Abstract
We report a case of early onset severe ovarian hyperstimulation syndrome (OHSS) presenting with oliguria in an antagonist cycle triggered with GnRH agonist and a freeze-all approach. Prophylactic measures in the form of GnRH antagonist, cabergolin and plasma expanders were given after oocyte retrieval. Twenty-four hours after oocyte retrieval patient developed oliguria and moderate ascites. She was managed in ICU with albumin and diuretics. She responded to conservative management and did not require paracentesis. Severe OHSS can occur in PCOS patients even after using a segmented approach i.e. GnRH agonist trigger with a ‘freeze all’ policy. Patients at risk of OHSS should be closely monitored following ovum pickup even when an agonist trigger has been given, for early detection and management.
KEY WORDS: Agonist trigger, in vitro fertilization, ovarian hyperstimulation syndrome
INTRODUCTION
Ovarian hyperstimulation syndrome (OHSS) is a serious often life-threatening, iatrogenic complication of assisted reproduction. Moderate to severe OHSS has been reported to occur in 0.2–2% of all ovarian stimulation cycles.[1] The syndrome is characterized by an acute shift of protein-rich fluid from the vascular compartment into the third space leading to hemoconcentration, decreased renal perfusion, oliguria, thrombo-embolism, eventually renal shut down, and death in the most extreme cases.
OHSS has been classified as early - occurring 3–5 days after the human chorionic gonadotrophin (hCG) trigger and late - occurring 5–7 days after embryo transfer. The exact pathophysiology of this syndrome is still not completely elucidated. Mediators implicated in the development of the syndrome include vascular endothelial growth factor (VEGF), interleukins (IL1, IL2, IL6, IL8, endothelin 1, and tumor necrosis factor -alpha), prostaglandins, and renin angiotensin aldosterone system.[2] VEGF is currently considered to be the most important mediator as it increases vascular permeability leading to the fluid shift which is responsible for the symptomatology of OHSS, that is, abdominal distension, respiratory distress, oliguria, and thromboembolic phenomenon.[3]
hCG is used to trigger final oocyte maturation in ovarian stimulation because of its similarity to luteinizing hormone (LH) and its long half-life. It has also been identified as the trigger for the development of OHSS.[4] hCG causes the stimulated enlarged ovaries to produce the angiogenic molecule VEGF.[5] Since, the advent of gonadotropin-releasing hormone (GnRH) antagonist protocols in in vitro fertilization (IVF) it has become possible to use GnRH agonist as an ovulation trigger. GnRH agonist trigger is effective in mounting an adequate LH surge for final oocyte maturation additionally it reduces the risk of OHSS.[6] Various other strategies have also been tried to prevent OHSS. Use of the agonist trigger with elective cryopreservation of all embryos - the segmentation approach - has been promoted as a way to have an OHSS free clinic.[7] Units having a good vitrification program have adopted this approach. Unfortunately, despite the great benefit of this approach a few cases have been reported in the literature recently where OHSS developed despite the use of the segmental approach.
We report a case of severe early OHSS despite the use of segmentation approach and OHSS prophylaxis, to highlight the importance of vigilance and early intervention.
CASE REPORT
A 33-year-old patient with polycystic ovary syndrome (PCOS) presented at our clinic with primary infertility for 9 years, in June 2014. PCOS had been diagnosed earlier based on the Rotterdam criteria.
Her menstrual cycles had been irregular since menarche, and she was only menstruating after progesterone withdrawal since a few years. Her tubal evaluation was normal. Husband's semen analysis revealed oligoasthenoteratozoospermia (count 5 million/ml, motility 25%, and 1% normal morphology).
She had previously undergone 2 cycles of intrauterine insemination and one cycle of controlled ovarian stimulation (COS) for IVF. During this cycle patient had been down-regulated with GnRH agonist and COS was started with 150 iu of recombinant follicle stimulating hormone (rFSH). Cycle was canceled due to lack of ovarian response after 8 days of stimulation.
On examination, her body mass index (BMI) was 28.6 kg/m2. She had mild hirsutism. Her hormonal profile showed an FSH of 4 miu/ml, LH - 7.4 miu/ml, E2-46.3 pg/ml, and antimullerian hormone (AMH) - 27 ng/ml. Her blood sugar, liver function tests, serum creatinine, and lipid profile was normal.
Baseline scan showed large PCO ovaries with >20 antral follicles per ovary, a typical cobweb appearance. The right ovarian measured – 47 mm × 26 mm × 37 mm, volume 25 cc3, and the left ovary measured 48 mm × 33 mm × 36 mm with a volume of 30 cc3. Endometrium was 16 mm thick, so an endometrial biopsy was taken. Histopathology revealed simple typical hyperplasia without malignancy.
She was advised IVF-intra-cytoplasmic sperm injection because of the presence of a male factor in addition to ovulatory dysfunction. Extensive counseling was done for OHSS risk and agonist trigger, and elective freezing of all embryos was discussed as a strategy to avoid development of OHSS.
The patient was stimulated under fixed day antagonist protocol with rFSH 200 iu s/c daily from the 2nd day of her periods. Dose of 200 iu was decided based on her last attempted IVF cycle and high BMI 29 kg/m2. Baseline antral follicle sizes were between 4 and 8 mm, and E2 was 35 pg/ml. On day 6 of stimulation, she had > 15 follicles 8–9 mm on both sides and E2 levels were 1030 pg/ml. GnRH antagonist Cetrorelix (Intas Pharmaceuticals) was started and stimulation continued with injection menopur 225 iu (Ferring pharmaceutical). On day 8 of stimulation all follicles were below 10–12 mm and hence the dose of gonadotropin was increased to 300 iu. E2 levels taken on day 8 were 3100 pg/ml. After increasing the dose, follicular growth was appreciated. Two days later on day 10, after 9 days of stimulation she had 6 follicles 16 mm average diameter, rest of the follicles more than 20 per ovary were in the range of 12–14 mm. The ovaries were large with the right ovary being >8 cm and left >10 cm. Some amount of pelvic free fluid had been appreciated since day 6, and this had increased marginally on the day of the trigger. There was no free fluid in the flanks or upper abdomen. The patient complained of pain in the lower abdomen and some nausea since day 8 of stimulation.
She received 1 mg leuprolide acetate (Sun Pharmaceuticals) for final oocyte maturation on stimulation day 10.
The total dose of gonadotrophin received by the patient was 2050 iu. On the day of her trigger, E2 was 10,045 pg/ml and progesterone was 3.5 ng/ml. A total of 44 oocytes were retrieved with 37 MII oocytes of which 20 fertilized. Blood was collected for evaluation of hematocrit, liver enzymes, and creatinine. At egg collection fluid in the pelvis was approximately 200–300 cc, upper abdomen, and flanks were found to be fluid free. There was no significant hemorrhage from the ovaries postretrieval.
Following oocyte pick-up (OPU) patient was kept prophylactically on dopamine agonist cabergolin (0.5 mg) once a day, GnRH antagonist 0.25 mg s/c twice a day and enoxaparin 20 mg s/c daily. She was also given prophylactic hydroxyl ethyl starch (HES) 500 ml. She was admitted overnight for observation because of the large ovaries and abdominal discomfort she was experiencing.
Twenty-four hours after OPU patient complained of pain, nausea, and abdominal distention. Her vitals were stable, but urine output was <30 ml/h (200 ml) in last 24 h despite adequate hydration and administration of HES. Ultrasound showed bilateral enlarged ovaries occupying most of the pelvis and abdomen up to an inch above the umbilicus. Nonechogenic fluid was now visible mainly in the upper abdomen, the pelvis, and most of the abdomen being occupied by the ovaries. Her abdominal girth had also increased by 3 cm and weight by 1 kg. She was given I/V albumin 100 ml and Lasix 40 mg. 100 ml of albumin was repeated after 4 h, and 500 ml of saline was also administered. This improved the output to 650 ml. The patient was moved to the Intensive Care Unit (ICU) for further management.
In ICU patient was managed with a continuous albumin drip and diuretic. Urinary output improved in the next 24 h, ascitic fluid showed a marginal increase beyond that seen at 36 h, bilateral pleural effusion was appreciated, and the liver enzymes were mildly altered. Creatinine and electrolyte levels were within range though sodium was borderline low. Since, there was not much increase in ascites tapping was avoided to prevent hemorrhage due to inadvertent injury to the greatly enlarged ovaries. Oxygenation was well maintained throughout. Coagulation profile remained normal. The patient improved with conservative management and was discharged from ICU after 3 days.
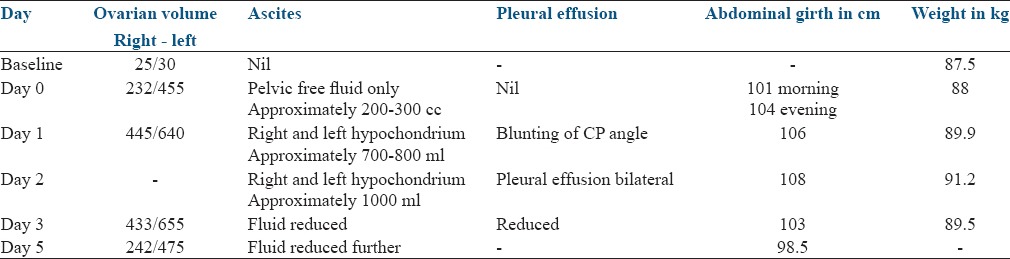
Details of investigations are given in Table 1. Details of ovarian size and fluid in third space are given in Table 2.
Table 1.
Blood biochemistry
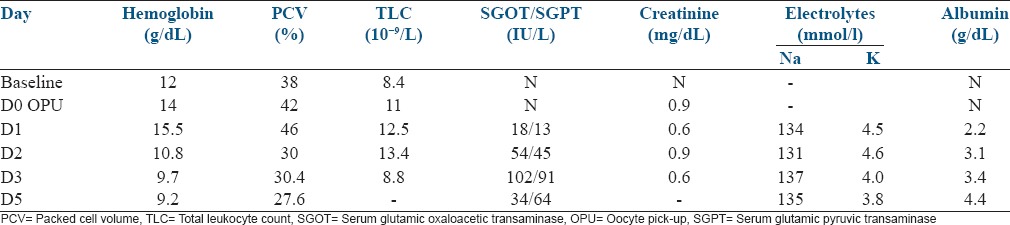
Table 2.
Ultrasound and clinical parameters
DISCUSSION
OHSS is a systemic clinical disorder arising from an exaggerated response to ovarian stimulation. It is mostly iatrogenic and exposes a young disease free woman to a potentially lethal complication. Advances in reproductive medicine have led to improved protocols (GnRH antagonist protocol) and drugs (cabergoline) to minimize this risk.
The use of GnRH agonist trigger as an alternative to hCG came as an important breakthrough in IVF.[8] Segal and Casper in 1992[9] demonstrated that using agonist trigger in IVF prevents OHSS. Subsequently many trials in self-cycles[10] and donor cycles showed the effectiveness of GnRH agonist trigger in OHSS prevention, an incidence of 0% OHSS was reported with agonist.[11] It has been suggested that the shorter duration of the agonist-induced LH surge leads to a significant reduction in the total amount of gonadotropins released. Supraphysiological steroid levels in COS with their negative impact on pituitary secretion of LH, coupled with the altered nature of the agonist-induced LH surge and the duration of LH receptor stimulation, has been proposed as a mechanism for the reduction of OHSS witnessed with the agonist trigger.[12]
The benefit of OHSS prevention comes with a disadvantage of a reduced pregnancy rates (PR) when an agonist trigger is used. This is a result of the letuolytic effect of the GnRH agonist combined with unfavorable endometrial changes.[13] Administration of hCG even in small a dose of 1500 iu improves implantation but leaves the patient vulnerable to OHSS. Seyhan et al. 2013[14] reported development of OHSS in 22% patients on agonist flare when 1500 iu of hCG was added to improve PR.
Devroy et al. 2011 proposed a segmentation approach to achieve an OHSS free clinic. This involved the use of an antagonist protocol with GnRH agonist trigger and elective cryopreservation of all embryos and subsequently embryo transfer in a natural or hormone replacement cycle. This approach has been used with great success and seemed finally to provide an answer to this dreaded complication.
We report a case of severe early OHSS in a PCOS patient where not only was a segmentation approach used, but OHSS prophylaxis was also initiated immediately after OPU. Surprisingly she developed oliguria within 24 h of oocyte collection even though there was only a moderate amount of ascites and the hematocrit was just marginally raised. Oliguria, a late manifestation in OHSS, exists in about 30% of cases, and renal failure secondary to hypoperfusion or to compressive obstruction occurs in about 1.4% of the severe forms of OHSS.[15]
Early oliguria seen in our patient could be the result of a number of factors working in tandem to reduce renal perfusion.
Hemoconcentration and hypovolemia due to fluid shift. Evbuomwan et al., 2000[16] carried out a longitudinal study of women undergoing superovulation to obtain data about hematocrit and osmolality before the onset of clinical symptoms of OHSS. They reported that in OHSS patient a significant increase in osmolality was observed, during the later stages of follicular growth, before hCG administration. They also observed a loss of around 20% of blood volume between days 2 and 4 after administration of hCG. These alterations in blood volume were not seen in patients with uncomplicated cycles (P < 0.006). Their observations suggest that alterations in osmoregulation and volume homeostasis occur much earlier than previously suspected
During oocyte recovery hemorrhage occurs due to pricking of vessels on the ovarian surface and within the follicles aspirated. A large number of aspirated follicles could have caused enough hemorrhage to reduce the circulatory volume further. The body compensatory mechanism would have prevented a drop in blood pressure and tachycardia
Raised intra-abdominal pressure due to a combination of large ovaries and moderate ascites could have resulted in abdominal compartment syndrome and obstructive uropathy leading to oliguria.[17]
Cabergoline a dopaminergic agent blocks binding of VEGF with VEGF receptor thereby preventing an increase in vascular permeability. It decreases the incidence and severity of the syndrome but cannot prevent it completely, especially if there is hCG in circulation.[18] VEGF expression in granulosa cells begins before hCG administration and is stimulated further by hCG exposure indicating thereby that the best time to start administration of dopaminergic agents is a few hours before giving the trigger. Starting cabergoline before trigger and giving it twice a day might have reduced the severity of OHSS in our patient.
Re-initiation of GnRH antagonist in the luteal phase to prevent severe early OHSS was proposed by Lainas et al. 2007.[19] The same author presented an observational cohort study in 2012[20] describing the outpatient management of severe early OHSS with luteal antagonist administration. It was demonstrated that luteal antagonist resulted in rapid resolution of severe OHSS as early as 2 days after initiation, with a significant decline of ovarian volume, hematocrit, and ascites, as well as steroid concentrations, eliminating the need for albumin infusion and in-patient care. In a more recent study, a significant decline in VEGF was also elucidated.[21]
We reinitiated antagonist from the day of oocyte retrieval hoping that it would initiate a quick leutolysis and decrease ovarian size. Having given an agonist trigger, we were not expecting the patient to go into OHSS. Within 24 h of OPU the patient developed oliguria and the ascitic fluid increased. Unlike the cohort described by Lainas et al. 2012 our patient required inpatient ICU care.
Early detection and aggressive management helped to correct the hematocrit and blood biochemistry within 24 h. Ascitic fluid starting reducing after 48 h and patient was comfortable without tapping. The ovarian size increased after OPU and started reducing only after 5 days.
This case underscores the importance of maintaining extreme care while stimulating PCOS patients even when a segmentation approach is planned. We should have started with a lower dose and made slower increments to avoid high levels of estradiol. Our patient also had a high AMH though not as high as the case described by Ling et al. 2014[22] and an ovarian volume of >20 cc3 bilaterally. Patients with high AMH levels are at an increased risk of hyperstimulation. It may be prudent to see if we can determine an association between AMH levels and ovarian volume and the risk of OHSS with agonist trigger, to identify this subgroup of patients.
The underlying cause of early OHSS with segmentation approach is unclear, but the possibility that there may be an FSH and LH receptor mutation has been considered. Gonadotrophin receptor mutation may increase the patient's sensitivity to FSH and LH/hCG. Montanelli et al. 2004[23] reported on familial recurrent spontaneous OHSS in two families who had mutations of the FSH receptor, resulting in abnormal sensitivity to hCG. Genetic studies on VEGF receptor may also reveal important information since it is important in the pathogenesis of OHSS. We believe our patient may be the carrier of such a mutation, and we are looking into the possibility of screening for this.
CONCLUSION
This case highlights the fact that an antagonist cycle with agonist trigger and freeze all policy in IVF does not necessarily eliminate OHSS. Even administering prophylaxis in the form of HES, cabergoline, and antagonist post OPU did not prevent the development of the syndrome. Early diagnosis and prompt management corrected the oliguria and mitigated the symptoms. Clinicians must be vigilant to pick out patients at risk and exercise extreme care and caution during stimulation. Starting with a smaller dose of FSH and avoiding early dose augmentation is essential to reduce the risk of OHSS. Currently, we have no means to identify this subgroup of patients. Very high AMH and a large ovarian volume may present a possibility of identifying women at risk, but the cut-off level needs to be determined.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Papanikolaou EG, Pozzobon C, Kolibianakis EM, Camus M, Tournaye H, Fatemi HM, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2006;85:112–20. doi: 10.1016/j.fertnstert.2005.07.1292. [DOI] [PubMed] [Google Scholar]
- 2.Manno M, Tomei F. Renin-angiotensin system activation during severe OHSS: Cause or effect? Fertil Steril. 2008;89:488. doi: 10.1016/j.fertnstert.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Busso CE, Garcia-Velasco J, Gomez R, Alvarez C, Simón C, Pellicer A. Symposium: Update on prediction and management of OHSS. Prevention of OHSS – Dopamine agonists. Reprod Biomed Online. 2009;19:43–51. doi: 10.1016/s1472-6483(10)60044-2. [DOI] [PubMed] [Google Scholar]
- 4.Gonen Y, Balakier H, Powell W, Casper RF. Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. J Clin Endocrinol Metab. 1990;71:918–22. doi: 10.1210/jcem-71-4-918. [DOI] [PubMed] [Google Scholar]
- 5.Humaidan P, Quartarolo J, Papanikolaou EG. Preventing ovarian hyperstimulation syndrome: Guidance for the clinician. Fertil Steril. 2010;94:389–400. doi: 10.1016/j.fertnstert.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: A prospective randomized controlled study. Fertil Steril. 2008;89:84–91. doi: 10.1016/j.fertnstert.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Devroey P, Polyzos NP, Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26:2593–7. doi: 10.1093/humrep/der251. [DOI] [PubMed] [Google Scholar]
- 8.Imoedemhe DA, Sigue AB, Pacpaco EL, Olazo AB. Stimulation of endogenous surge of luteinizing hormone with gonadotropin-releasing hormone analog after ovarian stimulation for in vitro fertilization. Fertil Steril. 1991;55:328–32. doi: 10.1016/s0015-0282(16)54125-9. [DOI] [PubMed] [Google Scholar]
- 9.Segal S, Casper RF. Gonadotropin-releasing hormone agonist versus human chorionic gonadotropin for triggering follicular maturation in in vitro fertilization. Fertil Steril. 1992;57:1254–8. [PubMed] [Google Scholar]
- 10.Humaidan P, Kol S, Papanikolaou EG. Copenhagen GnRH Agonist Triggering Workshop Group. GnRH agonist for triggering of final oocyte maturation: Time for a change of practice? Hum Reprod Update. 2011;17:510–24. doi: 10.1093/humupd/dmr008. [DOI] [PubMed] [Google Scholar]
- 11.Bodri D, Guillén JJ, Galindo A, Mataró D, Pujol A, Coll O. Triggering with human chorionic gonadotropin or a gonadotropin-releasing hormone agonist in gonadotropin-releasing hormone antagonist-treated oocyte donor cycles: Findings of a large retrospective cohort study. Fertil Steril. 2009;91:365–71. doi: 10.1016/j.fertnstert.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 12.Fatemi HM, Popovic-Todorovic B, Humaidan P, Kol S, Banker M, Devroey P, et al. Severe ovarian hyperstimulation syndrome after gonadotropin-releasing hormone (GnRH) agonist trigger and “freeze-all” approach in GnRH antagonist protocol. Fertil Steril. 2014;101:1008–11. doi: 10.1016/j.fertnstert.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Humaidan P, Bredkjaer HE, Bungum L, Bungum M, Grøndahl ML, Westergaard L, et al. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: A prospective randomized study. Hum Reprod. 2005;20:1213–20. doi: 10.1093/humrep/deh765. [DOI] [PubMed] [Google Scholar]
- 14.Seyhan A, Ata B, Polat M, Son WY, Yarali H, Dahan MH. Severe early ovarian hyperstimulation syndrome following GnRH agonist trigger with the addition of 1500 IU hCG. Hum Reprod. 2013;28:2522–8. doi: 10.1093/humrep/det124. [DOI] [PubMed] [Google Scholar]
- 15.Khalaf Y, Elkington N, Anderson H, Taylor A, Braude P. Ovarian hyperstimulation syndrome and its effect on renal function in a renal transplant patient undergoing IVF treatment: Case report. Hum Reprod. 2000;15:1275–7. doi: 10.1093/humrep/15.6.1275. [DOI] [PubMed] [Google Scholar]
- 16.Evbuomwan IO, Davison JM, Murdoch AP. Coexistent hemoconcentration and hypoosmolality during superovulation and in severe ovarian hyperstimulation syndrome: A volume homeostasis paradox. Fertil Steril. 2000;74:67–72. doi: 10.1016/s0015-0282(00)00573-2. [DOI] [PubMed] [Google Scholar]
- 17.Marak CP, Chopra A, Alappan N, Ponea AM, Guddati AK. Ovarian hyperstimulation syndrome as an etiology of obstructive uropathy. Case Rep Obstet Gynecol 2013. 2013:653704. doi: 10.1155/2013/653704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youssef MA, van Wely M, Hassan MA, Al-Inany HG, Mochtar M, Khattab S, et al. Can dopamine agonists reduce the incidence and severity of OHSS in IVF/ICSI treatment cycles?. A systematic review and meta-analysis. Hum Reprod Update. 2010;16:459–66. doi: 10.1093/humupd/dmq006. [DOI] [PubMed] [Google Scholar]
- 19.Lainas TG, Sfontouris IA, Zorzovilis IZ, Petsas GK, Lainas GT, Kolibianakis EM. Management of severe early ovarian hyperstimulation syndrome by re-initiation of GnRH antagonist. Reprod Biomed Online. 2007;15:408–12. doi: 10.1016/s1472-6483(10)60366-5. [DOI] [PubMed] [Google Scholar]
- 20.Lainas GT, Kolibianakis EM, Sfontouris IA, Zorzovilis IZ, Petsas GK, Tarlatzi TB, et al. Outpatient management of severe early OHSS by administration of GnRH antagonist in the luteal phase: An observational cohort study. Reprod Biol Endocrinol. 2012;10:69. doi: 10.1186/1477-7827-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lainas GT, Kolibianakis EM, Sfontouris IA, Zorzovilis IZ, Petsas GK, Lainas TG, et al. Serum vascular endothelial growth factor levels following luteal gonadotrophin-releasing hormone antagonist administration in women with severe early ovarian hyperstimulation syndrome. BJOG. 2014;121:848–55. doi: 10.1111/1471-0528.12572. [DOI] [PubMed] [Google Scholar]
- 22.Ling LP, Phoon JW, Lau MS, Chan JK, Viardot-Foucault V, Tan TY, et al. GnRH agonist trigger and ovarian hyperstimulation syndrome: Relook at freeze-all strategy. Reprod Biomed Online. 2014;29:392–4. doi: 10.1016/j.rbmo.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Montanelli L, Delbaere A, Di Carlo C, Nappi C, Smits G, Vassart G, et al. A mutation in the follicle-stimulating hormone receptor as a cause of familial spontaneous ovarian hyperstimulation syndrome. J Clin Endocrinol Metab. 2004;89:1255–8. [PubMed] [Google Scholar]


