Abstract
CONTEXT:
Several studies have reported that thrombophilia is responsible for recurrent pregnancy loss (RPL).
AIMS:
The aim of this study was to evaluate the prevalence and role of inherited thrombophilia in early pregnancy loss, specifically in the first trimester.
MATERIALS AND METHODS:
A total of 104 women (patients) with a history of two or more miscarriages during the first trimester of pregnancy and 110 women (controls) who had experienced two or more births without a miscarriage were included in this study. In both groups, we determined the biological activities of antithrombin III (ATIII) and protein C (PC) using the chromogenic method and the biological activity of protein S (PS) and the activated protein C resistance (APCR) were examined using a clotting method.
RESULTS:
In the patient group, deficiencies of ATIII, PC, and PS were detected in 3 (2.88%), 4 (3.85%), and 6 (5.77%) cases, respectively. In the control group, ATIII (0%) deficiencies were not detected, and deficiencies for PC (0.9%) and PS (0.9%) were each detected in 1 patient. APCR was detected in 9 patients (8.65%) and 4 control subjects (3.63%).
CONCLUSION:
Based on our results, we can conclude that thrombophilia is a causal factor for miscarriages in the first trimester of pregnancy, although there are the conflicting data in the literature.
KEY WORDS: Activated protein C resistance, antithrombin III, early pregnancy loss, protein C, protein S, repeated miscarriages, thrombophilia
INTRODUCTION
Many studies have demonstrated that acquired or inherited thrombophilia is associated with RPL.[1,2,3] The risk of fetal loss increases throughout pregnancy and may be higher in the second and third trimesters because the levels of some clotting factors (I, VII, VIII, and X) increase, protein S (PS) and fibrinolytic activity decrease, and resistance to activated protein C resistance (APCR) develops.[4] Mutations in the genes for Factor V Leiden (FVL), coagulation factor II (FII) (the prothrombin gene), and methylenetetrahydrofolate reductase (MTHFR) are among the most common inherited disorders responsible for inherited thrombophilia and spontaneous abortions.[5] Additionally, antithrombin III (ATIII), protein C (PC), or PS deficiencies are important causes of thrombophilia, which is associated with a high risk of venous thromboembolisms during pregnancy.[6] Women should be screened for thrombophilia because unexplained first pregnancy loss occurs in approximately 10% of pregnancies.[7,8] Recurrent pregnancy loss (RPL), with two or more losses occurs in up to 5% of women of reproductive age and is a major health problem.[9] Recurrent miscarriage status is typically defined as the loss of three or more consecutive pregnancies prior to 20 or even 28 weeks of pregnancy.[10] However, within this definition is a large, heterogeneous group of patients who exhibit many causes of miscarriage. A normal pregnancy outcome requires an efficient uteroplacental vascular system,[11,12] and a thrombosis in a decidual vessel may cause intrauterine growth retardation, fetal death, and possibly recurrent miscarriages.[13] Early pregnancy loss is the most common pregnancy complication and acquired, and inherited thrombophilia conditions are associated with a risk of pregnancy failure.[14,15] In this study, we analyzed the prevalence of APCR and deficiencies of major natural anticoagulant (ATIII, PC, and PS) in women with recurrent miscarriages in Kosovo. Although some data have indicated that inherited thrombophilia can cause more complications of pregnancy in the second and third trimesters of pregnancy, we studied women with a history of miscarriages in the first trimester of pregnancy.
MATERIALS AND METHODS
The study group (patient group) comprised 104 women with a history of two or more miscarriages during the first trimester of pregnancy, whereas the control group included 110 women who had pregnancy outcomes of two or more births without a miscarriage. The cases in the patient group were referred to our center between December 2012 and December 2014 for thrombophilia testing. The study included all women who had spontaneous abortions during the 2-year period (2012–2014) and did not exclude any patient who had not been previously examined for the most frequent causes of spontaneous abortions in the first trimester of pregnancy, including chromosomal disorders, infections, anatomical abnormalities, and endocrine dysfunction. The exclusion criterion for both groups was current pregnancy. Blood samples were taken with 3.2% sodium citrate in a 9:1 blood to citrate ratio and were centrifuged at 2000 rpm for 15 min to separate the plasma, which was quick-frozen and maintained at −35°C until testing. The study was approved by the Ethics Committee, and each patient and control provided written informed consent before inclusion in the study.
For all subjects, we performed the following screening tests for hemostasis: The Prothrombin Time, Activated Partial Thromboplastin Time, and Thrombin Time. ATIII and PC were measured by a chromogenic method using TEChrom AT (anti-Xa) and TEChrom PC (TECO GmbH, Neufahn NB Germany) and an automatic coagulometer (Coatron 6). PS and APCR were measured by a clotting method using the Coatron 6 and a semi-automatic coagulometer (Amelung KC4) with commercial reagents (TEClot Protein S and TEClot PCA Ratio, TECO GmbH).
Statistical analysis
The odds ratio (OR) and 95% confidence interval were estimated separately for each inhibitor, and APCR was analyzed with the Chi-square test in Instat 3.0 software (GraphPad Software, San Diego, CA, USA). Data were compared with the t-test and the difference was significant if P < 0.05.
RESULTS
Natural coagulation inhibitors (ATIII, PC, and PS) and APCR were evaluated in 104 women (the patient group) with a history of two or more miscarriages in the first trimester of pregnancy and in 110 women (the control group) with two or more normal births without a miscarriage. The average age of the patients was 31.31 years (standard deviation [SD] 5.2), with a range of 23–42 and median of 31.0, whereas the average age in the control group was 30.62 years (SD 3.65) with a range of 23–36 and a median of 32.0. In the patient group, 58 (55.77%) women had a history of 2 miscarriages, 34 (32.70%) had a history of 3 miscarriages and 12 (11.53%) had a history of more than 3 miscarriages.
The prevalence of ATIII, PC, and PS deficiencies and APCR are presented in Table 1. In the patient group, the prevalence of ATIII, PC, and PS deficiencies and APCR were as follows: 2.88% (3/104), 3.85% (4/104), 5.77% (6/10), and 8.65% (9/104), respectively. In the control group, the prevalence of ATIII deficiency was 0% (0/104), whereas a prevalence of 0.9% was found for both PC and PS deficiencies (1/110 each). The control group exhibited a greater prevalence of APCR (3.63% or 4/110). The ORs for PC, PS, and APCR deficiency and for total defects are 4.36 (0.48–39.7), 6.67 (0.78–56), 2.51 (0.77–8.41), and 5.78 (2.27–14.71), respectively.
Table 1.
The prevalence of antithrombin III, protein C and protein S deficiencies and the prevalence of APCR in the women with RLP and in the controls
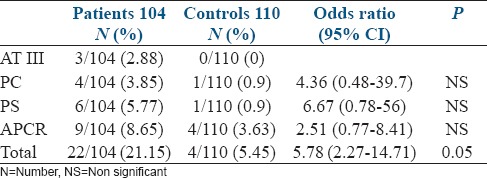
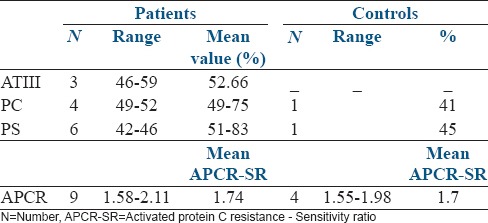
Table 2 presents the number of cases of both groups with ATIII, PC, and PS deficiencies and APCR as well the range and average value of the deficiencies. In both groups, we observed that heterozygote deficiencies were involved in ATIII, PC, and PS deficiencies. The same finding applies to the cases with APCR because the APCR-SR values in the patients ranged from 1.58 to 2.11, whereas the range in the control group was 1.55–1.98. With the methods we used, the normal values of APCR-SR are higher than 2.2 (>2.2), whereas the normal values for ATIII, PC, and PS are adjusted to 70–135%. Our results indicate that the mean values for ATIII, PC, and PS deficiencies and for APCR were not significantly different between the patients and controls. Additionally, a significant difference was not found between the groups for the prevalence of ATIII, PC, and PS deficiencies and APCR [Table 1]. However, a significant difference (P < 0.05) was found when comparing all of the cases with defects in ATIII, PC and/or PS and APCR (22/104 or 21.15%) in the patient group with all of the cases with those defects in the control group (6/110 or 5.45%).
Table 2.
The number of cases in both groups with a deficiency of ATIII, PC, and PS and APCR as well as the range and mean values of their deficiencies
DISCUSSION
Our study demonstrates that AT, PC, and PS deficiencies, as well as APCR, can be detected in healthy subjects and in patients with thromboembolic events. The prevalence of APCR (mainly its hereditary form - FVL) in the healthy population in different countries ranges from 2% to 15%.[16] Literature studies indicate that APCR is more frequent among the Caucasian population. Koster et al. reported that the prevalence of FVL in the general population in Europe is 3–7%.[17] According Herrmann et al., the percentage of FVL differs among countries as follows: Argentina 5.1%, Poland 5.0%, Germany 2.9%, Costa Rica 2.0%, Venezuela 1.6%, and India 1.3%.[18] The prevalence of FVL in Europe is similar to that reported in Turkey (5%), Iraq (3%), and Saudi Arabia (2.5%).[19,20,21] In some Asian countries, a much higher percentage of the prevalence of FVL has been reported: Lebanon 14.2%, Syria 13.6%, and Jordan 12.25%.[22] The prevalence of FVL in Canada is 5.3%.[23] The prevalence of AT, PC, and PS deficiencies in the general populations is much lower than the prevalence of FVL. In the study by Tait et al., which involved 10,000 blood donors, the prevalence of type-1 antithrombin deficiency was 0.02%,[24] whereas the prevalence of heterozygote PC deficiency in the general population is 0.3% (approximately 10 times more than the prevalence of AT).[25] The prevalence of PS deficiency in healthy individuals is 0.03–1.3%,[26] but the risks associated with PS deficiency are similar to those of PC deficiency.[27] The prevalence of AT, PC, and PS deficiencies and FVL are higher in patients with thromboembolic events compared with those in the healthy population. Literature studies indicate that FVL and AT deficiency are present in 20% and 0.5–1%, respectively, of patients with thromboembolic events, whereas both PC and PS exhibit a similar prevalence (3%) in the same patients.[16,17,25,27] Most studies have reported differing prevalence of ATIII, PC, and PS deficiencies as well as a differing prevalence of FVL in women with early and late pregnancy loss.
The aim of this study was to evaluate the prevalence and role of inherited thrombophilia in early pregnancy loss, specifically in the first trimester. We tested 104 RPL patients and 110 controls for ATIII, PC, and PS deficiencies and for the presence of APCR. Table 1 indicates that deficiencies for ATIII, PC, and PS were detected in 2.88%, 3.85%, and 5.76% of the patients, respectively, compared with 0% for ATIII and 0.9% for PC and PS in controls. Additionally, we found that APCR was more common in the patients (8.65%) than in the controls (3.63%). The prevalence of APCR in the controls is nearly identical to our previous results for APCR determined in 944 blood donors (537 males and 407 females) in Kosovo. In this previous study, the prevalence of APCR in the entire group was 3.4% (3.44% and 3.35% for males and females, respectively).[28] We did not perform genetic testing for ATIII, PC, or PS because of the lack of technology and material samples; therefore, we are uncertain whether the deficiencies that we identified in the present study were acquired or inherited. However, we excluded pregnant women, in whom reduced PS and the presence of acquired APCR are found, from both groups. In the patients with PC and PS deficiencies, we evaluated the Vitamin K-dependent factors of coagulation (FII, FVII, FIX, and FX) and obtained normal values. We did not have the equipment to specifically determine the prevalence of FVL in our cases with APCR. Based on other studies, FVL accounts for 95% of the cases of APCR, and the FVL allele is not detected in the remaining 5% of cases.[11]
Although we found that the overall percentages of cases with ATIII, PC and PS deficiencies and of the cases with APCR were higher in the patient group than in the control group, we are not certain whether the recurrent miscarriages in these patients were caused by a deficiency of ATIII, PC, or PS or by the presence of APCR because approximately 70% of first trimester spontaneous abortions (12 weeks) result from chromosomal disorders.[29] Additionally, a significant number of early pregnancy spontaneous abortions result from the presence of antiphospholipid antibodies, anatomical abnormalities, specific infections, and endocrine disorders.[30,31,32] There are contradictory hypotheses concerning the role of thrombophilia as a cause of abortions. According to many authors, abortions do not occur in all cases of thrombophilia. Sibai et al. found that approximately 0.2–1% of patients with a combined deficiency of PC and PS and 2–10% of women with an FVL mutation have normal pregnancy outcomes.[5] Rey et al. performed a meta-analysis of 31 studies and concluded that fetal loss is a well-established complication in women with thrombophilia. According these authors, FVL is associated with early RLP (OR: 2.01 [1.35–3.58]), late (OR: 7.83 [2.83–21.67]), and late nonrecurrent fetal loss (OR: 3.26 [1.82–5.83]).[33]
In a meta-analysis of the role of FVL in recurrent first trimester loss, Laghoff-Ross et al. reported that the typical OR was 1.67 (1.16–2.4).[34] A study by Rai et al. assessed the prevalence of APCR among 1111 consecutive Caucasian women with a history of either recurrent early miscarriage (three or more consecutive pregnancy losses at < 12 weeks gestation: n = 904 or at least one late miscarriage (>12 weeks gestation; n = 207). The authors reported that acquired APCR was significantly more common in women with recurrent early miscarriages (8.8%) and late miscarriages (8.7%) than in the controls (3.3%).[35] Furthermore, Jivraj et al. reported that FVL was associated with a nonsignificant trend toward a risk of miscarriage during a subsequent pregnancy in Caucasian women with a history of recurrent miscarriages.[36] The association with inherited thrombophilia has not been conclusively established because some studies have demonstrated an association between RPL and prothrombotic states.[33,37] Kutteh et al.[38] did not find this association. Furthermore, Cardona et al. reported a lack of association between RPL and prothrombotic states in non-Caucasian Columbian patients.[39] Kujovich reported that FVL is associated with a 2–3-fold increased relative risk of pregnancy loss and other obstetric complications.[40] Some evidence suggests that FVL is more responsible for late pregnancy loss than for early first trimester loss,[41,42] whereas other evidence has indicated that FVL is responsible for early first trimester loss.[3,33,43] The role of thrombophilia from other causes is less clear. In the Thrombosis: Risk and Economic Assessment of Thrombophilia Screening Study, which is a systematic review of thrombophilia in pregnancy that included a total of 79 studies (three randomized controlled trials, eight prospective cohorts, and 68 retrospective studies), Robertson et al. determined the risk of early pregnancy loss in thrombophilia patients. According these authors, the ORs for FVL (heterozygous), AT, PC, and PS deficiency were 1.68 (1.09–2.58), 0.88 (0.17–4.48), 2.29 (0.20–26.43), and 3.35 (0.35–35.72), respectively.[3] According to a meta-analysis by Rey et al., PC and ATIII deficiencies are not linked with fetal loss, whereas a PS deficiency is associated with late-term fetal loss.[33] Hansda and Roychowdhury reported very high percentages of PC and PS deficiencies (15.09% and 50.94%, respectively.) in Indian patients with RLP (P = 0.000).[44] Furthermore, Jyotsna et al. described a significant risk of RPL in pregnant Indian women with thrombophilias.[45] In the European Prospective Cohort on Thrombophilia study, which involved 1384 women, Preston et al. reported that of 843 women with thrombophilia, 571 of them had 1524 pregnancies, whereas of 541 control women, 395 had 1019 pregnancies. After analyzing the frequencies of miscarriage and stillbirth jointly and separately, the authors confirmed that the risk of fetal loss was increased in women with thrombophilia compared with controls (168/571 vs. 93/395; OR: 1.35 [1.01–1.82]).[12] In the same study, the authors reported increased fetal losses in women with familial thrombophilia, particularly in those with combined defects or isolated deficiencies of ATIII, PC, or PS. Additionally, the authors reported that the OR was higher for stillbirths than for miscarriages (3.6 [1.4–9.4] vs. 1.27 [0.94–1.71]), and a combined deficit of natural inhibitors of coagulation was more frequently a cause of stillbirths (OR: 14.3) than was an individual deficit of ATIII (OR: 5.2), PC (OR: 2.3), PS (OR: 3.3), or FVL (OR: 2.0).[12] Laghoff-Ross et al. reported that PS and ATIII are not associated with recurrent early fetal loss.[34] We did not find any case with a combined deficiency of ATIII, PC, or PS and APCR. A number of other inherited disorders are responsible for early pregnancy loss, such as mutations in the prothrombin gene and the MTHFR gene.[5] Based on the above literature data concerning the role of thrombophilia in RLP, Walker et al. have suggested that although it remains uncertain whether heritable thrombophilia causes recurrent miscarriage, it is still preferable to perform routine testing in women with recurrent miscarriage.[46] Most other authors suggest that thrombophilia is an important factor in early and late pregnancy losses and propose that women with unexplained pregnancy loss should be screened for thrombophilia.[7,8,45,47] However, routine thrombophilia screening is not recommended for all women attending the antenatal clinical examination.[34] Therefore, it is difficult to ascertain the true role of thrombophilia in the etiopathogenesis of spontaneous abortions in the first trimester of pregnancy due to conflicting data in the literature.
CONCLUSION
Based on our results, we conclude that thrombophilia is a causal factor for miscarriages in the first trimester of pregnancy, although there are the conflicting data in the literature. However, many authors suggest that other etiological factors are more responsible for early pregnancy loss in this period, including the following most frequently observed factors: Chromosomal disorders, antiphospholipid antibodies, anatomical abnormalities, specific infections, endocrine disorders, and other inherited and acquired disorders. Nevertheless, because RPL could be a consequence of thrombophilia, we recommended that women with unexpected RPL should be screened for thrombophilia. This is important because pregnant women in whom the thrombophilia is confirmed should be treated with unfractionated heparin throughout pregnancy and 4–6 weeks after delivery to prevent RLP.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111. doi: 10.1186/1477-7827-1-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates SM. Management of pregnant women with thrombophilia or a history of venous thromboembolism. Hematology Am Soc Hematol Educ Program. 2007;1:143–50. doi: 10.1182/asheducation-2007.1.143. [DOI] [PubMed] [Google Scholar]
- 3.Robertson L, Wu O, Langhorne P, Twaddle S, Clark P, Lowe GD, et al. Thrombophilia in pregnancy: A systematic review. Br J Haematol. 2006;132:171–96. doi: 10.1111/j.1365-2141.2005.05847.x. [DOI] [PubMed] [Google Scholar]
- 4.Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. 2003;16:153–68. doi: 10.1016/s1521-6926(03)00021-5. [DOI] [PubMed] [Google Scholar]
- 5.Sibai BM, How HY, Stella CL. Thrombophilia in pregnancy: Whom to screen, when to treat. OBG Manage. 2007;19:50–64. [Google Scholar]
- 6.Lim W, Eikelboom JW, Ginsberg JS. Inherited thrombophilia and pregnancy associated venous thromboembolism. BMJ. 2007;334:1318–21. doi: 10.1136/bmj.39205.484572.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner B. Thrombophilia and pregnancy loss in first intended pregnancy. J Thromb Haemost. 2005;3:2176–7. doi: 10.1111/j.1538-7836.2005.01609.x. [DOI] [PubMed] [Google Scholar]
- 8.Micco PD, Uva MD. Recurrent pregnancy loss and thrombophilia. Open Atheroscler Thromb J. 2009;2:33–5. [Google Scholar]
- 9.Sarig G, Younis JS, Hoffman R, Lanir N, Blumenfeld Z, Brenner B. Thrombophilia is common in women with idiopathic pregnancy loss and is associated with late pregnancy wastage. Fertil Steril. 2002;77:342–7. doi: 10.1016/s0015-0282(01)02971-5. [DOI] [PubMed] [Google Scholar]
- 10.Salat-Baroux J. Recurrent spontaneous abortions. Reprod Nutr Dev. 1988;28:1555–68. [PubMed] [Google Scholar]
- 11.Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412–24. doi: 10.1016/j.ajog.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Preston FE, Rosendaal FR, Walker ID, Briët E, Berntorp E, Conard J, et al. Increased fetal loss in women with heritable thrombophilia. Lancet. 1996;348:913–6. doi: 10.1016/s0140-6736(96)04125-6. [DOI] [PubMed] [Google Scholar]
- 13.Carp H, Salomon O, Seidman D, Dardik R, Rosenberg N, Inbal A. Prevalence of genetic markers for thrombophilia in recurrent pregnancy loss. Hum Reprod. 2002;17:1633–7. doi: 10.1093/humrep/17.6.1633. [DOI] [PubMed] [Google Scholar]
- 14.Bennett SA, Bagot CN, Arya R. Pregnancy loss and thrombophilia: The elusive link. Br J Haematol. 2012;157:529–42. doi: 10.1111/j.1365-2141.2012.09112.x. [DOI] [PubMed] [Google Scholar]
- 15.McNamee K, Dawood F, Farquharson RG. Thrombophilia and early pregnancy loss. Best Pract Res Clin Obstet Gynaecol. 2012;26:91–102. doi: 10.1016/j.bpobgyn.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Walker ID. Thrombophilia in pregnancy. J Clin Pathol. 2000;53:573–80. doi: 10.1136/jcp.53.8.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342:1503–6. doi: 10.1016/s0140-6736(05)80081-9. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann FH, Koesling M, Schroder W, Altman R, Jiménez Bonilla R, Lopaciuk S, et al. Prevalence of factor V Leiden mutation in various populations. Genet Epidemiol. 1997;14:403–11. doi: 10.1002/(SICI)1098-2272(1997)14:4<403::AID-GEPI5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Cadroy Y, Sié P, Boneu B. Frequency of a defective response to activated protein C in patients with a history of venous thrombosis. Blood. 1994;83:2008–9. [PubMed] [Google Scholar]
- 20.Al-Allawi NA, Jubrael JM, Hilmi FA. Factor V Leiden in blood donors in Baghdad (Iraq) Clin Chem. 2004;50:677–8. doi: 10.1373/clinchem.2003.029314. [DOI] [PubMed] [Google Scholar]
- 21.Dzimiri N, Meyer B. World distribution of factor V Leiden. Lancet. 1996;347:481–2. doi: 10.1016/s0140-6736(96)90064-1. [DOI] [PubMed] [Google Scholar]
- 22.Irani-Hakime N, Tamim H, Elias G, Finan RR, Daccache JL, Almawi WY. High prevalence of factor V mutation (Leiden) in the Eastern Mediterranean. Clin Chem. 2000;46:134–6. [PubMed] [Google Scholar]
- 23.Lee DH, Henderson PA, Blajchman MA. Prevalence of factor V Leiden in a Canadian blood donor population. CMAJ. 1996;155:285–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Tait RC, Walker ID, Perry DJ, Islam SI, Daly ME, McCall F, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol. 1994;87:106–12. doi: 10.1111/j.1365-2141.1994.tb04878.x. [DOI] [PubMed] [Google Scholar]
- 25.Koster T, Rosendaal FR, Briët E, van der Meer FJ, Colly LP, Trienekens PH, et al. Protein C deficiency in a controlled series of unselected outpatients: An infrequent but clear risk factor for venous thrombosis (Leiden Thrombophilia Study) Blood. 1995;85:2756–61. [PubMed] [Google Scholar]
- 26.Dykes AC, Walker ID, McMahon AD, Islam SI, Tait RC. A study of Protein S antigen levels in 3788 healthy volunteers: Influence of age, sex and hormone use, and estimate for prevalence of deficiency state. Br J Haematol. 2001;113:636–41. doi: 10.1046/j.1365-2141.2001.02813.x. [DOI] [PubMed] [Google Scholar]
- 27.Rosendaal FR. Risk factors for venous thrombotic disease. Thromb Haemost. 1999;82:610–9. [PubMed] [Google Scholar]
- 28.Mekaj Y, Zhubi B, Hoxha H, Belegu R, Mekaj A, Miftari E, et al. Prevalence of resistence to activated protein C (APC-resistance) in blood donors in Kosovo. Bosn J Basic Med Sci. 2009;9:329–34. doi: 10.17305/bjbms.2009.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatasaka HH. Recurrent miscarriage: Epidemiologic factors, definitions, and incidence. Clin Obstet Gynecol. 1994;37:625–34. doi: 10.1097/00003081-199409000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Battinelli EM, Marshall A, Connors JM. The role of thrombophilia in pregnancy. Thrombosis 2013. 2013:516420. doi: 10.1155/2013/516420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford HB, Schust DJ. Recurrent pregnancy loss: Etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2:76–83. [PMC free article] [PubMed] [Google Scholar]
- 32.Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66:24–9. [PubMed] [Google Scholar]
- 33.Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: A meta-analysis. Lancet. 2003;361:901–8. doi: 10.1016/S0140-6736(03)12771-7. [DOI] [PubMed] [Google Scholar]
- 34.Laghoff-Ross J, Paidas MJ, Ku DH, Arkel YS, Lockwood CJ. Inherited thrombophilias and early pregnancy loss. In: Mor G, editor. Immunology of Pregnancy. Ch 20. New York: Eurekah com and Springer Science+Business Media; 2006. [Google Scholar]
- 35.Rai R, Shlebak A, Cohen H, Backos M, Holmes Z, Marriott K, et al. Factor V Leiden and acquired activated protein C resistance among 1000 women with recurrent miscarriage. Hum Reprod. 2001;16:961–5. doi: 10.1093/humrep/16.5.961. [DOI] [PubMed] [Google Scholar]
- 36.Jivraj S, Makris M, Saravelos S, Li TC. Pregnancy outcome in women with factor V Leiden and recurrent miscarriage. BJOG. 2009;116:995–8. doi: 10.1111/j.1471-0528.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- 37.Pihusch R, Buchholz T, Lohse P, Rübsamen H, Rogenhofer N, Hasbargen U, et al. Thrombophilic gene mutations and recurrent spontaneous abortion: Prothrombin mutation increases the risk in the first trimester. Am J Reprod Immunol. 2001;46:124–31. doi: 10.1111/j.8755-8920.2001.460202.x. [DOI] [PubMed] [Google Scholar]
- 38.Kutteh WH, Park VM, Deitcher SR. Hypercoagulable state mutation analysis in white patients with early first-trimester recurrent pregnancy loss. Fertil Steril. 1999;71:1048–53. doi: 10.1016/s0015-0282(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 39.Cardona H, Castañeda SA, Cardona Maya W, Alvarez L, Gómez J, Gómez J, et al. Lack of Association between Recurrent Pregnancy Loss and Inherited Thrombophilia in a Group of Colombian Patients. Thrombosis 2012. 2012:367823. doi: 10.1155/2012/367823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kujovich JL. Factor V Leiden thrombophilia. Genet Med. 2011;13:1–16. doi: 10.1097/GIM.0b013e3181faa0f2. [DOI] [PubMed] [Google Scholar]
- 41.Dudding TE, Attia J. The association between adverse pregnancy outcomes and maternal factor V Leiden genotype: A meta-analysis. Thromb Haemost. 2004;91:700–11. doi: 10.1160/TH03-10-0637. [DOI] [PubMed] [Google Scholar]
- 42.Kist WJ, Janssen NG, Kalk JJ, Hague WM, Dekker GA, de Vries JI. Thrombophilias and adverse pregnancy outcome – A confounded problem! Thromb Haemost. 2008;99:77–85. doi: 10.1160/TH07-05-0373. [DOI] [PubMed] [Google Scholar]
- 43.Tal J, Schliamser LM, Leibovitz Z, Ohel G, Attias D. A possible role for activated protein C resistance in patients with first and second trimester pregnancy failure. Hum Reprod. 1999;14:1624–7. doi: 10.1093/humrep/14.6.1624. [DOI] [PubMed] [Google Scholar]
- 44.Hansda J, Roychowdhury J. Study of thrombophilia in recurrent pregnancy loss. J Obstet Gynaecol India. 2012;62:536–40. doi: 10.1007/s13224-012-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jyotsna PL, Sharma S, Trivedi SS. Coagulation inhibitors and activated protein C resistance in recurrent pregnancy losses in Indian women. Indian J Pathol Microbiol. 2011;54:752–5. doi: 10.4103/0377-4929.91507. [DOI] [PubMed] [Google Scholar]
- 46.Walker ID, Greaves M, Preston FE. Investigation and management of heritable thrombophilia. Br J Haematol. 2001;114:512–28. doi: 10.1046/j.1365-2141.2001.02981.x. [DOI] [PubMed] [Google Scholar]
- 47.Vora S, Shetty S, Salvi V, Satoskar P, Ghosh K. Thrombophilia and unexplained pregnancy loss in Indian patients. Natl Med J India. 2008;21:116–9. [PubMed] [Google Scholar]


