Abstract
OBJECTIVE:
To study the prevalence of metabolic syndrome (MBS) in Indian women and to see how does it correlate to body mass index (BMI) and polycystic ovarian syndrome (PCOS) in this population.
STUDY DESIGN:
Prospective cross-sectional observational study.
SETTING:
Infertility clinic of a tertiary center.
MATERIALS AND METHODS:
Two hundred women, 120 with PCOs and 80 age-matched controls were enrolled. The prevalence of MBS was studied in the women with and without and was co related to BMI by further subgrouping as team (BMI <23 kg/m3) and obese (BMI >23 kg/m2). The sample size was: team controls-40, obese controls-40, team PCOS-80. Each subject underwent a physical examination and laboratory evaluation for the diagnosis of MBS, which was defined according to the guidelines of National Cholesterol Education Program Adult Treatment Pamel (NCEP ATP III) 2005.
INTERVENTION:
None.
MAIN OUTCOME MEASURES:
Main Outcome Measures: Subjects with and without PCOs were compared with each other for the prevalence of MBS, and similarly team subjects were compared with obese subjects. Receiver operator characteristic (ROC) curves were obtained for both the PCOS and non PCOS population separately, co-relating the prevalence of MBS with BMI. These ROC curves were used to establish the cut off values of BMI, which could best predict the risk of MBS.
RESULTS:
The prevalence of MBS was significantly higher in the women with PCOS, as compared to age-matched controls. Similarly, when BMI was considered, MBS was more prevalent in overweight subjects than in lean subjects with or without PCOS. In subgroup analysis, the presence of PCOS had a lesser impact on the prevalence of MBS as compared to non-PCOS controls with higher BMI. The relative risk of MBS increased as follows: lean controls-1, lean PCOS-2.66, obese controls-5.33, and obese PCOS-6.5. The most appropriate cut-off level of BMI for predicting the risk of MBS in Indian women without PCOS seems to be 23 kg/m2, whereas, with PCOS, it was 22.5 kg/m2.
CONCLUSION:
MBS is more prevalent in women with PCOS. However, obesity is an independent and stronger risk factor for developing MBS. To reduce the risk of MBS and its related long-term health consequences, lifestyle modification is advisable above BMI of 23 kg/m2 in the normal population and 22.5 kg/m2 in women with PCOS.
KEY WORDS: Body mass index, metabolic syndrome, obesity, polycystic ovarian syndrome
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is common disorder, affecting approximately 5–10% of the women in reproductive age group.[1,2] It is characterized by chronic anovulation, hyperandrogenism, and polycystic ovaries. The other metabolic abnormalities associated with PCOS are obesity, dyslipidemia, insulin resistance, glucose intolerance, and hypertension, which confer an increased risk of long-term health consequences such as type II diabetes mellitus and cardiovascular risk.[3] Most of these metabolic features are also shared by the syndrome X or metabolic syndrome (MBS), which is associated with atherosclerosis, hypertension, dyslipidemia, coronary artery disease, and diabetes. Some of the factors affecting the prevalence of MBS are age,[4,5] obesity,[4] insulin resistance,[6] and underlying PCOS.[7]
Till date, there are few studies, mainly from the American population[8,9,10] and still fewer from the European continent,[11,12] which have addressed the prevalence of MBS in women with PCOS. There is a scarcity of data from Asian population.[13,14] Moreover, in most of these studies, the women with PCOS were found to have a higher body mass index (BMI) and waist circumference, as compared to the controls, which could have confounded in the higher prevalence of MBS.
This study was undertaken with an aim of comparing the prevalence and different characteristics of MBS in Indian women with PCOS and age-matched controls. To negate the confounding effect of the high BMI associated with PCOS, subgroups of lean and obese women were studied separately for the prevalence of MBS.
In order to reduce the serious long-term consequences related to MBS, we have attempted to find out the predictors of MBS, and the action points, at which screening for MBS and lifestyle modification would be beneficial, in respect to preventing, or modifying long-term morbidity.
MATERIALS AND METHODS
We performed a prospective cross-sectional observational study of Indian women with normal (BMI-19-23) and higher BMI (BMI >23 kg/m2). A total of 200 subjects were enrolled over a period of 18 months, 120 with a diagnosis of PCOS, and 80 age-matched controls. Proper approval was taken from the institutional review board regarding the materials and methods of the study.
All these women were taken from a tertiary care infertility center and presented with oligoovulatory or anovulatory cycles. PCOS was defined by the 2003 Rotterdam's criteria. Women with menstrual irregularity (fewer than eight cycles per year) and polycystic appearance of ovaries on ultrasound were both taken as inclusion criteria with or without clinical evidence of hyperandrogenism. Biochemical evidence was looked for only in cases, where it was required for the establishment of the diagnosis. Subjects were recruited from the infertility clinic of a tertiary care center. Controls were the women with normal and higher BMI, who had regular menstrual cycles, no evidence of polycystic ovaries on ultrasound, and no clinical evidence of hyperandrogenism.
Hypothyroidism and hyperprolactinemia were ruled out as a cause of menstrual irregularity.
Each subject underwent a physical examination and laboratory evaluation for the diagnosis of MBS. Serum triglyceride and high-density lipoprotein-cholesterol (HDL-C) levels were measured in fasting state. An oral glucose tolerance test (OGTT) along with measurement of serum insulin was done in all the patients with 75 g of glucose. All subjects who were enrolled in the study were enquired about past medical history, family history of diabetes mellitus and/or hypertension, and underwent a physical examination, including determination of clinical features of hyperandrogenism. Weight was measured in kilograms, and height was measured in centimeters to the nearest 0.5 cm. BMI was calculated. Blood pressure (BP) was measured in sitting position in left arm, and an average of two measurements was taken into consideration. Waist circumference was measured in the standing position, halfway between the lower ribs and the crest of the pelvis. Hip circumference was measured at the widest part of the hips. Waist to hip ratio was also calculated.
Results were analyzed in three steps, keeping the objectives of the study in mind.
In the first step of the analysis, the prevalence of MBS was calculated separately in women with and without PCOS and the results was compared by applying the Chi-square test. A two-tailed P < 0.05 was considered statistically significant.
MBS was defined as having three or more of the following abnormalities:[15]
Waist circumference in females >88 cm
Fasting serum glucose ≥100mg/dl
Fasting serum triglycerides ≥150 mg/dl
Serum HDL-C <50 mg/dl
BP ≥130/85 mmHg.
The anthropometric and biochemical parameters in the women with and without PCOS were evaluated and expressed as a mean ± standard deviation, and comparison was done by applying the student's t-test.
The second step of analysis, to eliminate high BMI as a confounding factor in the prevalence of MBS, the women with and without PCOS were further studied in subgroups groups based on BMI as follows:
Non-PCOS: Lean controls (n - 40) and obese controls (n - 40)
PCOS: Lean PCOS (n - 40) and obese PCOS (n - 80).
BMI of 23 kg/m2 was taken on the basis of the WHO expert consultation on BMI in the Asian population.[16]
Prevalence and characteristics of MBS were studied in each of the above-mentioned group. The prevalence of MBS in different groups was compared by using the Chi-square test (P < 0.05 as statistically significant). The student's t-test was used to compare the continuous variables between different groups.
The relative risk of MBS was calculated for each group, depicting its correlation with BMI and underlying PCOS.
The third step of analysis, to establish the cutoff level of BMI, above which lifestyle modification would be advisable. Receiver operator characteristics (ROC) curves were obtained for both the PCOS and non-PCOS population separately, co-relating the prevalence of MBS with BMI. Data analysis was done with statistical package for social sciences software (SPSS) version 17.0 (Chicago).
RESULTS
The anthropometric and metabolic parameters of Indian women with PCOS and the controls are compared in Table 1.
Table 1.
Anthropometric and metabolic parameters in women with and without PCOS, along with the prevalence of MBS
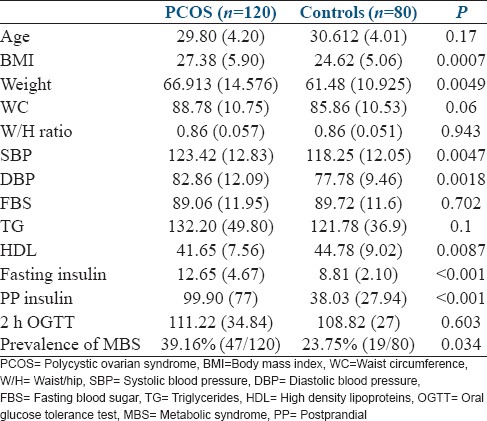
Women with PCOS had a significantly higher BMI, higher mean of systolic BP, diastolic BP, fasting insulin, and 2 h insulin levels (2 h after 75 g of glucose). The mean of HDL-C was significantly lower in women with PCOS. There was no statistical difference in the means of fasting blood sugar, 2 h OGTT values, and serum triglyceride levels.
The prevalence of MBS was significantly higher in women with PCOS [Table 1].
The prevalence of various components of MBS in women with and without PCOS are given in Table 2.
Table 2.
Prevalence of the components of MBS in PCOS and Controls
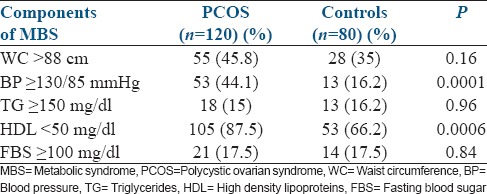
On comparing the prevalence of the components of MBS in women with PCOS to the controls, irrespective of their BMI, a significant difference was present in the prevalence of high BP (P - 0.0001) and low HDL-C (P - 0.0006), thus increasing cardiovascular risk in the women with PCOS.
For the second step of the analysis, women with and without PCOS were further divided into sub-groups based on BMI (lower or higher than 23 kg/m2) [Table 3].
Table 3.
Anthropometric and biochemical parameters, and prevalence of MBS in women with and without PCOS in relation to BMI
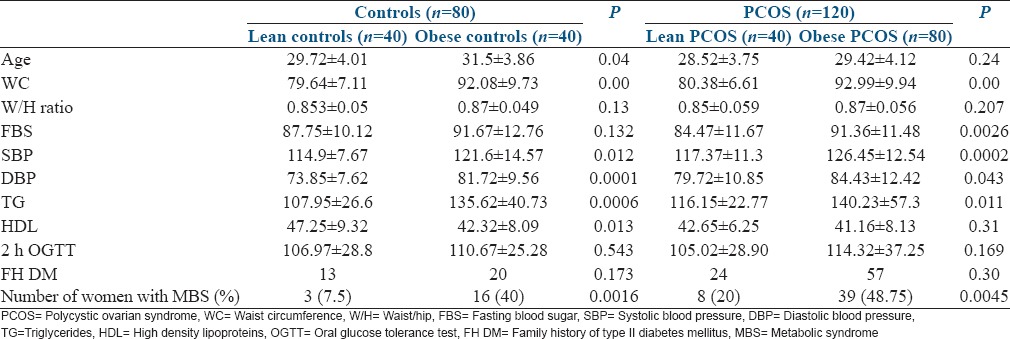
It is evident from Table 3 that most of the factors which favor the development of MBS were significantly higher in the obese subgroups, in both the PCOS and control groups.
The prevalence of MBS was also significantly higher in the obese subgroups as compared to their lean counterparts [Table 3].
Obesity had a strong impact on the prevalence of MBS in both PCOS and control groups. Further, to eliminate the effect of high BMI as a confounding factor in the prevalence of MBS, lean PCOS were compared with lean controls and obese PCOS were compared to obese controls [Table 4].
Table 4.
Anthropometric and biochemical parameters, and prevalence of MBS in women with normal and high BMI in relation to PCOS
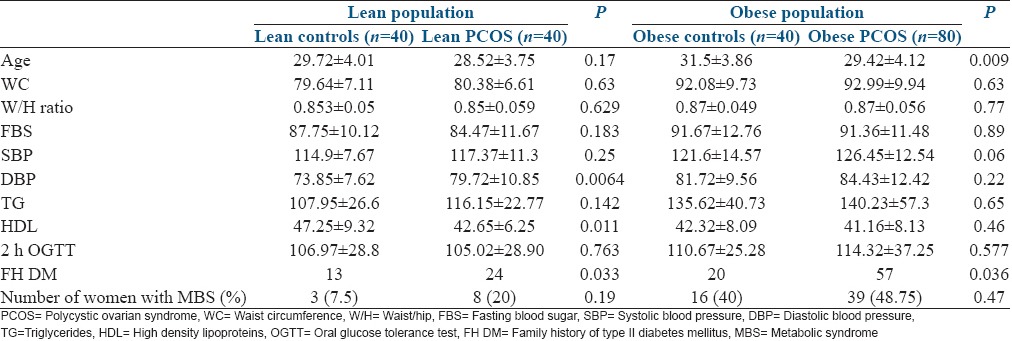
Within the lean population, the presence of PCOS had an impact only on diastolic BP and HDL as compared to the controls. All the other parameters were not significantly different in the PCOS and controls in the lean population. For the obese population (BMI >23 kg/m2), when the obese PCOS were compared with the obese controls, no statistically significant difference was found in the prevalence of any of the studied parameters.
The family history of type II diabetes mellitus was significantly higher in the women with PCOS in both lean and obese populations (P - 0.033 and 0.036, respectively).
It is evident that the women with PCOS had a higher prevalence of MBS, as compared to the controls both in lean and obese populations, although it failed to achieve statistical significance, probably due to a small sample size [Table 4]. Table 5 shows the relative risk of MBS in correlation with BMI and underlying PCOS. In the third step of the analysis, we intended to find out a cutoff level of BMI at which counseling should be done for lifestyle modification to reduce the risk of MBS. ROC curves were obtained for the prevalence of MBS in relation to BMI, for the women with and without PCOS separately [Figures 1 and 2].
Table 5.
Relative risk of MBS in relation to PCOS and high BMI
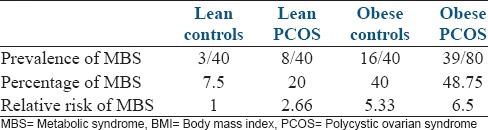
Figure 1.
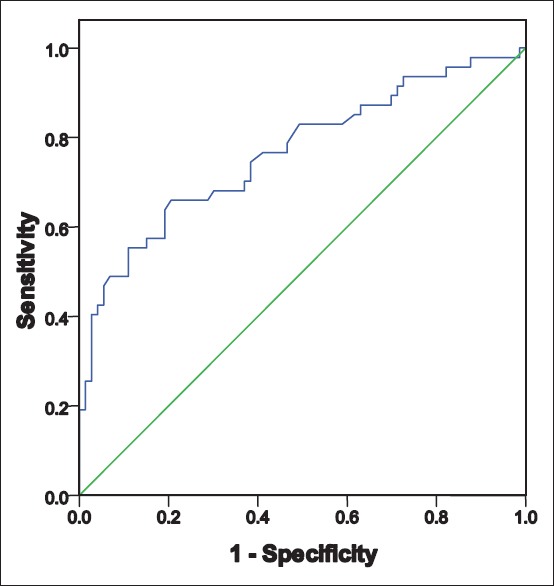
Women with polycystic ovarian syndrome: Receiver operator characteristics curve to predict metabolic syndrome in relation to body mass index area under the curve - 0.766 Sig - 0.000 95% confidence interval (0.675–0.875)
Figure 2.
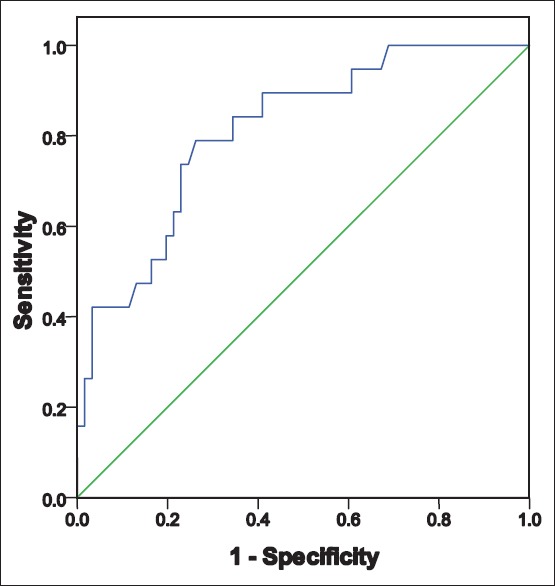
Controls (women without polycystic ovarian syndrome): Receiver operator characteristics curve to predict metabolic syndrome in relation to body mass index area under the curve-0.811 Sig- 0.000 95% confidence interval (0.706–0.917)
In both, women with and without PCOS, BMI could independently predict the risk of MBS (area under the curve [AUC] being 0.766 and 0.811). However, the prediction was stronger in the control group, as we can see that AUC 0.811 (for ROC of women without PCOS) is more than AUC 0.766 (for ROC of women with PCOS). More the AUC, higher the prediction and association. This is probably because PCOS itself is a risk factor for the development of MBS and independently influences its prevalence.
The analysis of the coordinates of the ROC curves for women with and without PCOS is given in Figures 3 and 4. To avoid missing out on women with actual risk of MBS, the cut-off level of BMI should have high sensitivity with a balanced (or lower) specificity. On analyzing the coordinates of the ROC curves, the most appropriate cut-off level of BMI for predicting MBS in women without PCOS seems to be 23 kg/m2, with a sensitivity of about 90% and a specificity of about 60% [Figure 4]. In order to achieve the same sensitivity for the risk of MBS, the cut-off levels of BMI in women with PCOS had to be brought down to 22.5 kg/m2 [Figure 3]. This clearly indicates that the women with PCOS have a risk of developing MBS at a lower level of BMI as compared to the controls and should be advised lifestyle modification at a lower BMI in order to minimize the risk.
Figure 3.
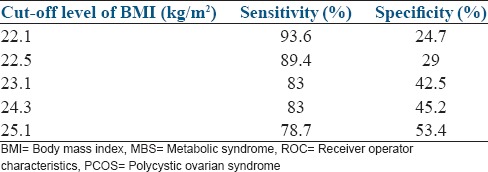
Co-ordinates of the ROC curve (BMI vs MBS) in women with PCOS
Figure 4.
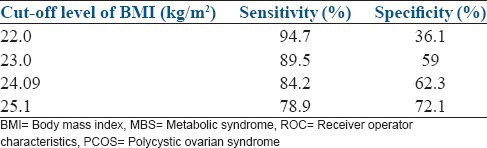
Co-ordinates of the ROC curve (BMI vs MBS) in women without PCOS (controls)
DISCUSSION
In this study, the prevalence of MBS was significantly higher in the women with PCOS as compared to the controls, which is favorably comparable to the studies from the USA[8,9] and Indian population [Table 6].[14]
Table 6.
Prevalence of MBS in different studies
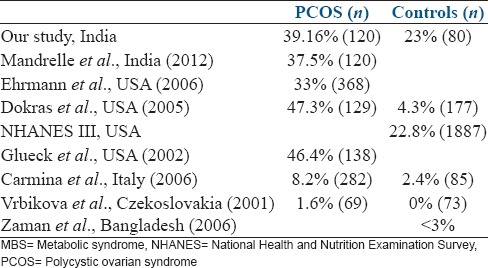
In contradiction to the present study, the prevalence of MBS (in women with and without PCOS) is significantly lower in the studies from European population.[11,12] Vrbikova reported the prevalence of MBS as 1.6% in women with PCOS and 0% in controls from Czech population. A logical explanation to this difference could be a lower BMI (23 and 21.9 kg/m2 for PCOS and controls) and waist circumference (74 and 68.2 cm in PCOS and controls) in the studied Czech population, as compared to our study population. Similarly, Zaman et al. observed that the prevalence of MBS in rural Bangladeshi population is <3%.[13] This could be explained by the fact that their study group was primarily an agricultural population with high level of physical activity, as compared to the controls in our present study. This difference in the prevalence of MBS can further be explained by the high levels of triglycerides and fasting blood sugar, a lower concentration of HDL-C and higher prevalence of insulin resistance in the Bangladeshi immigrants to the UK, which could be attributed to increased stress, smoking, and physical inactivity.[17] The prevalence of MBS in the Indian population, as reported by different authors varies between 30.9% and 41.1%.[18,19] However, in both of these studies, neither the women with PCOS have been excluded from the studied population, nor has BMI has been addressed in relation to the prevalence of MBS. Thus, the prevalence of PCOS and high BMI could have confounded in the development of MBS, explaining the differently reported prevalence in different studies.
The women with PCOS had a higher BMI, waist circumference, systolic and diastolic BPs, and lower HDL-C levels, as compared to the controls. These observations were in accordance with other studies.[12,20,21,22]
From Table 7, it is clearly evident that the prevalence of MBS in a given population of PCOS is dependent on the anthropometric and biochemical parameters prevalent in that given population, and waist circumference is one of these important factors.
Table 7.
Prevalence of individual components of MBS (expressed as percentage)
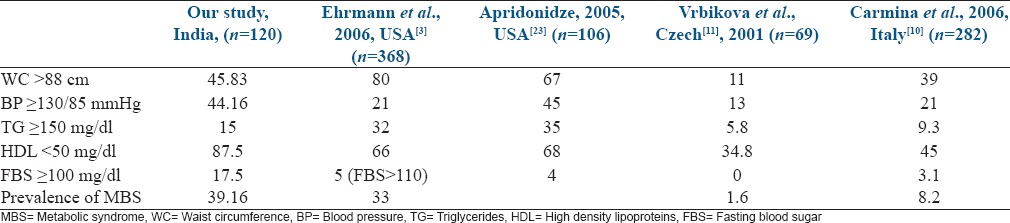
In this present study, we analyzed PCOS and high BMI separately as risk factors contributing to the development of MBS and found that both are independent risk factors, obesity being a stronger one. This is in accordance with the study of St-Onge et al., where MBS in the general population increased in a graded fashion as the BMI increased slightly, even to the high normal range.[24] Similarly Conway et al. also observed that hyperinsulinemia is more prevalent in women with PCOS, but the addition of obesity as a risk factor is associated with higher systolic BP, serum triglyceride, and plasma glucose concentration than lean women with PCOS and controls.[20]
It is known that PCOS, age, BMI and associated hyperinsulinemia are the common risk factors for the development of MBS.[4,5,6,7] Among these, BMI is the only risk factor which is modifiable, and its reduction may also partly correct the element of hyperinsulinemia. Thus, we attempted to find out a cut-off point of BMI, above which the risk of MBS increases and lifestyle modification becomes advisable. According to the 1993, WHO expert committee, a BMI of 25 kg/m2 was selected as a cut-off between normal and overweight.[25] However, there was an increasing evidence of high prevalence of type II diabetes mellitus and cardiovascular risk factors in parts of Asia where the average BMI is below the cut-off point of 25 kg/m2. This led WHO to convene another expert consultation on BMI classifications, and a cut-off of 23 kg/m2 was chosen as a public health action point.[16] According to the present study, a BMI of 23 kg/m2, when chosen as a cut-off point, had a sensitivity of approximately 90% in diagnosing the individuals at risk of MBS in a normal population, whereas, to maintain the same sensitivity in the women with PCOS, the cut-off had to be brought down to 22.5 kg/m2. This indicates the higher risk of developing MBS in women with PCOS at a lower BMI as compared to normal population and a need for lifestyle modification at a lower BMI, to reduce the long-term health consequences. However, larger multicentric studies with a bigger sample size are required for further supporting the findings of this study.
CONCLUSIONS
MBS is more prevalent in women with PCOS as compared to controls. Thus, PCOS is a risk factor for the development of MBS. However, obesity is an independent and stronger risk factor, as compared to PCOS. In order to reduce the risk of MBS and the long-term health consequences which may be related to it, lifestyle modification is advisable above a BMI of 23 kg/m2 in the normal population and 22.5 kg/m2 in women with PCOS.
Future prospects
Long-term prospective studies are required to see how this increased prevalence of MBS in PCOS and in slightly overweight individuals, translates to end-point cardiovascular events. The impact of weight loss and physical activity in this population group needs to be studied prospectively.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would offer gratitude to Dr. M. Kochar, Dr. S. Talwar, Dr. S. Mittal and Dr. N. Tiwari for their generous help and constant inspiration. We are thankful to Dr. R. Satwik for helping in the statistical analysis. Dr. D. Inamdar is appreciated for the final editing of this article. The authors would like to thank all the women who participated in this study.
REFERENCES
- 1.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: A prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Vignesh JP, Mohan V. Polycystic ovary syndrome: A component of metabolic syndrome? J Postgrad Med. 2007;53:128–34. doi: 10.4103/0022-3859.32217. [DOI] [PubMed] [Google Scholar]
- 4.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427–36. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez Cosmea A, López Fernández V, Suárez García S, Arias García T, Prieto Díaz MA, Díaz González L. Differences in the prevalence of metabolic syndrome according to the ATP-III and WHO definitions. Med Clin (Barc) 2005;124:368–70. doi: 10.1157/13072570. [DOI] [PubMed] [Google Scholar]
- 6.Seidell JC. Obesity, insulin resistance and diabetes – A worldwide epidemic. Br J Nutr. 2000;83(Suppl 1):S5–8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]
- 7.Lobo RA, Carmina E. The importance of diagnosing the polycystic ovary syndrome. Ann Intern Med. 2000;132:989–93. doi: 10.7326/0003-4819-132-12-200006200-00010. [DOI] [PubMed] [Google Scholar]
- 8.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. PCOS/Troglitazone Study Group. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 9.Dokras A, Bochner M, Hollinrake E, Markham S, Vanvoorhis B, Jagasia DH. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005;106:131–7. doi: 10.1097/01.AOG.0000167408.30893.6b. [DOI] [PubMed] [Google Scholar]
- 10.Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–15. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 11.Carmina E, Napoli N, Longo RA, Rini GB, Lobo RA. Metabolic syndrome in polycystic ovary syndrome (PCOS): Lower prevalence in southern Italy than in the USA and the influence of criteria for the diagnosis of PCOS. Eur J Endocrinol. 2006;154:141–5. doi: 10.1530/eje.1.02058. [DOI] [PubMed] [Google Scholar]
- 12.Vrbíková J, Vondra K, Cibula D, Dvoráková K, Stanická S, Srámková D, et al. Metabolic syndrome in young Czech women with polycystic ovary syndrome. Hum Reprod. 2005;20:3328–32. doi: 10.1093/humrep/dei221. [DOI] [PubMed] [Google Scholar]
- 13.Zaman MM, Ahmed J, Choudhury SR, Numan SM, Islam MS, Parvin K. Prevalence of metabolic syndrome in rural Bangladeshi women. Diabetes Care. 2006;29:1456–7. doi: 10.2337/dc06-0431. [DOI] [PubMed] [Google Scholar]
- 14.Mandrelle K, Kamath MS, Bondu DJ, Chandy A, Aleyamma T, George K. Prevalence of metabolic syndrome in women with polycystic ovary syndrome attending an infertility clinic in a tertiary care hospital in south India. J Hum Reprod Sci. 2012;5:26–31. doi: 10.4103/0974-1208.97791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 16.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 17.Bhopal R, Unwin N, White M, Yallop J, Walker L, Alberti KG, et al. Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: Cross sectional study. BMJ. 1999;319:215–20. doi: 10.1136/bmj.319.7204.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta R, Deedwania PC, Gupta A, Rastogi S, Panwar RB, Kothari K. Prevalence of metabolic syndrome in an Indian urban population. Int J Cardiol. 2004;97:257–61. doi: 10.1016/j.ijcard.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Metabolic syndrome in urban Asian Indian adults – A population study using modified ATP III criteria. Diabetes Res Clin Pract. 2003;60:199–204. doi: 10.1016/s0168-8227(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 20.Conway GS, Agrawal R, Betteridge DJ, Jacobs HS. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;37:119–25. doi: 10.1111/j.1365-2265.1992.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 21.Talbott E, Clerici A, Berga SL, Kuller L, Guzick D, Detre K, et al. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: Results of a case-control study. J Clin Epidemiol. 1998;51:415–22. doi: 10.1016/s0895-4356(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 22.Talbott E, Guzick D, Clerici A, Berga S, Detre K, Weimer K, et al. Coronary heart disease risk factors in women with polycystic ovary syndrome. Arterioscler Thromb Vasc Biol. 1995;15:821–6. doi: 10.1161/01.atv.15.7.821. [DOI] [PubMed] [Google Scholar]
- 23.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–35. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 24.St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans: New definition of the metabolically obese, normal-weight individual. Diabetes Care. 2004;27:2222–8. doi: 10.2337/diacare.27.9.2222. [DOI] [PubMed] [Google Scholar]
- 25.Geneva: World Health Organization; 1995. WHO. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Consultation. WHO Technical Report Series Number. 854. [PubMed] [Google Scholar]


