Abstract
The objective is to study the FSH receptor (FSHR) for mutations in a case of spontaneous ovarian hyperstimulation syndrome (sOHSS). This is a single case study and it examined patient who presented with spontaneous critical OHSS in early pregnancy and had successful good obstetric outcome. Intervention of this study was analysis of blood for genetic analysis of FSHR postdelivery. The main outcome measure noted was FSHR mutation. The study resulted in a novel, here though unreported, heterozygous mutation in FSHR gene at nucleotide position 1346 (AC1346T to AAT) in exon 10 yielding a threonine to asparagine (Thr449Asn) substitution in the transmembrane domain helix 3 of the FSHR. To conclude FSHR gene analysis can add to our understanding of sOHSS.
KEY WORDS: FSH receptor genetic analysis, FSHR mutation, spontaneous ovarian hyperstimulation syndrome
INTRODUCTION
Spontaneous ovarian hyperstimulation syndrome (sOHSS) is now a recognized entity, defined as OHSS appearing in patients with no history of stimulatory infertility treatment. OHSS, which otherwise may occur in normal pregnancies (Type I OHSS), is now reported to be associated with mutated FSH receptor (FSHR) gene, which may lead to recurrent sOHSS in patients presenting the mutations. Type II is secondary to high levels of human chorionic gonadotropin (hCG), while Type III coexists with hypothyroidism.[1,2] Very few cases have been reported in literature.[3]
Reported here is a case of sOHSS with a novel FSHR gene mutation.
CASE REPORT
A 29-year-old female married since 1 year, primigravida with 3 months amenorrhea and ultrasonographic gestational age of 8 weeks and 5 days, presented with acute onset of abdominal distension, vulval edema and breathlessness. There was no vomiting, trauma, fever, bleeding, or spotting per vaginum. She had regular past menstrual cycles and no symptoms suggestive of renal, cardiac, or liver disorders. Ultrasonography done a few weeks prior to presentation revealed a normal intrauterine pregnancy and adnexae [Figure 1].
Figure 1.
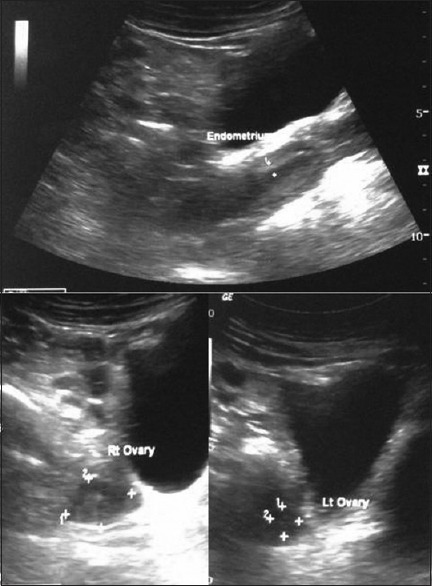
Ultrasonography images showing normal endometrial cavity (intrauterine pregnancy) and bilateral ovaries
The patient was conscious, cooperative, afebrile, with no tachycardia and normotensive. Cardiovascular system examination was normal. Breath sounds were reduced on the right side. Abdominal examination revealed shiny skin, tense ascites with no guarding, rigidity, or tenderness. Vaginal examination revealed an anteverted uterus with bilateral nontender forniceal fullness. History was reconfirmed; patient had not sought any medical advice or treatment for fertility enhancement.
Blood investigations revealed hyponatremia (125 meq/l) and hypoalbuminemia (3.4 gm/dl). Hemoglobin, white blood cell count, platelet count, coagulation profile, liver enzymes, blood urea nitrogen, and serum creatinine were normal. Estradiol was elevated at 12927 pg/dl (normal range 188-2497 pg/dl). Thyroid profile and β-hCG were normal. Ultrasonography revealed a multiloculated cystic lesion of size 17 cm × 11 cm with vascularity within the wall of cystic lesion. Ascites and bilateral pleural effusion were present [Figure 2]. Features of OHSS were confirmed on magnetic resonance imaging (MRI) [Figure 3].
Figure 2.
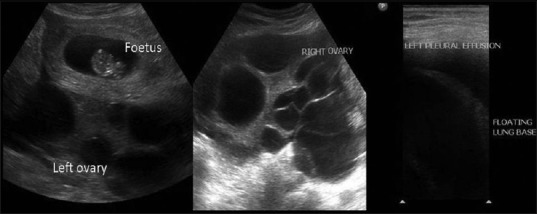
Ultrasonography B-mode images show intrauterine fetus with multiple cysts involving both the right and left ovary. There is mild free fluid in the intra-peritoneal cavity with left pleural effusion
Figure 3.
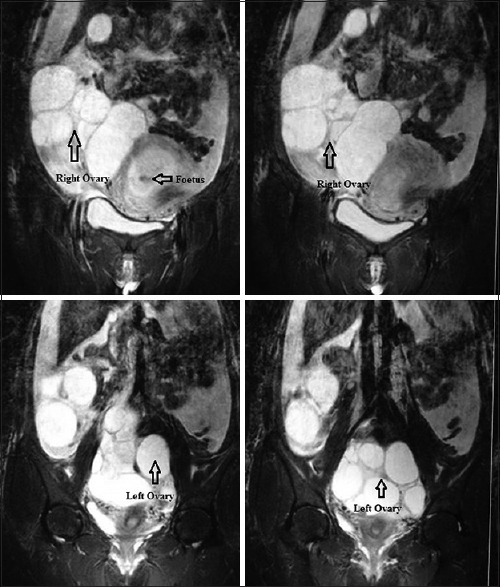
T2-weighted magnetic resonance imaging pelvic coronal images show T2-hypo intense lesion in the intrauterine cavity suggestive of fetus. There are hyper intense multiple cysts seen without any mural nodule arising from both right (in right iliac fossa) and left ovary (in pouch of Douglas)
Vital parameters, intake-output, and abdominal girth were monitored. Additional intravenous fluids were omitted, oral fluids were encouraged, and diuretics were initially required. Hyponatremia gradually improved. In view of severe hypoalbuminemia, intravenous albumin infusion was instituted (25 g in 0.1 L solution per day for 7 days). No interventional procedure was done for ascites and pleural effusion, both of which reduced, along with the adnexal mass. Unfractionated heparin 5000 U subcutaneous injection twice daily was instituted and continued for 9 days. She was discharged after 2 weeks of inpatient stay.
The remainder of the antenatal period was uneventful. Regular ultrasonographic monitoring showed further reduction in size of adnexal mass. At 40 weeks’ gestation, patient underwent cesarean section in view of nonprogress of labor and delivered a healthy male of 2.85 kg. Intraoperatively, an left ovarian simple cyst of 5 cm × 5 cm was observed, for which no intervention was done. Postoperative MRI revealed simple left ovarian cyst of 2 cm × 2 cm, right side normal and involuting uterus [Figure 4].
Figure 4.
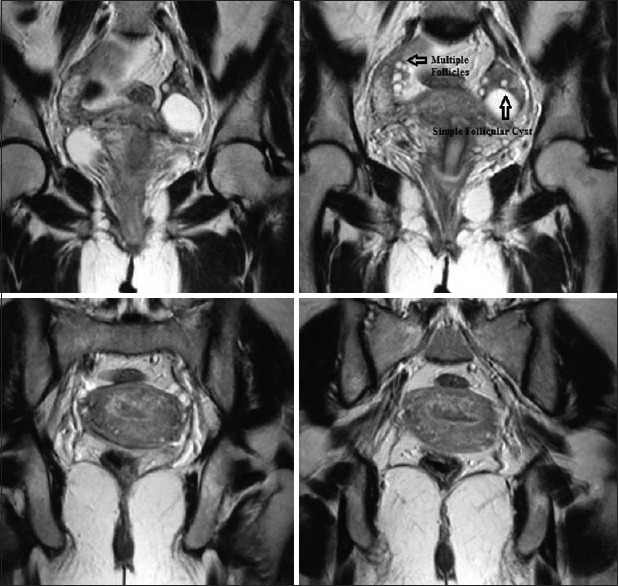
T2-weighted magnetic resonance imaging pelvis images 6 weeks postpartum show complete resolution of bilateral cystic lesions with a single follicular cyst in the left ovary and multiple small follicles in the right ovary
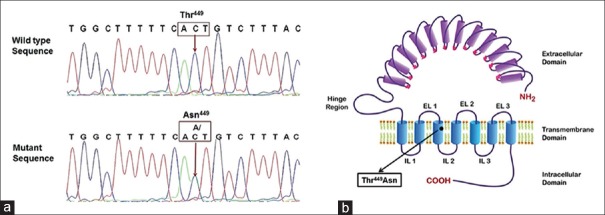
To investigate further, if ovarian hyperstimulation observed in this patient was due to any mutation in FSHR gene, the patient was called back after delivery. The entire exonic region of FSHR gene was screened for known/novel mutations as described earlier.[4] To our interest, we identified a novel heterozygous mutation in FSHR gene leading to a change at amino acid position 449 [Figure 5]. This was observed to be a transversion at nucleotide position 1346 (AC1346T to AAT) in exon 10 yielding a threonine to asparagine (Thr449Asn) substitution in the transmembrane domain helix 3 of the FSHR. The Genbank accession number for this novel mutation (T449N) is KP340897.
Figure 5.
(a) Electropherogram of 5’-3’ strand of the exon 10 of FSHR gene, showing a wild type sequences (upper panel) of a control subject and heterozygous mutant sequence (lower panel) resulting in change of nucleotide ‘C’ to ‘A/C’ (1346 position) leading to Thr449Asn substitution identified in patient who developed spontaneous ovarian hyperstimulation syndrome during pregnancy. (b) Schematic representation of FSHR showing the position of naturally occurring novel mutationThr449Asn
DISCUSSION
OHSS is usually a complication of gonadotropin stimulation during controlled ovarian stimulation protocols. The majority of OHSS cases are usually iatrogenic and are induced by exogenous hormonal therapy. The pathophysiology is one of the fluid extravasations, and the mediator appears to vascular endothelial growth factor.[5] With respect to the severity of clinical picture, it can be classified into:
Grade 1: Abdominal distension and discomfort
Grade 2: Grade 1 + nausea, vomiting, and/or diarrhea, ovaries 5–12 cm
Grade 3: Grade 2 and ultrasonic evidence of ascites
Grade 4: Grade 3 and/or hydrothorax or dyspnea
Grade 5: All above with hemoconcentration, coagulation abnormalities, and diminished renal perfusion (critical OHSS).
However, it is now being recognized and slowly coming to light, in the form of individual case reports that a similar clinical picture can occur in patients, unrelated to exogenous hormone administration.[6,7,8,9,10,11]
After a sufficient number of cases of sOHSS were reported based on the underlying primary abnormality and the triggers leading to the sOHSS, De Leener et al., have proposed a 4-group classification for this abnormality, which is shown in Table 1.
Table 1.
Classification of FSH receptor mutation
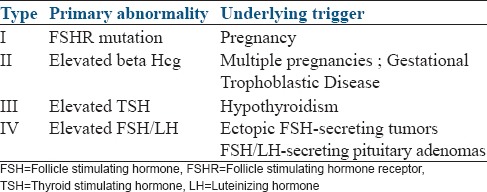
Our patient was classified as Type 1 critical sOHSS in pregnancy and managed as per existing guidelines. Though the reports of successful completion of pregnancies complicated by sOHSS have been few,[3,12,13,14] the conservative management recommended by the existing guidelines[15] is validated by these few successful outcomes. This is further reinforced by our case.
The understanding of the molecular basis of sOHSS is very recent, evident by the fact that the first recognition of a mutation in the FSHR gene resulting in OHSS was described very recently.[2] Interestingly, all the activating mutations of FSHR gene reported in women till date are associated with sOHSS leading to the activation of the receptor.[1,2,16,17,18,19]
These mutations have been reported to cause loss of ligand binding specificity and increased response to hCG (especially in first trimester when the levels of hCG are high) resulting in hyperstimulation.[20]
A naturally occurring heterozygous novel mutation in FSHR gene was identified in our patient. This mutation leads to the substitution of threonine to asparagine at amino acid position 449 in FSHR. However, the functional analysis of this mutation needs to be investigated to understand the pathophysiology at the molecular level. In the wake of this finding, the patient has been counseled regarding the risk of recurrence in subsequent pregnancies.
CONCLUSION
At this point in time, much is yet to be understood about sOHSS and the molecular mechanisms behind it, and hence the reason of presenting this case report: A novel mutation in a condition, not fully understood.
The general gynecologist/obstetrician should have a high index of suspicion for sOHSS,[3] as this entity can have several implications. Genetic analysis should be considered in all patients with sOHSS to improve our understanding of this entity, paving way for an earlier recognition and improved management of this condition.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.De Leener A, Montanelli L, Van Durme J, Chae H, Smits G, Vassart G, et al. Presence and absence of follicle-stimulating hormone receptor mutations provide some insights into spontaneous ovarian hyperstimulation syndrome physiopathology. J Clin Endocrinol Metab. 2006;91:555–62. doi: 10.1210/jc.2005-1580. [DOI] [PubMed] [Google Scholar]
- 2.De Leener A, Caltabiano G, Erkan S, Idil M, Vassart G, Pardo L, et al. Identification of the first germline mutation in the extracellular domain of the follitropin receptor responsible for spontaneous ovarian hyperstimulation syndrome. Hum Mutat. 2008;29:91–8. doi: 10.1002/humu.20604. [DOI] [PubMed] [Google Scholar]
- 3.Sridev S, Barathan S. Case report on spontaneous ovarian hyperstimulation syndrome following natural conception associated with primary hypothyroidism. J Hum Reprod Sci. 2013;6:158–61. doi: 10.4103/0974-1208.117164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achrekar SK, Modi DN, Meherji PK, Patel ZM, Mahale SD. Follicle stimulating hormone receptor gene variants in women with primary and secondary amenorrhea. J Assist Reprod Genet. 2010;27:317–26. doi: 10.1007/s10815-010-9404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): A review. Hum Reprod Update. 2002;8:559–77. doi: 10.1093/humupd/8.6.559. [DOI] [PubMed] [Google Scholar]
- 6.Irvine LM. Spontaneous ovarian hyperstimulation syndrome (OHSS): A rare but important differential diagnosis for abdominal distension in early pregnancy. J Obstet Gynaecol. 2011;31:338–9. doi: 10.3109/01443615.2011.560299. [DOI] [PubMed] [Google Scholar]
- 7.Chae HD, Park EJ, Kim SH, Kim CH, Kang BM, Chang YS. Ovarian hyperstimulation syndrome complicating a spontaneous singleton pregnancy: A case report. J Assist Reprod Genet. 2001;18:120–3. doi: 10.1023/A:1026543027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oztekin O, Soylu F, Tatli O. Spontaneous ovarian hyperstimulation syndrome in a normal singleton pregnancy. Taiwan J Obstet Gynecol. 2006;45:272–5. doi: 10.1016/S1028-4559(09)60241-2. [DOI] [PubMed] [Google Scholar]
- 9.He C, Huang H, Song Y. Spontaneous and severe ovarian hyperstimulation syndrome after delivery: A case report. Gynecol Endocrinol. 2008;24:450–1. doi: 10.1080/09513590802246075. [DOI] [PubMed] [Google Scholar]
- 10.Panagiotopoulou N, Byers H, Newman WG, Bhatia K. Spontaneous ovarian hyperstimulation syndrome: Case report, pathophysiological classification and diagnostic algorithm. Eur J Obstet Gynecol Reprod Biol. 2013;169:143–8. doi: 10.1016/j.ejogrb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal NR, Gupta G, Verma K, Varyani N. Spontaneous ovarian hyperstimulation syndrome in a triplet pregnancy. Case Rep Crit Care 2012. 2012:189705. doi: 10.1155/2012/189705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei R, Xiao S, Xue M, Deng X. Successful delivery in a women with natural pregnancy and severe ovarian hyperstimulation syndrome: A case report and literature review. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:1225–8. [PubMed] [Google Scholar]
- 13.Di Carlo C, Savoia F, Gargano V, Sparice S, Bifulco G, Nappi C. Successful pregnancy complicated by spontaneous, familial, recurrent ovarian hyperstimulation syndrome: Report of two cases. Gynecol Endocrinol. 2013;29:897–900. doi: 10.3109/09513590.2013.825713. [DOI] [PubMed] [Google Scholar]
- 14.Francisco C, Júlio C, Pinto G, Martins AT, Ferreira A, Martins L. Ovarian hyperstimulation syndrome in a spontaneous pregnancy. Acta Med Port. 2011;24(Suppl 3):635–8. [PubMed] [Google Scholar]
- 15.Jenkins JM, Drakeley AJ, Mathur RS, editors. The Management of Ovarian Hyperstimulation Syndrome. RCOG Green Top Guideline no. 5, 2006 Reconfirmed. [Last accessed 2015 Nov 03];2011 Available from : https://www.rcog.org.uk/globalassets/documents/guidelines/gtg5_230611.pdf . [Google Scholar]
- 16.Smits G, Olatunbosun O, Delbaere A, Pierson R, Vassart G, Costagliola S. Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. N Engl J Med. 2003;349:760–6. doi: 10.1056/NEJMoa030064. [DOI] [PubMed] [Google Scholar]
- 17.Vasseur C, Rodien P, Beau I, Desroches A, Gérard C, de Poncheville L, et al. A chorionic gonadotropin-sensitive mutation in the follicle-stimulating hormone receptor as a cause of familial gestational spontaneous ovarian hyperstimulation syndrome. N Engl J Med. 2003;349:753–9. doi: 10.1056/NEJMoa030065. [DOI] [PubMed] [Google Scholar]
- 18.Casas-González P, Scaglia HE, Pérez-Solís MA, Durand G, Scaglia J, Zariñán T, et al. Normal testicular function without detectable follicle-stimulating hormone. A novel mutation in the follicle-stimulating hormone receptor gene leading to apparent constitutive activity and impaired agonist-induced desensitization and internalization. Mol Cell Endocrinol. 2012;364:71–82. doi: 10.1016/j.mce.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Montanelli L, Van Durme JJ, Smits G, Bonomi M, Rodien P, Devor EJ, et al. Modulation of ligand selectivity associated with activation of the transmembrane region of the human follitropin receptor. Mol Endocrinol. 2004;18:2061–73. doi: 10.1210/me.2004-0036. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser UB. The pathogenesis of the ovarian hyperstimulation syndrome. N Engl J Med. 2003;349:729–32. doi: 10.1056/NEJMp038106. [DOI] [PubMed] [Google Scholar]