Abstract
Aims
Stent underexpansion and malapposition are associated with adverse outcomes following percutaneous coronary intervention, but detection and treatment can be challenging in the presence of extensive coronary artery calcification. Frequency domain optical coherence tomography (FD-OCT) is a novel intravascular imaging technique with greater spatial resolution than intravascular ultrasound (IVUS) but its role in the presence of extensive coronary calcification remains unclear. We sought to determine the utility of FD-OCT compared to IVUS imaging to guide percutaneous coronary intervention in patients with severe calcific coronary artery disease.
Methods
18 matched IVUS and FD-OCT examinations were evaluated following coronary stent implantation in 12 patients (10 male; mean age 70±7 years) undergoing rotational atherectomy for symptomatic calcific coronary artery disease.
Results
In-stent luminal areas were smaller (minimum in-stent area 6.77±2.18 vs 7.19±2.62 mm2, p<0.05), while reference lumen dimensions were similar with FD-OCT compared with IVUS. Stent malapposition was detected in all patients by FD-OCT and in 10 patients by IVUS. The extent of stent malapposition detected was greater (20% vs 6%, p<0.001) with FD-OCT compared to IVUS. Postdilation increased the in-stent luminal area (minimum in-stent area: 8.15±1.90 vs 7.30±1.62 mm2, p<0.05) and reduced the extent of stent malapposition (19% vs 34%, p<0.005) when assessed by FD-OCT, but not IVUS.
Conclusions
Acute stent malapposition occurs frequently in patients with calcific coronary disease undergoing rotational atherectomy and stent implantation. In the presence of extensive coronary artery calcification, FD-OCT affords enhanced stent visualisation and detection of malapposition, facilitating improved postdilation stent apposition and minimal luminal areas.
Trial Registration number
Keywords: INTERVENTIONAL CARDIOLOGY
Key questions.
What is already known about this subject?
Stent malapposition and underexpansion are major risk factors for stent thrombosis and restenosis.
Intravascular imaging using ultrasound is useful to help identify stent malapposition and underexpansion and facilitates optimal stent placement.
The presence of extensive calcification limits the value of intravascular ultrasound reflecting the ultrasound waves and causing artefacts.
Optical coherence tomography is a novel intravascular imaging modality with 10-fold greater axial resolution than intravascular ultrasound.
It is not yet clear whether optical coherence tomography is superior to intravascular ultrasound in the presence of extensive vascular calcification.
What does this study add?
Coronary stent malapposition and underexpansion are common in patients with extensive vascular calcification undergoing percutaneous coronary intervention.
Optical coherence tomography is superior to intravascular ultrasound in the detection of stent underexpansion and malapposition in patients with extensive coronary artery calcification.
Stent postdilation reduced the extent of stent malapposition as assessed by optical coherence tomography.
How might this impact on clinical practice?
Choosing the most appropriate intravascular imaging modality in patients with extensive coronary artery calcification should facilitate the detection of stent underexpansion and malapposition, permit optimal stent implantation and, ultimately reduce the risk of stent thrombosis and restenosis.
Ensuring adequate coronary stent expansion and apposition at implantation are key factors in the prevention of in-stent restenosis and stent thrombosis.1–4 This can be challenging in patients with extensive coronary artery calcification where vascular calcification may limit equipment delivery, lesion preparation and ultimately, stent expansion and apposition to the vessel wall. The presence of extensive vascular calcification also limits angiographic visualisation impairing lesion assessment and detection of stent underexpansion and malapposition.
Intravascular ultrasound (IVUS) imaging is often used in conjunction with fluoroscopy to assess coronary stent implantation and guide percutaneous coronary intervention.5 However, ultrasound itself penetrates calcium poorly and this, combined with the potential for artefact, such as reverberation or reflection,6 limits the value of IVUS in the assessment of heavily calcified coronary arteries.
Fourier domain optical coherence tomography (FD-OCT) is a novel near-infrared intravascular imaging modality with ∼10-fold greater axial resolution (∼15 μm) than IVUS.7 8 Assessment of coronary artery dimensions by FD-OCT is accurate9 and reproducible.10 Postmortem data11 12 and case reports suggest that FD-OCT may afford enhanced visualisation in heavily calcified vessels when compared to IVUS,13 14 but this hypothesis remains to be tested systematically in a clinical study. The aim of this study was to compare FD-OCT with IVUS imaging in patients with extensive coronary artery calcification undergoing rotational atherectomy and coronary stent implantation.
Methods
Twelve patients undergoing percutaneous coronary intervention with adjunctive rotational atherectomy for undilatable calcific coronary artery disease at the Edinburgh Heart Centre were enrolled. Rotational atherectomy was performed using the Rotablator (Boston Scientific, Fremont, California, USA) and conventional techniques.15 Operators were encouraged to use a maximum burr/vessel ratio of 0.5 and rotational burr speed ranged between 160 000 and 190 000 rotations per minute. Intracoronary verapamil was administered during rotablation and temporary pacing wires were only inserted when clinically indicated. Operators were encouraged but not mandated to postdilate with a non-compliant balloon matched at least 1:1 with the proximal reference vessel. Patients were only included in the postdilation analysis if IVUS and FD-OCT data were available.
All patients were loaded and established on maintenance dose aspirin (75 mg) and clopidogrel (75 mg) prior to the procedure. Unfractionated heparin was administered as an initial bolus of 70 IU/kg with additional heparin being administered as guided by the activated clotting time (target 250–300 s). This study was performed with the approval of the West of Scotland Research Ethics Committee and written informed consent was obtained from all patients.
Imaging acquisition and analysis
In all patients, paired FD-OCT and IVUS automated pullback assessments were performed immediately following coronary stent implantation. In six patients, further paired FD-OCT and IVUS pullbacks were obtained immediately following high-pressure postdilation of the stent. Intracoronary nitroglycerin (200 μg) was administered immediately prior to each imaging pullback. Imaging data were stored digitally and analysed offline.
Intravascular ultrasound
IVUS imaging was performed using a 40 MHz Atlantis SR Pro catheter (Boston Scientific, Fremont, California, USA) and an automated pullback at 0.5 mm/s to include the stented segment and at least 5 mm reference at either end.
Optical coherence tomography
Fourier domain OCT was performed using a FastView OFDI imaging catheter (Terumo, Tokyo, Japan) with an automated pullback at 20 mm/s. Intracoronary injection of undiluted X-ray contrast medium (omnipaque 300) at 4–5 mL/s was used to achieve a blood-free field of view.
Image analysis
Cross-sectional images were evaluated by two experienced operators using validated software (Analyze 11.0, Mayo Clinic, Minnesota, USA). Luminal areas and diameters were assessed at 0.15 mm intervals. Matched stented segments were defined for IVUS and FD-OCT images using proximal and distal stent edges and side branches as reference landmarks.
Malapposition detected by IVUS was defined as clear separation with visible blood speckle between at least one stent strut and the vessel wall.16 Malapposition detected by FD-OCT was defined as a distance between stent strut and vessel wall (chord length) of >1.5 times the manufacturers stated stent strut thickness. For IVUS and FD-OCT, 360° chords were generated based on the identified lumen and stent contours to identify the per cent of stent perimeter identified as malapposed and the maximum malapposition area. Given the thickness of each cross-sectional slice and the total number of analysed slices containing circumferential stent, we calculated the amount of malapposed stent expressed as a percentage of the total stent surface area for each patient.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism 6. Data are expressed as mean±SD, median (range) or n (%) as appropriate. Between and within comparisons for matched IVUS and FD-OCT data were made using paired t test and further examined by correlation analysis and Bland-Altman plots. Two-sided p<0.05 was taken as statistical significance.
Results
Eighteen paired IVUS and FD-OCT pullbacks were performed in 12 patients. In six patients, paired IVUS and FD-OCT pullbacks performed immediately following stent implantation and following stent postdilation were available. Patients were predominantly male with a mean age of 70 years (table 1) and principally received a single long drug-eluting stent following rotablation (table 2).
Table 1.
Patient demographics
| N=12 | |
|---|---|
| Age, years | 70±7 |
| Male, n (%) | 10 (83) |
| Acute presentation, n (%) | 4 (33) |
| Previous MI, n (%) | 3 (25) |
| Hypertension, n (%) | 9 (75) |
| Hyperlipidemia, n (%) | 12 (100) |
| Diabetes mellitus, n (%) | 2 (17) |
| Previous CABG, n (%) | 3 (25) |
| History of cigarette smoking, n (%) | 7 (58) |
CABG, coronary artery bypass graft; MI, myocardial infarction.
Table 2.
Procedural characteristics
| N=12 | |
|---|---|
| Radial/femoral access, n | 10/2 |
| Guide catheter size, F | 6 (6–7.5) |
| Largest rotablation burr used, mm | 1.75 (1.25–2.0) |
| Total burr duration, seconds | 55±17 |
| Heparin dose, IU | 7042±2050 |
| Procedural success, n | 12 (100%) |
| Number of stents/patient, n | 1.1±0.3 |
| Number of drug eluting stents/patient, n | 0.9±0.5 |
| Mean stent diameter, mm | 3.3±0.5 |
| Total stent length, mm | 27±15 |
| Mean stent deployment pressure, atm | 13±3 |
| Postdilation balloon diameter (n=10), mm | 3.8±0.8 |
| Maximum postdilation balloon pressure (n=10), atm | 16±5 |
Data are presented as median (range), mean±SD or n (%).
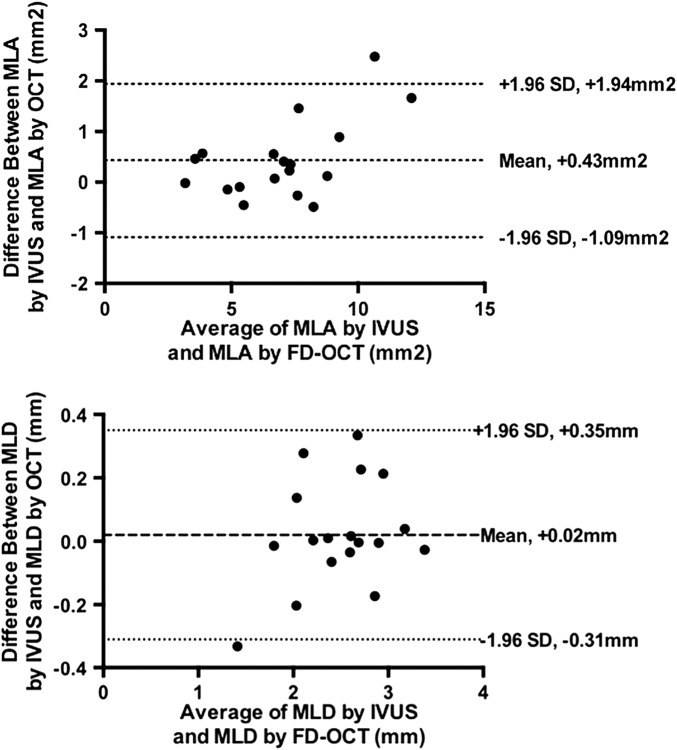
Reference lumen areas were similar but in-stent luminal areas as determined by FD-OCT were smaller (minimum in-stent luminal area: 6.77±2.18 vs 7.19±2.62 mm2, p<0.05) when compared to IVUS (tables 2 and 3). There was a good correlation between FD-OCT and IVUS measurements (minimum luminal diameter, r=0.95, p<0.0001; and minimum luminal area, r=0.96, p<0.0001, respectively). The mean differences between FD-OCT and IVUS were 0.02±0.17 mm for minimum luminal diameter and 0.42±0.77 mm2 for minimum luminal area, respectively, (figure 1).
Table 3.
Comparison of IVUS and FD-OCT assessments following rotational atherectomy (n=18)
| IVUS | FD-OCT | Difference (IVUS—FD-OCT) | p Value | |
|---|---|---|---|---|
| Mean reference lumen diameter, mm | 3.64±0.80 | 3.38±0.66 | 0.26±0.34 | 0.330 |
| Mean reference lumen area, mm2 | 10.77±1.14 | 9.49±3.59 | 1.28±1.69 | 0.388 |
| In-stent diameter, mm | ||||
| Minimum | 2.51±0.53 | 2.48±0.48 | 0.02±0.17 | 0.586 |
| Maximum | 4.38±0.82 | 3.81±0.67 | 0.57±0.28 | <0.001 |
| Mean | 3.38±0.62 | 3.25±0.60 | 0.13±0.17 | <0.005 |
| In-stent area, mm2 | ||||
| Minimum | 7.19±2.62 | 6.77±2.18 | 0.42±0.77 | <0.05 |
| Maximum | 12.81±4.61 | 10.48±3.65 | 2.33±2.25 | <0.001 |
| Mean | 9.54±3.48 | 8.78±2.91 | 0.82±1.15 | <0.01 |
| Percentage of stent malapposed, % | 5.5±5.0 | 19.6±15.1 | −14.1±12.4 | <0.001 |
| Max stent malapposition, distance in mm | 0.57±0.32 | 1.10±0.34 | −0.53±0.33 | <0.001 |
| Max stent malapposition, area in mm2 | 0.88±1.09 | 2.65±1.88 | −1.77±1.35 | <0.001 |
FD-OCT, frequency domain optical coherence tomography; IVUS, intravascular ultrasound.
Figure 1.
Bland Altman analyses comparing minimum luminal area (MLA; upper panel) and diameter (MLD; lower panel) obtained using FD-OCT and IVUS. FD-OCT, frequency domain optical coherence tomography; IVUS, intravascular ultrasound.
Stent malapposition was detectable in all 12 patients (100%) using FD-OCT, but in only 10 patients (83%) using IVUS. The extent of stent malapposition detected with FD-OCT was greater than that detected by IVUS (20% vs 6%, p<0.001, expressed as per cent of total stent surface area; table 3). The maximum distance and maximum area of malapposition detected using FD-OCT were greater than those obtained with IVUS (1.1±0.34 mm vs 0.57±0.32 mm, p<0.001 and 2.65±1.88 mm2 vs 0.88±1.09 mm2, p<0.001, respectively; table 3).
An increase in-stent luminal areas (minimum in-stent luminal area: 8.15±1.90 vs 7.30±1.62 mm2, p<0.05) and a reduction in the extent of stent malapposition (19% vs 34%, p<0.005, expressed as % of total stent surface area) were observed following postdilation when assessed with FD-OCT, but not IVUS (table 4).
Table 4.
Effect of postdilation on stent dimensions assessed by FD-OCT and IVUS (N=6)
| IVUS |
FD-OCT |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | p Value | Pre | Post | p Value | |
| In-stent diameter, mm | ||||||
| Minimum | 2.55±0.42 | 2.70±0.50 | 0.057 | 2.60±0.41 | 2.82±0.34 | 0.036 |
| Maximum | 4.43±0.63 | 4.77±0.85 | 0.152 | 3.84±0.48 | 4.25±0.65 | 0.031 |
| In-stent area, mm2 | ||||||
| Minimum | 7.73±2.20 | 7.95±2.72 | 0.551 | 7.30±1.62 | 8.15±1.90 | 0.023 |
| Maximum | 13.02±3.41 | 14.77±5.45 | 0.184 | 10.72±2.55 | 13.00±3.62 | 0.040 |
| Percentage of stent malapposed (%) | 7±7 | 7±4 | 0.984 | 34±12 | 19±10 | 0.004 |
FD-OCT, frequency domain optical coherence tomography; IVUS, intravascular ultrasound.
Discussion
This is the first clinical study to examine systematically the utility of FD-OCT and IVUS in the setting of extensive coronary artery calcification. Consistent with published data in more conventional atherosclerotic populations,9 10 17 FD-OCT generally described smaller lumen dimensions and detected acute stent malapposition more frequently when compared to IVUS. Indeed, acute stent malapposition was detectable to some extent in all patients using FD-OCT, with the amount of stent malapposed ranging from 1% to 53% of the total stent surface area. Finally, high-pressure postdilation with a non-compliant balloon was associated with a significant reduction in acute stent malapposition as detected by FD-OCT.
Our findings that in-stent diameters and luminal areas were smaller with FD-OCT compared to IVUS are largely in keeping with previous clinical studies.9 17–20 Moreover, comparing dimensions obtained using FD-OCT with those obtained using IVUS, the mean differences in lumen area observed in this study are consistent with previous work.9 10 20 The smaller catheter size and faster pullback speed with FD-OCT have been proposed as a potential explanation for these differences.17 However, a recent study by Kubo et al demonstrated that vessel measurements obtained using IVUS overestimated vessel dimensions, while enhanced delineation of the lumen-vessel interface and visualisation of stent struts obtained with FD-OCT led to a more accurate assessment of vessel and stent dimensions.9 This is particularly relevant in heavily calcified vessels where acoustic shadow and reflection may interfere with IVUS assessment (figure 2).6 Consistent with this hypothesis, previous work has demonstrated that vessel dimensions obtained using FD-OCT are highly reproducible10 and more accurate than IVUS.9 21 Indeed, in phantom models and in human arteries in vitro, IVUS overestimates vessel luminal area by up to 16% and 14%, respectively,9 22 in contrast to QCA measurement of coronary arteries, which underestimates vessel dimensions when compared to FD-OCT.9
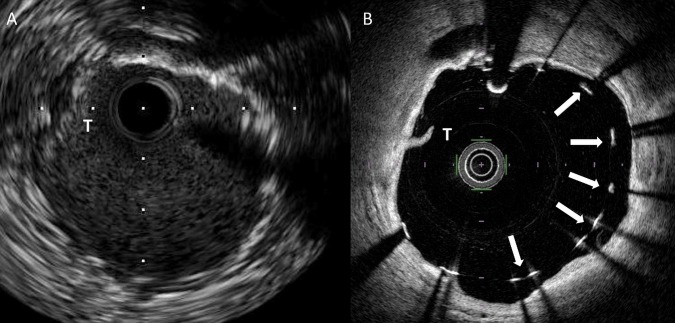
Figure 2.
(A) Matched IVUS and (B) FD-OCT cross-sectional images following coronary stent implantation in a patient treated with rotational atherectomy. An arc of calcification is visible in the vessel wall from the 12 o'clock position around to the 6 o'clock position. Malapposed stent struts are clearly visible in this area with FD-OCT (panel B, arrows) but the interface between stent strut and vessel wall is poorly delineated in the corresponding IVUS image (panel A). An area of thrombus adherent to the luminal surface of the stent is also visible (T). FD-OCT, frequency domain optical coherence tomography; IVUS, intravascular ultrasound.
In keeping with more accurate delineation of the stent and vessel interface, we found that FD-OCT detected stent malapposition more frequently than IVUS (figure 2). Indeed, following stent deployment but prior to postdilation, stent malapposition was detected in all patients with FD-OCT but in only 83% patients with IVUS. With FD-OCT, the extent of stent malapposition detected (expressed as % of the total stent surface area) ranged from 1% to 53%, compared with 1–21% for IVUS. The extent of malapposition is in keeping the underlying nature of calcific coronary artery disease, where even with the use of ablative procedures such as rotational atherectomy and high-pressure postdilation, stent strut malapposition frequently persists.13 The circumferential extent but not the depth of calcification in the vessel wall as defined by IVUS has previously been shown to predict stent malapposition.23
While the resolution of FD-OCT permits stent strut level analysis,24 this is not the case for IVUS. Previous studies have employed a number of techniques to permit comparison between these two imaging techniques: for example, expressing malapposition as mean malapposed area per cross-sectional slice17 or in binary form as the presence or absence of any stent malapposition.9 More refined methods include an attempt to quantify the extent of malapposition, either as the maximum number of consecutive frames with malapposed struts25 or as the percentage malapposed struts expressed as per cent of total number of struts.26 To address this issue and allow a clinically meaningful comparison between the techniques, we calculated the surface area of the stent that was malapposed and expressed this as a percentage of the total stent surface area. We believe this affords a robust and clinically meaningful method by which to compare stent malapposition as detected using these two techniques.
In this study, postdilation of the implanted stent with a non-compliant balloon inflated to high pressure was associated with a significant increase in the minimal in-stent luminal diameter and area, and a halving in the extent of malapposition observed using FD-OCT. While high-pressure postdilation of coronary stents has yielded mixed outcomes in clinical studies,27–29 our data provide some evidence to support this strategy as routine in patients with extensive coronary calcification where stent malapposition is a frequent finding. Although, mean stent and lumen dimensions obtained using IVUS were numerically greater following postdilation, this did not achieve statistical significance. As discussed above, we believe that this reflects the difficulties in delineating the stent-lumen interface in the presence of extensive vascular calcification.
Clinical relevance
Our findings suggest that in patients with extensive coronary artery calcification, FD-OCT is superior to IVUS at detecting acute stent underexpansion and malapposition. This, in combination with a more rapid pullback speed (up to 40 mm/s) of FD-OCT resulting in less ischaemic burden,9 and previous data suggesting more accurate assessment of lesion dimensions with FD-OCT, support a clinical utility for FD-OCT in the evaluation of percutaneous coronary intervention in patients with extensive coronary artery calcification. Most of the contemporary data supporting a role for intravascular imaging in this area relate to IVUS30–32 reflecting the temporal evolution of these two technologies. However, in a recent non-randomised case–control study, Prati et al demonstrated a lower incidence of cardiac death and myocardial infarction (6.6% vs 13%, p=0.006) in patients undergoing percutaneous coronary intervention guided by fluoroscopy and FD-OCT compared to fluoroscopic guidance alone.33 Large-scale randomised clinical trials are required before we can be sure our findings with FD-OCT will translate into improved clinical outcomes for patients.
Limitations
By definition, patients included in this study had undilatable calcific coronary artery lesions preventing delivery of the imaging catheter to the area of interest prior to atherectomy and treatment. This, combined with the need for a rapid intracoronary injection of contrast during FD-OCT imaging and the associated potential for propagation of any atherectomy-induced dissection, meant that intravascular imaging was performed only following rotational atherectomy and stent implantation.
In the current study, reference vessel dimensions were numerically smaller with FD-OCT compared to IVUS but this did not achieve statistical significance, perhaps reflecting the sample size. We elected to pool images obtained following stent implantation with those obtained following postdilation to maximise study power. While we accept this may be a potential source of bias, we believe that the postdilation intervention was sufficient to justify treating each run as a separate data set.
In summary, we have performed a systematic evaluation of the clinical utility of FD-OCT and IVUS in the presence of extensive coronary artery calcification. Our findings suggest that acute stent malapposition occurs frequently in this setting and that FD-OCT affords enhanced stent visualisation and detection of stent malapposition, facilitating stent postdilation and leading to improved stent apposition and minimal luminal areas.
Footnotes
Contributors: IG, PA, NGU and NLC contributed to the conception and design of the study were involved in data acquisition, analysis and interpretation, drafted the manuscript and approved the final version for publication. CG was involved in data analysis and interpretation, contributed to manuscript drafting and approved the final version for publication. JCS, MWB, PH, NM and DEN were involved in data acquisition, contributed to the draft manuscript and approved the final version for publication. All of the authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was funded by the Edinburgh and Lothians Health Foundation. NLMC is supported by a National Health Service Research Scotland Career Researcher Award.
Competing interests: None declared.
Ethics approval: West of Scotland Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Uren NG, Schwarzacher SP, Metz JA et al. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur Heart J 2002;23:124–32. 10.1053/euhj.2001.2707 [DOI] [PubMed] [Google Scholar]
- 2.Hong MK, Mintz GS, Lee CW et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J 2006;27:1305–10. 10.1093/eurheartj/ehi882 [DOI] [PubMed] [Google Scholar]
- 3.Doi H, Maehara A, Mintz GS et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv 2009;2:1269–75. 10.1016/j.jcin.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 4.Attizzani GF, Capodanno D, Ohno Y et al. Mechanisms, pathophysiology, and clinical aspects of incomplete stent apposition. J Am Coll Cardiol 2014;63:1355–67. 10.1016/j.jacc.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 5.Klersy C, Ferlini M, Raisaro A et al. Use of IVUS guided coronary stenting with drug eluting stent: a systematic review and meta-analysis of randomized controlled clinical trials and high quality observational studies. Int J Cardiol 2013;170:54–63. 10.1016/j.ijcard.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 6.Mintz GS, Anderson WD, Bailey SR et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents developed in collaboration with the European Society of Cardiology endorsed by the Society of Cardiac Angiography and Interventions. Eur J Echocardiogr 2001;2:299–313. 10.1053/euje.2001.0133 [DOI] [PubMed] [Google Scholar]
- 7.Prati F, Regar E, Mintz GS et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010;31:401–15. 10.1093/eurheartj/ehp433 [DOI] [PubMed] [Google Scholar]
- 8.Bezerra HG, Costa MA, Guagliumi G et al. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv 2009;2:1035–46. 10.1016/j.jcin.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubo T, Akasaka T, Shite J et al. OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study. JACC Cardiovasc Imaging 2013;6:1095–104. 10.1016/j.jcmg.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 10.Okamura T, Gonzalo N, Gutierrez-Chico JL et al. Reproducibility of coronary Fourier domain optical coherence tomography: quantitative analysis of in vivo stented coronary arteries using three different software packages. EuroIntervention 2010;6:371–9. 10.4244/EIJV6I1A62 [DOI] [PubMed] [Google Scholar]
- 11.Mehanna E, Bezerra HG, Prabhu D et al. Volumetric characterization of human coronary calcification by frequency-domain optical coherence tomography. Circ J 2013;77:2334–40. 10.1253/circj.CJ-12-1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kume T, Okura H, Kawamoto T et al. Assessment of the coronary calcification by optical coherence tomography. EuroIntervention 2011;6:768–72. 10.4244/EIJV6I6A130 [DOI] [PubMed] [Google Scholar]
- 13.Tanigawa J, Barlis P, Di Mario C. Heavily calcified coronary lesions preclude strut apposition despite high pressure balloon dilatation and rotational atherectomy: in-vivo demonstration with optical coherence tomography. Circ J 2008;72:157–60. 10.1253/circj.72.157 [DOI] [PubMed] [Google Scholar]
- 14.Girassolli A, Carrizo S, Jimenez-Valero S et al. Utility of optical coherence tomography and intravascular ultrasound for the evaluation of coronary lesions. Rev Port Cardiol 2013;32:925–9. 10.1016/j.repc.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 15.Tomey MI, Kini AS, Sharma SK. Current status of rotational atherectomy. JACC Cardiovasc Interv 2014;7:345–53. 10.1016/j.jcin.2013.12.196 [DOI] [PubMed] [Google Scholar]
- 16.Mintz GS, Nissen SE, Anderson WD et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2001;37:1478–92. 10.1016/S0735-1097(01)01175-5 [DOI] [PubMed] [Google Scholar]
- 17.Bezerra HG, Attizzani GF, Sirbu V et al. Optical coherence tomography versus intravascular ultrasound to evaluate coronary artery disease and percutaneous coronary intervention. JACC Cardiovasc Interv 2013;6:228–36. 10.1016/j.jcin.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi T, Terashima M, Akasaka T et al. Safety and feasibility of an intravascular optical coherence tomography image wire system in the clinical setting. Am J Cardiol 2008;101:562–7. 10.1016/j.amjcard.2007.09.116 [DOI] [PubMed] [Google Scholar]
- 19.Capodanno D, Prati F, Pawlowsky T et al. Comparison of optical coherence tomography and intravascular ultrasound for the assessment of in-stent tissue coverage after stent implantation. EuroIntervention 2009;5:538–43. 10.4244/EIJV5I5A88 [DOI] [PubMed] [Google Scholar]
- 20.Okamura T, Onuma Y, Garcia-Garcia HM et al. First-in-man evaluation of intravascular optical frequency domain imaging (OFDI) of Terumo: a comparison with intravascular ultrasound and quantitative coronary angiography. EuroIntervention 2011;6:1037–45. 10.4244/EIJV6I9A182 [DOI] [PubMed] [Google Scholar]
- 21.Tahara S, Bezerra HG, Baibars M et al. In vitro validation of new Fourier-domain optical coherence tomography. EuroIntervention 2011;6:875–82. 10.4244/EIJV6I7A149 [DOI] [PubMed] [Google Scholar]
- 22.Chae JS, Brisken AF, Maurer G et al. Geometric accuracy of intravascular ultrasound imaging. J Am Soc Echocardiogr 1992;5:577–87. 10.1016/S0894-7317(14)80323-4 [DOI] [PubMed] [Google Scholar]
- 23.Lindsay AC, Paulo M, Kadriye K et al. Predictors of stent strut malapposition in calcified vessels using frequency-domain optical coherence tomography. J Invasive Cardiol 2013;25:429–34. [PubMed] [Google Scholar]
- 24.Barlis P, Dimopoulos K, Tanigawa J et al. Quantitative analysis of intracoronary optical coherence tomography measurements of stent strut apposition and tissue coverage. Int J Cardiol 2010;141:151–6. 10.1016/j.ijcard.2008.11.204 [DOI] [PubMed] [Google Scholar]
- 25.Kawamori H, Shite J, Shinke T et al. Natural consequence of post-intervention stent malapposition, thrombus, tissue prolapse, and dissection assessed by optical coherence tomography at mid-term follow-up. Eur Heart J Cardiovasc Imaging 2013;14:865–75. 10.1093/ehjci/jes299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Im E, Kim BK, Ko YG et al. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. Circ Cardiovasc Interv 2014;7:88–96. 10.1161/CIRCINTERVENTIONS.113.000797 [DOI] [PubMed] [Google Scholar]
- 27.Dirschinger J, Kastrati A, Neumann FJ et al. Influence of balloon pressure during stent placement in native coronary arteries on early and late angiographic and clinical outcome: a randomized evaluation of high-pressure inflation. Circulation 1999;100:918–23. 10.1161/01.CIR.100.9.918 [DOI] [PubMed] [Google Scholar]
- 28.Frobert O, Sarno G, James SK et al. Effect of stent inflation pressure and post-dilatation on the outcome of coronary artery intervention. A report of more than 90,000 stent implantations. PLoS ONE 2013;8:e56348 10.1371/journal.pone.0056348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg SL, Di Mario C, Hall P et al. Comparison of aggressive versus nonaggressive balloon dilatation for stent deployment on late loss and restenosis in native coronary arteries. Am J Cardiol 1998;81:708–12. 10.1016/S0002-9149(97)01017-5 [DOI] [PubMed] [Google Scholar]
- 30.Casella G, Klauss V, Ottani F et al. Impact of intravascular ultrasound-guided stenting on long-term clinical outcome: a meta-analysis of available studies comparing intravascular ultrasound-guided and angiographically guided stenting. Catheter Cardiovasc Interv 2003;59:314–21. 10.1002/ccd.10537 [DOI] [PubMed] [Google Scholar]
- 31.Ahn JM, Kang SJ, Yoon SH et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol 2014;113:1338–47. 10.1016/j.amjcard.2013.12.043 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Farooq V, Garcia-Garcia HM et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention 2012;8:855–65. 10.4244/EIJV8I7A129 [DOI] [PubMed] [Google Scholar]
- 33.Prati F, Di Vito L, Biondi-Zoccai G et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention 2012;8:823–9. 10.4244/EIJV8I7A125 [DOI] [PubMed] [Google Scholar]