Abstract
BACKGROUND:
EmbryoGlue is a hyaluronan-enriched embryo transfer (ET) medium which aids in implantation of embryos, hence, improves pregnancy rates in in-vitro fertilization-ET cycles (IVF-ET).
AIM:
To evaluate the role of EmbryoGlue in improving implantation and pregnancy rates.
DESIGN AND SETTING:
A prospective case–control study conducted at assisted reproductive center of a tertiary care hospital.
METHOD:
In 42 women undergoing IVF, embryos were transferred into 50 μL of EmbryoGlue for 10 min prior to transfer inside uterine cavity. In the control group (n = 42), embryos were transferred to conventional blastocyst culture medium. Statistical analysis was performed using SPSS IBM version 19.0.
RESULTS:
Clinical pregnancy rate in the study group was 7% higher than the control group. The difference, however, was not statistically significant. In addition, no improvement in implantation rates was observed in the study group. However, significant difference (P = 0.04) in clinical pregnancy rate was observed with the EmbryoGlue in patients with previous IVF failure. In the study group, 50% patients (6/12) with previous IVF failure had successful implantation, but in the control group none of the patients (0/11) with previous implantation failure could achieve pregnancy.
CONCLUSION:
It is difficult to conclude a favourable role of EmbryoGlue in IVF-ET cycles with a good prognosis. However, in patients with recurrent implantation failure, it may be considered as a useful transfer medium.
KEY WORDS: Embryo transfer, hyaluronan, implantation, in-vitro fertilization, pregnancy, recurrent implantation failure
INTRODUCTION
Assisted reproductive technologies have become an integral part of infertility treatment in the present decade. To increase the efficacy of in-vitro fertilization/embryo transfer (IVF-ET) cycles, improvements have been made in techniques of ovarian stimulation, fertilization, culture and transfer of embryos. Despite these advances, implantation of embryos still presents a challenge to the clinician. Implantation is a multistage process which includes zona hatching, apposition and adhesion of blastocyst followed by an invasion of trophoblast into the uterine endometrium. There are several reasons why embryos fail to implant despite a favourable transfer. One of the reasons for unsuccessful implantation being failure to develop a sticky matrix between blastocyst and endometrium. Various modifications have been made in ET medium to improve implantation and pregnancy rates. Protein supplementation has been widely used in ET medium, most common of which is albumin.[1] Albumin is present abundantly in the female reproductive tract and serves as a source of energy, hormones, vitamins, and metals. In addition, albumin not only provides viscosity to culture medium, but also acts as a lubricant promoting easy handling and preventing adherence of embryos to the culture dish.[2] Hyaluronan (HA) supplementation of culture medium was introduced to improve implantation and pregnancy rate in IVF-ET cycles.[3] HA, a glycosaminoglycan, is present in the oviduct and uterine fluid and increases at the time of implantation. EmbryoGlue is a human ET medium, which contains high concentration of HA (0.5 mg/mL) and low concentration of recombinant human albumin (rHA = 2.5 mg/mL). Standard blastocyst culture medium (G-2™, Vitrolife, Sweden) is supplemented with 10 mg/mL of rHA and a lower concentration of HA (0.125 mg/mL) compared to EmbryoGlue. G-2™ medium also contains ethylenediaminetetraacetic acid (EDTA), which is absent in EmbryoGlue. EmbryoGlue is supplemented with both nonessential and essential amino acids, whereas G-2™ contains only nonessential amino acids. EDTA is required for cleavage stage embryos, but inhibits blastocyst development.[4] Similarly, only nonessential amino acids promote the development of cleavage stage embryos, whereas both essential and nonessential amino acids are required for blastocyst development.[5,6] Hence, EmbryoGlue is thought to be the ideal medium for blastocyst development. Accordingly, the present case–control clinical trial was undertaken to evaluate the efficacy of EmbryoGlue as a human ET medium and to compare the pregnancy outcomes versus those of G-2™ medium.
MATERIALS AND METHODS
Ethical approval was taken from the Institute's Ethics Committee prior to the initiation of the study.
Type of study
Prospective, case–control clinical trial.
Study population and site
Women in the reproductive age group undergoing IVF at Assisted Reproductive Unit in the Department of Obstetrics and Gynaecology at a tertiary care center were enrolled in this study.
Study duration
The study was conducted over a period of 3 months between March and May 2014.
Sample size
Eighty-four women undergoing IVF were included in this study. In 42 women undergoing IVF or intracytoplasmic sperm injection (ICSI), embryos were transferred to HA-enriched transfer medium. In the control group (n = 42), embryos were transferred to conventional blastocyst culture medium. Women aged >35 years, those with poor ovarian reserve, and possible causes for failure of implantation such as diabetes mellitus, hypertension, and autoimmune diseases were excluded from the study.
Ovarian stimulation
For ovarian stimulation, we either used the gonadotropin-releasing hormone-agonist (GnRH) “long protocol” or flexible GnRH-antagonist regimen. In the long protocol, pituitary down-regulation was achieved with injectable GnRH-agonist, leuprolide acetate (Luprofact; Bayer Zydus, Mumbai, India) 1 mg/day subcutaneous (s.c) from the midluteal phase prior to the treatment cycle. Gonadotropins, most commonly Recombinant Follicle Stimulating Hormone (Gonal F; Merck Serono, Italy) injected s.c. or rarely intramuscular (i.m.) human menopausal gonadotropin (Humog; Bharat Serums and Vaccines Limited, Ambernath) was used for ovarian stimulation after adequate down-regulation was achieved, that is, serum estradiol <30 pg/ml and luteinizing hormone <2 IU/L. During stimulation, the dose of leuprolide acetate was reduced to 0.5 mg/day. In the flexible GnRH-antagonist protocol, Cetrorelix acetate (Cetrotide; Merck Serono, Italy) 0.25 mg/day was administrated s.c in the stimulated cycle when the diameter of leading follicle reached 14 mm. Recombinant human chorionic gonadotropin (hCG, Ovitrelle; Merck Serono, Italy) 250 μg was administered s.c for inducing ovulation when 2–3 follicles were more than 18 mm in size. Oocyte retrieval was performed 34–36 h after hCG administration under ultrasound guidance. Retrieved oocytes were fertilized either by conventional IVF or ICSI technique. Embryos were cultured in sequential media using the “G-series” produced by Vitrolife (Goteborg, Sweden).
Embryo transfer
Embryos were transferred on day 2–5 depending on the number and quality of embryos. The best or good quality embryos were selected for intrauterine transfer (maximum 3–4 embryos in each ET cycle). In the control group, the selected embryos were transferred into 50 μl of G-2™ culture medium, whereas in the test group, they were transferred into 50 μl of EmbryoGlue (Vitrolife, Sweden). The embryos were incubated for at least 10 min in the transfer medium in an environment of 6% CO2 at 37°C prior to transfer into uterine cavity. ET was performed using a Labotect catheter (Labotect, Germany). Luteal phase support in the form of progesterone 100 mg i.m. or vaginal pessary 300 mg twice daily was administered to both the groups.
Outcome measures
To calculate the fertilization rate, we divided the number of fertilized zygotes by the total number of oocytes in conventional IVF or injected metaphase II oocytes in ICSI. We also checked the serum beta-hCG in all women on day 14 of transfer. Those with positive beta-hCG underwent sonography for confirmation of clinical pregnancy. The number of gestational sacs divided by the number of embryos transferred was used to calculate the implantation rate. Follow-up of pregnancy was done until 20 weeks period of gestation.
Data analysis
Statistical analysis was performed using SPSS IBM version 19.0 (Armonk, NY: IBM Corp). Descriptive statistics such as mean, median, standard deviation, and range values were calculated. Mean values of normally distributed data were compared using Student's t-independent test. For nonnormal distributed data, median values were compared using Mann–Whitney U-test. Frequency data of categorical variables were compared using Chi-square/Fisher's exact test as appropriate. For all statistical tests, P < 0.05 was considered for statistical significance. Main outcome measures were clinical pregnancy rate and implantation rate.
RESULTS
A total of 84 patients undergoing IVF-ET cycle participated in this study. The overall clinical pregnancy rate was 27.4%. In 42 patients, HA-enriched EmbryoGlue was used as a transfer medium and embryos of similar number of age-matched controls were transferred to conventional G-2™ medium. Patient characteristics were as depicted in Table 1. There was no significant difference in between the two groups in relation to mean age, indication of infertility, number of days of stimulation, total dose of gonadotropins, serum estradiol levels on day of trigger, and number of oocytes collected. The frequency of IVF and ICSI cycles was similar in both the groups. There was also no difference among the number of oocytes fertilized by IVF/ICSI, the fertilization rate, and the mean number of embryos transferred. The clinical pregnancy rate was slightly higher in the study group compared to the control group (31% vs. 23.8%). The difference, however, was not statistically significant [Table 2]. There was no difference in the rate of multiple pregnancy and implantation rate between the two groups. In the study group, nine patients who conceived had day 5 embryos transferred, whereas four had day 3. In the control group, six pregnancies were with day 5 embryos and four with day 3. The difference in pregnancy rate between day 5 and day 3 embryos was not significant. The pregnant patients were followed until 20 weeks period of gestation. One patient in the study group had a missed abortion at 8 weeks period of gestation. Rest of the pregnancies were uneventful until the follow-up period. In the study group, there were 12 patients with recurrent implantation failure, out of which 6 (50%) patients had successful implantation. Whereas in the control group, there were 11 patients with recurrent implantation failure and none could achieve pregnancy. The difference in implantation rate was significant (P = 0.04) between the two groups in patients with prior implantation failure.
Table 1.
Patient characteristics in both the groups
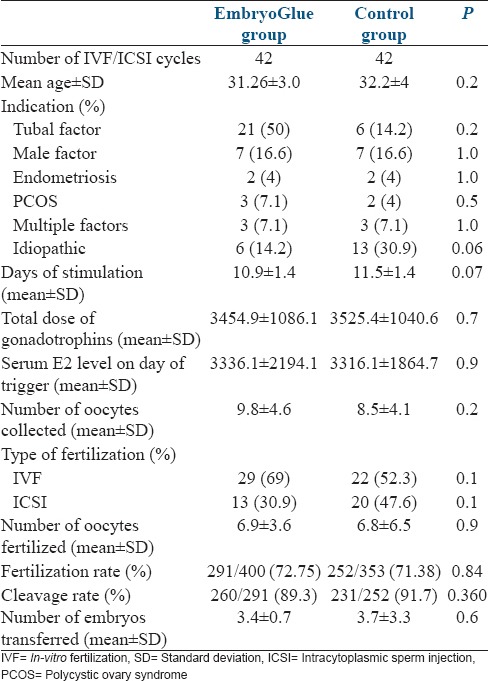
Table 2.
Clinical outcome in two groups

DISCUSSION
HA-enriched culture medium is hypothesized to be best suited for blastocyst development. EmbryoGlue contains higher concentration of HA and lower concentration of albumin. HA is a high molecular weight linear polysaccharide, comprised of repetitive units of n-acetyl-D-glucosamine and d-glucuronic acid.[7] HA interacts in autocrine and paracrine manner and also with certain cell surface receptors such as CD 44 expressed on preimplantation embryo and in the endometrium.[8] HA is known to be involved in physiological processes such as embryological development, migration, adhesion, proliferation, and differentiation of cells. It also indirectly promotes angiogenesis.[1] By virtue of these properties, it improves apposition and attachment of embryos which are key steps in the process of implantation. It also increases viscosity, which promotes easy handling, facilitating ET process, and by virtue of apposition, it prevents expulsion of embryos from uterine cavity posttransfer. Simon et al.[1] found better pregnancy and implantation rate with HA-enriched media compared to albumin supplementation, though the difference was not significant and the sample size was small. Several other studies have also demonstrated higher pregnancy and implantation rate with HA-enriched media.[9,10,11,12]
In our study, there was no significant difference in pregnancy and implantation rate with EmbryoGlue. However, the clinical pregnancy rate was 7% higher in the glue group. Similar finding of higher clinical pregnancy, but without statistical significance has been demonstrated in several studies which could be due to small sample sizes in these studies.[13,14,15,16] Fancsovits et al.[16] used HA-enriched media in 290 patients and found 3% higher clinical pregnancy rate in the study group, though the difference was not significant. Addition of HA is supposed to improve implantation of embryos; hence, if more number of embryos are transferred, chances of multiple pregnancy increases. Majority of the studies did not report significant difference in multiple pregnancy rate in between the two groups.[1,9,17] However, Urman et al.[10] reported 15% higher multiple pregnancy rate with HA-enriched media. We did not find any significant difference in multiple pregnancy rate between the two groups in our study.
When patients with previous IVF failure were evaluated in the study group, it was found that 50% had successful implantation with HA-enriched media compared to the control group. Our finding of improved implantation in recurrent IVF failure is in agreement with few other studies which included patients with recurrent IVF failure.[14,18] Beneficial effect of HA-enriched media in patients of advanced age and recurrent implantation failure has been demonstrated by Urman et al.[10] We excluded patients >35 years; hence, we cannot comment on implantation rate in elderly patients.
We can conclude that the use of EmbryoGlue is advantageous in patients with recurrent implantation failure. However, a large randomized control trial is required to evaluate its role in all patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the colleagues who supported us while conducting this study. We also acknowledge the patients who participated in the study.
REFERENCES
- 1.Simon A, Safran A, Revel A, Aizenman E, Reubinoff B, Porat-Katz A, et al. Hyaluronic acid can successfully replace albumin as the sole macromolecule in a human embryo transfer medium. Fertil Steril. 2003;79:1434–8. doi: 10.1016/s0015-0282(03)00349-2. [DOI] [PubMed] [Google Scholar]
- 2.Leese HJ. The formation and function of oviduct fluid. J Reprod Fertil. 1988;82:843–56. doi: 10.1530/jrf.0.0820843. [DOI] [PubMed] [Google Scholar]
- 3.Stojkovic M, Kölle S, Peinl S, Stojkovic P, Zakhartchenko V, Thompson JG, et al. Effects of high concentrations of hyaluronan in culture medium on development and survival rates of fresh and frozen-thawed bovine embryos produced in vitro. Reproduction. 2002;124:141–53. [PubMed] [Google Scholar]
- 4.Gardner DK, Lane MW, Lane M. EDTA stimulates cleavage stage bovine embryo development in culture but inhibits blastocyst development and differentiation. Mol Reprod Dev. 2000;57:256–61. doi: 10.1002/1098-2795(200011)57:3<256::AID-MRD7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Lane M, Gardner DK. Nonessential amino acids and glutamine decrease the time of the first three cleavage divisions and increase compaction of mouse zygotes in vitro. J Assist Reprod Genet. 1997;14:398–403. doi: 10.1007/BF02766148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane M, Hooper K, Gardner DK. Effect of essential amino acids on mouse embryo viability and ammonium production. J Assist Reprod Genet. 2001;18:519–25. doi: 10.1023/A:1016657228171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott JE, Heatley F. Biological properties of hyaluronan in aqueous solution are controlled and sequestered by reversible tertiary structures, defined by NMR spectroscopy. Biomacromolecules. 2002;3:547–53. doi: 10.1021/bm010170j. [DOI] [PubMed] [Google Scholar]
- 8.Campbell S, Swann HR, Aplin JD, Seif MW, Kimber SJ, Elstein M. CD44 is expressed throughout pre-implantation human embryo development. Hum Reprod. 1995;10:425–30. doi: 10.1093/oxfordjournals.humrep.a135955. [DOI] [PubMed] [Google Scholar]
- 9.Friedler S, Schachter M, Strassburger D, Esther K, Ron El R, Raziel A. A randomized clinical trial comparing recombinant hyaluronan/recombinant albumin versus human tubal fluid for cleavage stage embryo transfer in patients with multiple IVF-embryo transfer failure. Hum Reprod. 2007;22:2444–8. doi: 10.1093/humrep/dem220. [DOI] [PubMed] [Google Scholar]
- 10.Urman B, Yakin K, Ata B, Isiklar A, Balaban B. Effect of hyaluronan-enriched transfer medium on implantation and pregnancy rates after day 3 and day 5 embryo transfers: A prospective randomized study. Fertil Steril. 2008;90:604–12. doi: 10.1016/j.fertnstert.2007.07.1294. [DOI] [PubMed] [Google Scholar]
- 11.Hambiliki F, Ljunger E, Karlström PO, Stavreus-Evers A. Hyaluronan-enriched transfer medium in cleavage-stage frozen-thawed embryo transfers increases implantation rate without improvement of delivery rate. Fertil Steril. 2010;94:1669–73. doi: 10.1016/j.fertnstert.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa K, Takahashi C, Nishi Y, Jyuen H, Sugiyama R, Kuribayashi Y, et al. Hyaluronan-enriched transfer medium improves outcome in patients with multiple embryo transfer failures. J Assist Reprod Genet. 2012;29:679–85. doi: 10.1007/s10815-012-9758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loutradi KE, Prassas I, Bili E, Sanopoulou T, Bontis I, Tarlatzis BC. Evaluation of a transfer medium containing high concentration of hyaluronan in human in vitro fertilization. Fertil Steril. 2007;87:48–52. doi: 10.1016/j.fertnstert.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 14.Korosec S, Virant-Klun I, Tomazevic T, Zech NH, Meden-Vrtovec H. Single fresh and frozen-thawed blastocyst transfer using hyaluronan-rich transfer medium. Reprod Biomed Online. 2007;15:701–7. doi: 10.1016/s1472-6483(10)60538-x. [DOI] [PubMed] [Google Scholar]
- 15.Hazlett WD, Meyer LR, Nasta TE, Mangan PA, Karande VC. Impact of EmbryoGlue as the embryo transfer medium. Fertil Steril. 2008;90:214–6. doi: 10.1016/j.fertnstert.2007.05.063. [DOI] [PubMed] [Google Scholar]
- 16.Fancsovits P, Lehner A, Murber A, Kaszas Z, Rigo J, Urbancsek J. Effect of hyaluronan-enriched embryo transfer medium on IVF outcome: A prospective randomized clinical trial. Arch Gynecol Obstet. 2015;291:1173–9. doi: 10.1007/s00404-014-3541-9. [DOI] [PubMed] [Google Scholar]
- 17.Sifer C, Mour P, Tranchant S, Visentin E, Hafhouf E, Sermondade N, et al. Is there an interest in the addition of hyaluronan to human embryo culture in IVF/ICSI attempts? Gynecol Obstet Fertil. 2009;37:884–9. doi: 10.1016/j.gyobfe.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Valojerdi MR, Karimian L, Yazdi PE, Gilani MA, Madani T, Baghestani AR. Efficacy of a human embryo transfer medium: A prospective, randomized clinical trial study. J Assist Reprod Genet. 2006;23:207–12. doi: 10.1007/s10815-006-9031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


