate for infertility in non-obese women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2013;19:2–11. doi: 10.1093/humupd/dms036. [DOI] [PubMed] [Google Scholar]
- 27.Palomba S, Orio F, Jr, Falbo A, Manguso F, Russo T, Cascella T, et al. Prospective parallel randomized, double blind, double-dummy controlled clinical trial comparing clomiphene citrate and metformin as the first line treatment for ovulation induction in non obese anovulatory women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4068–74. doi: 10.1210/jc.2005-0110. [DOI] [PubMed] [Google Scholar]
- 28.Neveu N, Granger L, St-Michel P, Lavoie HB. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction and achievement of pregnancy in 154 women with polycystic ovary syndrome. Fertil Steril. 2007;87:113–20. doi: 10.1016/j.fertnstert.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 29.Ayaz A, Alwan Y, Farooq MU. Efficacy of combined metformin-clomiphene citrate in comparison with clomiphene citrate alone in infertile women with polycystic ovarian syndrome (PCOS) J Med Life. 2013;6:199–201. [PMC free article] [PubMed] [Google Scholar]
- 30.Leanza V, Coco L, Grasso F, Leanza G, Zarbo G, Palumbo M. Ovulation induction with clomiphene citrate and metformin in women with polycystic ovary syndrome. Minerva Ginecol. 2014;66:299–301. [PubMed] [Google Scholar]


