Abstract
Background:
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy, but not adequately studied in India.
Objectives:
To study clinical tests, nerve conduction studies (NCS), ultrasonography (USG), and magnetic resonance imaging (MRI) in diagnosing CTS.
Materials and Methods:
We diagnosed CTS in 54 patients (93 hands) out of 60 screened patients with symptoms compatible with CTS, including 19 control patients (23 hands). We conducted provocative tests and calculated Boston Carpal tunnel Questionnaire (BCTQ) symptom (S) and function (F) scores. NCS positive patients were classified into mild, mild-to-moderate, moderate, severe, and all-CTS groups. Median nerve anteroposterior, transverse, circumference (CIR), and cross-sectional area (CSA) at inlet (I), middle (M), and outlet (O) each was measured by USG in all patients. MRI was done in 26 patients (39 hands).
Results:
Phalen, hand elevation and pressure provocation tests had higher sensitivity, Tinel's test had higher specificity and tethered median nerve and tourniquet tests had low sensitivity and moderate specificity. USG had low sensitivity but high specificity, and MRI had moderate sensitivity. USG in patients compared to controls was significantly abnormal in CSA-I, CIR-I, and CSA-O. Significant correlation was found between BCTQ-S and NCS and BCTQ-S and CIR-O. CIR-M, CIR-O, CSA-M, and CSA-I had correlation with NCS. MRI was significant in moderate and in moderate + severe groups combined and associated pathologies were detected in 59% patients.
Conclusion:
NCS remain gold standard but USG and MRI help increase sensitivity and detect mass lesions amenable to surgery.
Keywords: Boston Carpal Tunnel Questionnaire, carpal tunnel syndrome, magnetic resonance imaging, nerve conduction study, ultrasonography
Introduction
Carpal tunnel syndrome (CTS) accounts for about 90% of all entrapment neuropathy.[1] It affects nearly 1–3.5 patients per 100,000 person years.[2,3] The incidence and prevalence varies, 0.125–1% and 5–16%, depending upon the criteria used for the diagnosis.[1,4] The usual predisposing factors are diabetes mellitus, obesity, hyperlipidemia, rheumatoid arthritis, hypothyroidism, recurrent twisting turning of hands while working, work with vibrating tools and postpartum period. CTS is primarily a clinical diagnosis and is clinically detected by stress tests such as Phalen test, Tinel's test, hand elevation test, pressure provocation test, tethered median nerve stress test, tourniquet test, and others. Nerve conduction study (NCS) is considered as gold standard for diagnosis. However, recent years have shown a surge in research papers on the utility of ultrasonography (USG) in diagnosis of CTS. Magnetic resonance imaging (MRI) too has been studied in this field, and has shown some importance. However not much study of has been done on this topic in India. We undertook this study to study the efficacy of clinical tests, NCS, USG, and MRI in diagnosing CTS, and the correlation between them. We also planned to study the correlation between Boston Carpal Tunnel Questionnaire Symptom (BCTQ-S) and function (BCTQ-F) score with NCS and USG.
Materials and Methods
This prospective study was performed over 24 months from August 2012 to July 2014. Patients attending Outdoor Department of Neurology Department and referred from Medicine and Orthopedics Outdoor Departments were included in the study. Patients were screened for symptoms suggestive of CTS and clinical provocative tests of CTS were done. Phalen test, Tinel's test, hand elevation test, pressure provocation test, tethered median nerve stress test, and tourniquet test were performed in all patients. Of a total of 60 patients (100 hands) screened with symptoms of upper limb paresthesias, 54 patients (93 hands) were diagnosed as having CTS clinically. Twenty-three control hands were included, of which 4 patients (8 hands) were completely asymptomatic, whereas 15 had unilateral CTS and opposite hand (15 hands in all) was taken as control. Then, patients were subjected to NCS on Medelec Synergy Machine, Oxford, UK measuring sensory and motor latency, amplitude, and velocity. Patients were also subjected to orthodromic mixed median nerve study, median to ulnar nerve, and median to radial nerve comparative study. All NCS positive patients were classified into subgroups for sake of analysis as follows: Mild-prolonged sensory latency and/or decreased sensory velocity, normal sensory amplitude and motor study; mild-moderate - prolonged latency and/or decreased velocity in sensory and motor nerves, with normal amplitude; moderate - above with mildly decrease sensory and/or motor amplitude; severe - absent sensory potentials or severely reduced sensory and/or motor amplitudes. USG was performed by Aloka Prosound α6 machine using a 10 MHz linear probe measuring anterior-posterior diameter, transverse diameter, circumference (CIR), and cross-section area (CSA) each, at the inlet (I), middle (M), and outlet (O) of the carpal tunnel. MRI of wrists for carpal tunnel and median nerve was done in 26 of the patients (39 hands) on a Philips Achieva, Netherland 1.5-Tesla MRI machine.
Statistical analysis
For statistical analysis the data was entered in Microsoft Excel format and analyzed using Stata for Windows, StataCorp USA, Version 11.1 and Statistical Package for the Social Sciences software (SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.; Now IBM SPSS Statistics). Data were expressed in mean, percentage, and standard deviation. Pearson's correlation coefficient was used for comparing BCTQ-S and BCTQ-F with both NCS and USG and between NCS and USG. USG of cases and controls were compared by Kruskal-Wallis Test, and comparison of MRI among the NCS severity groups was carried out by Fischer's exact test. Informed consent was obtained from all patients and the study was performed with approval of the Institutional Ethics Committee.
Results
Baseline characteristics of the study group are given in Table 1. The patients’ mean age was 43.9 ± 14 years, and most of them were females, with male to female ratio being 13:79. The most common risk factor was obesity (15%) followed by hypothyroidism (12.9%), diabetes mellitus (10.75%), work related (8.6%), and postpartum state (7.53%). Rheumatoid arthritis was associated in only one patient (1.1%). Among clinical tests, Phalen's test and hand elevation tests had high sensitivities (84.9% each) and specificities (74–83%), whereas Tinel's test had a moderate sensitivity (78.5%) but high specificity (91%). Pressure provocation test had high sensitivity (81.7%) but moderate specificity (69.6%), while tethered median nerve test and tourniquet tests had only moderate sensitivities (~70%) and specificities (65–70%). Among the subgroups, Phalen test had greater sensitivity in mild-moderate, moderate, and severe groups, while Tinel's had greater sensitivity in mild group. Rest of the tests had greater sensitivities in mild and mild-moderate groups [Table 2]. USG had a sensitivity and specificity of 30.1% and 91.3% respectively, while MRI had a sensitivity of 53.8%. USG in patients was significantly abnormal compared to controls in CSA-I and CIR-I in all patient groups, and also in CSA-O in all-CTS (ACTS) group by Kruskal-Wallis test. Pearson's correlation between BCTQ-S and BCTQ-F with both, NCS and USG and also between NCS and USG were calculated. Symptoms score in BCTQ showed significant correlation with sensory latency, sensory amplitude, and sensory velocities in most groups, while moderate and severe groups also had correlation with motor amplitude. Function score showed significant correlation with sensory latency, sensory amplitude, and motor amplitude [Figure 1]. Symptom score compared to USG showed significant correlation only in mild group in CIR-O, while function score showed no correlation with USG [Table 3]. Comparing USG with nerve conduction velocity showed significant correlation between CIR-M with sensory latency in ACTS group and between CIR-O with sensory latency in mild and sensory amplitude in moderate groups. CSA-M correlated with sensory latency in mild-moderate and ACTS groups. CSA-I correlated with sensory latency, motor latency, and motor amplitude in ACTS group [Table 4 and Figure 2]. MRI between moderate and nonmoderate groups was statistical significant by Fischer's exact test, as was the combined moderate and severe groups compared to the mild and mild-moderate groups [Table 5].
Table 1.
Clinical profile of study population with risk factors and hand involvement in CTS
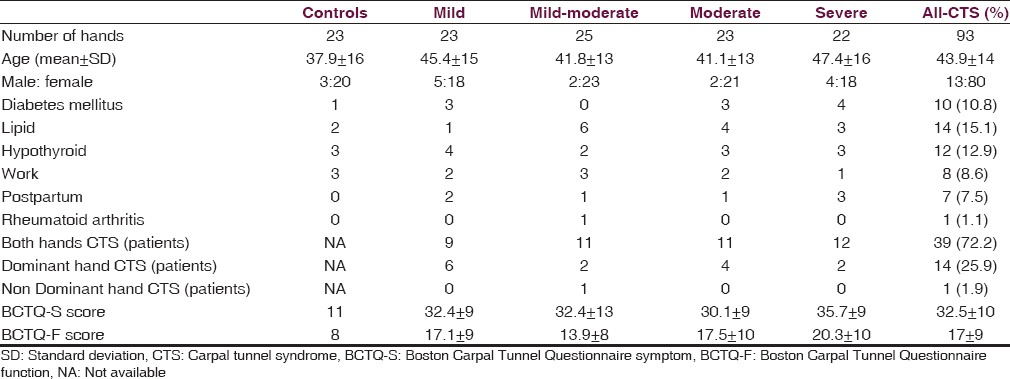
Table 2.
Sensitivity and specificity of clinical tests in diagnosing CTS
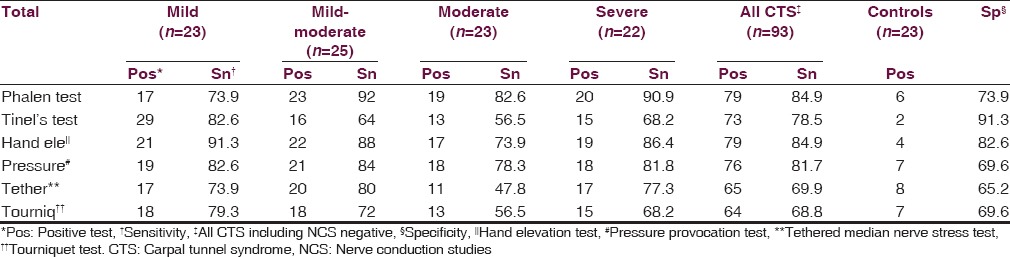
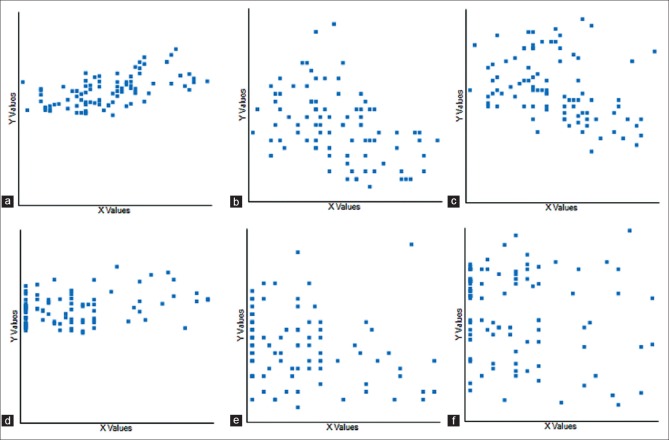
Figure 1.
Correlation between Boston Carpal Tunnel Questionnaire Symptom (S) and Function (F) scores and nerve conduction study in all-carpal tunnel syndrome patient group. (a) Boston Carpal Tunnel Questionnaire-S with SL in all-carpal tunnel syndrome group. (b) Boston Carpal Tunnel Questionnaire-S with SA in all-carpal tunnel syndrome group. (c) Boston Carpal Tunnel Questionnaire-S with SV in all-carpal tunnel syndrome group. (d) Boston Carpal Tunnel Questionnaire-F with SL in all-carpal tunnel syndrome group. (e) Boston Carpal Tunnel Questionnaire-F with SA in all-carpal tunnel syndrome group. (f) Boston Carpal Tunnel Questionnaire-F with MA in all-carpal tunnel syndrome group (SL = sensory latency, SA = sensory amplitude, SV = sensory velocity, and MA = motor amplitude)
Table 3.
Pearson's correlation (r) between BCTQ-S score and BCTQ-F score with NCS and USG
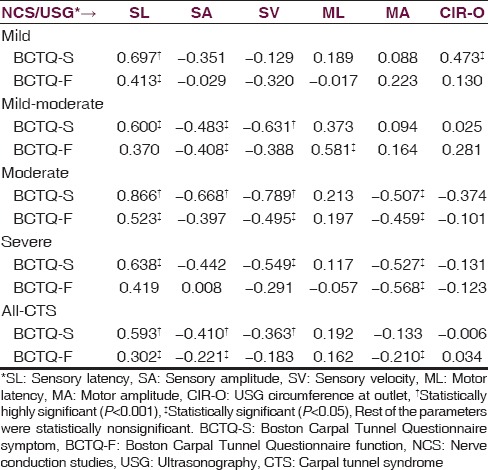
Table 4.
Pearson's correlation (r) between NCS and USG
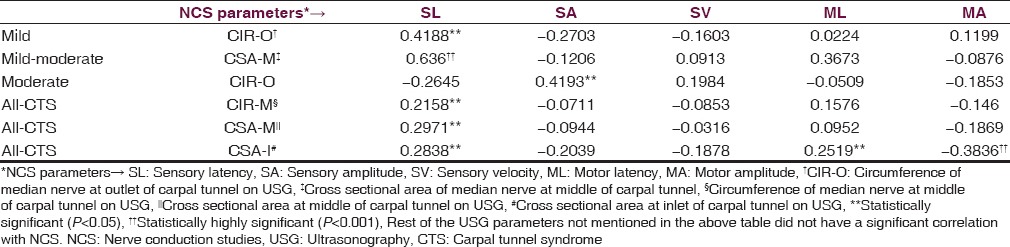
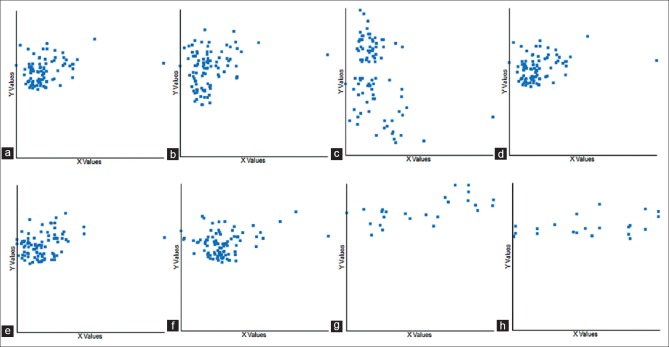
Figure 2.
Correlation between ultrasonography and nerve conduction study. (a) Cross-sectional area at inlet of carpal tunnel with SL in all-carpal tunnel syndrome group. (b) Cross-sectional area at inlet of carpal tunnel with ML in all-carpal tunnel syndrome group. (c) Cross-sectional area at inlet of carpal tunnel with MA in all-carpal tunnel syndrome group. (d) SL with cross-sectional area at inlet of carpal tunnel in all-carpal tunnel syndrome group. (e) SL with cross-sectional area at middle of carpal tunnel in all-carpal tunnel syndrome group. (f) SL with circumference at middle of carpal tunnel in all-carpal tunnel syndrome group. (g) SL with cross-sectional area at middle of carpal tunnel in mild-moderate carpal tunnel syndrome group. (h) SL with circumference at outlet of carpal tunnel in mild carpal tunnel syndrome group (SL = sensory latency, ML = motor latency, and MA = motor amplitude)
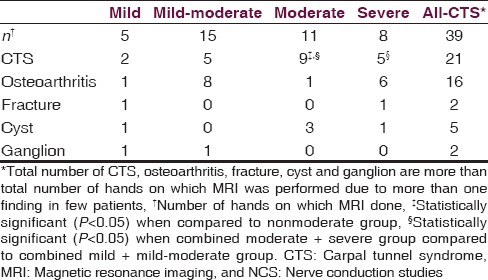
Table 5.
MRI diagnosis distributed as per NCS severity groups
Discussion
Most of the studies done till date have taken into account comparison between only NCS and CSA or bowing of the flexor retinaculum on USG.[5,6,7,8,9,10,11,12,13,14] We thus conducted this study to evaluate all available diagnostic parameters, that is, clinical tests, NCS, USG, and MRI and compare between them. We also compared them with BCTQ as previous studies have conflicting results, some showing a correlation while others not.[9,15,16]
Median nerve CSA has been the most studied USG parameter. Other include bowing of the flexor retinaculum, change in CSA of median nerve and flattening ratio. Various ranges for “normal” USG parameters have been reported with CSA ranging from 9 mm2 to 15 mm2. However, a lack of a consensus leads to difficulties in using USG as a diagnostic modality. Also the ideal site of CSA measurement is of debate. Sensitivity of CSA in diagnosing CTS ranges from 48% to 89% and specificity from 47% to 100%.[5,6,7,8,9,10,11,12,13,14] USG becomes more sensitive for moderate and severely affected patients compared to mildly affected ones. However, it has been reported to be positive in up to 30% of patients with false negative NCS.[7] Herein lays the importance of USG in detecting CTS.
NCS tend to become abnormal after significant compression leads to ischemic demyelination of the median nerve. This occurs first in the fast conducting fibers which travel deep to the flexor retinaculum. Thus, routine NCS measuring superficial sensory branch of median nerve may fail to pick up the pathology. Thus as per the AAEM guidelines, orthodromic mixed nerve studies and comparative studies (median to ulnar digit 4, median to radial thumb) should be done. These techniques increase the sensitivity and specificity of diagnosing CTS. Thus, NCS has been considered as gold standard. However, many patients experience discomfort during NCS and the same are more willing to undergo USG or MRI instead. In addition, adding USG in the diagnostic armamentarium helps increase sensitivity of NCS from 76% to 84% but decreases the specificity from 97% to 84%,[8] Mondelli et al.[9] reported combined sensitivity of 76%.
Our study showed significant difference between patients and controls in CSA-I in all the groups, including mild, mild-moderate, moderate, severe, and ACTS groups, and between CSA-O and ACTS group. A new finding noted in our study which has not been studied previously is the median nerve CIR. We found a significant difference in CIR-I of patients with CTS compared to controls in all the groups, including mild, mild-moderate, moderate, severe, and ACTS groups.
Comparison between USG and NCS in our study showed the overall significant correlation of both, area and CIR, with NCS. Among the subgroups, correlation was found between CIR-M and sensory latency in ACTS group, between CIR-O and sensory latency in mild group, and sensory amplitude in moderate group. Also, CSA-M correlated with sensory latency in mild-moderate group and in ACTS group, and CSA-I correlated with sensory latency, motor latency, and motor amplitude in ACTS group. Other studies also show a significant correlation between CSA and NCS, but have not studied CIR of the median nerve.[5,6,7,8,9,10,11,12,13,14]
Some studies have shown a correlation between BCTQ and NCS or USG,[15,16] whereas few have shown no correlation.[9] Our study showed a significant correlation with both. BCTQ symptom score correlated with sensory latency in mild group, sensory latency and velocity in severe group, and with sensory latency, amplitude and velocity in mild-moderate, moderate and ACTS group. A correlation was also found with motor amplitude in moderate and severe groups. The function score correlated with sensory latency in mild, moderate and ACTS groups, with sensory amplitude in mild-moderate and ACTS groups and sensory velocity in moderate group. Function score also correlated with motor latency in mild-moderate group and with motor amplitude in moderate, severe and ACTS group. In contrast, USG showed significant correlation only between CIR-O and symptom score, while no correlation was found between function score and any of the USG parameters.
MRI was performed in 39 of our CTS patients detected CTS in 21 patients (53.85%). Median nerve swelling and T2-weighted hyperintensity of median nerve and thenar muscles is considered as CTS on MRI[17,18,19] [Figure 3]. Other parameters considered likely suggestive of CTS were flattening of median nerve and to measure differing canal size. An important indication for the use of these MRI is preoperatively, and also for recurrent CTS after surgical release to look for real canal widening, inflammation, incomplete resection of ligament, and scarring or algodystrophy.[20,21] Our study documented associated pathologies such as osteoarthritis (41.03%), cyst (12.82%), ganglion (5.13%), and incidental fracture (5.13%). Thus, the overall sensitivity of MRI seems low. However, the associated pathologies (total 58.97% of MRIs done) detected were significant as they could not be detected on NCS or USG. This is important whenever surgical intervention, especially endoscopic intervention is planned for. As mentioned previously, MRI also plays a role in patients who have previously undergone carpal tunnel release operation and develop recurrent symptoms, to know the underlying anatomy and deformities, if any.
Figure 3.
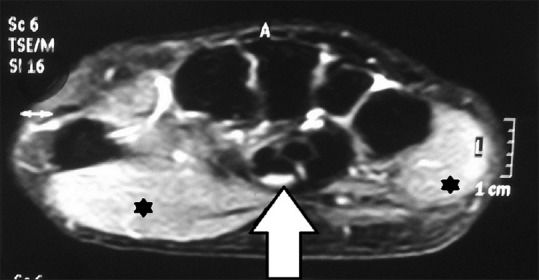
Magnetic resonance imaging of carpal tunnel showing T2-weighted hyperintensity of median nerve (arrow) and thenar and hypothenar muscles (asterisks)
There are a few limitations of our study. One limitation of this study was that MRI was not done in controls, due to financial constraints. Thus, we were unable to find the combined sensitivity of NCS, USG, and MRI. For the same reason, specificity of MRI could not be calculated either. Also, among our controls we included both completely asymptomatic patients (8 hands) and the asymptomatic hand of patients with unilateral CTS (15 hands). Some consider inclusion of asymptomatic hand as a control to be beneficial as it helps eliminate the bias of the normal anatomical variation in median nerve and the carpal tunnel structures. However, others are of the opinion that inclusion of asymptomatic hand leads to selection bias.[22] Although some may consider this as a limitation of our study, we have deliberately included such controls in order to eliminate this controversy and to derive an overall unbiased result.
Conclusions
Clinical tests even today have a role as Phalen test, hand elevation test, and pressure provocation test have a high sensitivity, whereas Tinel's test has a high specificity, which approach that seen with NCS, and may even exceed that of USG and MRI. Also, although even today NCS may be the gold standard for diagnosing, USG and MRI play a complimentary role in NCS negative patients and those planning for surgery or having recurrent symptoms even after surgery. MRI is particularly abnormal only in moderate and moderate to severely affected patients. In our study, we also found that the median nerve CIR is an important measurement and further larger multi-centric studies may show whether it is significant or not.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Basavraj Banakar, Janardan Sharma, Mohammad Yasin, Ankit Shah, Kirti Sachdev
References
- 1.Aroori S, Spence RA. Carpal tunnel syndrome. Ulster Med J. 2008;77:6–17. [PMC free article] [PubMed] [Google Scholar]
- 2.Cranford CS, Ho JY, Kalainov DM, Hartigan BJ. Carpal tunnel syndrome. J Am Acad Orthop Surg. 2007;15:537–48. doi: 10.5435/00124635-200709000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Kotwal PP, Varshney MK. Carpal tunnel syndrome: Controversies and Consensus. Pb J Orthop. 2009;XI:32–7. [Google Scholar]
- 4.Tanaka S, Wild DK, Seligman PJ, Behrens V, Cameron L, Putz-Anderson V. The US prevalence of self-reported carpal tunnel syndrome: 1988 National Health Interview Survey data. Am J Public Health. 1994;84:1846–8. doi: 10.2105/ajph.84.11.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotevoglu N, Gülbahce-Saglam S. Ultrasound imaging in the diagnosis of carpal tunnel syndrome and its relevance to clinical evaluation. Joint Bone Spine. 2005;72:142–5. doi: 10.1016/j.jbspin.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Prime MS, Palmer J, Khan WS, Goddard NJ. Is there light at the end of the tunnel?. Controversies in the diagnosis and management of carpal tunnel syndrome. Hand (N Y) 2010;5:354–60. doi: 10.1007/s11552-010-9263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyuncuoglu HR, Kutluhan S, Yesildag A, Oyar O, Guler K, Ozden A. The value of ultrasonographic measurement in carpal tunnel syndrome in patients with negative electrodiagnostic tests. Eur J Radiol. 2005;56:365–9. doi: 10.1016/j.ejrad.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Nakamichi K, Tachibana S. Ultrasonographic measurement of median nerve cross-sectional area in idiopathic carpal tunnel syndrome: Diagnostic accuracy. Muscle Nerve. 2002;26:798–803. doi: 10.1002/mus.10276. [DOI] [PubMed] [Google Scholar]
- 9.Mondelli M, Filippou G, Gallo A, Frediani B. Diagnostic utility of ultrasonography versus nerve conduction studies in mild carpal tunnel syndrome. Arthritis Rheum. 2008;59:357–66. doi: 10.1002/art.23317. [DOI] [PubMed] [Google Scholar]
- 10.Duncan I, Sullivan P, Lomas F. Sonography in the diagnosis of carpal tunnel syndrome. AJR Am J Roentgenol. 1999;173:681–4. doi: 10.2214/ajr.173.3.10470903. [DOI] [PubMed] [Google Scholar]
- 11.Swen WA, Jacobs JW, Bussemaker FE, de Waard JW, Bijlsma JW. Carpal tunnel sonography by the rheumatologist versus nerve conduction study by the neurologist. J Rheumatol. 2001;28:62–9. [PubMed] [Google Scholar]
- 12.Lee D, van Holsbeeck MT, Janevski PK, Ganos DL, Ditmars DM, Darian VB. Diagnosis of carpal tunnel syndrome. Ultrasound versus electromyography. Radiol Clin North Am. 1999;37:859–72, x. doi: 10.1016/s0033-8389(05)70132-9. [DOI] [PubMed] [Google Scholar]
- 13.Bayrak IK, Bayrak AO, Tilki HE, Nural MS, Sunter T. Ultrasonography in carpal tunnel syndrome: Comparison with electrophysiological stage and motor unit number estimate. Muscle Nerve. 2007;35:344–8. doi: 10.1002/mus.20698. [DOI] [PubMed] [Google Scholar]
- 14.El Miedany YM, Aty SA, Ashour S. Ultrasonography versus nerve conduction study in patients with carpal tunnel syndrome: Substantive or complementary tests? Rheumatology (Oxford) 2004;43:887–95. doi: 10.1093/rheumatology/keh190. [DOI] [PubMed] [Google Scholar]
- 15.Giannini F, Cioni R, Mondelli M, Padua R, Gregori B, D’Amico P, et al. A new clinical scale of carpal tunnel syndrome: Validation of the measurement and clinical-neurophysiological assessment. Clin Neurophysiol. 2002;113:71–7. doi: 10.1016/s1388-2457(01)00704-0. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Kim TK, Yoon ES, Dhong ES. Correlation of high-resolution ultrasonographic findings with the clinical symptoms and electrodiagnostic data in carpal tunnel syndrome. Ann Plast Surg. 2005;54:20–3. doi: 10.1097/01.sap.0000141942.27182.55. [DOI] [PubMed] [Google Scholar]
- 17.Jarvik JG, Comstock BA, Heagerty PJ, Haynor DR, Fulton-Kehoe D, Kliot M, et al. Magnetic resonance imaging compared with electrodiagnostic studies in patients with suspected carpal tunnel syndrome: Predicting symptoms, function, and surgical benefit at 1 year. J Neurosurg. 2008;108:541–50. doi: 10.3171/JNS/2008/108/3/0541. [DOI] [PubMed] [Google Scholar]
- 18.Kleindienst A, Hamm B, Hildebrandt G, Klug N. Diagnosis and staging of carpal tunnel syndrome: Comparison of magnetic resonance imaging and intra-operative findings. Acta Neurochir (Wien) 1996;138:228–33. doi: 10.1007/BF01411366. [DOI] [PubMed] [Google Scholar]
- 19.Radack DM, Schweitzer ME, Taras J. Carpal tunnel syndrome: Are the MR findings a result of population selection bias? AJR Am J Roentgenol. 1997;169:1649–53. doi: 10.2214/ajr.169.6.9393185. [DOI] [PubMed] [Google Scholar]
- 20.Bordalo-Rodrigues M, Amin P, Rosenberg ZS. MR imaging of common entrapment neuropathies at the wrist. Magn Reson Imaging Clin N Am. 2004;12:265–79, vi. doi: 10.1016/j.mric.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Altinok T, Baysal O, Karakas HM, Sigirci A, Alkan A, Kayhan A, et al. Ultrasonographic assessment of mild and moderate idiopathic carpal tunnel syndrome. Clin Radiol. 2004;59:916–25. doi: 10.1016/j.crad.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Padua L, Pasqualetti P, Rosenbaum R. One patient, two carpal tunnels: Statistical and clinical analysis – by hand or by patient? Clin Neurophysiol. 2005;116:241–3. doi: 10.1016/j.clinph.2004.08.006. [DOI] [PubMed] [Google Scholar]