Abstract
The arcuate-nucleus of the hypothalamus is essential for metabolic-homeostasis and responds to leptin by producing several neuropeptides including proopiomelanocortin (POMC). We previously reported that high-dose erythropoietin (Epo)-treatment in mice while increasing hematocrit, reduced body-weight, fat-mass, and food intake, and increased energy-expenditure. Moreover, we showed that mice with Epo receptor (EpoR) restricted to erythroid cells (ΔEpoRE) became obese and exhibited decreased energy-expenditure. Epo/EpoR-signaling was found to promote hypothalamus POMC-expression independently from leptin. Herein we used wild-type (WT) and ΔEpoRE-mice and hypothalamus-derived neural-culture system to study the signaling pathways activated by Epo in POMC neurons. We show that Epo-stimulation activated STAT3-signaling and up-regulated POMC expression in WT neural cultures. ΔEpoRE-mice hypothalamus showed reduced POMC levels, and lower STAT3-phosphorylation, with and without leptin-treatment, compared to in vivo and ex vivo WT controls. Collectively, these data show that Epo regulates hypothalamus POMC-expression via STAT3-activation, and provide a previously unrecognized link between Epo- and leptin-response.
Keywords: Erythropoietin, POMC, Hypothalamus and Neuroendocrinology, Gene Expression
3. Introduction
The hematopoietic cytokine erythropoietin (Epo), the primary regulator of erythrocyte production, is mainly produced in the kidneys in a hypoxia-dependent manner. Epo receptor (EpoR) expression is highest in erythroid progenitors, and its deletion in mice (EpoR−/−) is embryonic lethal due to severe anemia (Wu, et al. 1995). In addition, EpoR-signaling in non-hematopoietic cells has pleiotropic effects beyond erythropoiesis (Alnaeeli, et al. 2012; Noguchi, et al. 2008). In the brain, Epo has been associated with neurogenesis, neuroprotection, neural progenitor cell migration, and memory retrieval (Chen, et al. 2007; Mengozzi, et al. 2012; Miskowiak, et al. 2007; Shingo, et al. 2001; Tsai, et al. 2006; Wang, et al. 2008; Wang, et al. 2006). Epo-treatment in mice increases hematocrit and can also increase energy-expenditure, reduce blood glucose levels, and reduce appetite and body fat mass (Foskett, et al. 2011; Hojman, et al. 2009; Katz, et al. 2010; Teng, et al. 2011). Expression of an erythroid tissue-specific EpoR-transgene rescued the embryonic lethal phenotype in EpoR−/− mice (Suzuki, et al. 2002). This mouse model has EpoR expression restricted to erythroid tissue (ΔEpoRE) (Suzuki et al. 2002). Previously, we reported that ΔEpoRE-mice exhibit increased body weight from the first postnatal week, show decreased energy-expenditure, and develop metabolic syndromes with age via effects on white adipose tissue and hypothalamus (Teng et al. 2011).
The hypothalamus is a major central neuroendocrine regulation center involved in the control of energy-homeostasis via orexigenic agouti-related peptide (AgRP)/neuropeptideY (NPY)-producing neurons and anorexigenic proopiomelanocortin (POMC)-producing neurons (Bouyer and Simerly 2013). Ablation of POMC neurons and loss of POMC-derived transmitters lead to obesity (Yaswen, et al. 1999), further underscoring the importance of POMC neurons in regulation of energy-homeostasis. Considering our reported findings showing high EpoR expression in hypothalamus, regulation of appetite and energy-expenditure by exogenous Epo-treatment in mice, and POMC induction in response to Epo-treatment (Foskett et al. 2011; Teng et al. 2011), here we examine regulation of hypothalamus POMC neuron function/response to Epo/EpoR-signaling.
Leptin, a hormone secreted by adipose tissue into the circulation, binds its long-form receptor (LepRb) in the arcuate nucleus of the hypothalamus, and activates signal transducer and activator of transcription 3 (STAT3), which positively regulates POMC expression (Bates, et al. 2003). The largest population of LepRb-expressing neurons is in the hypothalamus (Zuure, et al. 2013). Mice lacking leptin, LepRb, or STAT3 genes become obese with a concomitant decrease in POMC expression (Diano, et al. 2011; Gao, et al. 2004; Harlan, et al. 2011). Conversely, leptin-treatment in mice reduced food intake and increased oxygen consumption (Coppari, et al. 2005). Interestingly, Epo and leptin are both members of the hematopoietic cytokine superfamily and have similar protein structure (Fruhbeck 2006; Ouyang and He 2003), receptor structure (Constantinescu, et al. 2001; Mancour, et al. 2012), and signaling mechanism (Banks, et al. 2000; Zhang, et al. 2014), and stimulate anorexic response after administration (Balthasar, et al. 2004; Teng et al. 2011). Therefore, here we investigate potential links between Epo and leptin action, regulation of hypothalamus POMC neuron function/response to Epo/EpoR signaling, and potential cross talk between Epo- and leptin-signaling in POMC neurons.
4. Materials and Methods
4.1. Animal studies
ΔEpoRE-mice were generated using the TgEpoR transgene consisting of EpoR cDNA driven by the GATA-1 locus erythroid regulatory domain (Suzuki et al. 2002) bred onto an EpoR-null (EpoR−/−) background. This rescues the EpoR−/− phenotype of severe anemia and death in utero (Suzuki et al. 2002). These ΔEpoRE-mice can survive through adulthood and their hematocrits are normal. The ΔEpoRE mice are on a C57BL/6 background and age-matched C57BL/6 wild-type (WT) mice were used as control. For Epo treatment (3000 U/kg body weight; Epoetin alpha, Amgen), mice received subcutaneous injection three-times at 9:30 AM per week. For leptin treatment (5 mg/kg body weight; Peprotech), mice received intraperitoneal injection twice daily (9:30 AM and 5:30 PM) for three consecutive days. Mice were housed in individual cages for body weight and food intake studies. Mice were kept on a 12-h light/dark cycles and fed normal chow diet. Studies were conducted following National Institutes of Health guidelines under institution approved animal protocol.
4.2. Neural progenitor cell (NPC)-cultures
Embryos at 15.5 days (E15.5) were harvested from pregnant mice. The developing hypothalamus and cortex were dissected and transferred separately to serum-free media containing DMEM (with L-glutamine)/F12 (1:1) (Invitrogen) supplemented with B27 (Invitrogen), EGF (PeproTech, 20ng/ml), FGF-2 (PeproTech, 20 ng/ml), and 0.5 units/ml Pen-Strep (Invitrogen). Hypothalamus and cortex tissues were mechanically dissociated into single cell suspension with a pipette. Cell viability was assessed using trypan blue exclusion. Dissociated cells were cultured as an adherent monolayer and seeded on tissue culture plates pre-coated with 15 μg/ml poly-L-ornithine (PLO) (Sigma) and 1 μg/ml laminin (Sigma) with a seeding density of 1 × 105 cells /cm2. After 7 days, cells reached around 90% confluency and were passaged at 1:4 to new plates and designated as passage 1. Expansion cultures were passaged every 5 days regularly from first passage onward at 1:4 ratio and recorded as passage 2 through passage 10.
4.3. Quantitative RT-PCR
Total RNA was extracted using RNeasykit (Qiagen) and reverse-transcribed using Molony Murine Leukemia virus (MuLV) reverse-transcriptase and the reagents from GeneAmp RNA PCR (Applied Biosystems). The SYBR green-I (Life Technologies)-based relative quantitative real time PCR was performed on a 7900HT Thermocycler (Applied Biosystems). All primers were designed to span exon junctions in order to prevent the amplification of contaminating genomic DNA and β-Actin mRNA expression was used as a reference. The primer sequences are as follows: mEpoR-forward, ACGCTTGGAAGACTTGGTGTG; mEpoR-reverse, TGTTGGCAGTGAACACCAGAA; POMC-forward, TTTCCTGGCAACGGAGATGA; POMC-reverse, CCACCGTAACGCTTGTCCTT; NPY-forward, TCGTGTGTTTGGGCATTCTG; NPY-reverse, TCTGGTGATGAGATTGATGTAGTG; AgRP-forward, TGACTGCAATGTTGCTGAGTTGTG; AgRP-reverse, CTAGGTGCGACTACAGAGGTTCG; β-Actin-forward, CAACGGCTCCGGCATGTGCAAAG, β-Actin-reverse, GGTGTGATGGTGGGAATGGGTCAG; nestin-forward, AGAGATTGGAAGGCCGCTGG; nestin-reverse, CACCTTCCAGGATCTGAGCGA; β-tubulin-forward, GGGCCAAGTTCTGGGAGGTCATC; β-tubulin-reverse, GCACATACTTGTGAGAGGAGGCC.
4.4. Western blotting
For Western blot analysis, NPC-cultures were treated with Epo for 30 min to detect phosphorylated-STAT3 (p-STAT3), phosphorylated-JAK2 (p-JAK2), and phosphorylated-Akt (p-Akt) and for 24 h to detect POMC expression. Cells were lysed in RIPA buffer and protein was resolved on the basis of size in a NuPAGE electrophoresis system (Life Technologies). The primary antibodies used were anti-p-STAT3-Tyr705 (9131), anti-STAT3 (4904), anti-p-JAK2-Tyr1007 (4406), anti-JAK2 (3230), anti-β-Actin (4967) (from Cell Signaling), and anti-POMC (ab14064), and anti-GAPDH (ab9485) (from Abcam). All antibodies were used at 1:1000 diltuion. Either β-Actin or GAPDH protein expression was used to confirm equal protein loading between lanes.
4.5. Immunofluorescence analysis
After overnight fasting, mice were treated either with leptin, Epo or saline at 9:30 AM. After 90 minutes, animals were anesthetized with isoflurane and perfused with ice-cold PBS via heart and then fixed with 100 ml of 4% paraformaldehyde (PFA) solution (Sigma). Brain was removed, post-fixed overnight in 4% PFA, followed by overnight cryoprotection in 30% sucrose, and cut in 10 μm coronal sections, collected in five series. Immunostaining was done on serial sections with α-MSH antibody (20074, Immunostar) at 1:4000 dilution and counterstained with DAPI for nuclear staining. Alexa Fluor 488-conjugated goat anti-rabbit IgG (Abcam) was used as a secondary antibody. The saline-treated group served as a negative control and tested antibody specificity. For staining NPC cultures, passage 3 cells were grown in cover slips coated with poly-L-ornithine and laminin as described above for NPC culture, and fixed with 4% PFA for 20 minutes. Due to variability in commercial lots of EpoR polyclonal antibody, we used the monoclonal antibody developed at Genetics Institute against the EpoR extracellular domain (mh2er.16.5.1) that previously provided consistent results for immunohistochemistry and has been characterized for specificity (Anagnostou, et al. 1994; D'Andrea, et al. 1993; Teng et al. 2011). Anti-EpoR antibody was used at 1:25 dilution. Passage 1 NPC cells were also used as a negative control for EpoR staining. To detect POMC expression cells were stained using POMC antibody (H-029-30, Phoenix Pharmaceuticals) at 1:1000 dilution. For fluorescent detection of POMC and EpoR, Alexa488-conjugated goat anti-rabbit IgG and Alexa594-conjugated goat anti-mouse IgG, respectively (Abcam) were used.
4.6. Statistical analysis
Differences among groups were analyzed by ANOVA with post-hoc comparison by Student's t-test for continuous variables and chi-square or Fisher's exact test for nominal variables, as appropriate, followed by Bonferroni correction for multiple comparisons. All results are reported as mean±SD from at least 3 independent experiments. In all cases, p<0.05 was considered to be statistically significant.
5. Results
5.1. Primary hypothalamus NPC-cultures express POMC and EpoR
We previously reported that EpoR protein in mouse hypothalamus localizes to POMC expressing neurons and Epo directly regulates POMC expression in these neurons (Teng et al. 2011). To study Epo/EpoR-mediated regulation of POMC in mouse hypothalamus, we established NPC-cultures from E15.5 mouse hypothalamus. For comparison, primary NPCs derived from E15.5 hippocampus and cortex were used, because these brain regions also express EpoR (Chen et al. 2007; Yu, et al. 2002). POMC expression in the respective cultures, determined by quantitative RT-PCR, revealed that POMC mRNA was mainly expressed in hypothalamus-derived NPC-cultures and ten-times higher than levels detected in cultures derived from cortex or hippocampus (Figure 1A). In contrast, EpoR mRNA expression was readily detected in NPC-cultures from each of these three regions with expression highest in hippocampus with levels in the hypothalamus one-half to one-third the level in hippocampus or cortex (Figure 1B).
Figure 1. Gene expression in primary neural progenitor cell (NPC)-cultures from wild-type (WT)-hypothalamus.
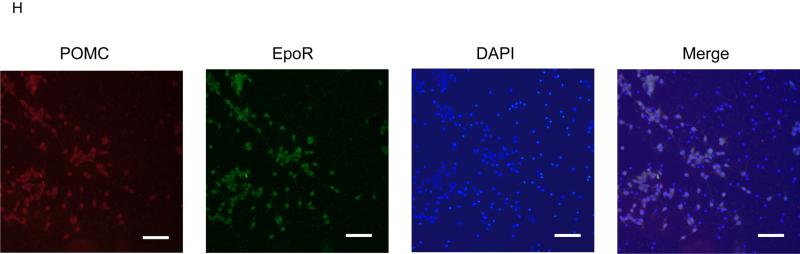
(A-B) POMC (A) and EpoR (B) mRNA levels in NPCs derived from embryonic day E15.5 cortex (cor), hippocampus (hip), and hypothalamus (hyp) from WT-mice as measured by quantitative real time RT-PCR and normalized to β-Actin mRNA levels. (C-F) Quantification of nestin (C), β-tubulin (D), POMC (E), and EpoR (F) mRNA levels in hypothalamus NPC culture at passages 1, 3, and 10 relative to β-Actin mRNA levels as determined by real time RT-PCR. Reactions were run in triplicate and results are representative of three independent experiments (G) Western blotting for POMC (top panel) and β-Actin (middle panel) protein expression, and quantitative densitometry analysis (lower panel) at passages 1, 3, 5, and 8 of hypothalamus NPC cultures are shown. Densitometry data is representative of three independent experiments. Statistical significance is indicated by *p<0.05, **p<0.01, and ***p<0.001, (one-way ANOVA followed by Bonferroni correction). (H) Double fluorescent immunostaining for POMC (green) and EpoR (red) on hypothalamus NPC cultures at passage 3 and stained with DAPI staining (blue) for nuclei and merged for triple stained image. Scale bar represents100 μm.
NPC-cultures as an adherent monolayer are prone to density-dependent differentiation. Adherent NPC-cultures were passaged when confluence was >90% to promote density-dependent differentiation. With increasing passage, nestin mRNA expression decreased by more than an order of magnitude (Figure 1C), while β-tubulin mRNA expression increased between passage 1 and passage 3 (Figure 1D), indicating a shift from NPCs to immature neurons, concomitant with an increase in POMC and EpoR mRNA expression (Figure 1E-F). POMC protein production examined by Western blotting increased between passage 1 and passage 3, was maximal between passages 3 and 5, and then decreased by passage 8 (Figure 1G). These data suggest that the increase in POMC expression and protein production in these cultures correlates with a shift in nestin-expressing NPCs toward β-tubulin- and POMC-expressing immature neurons with a maximal shift occurring between passages 3 and 5. At passage 10, EpoR mRNA continued to be expressed but β-tubulin and POMC mRNA levels declined (Figure 1D-E). Immunostaining of passage 3 cells also showed colocalization of POMC and EpoR protein expression and the presence of cells that did not stain for either, suggesting EpoR expression is specific for POMC neurons in this population of cells (Figure 1H). These POMC- and EpoR-expressing cells comprised approximately 50% of total cell population in culture at this stage. Moreover, there were no cells that expressed EpoR only, and not POMC. Immunostaining of passage 1 cells with anti-POMC and anti-EpoR antibodies served as negative controls and confirmed the specificity of these antibodies. The apparent patterns of cytoplasmic anti-EpoR staining in POMC neurons may relate to the processing and trafficking of EpoR, and the reported observations that only a minor fraction of the Golgi-processed receptor is on the cell surface and a few high affinity Epo surface binding receptors in neuronal cells are sufficient for Epo activated cell signaling (Hilton, et al. 1995; Um, et al. 2007). In all subsequent experiments, cells from passages 3-5 which showed optimum POMC and EpoR expression, were used. In cortex-derived NPC-cultures, the low level of POMC expression did not increase with time in passage indicating that cortex-derived NPCs did not exhibit the ability to self-differentiate into POMC-expressing neurons (data not shown).
5.2. Epo increases POMC expression in hypothalamus NPC-cultures via JAK2/STAT3 pathway
Epo-treatment of hypothalamus NPC-cultures increased POMC mRNA expression in a dose-dependent manner as determined by quantitative RT-PCR (Figure 2A). Similar increase in POMC protein expression was also observed as determined by Western blotting (Figure 2B). In addition to regulating POMC, Epo-treatment increased JAK2- and STAT3-phosphorylation in NPC-cultures (Figure 2C). Pre-treatment with STAT3-inhibitor WP1066 (3 μM) for 30 min was sufficient to abrogate Epo-induction of POMC (Figure 2D). While β-tubulin expression did not change with Epo-treatment, there was a modest decrease (10% or less) in β-tubulin with WP1066 treatment with and without Epo (Figure 2F). These results suggest that STAT3-phosphorylation is required for Epo induction of POMC and that inhibition of STAT3-phosphorylation by WP1066 decreases POMC expression by reducing the amount of POMC expression with minimal change in β-tubulin and the proportion of cells with the potential to express POMC. Epo-treatment of hypothalamus NPC-cultures at 10 and 20 U/ml also resulted in a small but significant increase in cell number in a dose-dependent manner at both 32h and 48h of treatment (Figure 2F).
Figure 2. Epo treatment induces proliferation, POMC expression, and JAK2- and STAT3-signaling in WT hypothalamus NPC-culture.
(A-B) Dose-dependent increase in POMC mRNA levels relative to untreated cells and adjusted to β-Actin mRNA levels (A), and POMC protein normalized to β-Actin after 24 h of Epo-treatment (B) were determined. Densitometry data is representative of three independent experiments. (C) Western blotting for phosphorylated-JAK2 (p-JAK2), total JAK2 (t-JAK2), phosphorylated-STAT3 (p-STAT3), total STAT3 (t-STAT3), and β-Actin protein in control- and Epo-treated (20 U/ml) NPC cultures, and quantitative densitometry analysis of band intensity ratio of p-STAT3 and t-STAT3 are shown. Densitometry data is representative of three independent experiments. (D-E) Level of POMC (D) and β-tubulin (E) mRNA as measured by real time RT-PCR analysis of NPCs after saline-treatment (Cont), Epo-treatment, Epo with STAT3-inhibitor WP1066, and WP1066 only. The mRNA expression levels are relative to saline–treated (Cont.) cells and adjusted to β-Actin mRNA levels, and represents mean ± SD from triplicate reactions. (F) Proliferation of NPC cultures after 32 h and 48 h of Epo-treatment at 0, 10, and 20 U/ml doses. Relative cell numbers are representative of triplicate treatments for each dose. Statistical significance is indicated by *p<0.05, and **p<0.01, (one-way ANOVA followed by Bonferroni correction).
5.3. Loss of EpoR-signaling in hypothalamus NPC-cultures affects their leptin response
We previously reported that leptin is not required for the effects of exogenous Epo-treatment on body weight and fat mass loss (Teng et al. 2011). However, Epo signaling in NPC is mediated via STAT3-pathway (Figure 2), which is also activated in the arcuate nucleus after leptin treatment. Whether Epo/EpoR-signaling affects leptin-response in hypothalamus was unknown. To investigate this question, hypothalamus-derived NPC-cultures were generated from WT- and ΔEpoRE-mice. With leptin- and Epo-treatment, WT cultures showed an increase in STAT3-phosphorylation (Figure 3A). In contrast, NPC-cultures from ΔEpoRE-mice did not show the increase in STAT3-phosphorylations expected with Epo-treatment (Figure 3A). Interestingly, ΔEpoRE-hypothalamus cultures also did not exhibit the expected increase in STAT3-phosphorylation with leptin-treatment (Figure 3A). Of note, baseline levels of STAT3-phosphorylation were lower in ΔEpoRE-hypothalamus NPC-cultures compared to those from WT-hypothalamus (comparing saline treatment lanes, Figure 3A). Moreover, Epo and leptin combination treatments were not more effective than Epo or leptin treatment alone in inducing STAT3-phosphorylation (Figure 3A). Consistent with STAT3-activation, 24 hours treatment with either leptin or Epo increased POMC mRNA expression in WT-hypothalamus cultures by 2- to 2.5-fold, with combination-treatment no better than individual-treatment conditions (Figure 3B). In ΔEpoRE-hypothalamus cultures, baseline POMC mRNA expression appeared to be 4-fold lower than WT cultures, but was induced approximately 2-fold by leptin or the combination of leptin plus Epo-treatment, but not by Epo alone (Figure 3B). Note that even with leptin-treatment, POMC expression in ΔEpoRE cultures was only half the level of control-treated WT cultures (Figure 3B), and no significant induction in STAT3-phosphorylation was detected (Figure 3A). Conversely, NPY and AgRP mRNA expression did not show any difference between WT and ΔEpoRE hypothalamus NPC-cultures, and was reduced similarly by leptin treatment only (Figures 3C and D). Combination treatment or Epo alone did not affect either NPY or AgRP mRNA expression (Figures 3C and D). These data support the previous finding that EpoR expression is specific for POMC neurons (Teng et al. 2011) and lack of EpoR neither affect NPY and AgRP expression nor its response after leptin treatment. These observations collectively point to an important and previously unrecognized role for EpoR-signaling to contribute to leptin-response in hypothalamus NPC ex vivo. Interestingly, LepRb mRNA levels showed a small but significant reduction in ΔEpoRE cultures compared to the WT (Figure 3E), but cell proliferation during the NPC culture is not affected by loss of EpoR (Figure 3F).
Figure 3. EpoR-signaling is required for optimum leptin response in NPC cultures.
(A) Western blotting and quantitative densitometry analysis were used to determine levels of phosphorylated-STAT3, total-STAT3, and β-Actin protein levels (A), in WT and ΔEpoRE NPC cultures after control saline-treatment, Epo-treatment, Epo- and leptin-treatment, and leptin-treatment. Epo and leptin was used at 20 U/ml and 1000 U/ml, respectively and densitometry analysis is representative of 3 independent experiments (B-D) POMC (B), NPY (C), and AgRP (D) mRNA expression in WT and ΔEpoRE NPC-cultures were determined after Epo-only, Epo and leptin, and leptin-only treatments, relative to control saline-treated WT cells. (E) LepRb mRNA expression in WT and ΔEpoRE NPC cultures. Gene expressions were adjusted to β-Actin mRNA levels and real-time PCR reactions were run in triplicate. (F) Fold change in numbers of NPC from WT and ΔEpoRE E15.5 embryos. P0 represents cell proliferation from day 1 to day 7 of culture, and P1-P5 represents passages 1-5 after every 5 days. Fold change is representative of three independent experiments. Statistical significance is indicated by **p<0.01 and ***p<0.001 (one-way ANOVA followed by Bonferroni correction).
5.4. Absence of hypothalamus EpoR signaling reduces in vivo STAT3-phosphorylation and POMC production, blunts leptin-induced STAT3-activation but not the leptin-induced relative increase of POMC
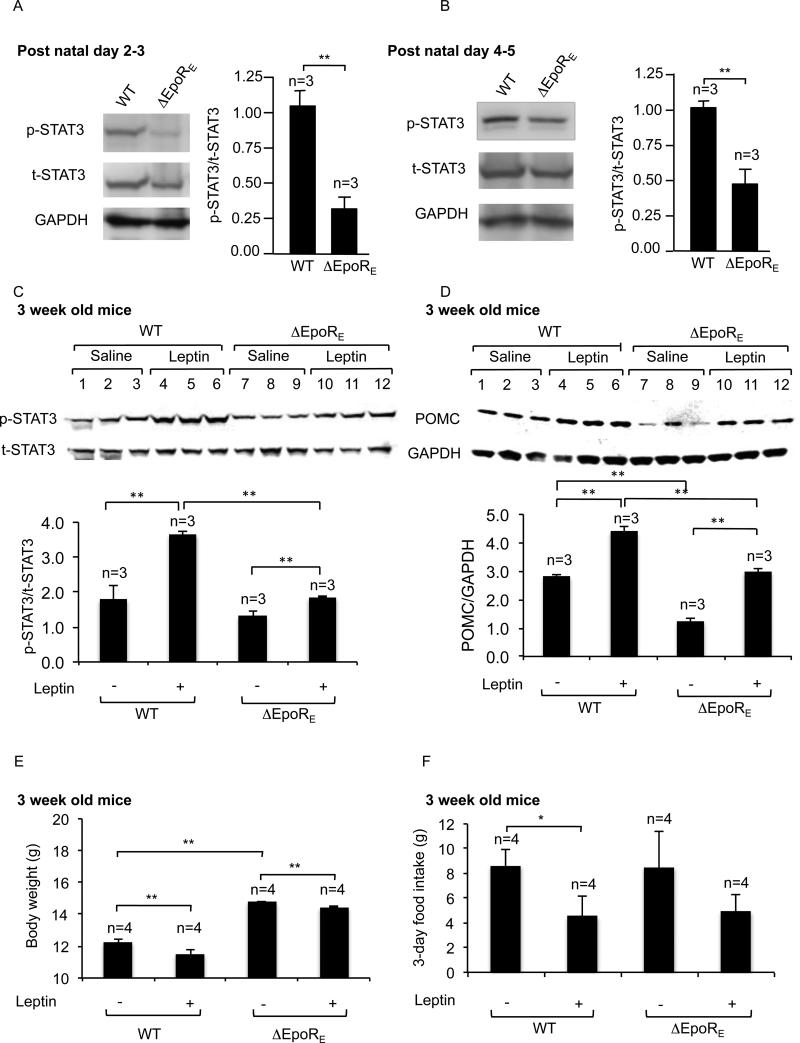
Ex vivo hypothalamus NPC-cultures provided evidence for Epo-stimulated STAT3-activation (Figure 2 and 3). Loss of endogenous Epo-activity in ΔEpoRE-hypothalamus should also reduce STAT3-phosphorylation in vivo. ΔEpoRE-mice show age-dependent obesity, and at 8 months are 30% or greater in body mass versus WT due to increased fat mass with a corresponding increase in circulating leptin levels (Teng et al. 2011). To avoid this apparent obesity related increase in leptin level and the expected change in STAT3-phosphorylation related to increased leptin-signaling, we examined hypothalamus STAT3-phosphorylation in young mice prior to development of fat tissue. At postnatal day 2- 3, STAT3-phosphorylation in the hypothalamus of ΔEpoRE-mice was reduced by 3-fold compared with WT-mice while levels of total-STAT3 were comparable (Figure 4A). The difference in STAT3-phosphorylation was also evident in ΔEpoRE-mice at postnatal day 4- 5- (Figure 4B).
Figure 4. ΔEpoRE-mice show blunted STAT3-activation but equivalent POMC induction in the hypothalamus after leptin treatment, compared to WT-mice.
(A-B) Western blotting and quantitative densitometry analysis (from 3 pups each of WT and ΔEpoRE) were used to compare phosphorylated-STAT3 (p-STAT3), total-STAT3 (t-STAT3), and GAPDH protein levels in WT and ΔEpoRE pups at postnatal day 2-3 (A) and postnatal day 4-5 (B). (C-D) Western blotting and quantitative densitometry analysis demonstrating phosphorylated-STAT3 (p-STAT3) and total-STAT3 (t-STAT3) (C), POMC and GAPDH (D) in 3 week-old female WT and ΔEpoRE mice 45 mins after treated with leptin (5 mg/kg body weight) or saline (n=3 for each treatment group). (E-F) Body weight (E), and food intake (F) were determined in WT and ΔEpoRE mice after saline- or leptin-treatment twice daily for 3 days (n=4 for each treatment group). Statistical significance is indicated by *p<0.05, and **p<0.01 001 (one-way ANOVA followed by Bonferroni correction).
Regulation of body weight involves a balance between energy intake and energy-expenditure. Leptin has the ability to decrease food intake, increase energy-expenditure and promote body weight loss. We investigated whether the changed energy balance of ΔEpoRE-mice involved alterations in leptin-mediated signaling in the hypothalamus (Figures 4C-F). 3-week old mice were used to eliminate possible leptin resistance associated with older, obese ΔEpoRE-mice, and also to ensure complete development of neurons of the arcuate nucleus. ΔEpoRE-hypothalamus showed a lower level of STAT3-phosphorylation compared to WT-hypothalamus (Figure 4C). However, the difference is reduced compared with mice at postnatal days 2-3 (Figure 4A) and days 4-5 old (Figure 4B). This suggests that ΔEpoRE-mice by 3 weeks develop a compensatory mechanism that restores STAT3-phosphorylation closer to normal WT-levels. In contrast to ex vivo ΔEpoRE-hypothalamus cultures that show no leptin-induced STAT3-phosphorylation (Figure 3A), ΔEpoRE-mice exhibited a blunted in vivo leptin-induced STAT3-phosphorylation in hypothalamus compared with WT-mice (Figure 4C). This suggests that, in vivo, other mechanisms attempt to compensate for the apparent loss of direct leptin induction of STAT3-phosphorylation in hypothalamus NPC derived cells.
In vivo, baseline POMC protein levels were lower in ΔEpoRE-mice, analogous to the reduced POMC mRNA in ΔEpoRE-hypothalamus cultures (Figure 3B), and leptin could induce POMC expression only up to baseline WT levels (Figure 4D). These data suggest that in vivo leptin response in ΔEpoRE-hypothalamus is blunted with respect to STAT3-phosphorylation, and the reduction in baseline STAT3-activation results in a lower level of POMC in ΔEpoRE-mice, compared with WT-mice without and with leptin-treatment. Leptin induction of POMC in both WT- and ΔEpoRE-mice was also reflected in a small but significant decrease in body weight after leptin-treatment in WT-mice (6%) and in an apparent smaller decrease in ΔEpoRE-mice (2.5%) (Figure 4E). Food intake for 3 days was significantly lower in leptin-treated WT mice, and also showed a reduced trend in ΔEpoRE mice (Figure 4F). In summary, these data suggest that loss of EpoR in young mice reduces baseline hypothalamus STAT3-activation and lowers POMC expression in the hypothalamus. Moreover, leptin sensitivity with respect to body weight loss and reduced food intake are still evident in ΔEpoRE-mice.
Finally, immunohistochemical staining of brain sections displaying the arcuate nucleus region of the hypothalamus also showed lower staining for α-MSH, a cleavage product of POMC, in ΔEpoRE-mice compared to WT-mice (Figure 5A,B). Both Epo and leptin treatment of WT mice induced α-MSH expression (Figure 5 C,E). As expected, the ΔEpoRE-mice brain sections showed higher immunoreactivity to α-MSH only after leptin treatment and not Epo treatment (Figure 5 D, F).
Figure 5. Induction of α-MSH, a POMC product, in the arcuate nucleus region of the mouse brain after either saline-, Epo-, or leptin-treatment.
Representative fluorescence photomicrographs for α-MSH immunoreactivity in brain sections prepared from the mouse mediobasal hypothalamus consisting of the arcuate nucleus region are shown. 3-week old female WT (A, C, E) or ΔEpoRE (B, D, F) mice were treated with either saline (A, B), Epo (3000 U/kg body weight) (C, D), or Leptin (5 mg/kg body weight) (E, F). Scale bar represents 100 μm. 3V, third ventricle, ARC, arcuate nucleus, and ME, median eminence.
6. Discussion
EpoR is expressed in the POMC neurons of the hypothalamus, a master regulatory site of appetite and energy-expenditure, and the increased POMC expression in Epo-treated WT-mice suggested that Epo could directly stimulate these POMC neurons (Teng et al. 2011), although the detailed mechanism of POMC neuron response to Epo was uncertain. We previously showed that Epo-treatment in WT-mice decreases body weight and fat mass by reducing food intake and increasing energy-expenditure (Foskett et al. 2011; Teng et al. 2011). While EpoR is expressed in several brain regions including hypothalamus, hippocampus and cortex, NPC-cultures from the hypothalamus show unique induction of POMC expression with Epo-treatment. Using hypothalamus-derived NPC-cultures, we studied the signaling pathways activated by Epo in POMC neurons and investigated the crosstalk between LepRb- and EpoR-signaling.
Epo and leptin are both members of the class-I cytokine superfamily (Fruhbeck 2006; Ouyang and He 2003) and act through the JAK/STAT signaling pathway (Constantinescu et al. 2001; Mancour et al. 2012). Leptin stimulation of POMC expression in the hypothalamus is mediated via STAT3-activation (Bates et al. 2003) and we found that Epo-stimulated increase of POMC is also dependent on STAT3-activation. The requirement for EpoR for normal hypothalamus response is further confirmed by analysis of hypothalamus from ΔEpoRE-mice with EpoR expression restricted to erythroid tissue. Neonatal ΔEpoRE-mice show reduced STAT3-phosphorylation and lower level of POMC expression in the hypothalamus compared with WT-mice. In addition, corresponding hypothalamus NPC-cultures from ΔEpoRE-mice lack activation of STAT3 or induction of POMC with Epo-treatment. Evidence that loss of EpoR may blunt the leptin response is further suggested by ex vivo findings from ΔEpoRE-hypothalamus cultures that also exhibit loss of leptin-induced increase in STAT3-phosphorylation. These data provide evidence that endogenous Epo-signaling in WT-hypothalamus contributes to STAT3-phosphorylation while loss of Epo-signaling reduces endogenous baseline level of STAT3-activation and POMC-production. The reduction in endogenous STAT3 activity in ΔEpoRE-mice is consistent with the severe obese phenotype observed in mice with neural-specific disruption of STAT3 that underscore its importance in the central nervous system in regulating energy-homeostasis (Gao et al. 2004). Interestingly, with leptin-treatment in the ΔEpoRE-mice, induction of STAT3-phosphorylation in the hypothalamus is reduced to half that in treated WT-mice. While the low level of POMC expression in ΔEpoRE-hypothalamus is increased with leptin-treatment and fold-induction of POMC in ΔEpoRE- and WT-hypothalamus are similar, the level of POMC remains 50% or less than treated WT-hypothalamus. The lack of leptin induced STAT3-activation observed in ΔEpoRE-hypothalamus cultures suggests that other Epo-independent signaling pathways compensate in part to provide leptin induced POMC expression in ΔEpoRE-hypothalamus. Epo influence on leptin-responsive weight loss is also suggested by the lower percentage weight decrease in ΔEpoRE-mice.
The finding that leptin requires functional EpoR for normal levels of STAT3-activation and POMC-induction in hypothalamus provides an explanation for the low POMC levels in hypothalamus of ΔEpoRE-mice despite elevated circulating leptin levels and for the resultant altered energy-homeostasis (Teng et al. 2011), and suggests disruption of critical leptin regulation of energy efficiency in the hypothalamus. However, a combination of Epo/leptin-treatment did not induce higher POMC expression, which raises the possibility that Epo may prime POMC neurons in the hypothalamus for subsequent leptin action, and the kinetics of treatment is critical to see a combined Epo/leptin effect on POMC expression. Although lack of EpoR does not affect NPC proliferation in culture, but LepRb expression is modestly reduced in ΔEpoRE NPC cultures. This raises the possibility that EpoR signaling is required for the complete maturation of POMC neurons without affecting its numbers.
These data suggest that direct response by the hypothalamus to Epo-treatment contributes, in part, to regulation of body weight and fat mass via STAT3-activation and POMC induction. The ΔEpoRE-mice provide further evidence that increased metabolic load of Epo-stimulated erythropoiesis cannot explain the body weight reduction after Epo-treatment in WT-mice. Specifically, ΔEpoRE-mice treated with Epo exhibit the expected increase in hematocrit, but without the significant reduction in body mass observed in WT-mice (Teng et al. 2011). Finally, transgene-driven expressions of EpoR in ΔEpoRE mice hematopoietic cells and spleen are comparable to endogenous EpoR levels and also ΔEpoRE mice do not show any difference in hematocrit levels (Suzuki et al. 2002; Teng et al. 2011).
Leptin receptor is distributed in the arcuate nucleus and other regions of the hypothalamus and in extra-hypothalamic autonomic control sites as well as cortex and hippocampus (Caron, et al. 2010), indicating that leptin response associated with energy balance involves multiple regions of the brain. EpoR is also expressed in various sites in the brain, such as cortex and hippocampus that are also linked to food intake (Morton, et al. 2006; Volkow, et al. 2011), raising the possibility that Epo-activity in the brain beyond POMC neurons may modulate metabolic activity. Although AgRP/NPY neurons in the arcuate nucleus of the hypothalamus also contribute to leptin regulation of POMC, EpoR expression was not detected in AgRP/NPY neurons (Teng et al. 2011). Moreover, we did not see any difference in AgRP and NPY expression between WT and ΔEpoRE hypothalamus NPC-cultures, and was reduced after leptin treatment only. This also suggests that the effect we see in ΔEpoRE mice is specifically due to Epo-mediated regulation of POMC neurons and not due to secondary effects on AgRP and NPY neurons.
Similar to acute leptin administration (Hill, et al. 2008), exogenous Epo-treatment in WT-mice also results in a reduction in food intake and body weight, and an increase in physical activity with activation of POMC neurons (Teng et al. 2011). The uncoupling of food intake and energy-expenditure has been observed in POMC neurons, and selective deletion of leptin receptor in POMC neurons fails to affect food intake (Balthasar et al. 2004). It has been argued that neural control over feeding behavior has several layers of redundancy (Schwartz, et al. 2003). It should be noted that although the knock-out of anorexigenic neuropeptide-expressing POMC gene causes increased food intake (Yaswen et al. 1999), the knock-out of orexigenic NPY gene does not affect daily food intake (Erickson, et al. 1996). In this regard, we observed that the ΔEpoRE-mice that show a 50% reduction in POMC expression in the hypothalamus develop obesity, with reduced physical activity, but no detectable increase in food intake (Teng et al. 2011).
Endogenous Epo is present in brain and cerebral spinal fluid (Chavez, et al. 2006), in addition to the adult kidney, Epo can be produced in an oxygen-dependent manner by astrocytes and neurons (Chavez et al. 2006) and provide a local source for Epo-production to stimulate the EpoR-expressing neural cells in the hypothalamus. Interestingly, hypothalamic astrocytes have also been suggested to influence metabolic status and obesity (Fuente-Martin, et al. 2012). High fat-diet feeding in mice results in inflammatory response and increase in pro-inflammatory markers in astrocytes, together with a decrease in POMC neurons in the arcuate nucleus (Thaler, et al. 2012).
Overall, in this study we identified a previously unknown mechanism of EpoR-dependent regulation of POMC expression via STAT3-activation and LepRb-signaling in the hypothalamus. We further explored the molecular links underlying the contribution of loss of EpoR in non-hematopoietic tissue to the obese phenotype in ΔEpoRE mice. Our study also identified the importance of endogenous Epo-signaling in regulation of energy-homeostasis.
Acknowledgments
8. Funding:
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, USA.
Footnotes
9. Author contributions:
S.D. and X.L. designed and performed experiments; S.D. wrote the paper; R.T., M.A., Z.C., H.M.R, contributed by technical assistance, advice, and manuscript revision; C.T.N developed the concept, supervised the study, and wrote the paper.
7. Declaration of Interest
The authors declare no conflict of interest
References
- Alnaeeli M, Wang L, Piknova B, Rogers H, Li X, Noguchi CT. Erythropoietin in brain development and beyond. Anat Res Int. 2012;2012:953264. doi: 10.1155/2012/953264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr., Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Banks AS, Davis SM, Bates SH, Myers MG., Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Bouyer K, Simerly RB. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. J Neurosci. 2013;33:840–851. doi: 10.1523/JNEUROSCI.3215-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol. 2010;518:459–476. doi: 10.1002/cne.22219. [DOI] [PubMed] [Google Scholar]
- Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Asavaritikrai P, Prchal JT, Noguchi CT. Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J Biol Chem. 2007;282:25875–25883. doi: 10.1074/jbc.M701988200. [DOI] [PubMed] [Google Scholar]
- Constantinescu SN, Keren T, Socolovsky M, Nam H, Henis YI, Lodish HF. Ligand-independent oligomerization of cell-surface erythropoietin receptor is mediated by the transmembrane domain. Proc Natl Acad Sci U S A. 2001;98:4379–4384. doi: 10.1073/pnas.081069198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr., et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- D'Andrea AD, Rup BJ, Fisher MJ, Jones S. Anti-erythropoietin receptor (EPO-R) monoclonal antibodies inhibit erythropoietin binding and neutralize bioactivity. Blood. 1993;82:46–52. [PubMed] [Google Scholar]
- Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, Kim E, Suyama S, Kelly K, Gyengesi E, Arbiser JL, et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011;17:1121–1127. doi: 10.1038/nm.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Foskett A, Alnaeeli M, Wang L, Teng R, Noguchi CT. The effects of erythropoietin dose titration during high-fat diet-induced obesity. J Biomed Biotechnol. 2011;2011:373781. doi: 10.1155/2011/373781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuente-Martin E, Garcia-Caceres C, Granado M, de Ceballos ML, Sanchez-Garrido MA, Sarman B, Liu ZW, Dietrich MO, Tena-Sempere M, Argente-Arizon P, et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest. 2012;122:3900–3913. doi: 10.1172/JCI64102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton DJ, Watowich SS, Murray PJ, Lodish HF. Increased cell surface expression and enhanced folding in the endoplasmic reticulum of a mutant erythropoietin receptor. Proc Natl Acad Sci U S A. 1995;92:190–194. doi: 10.1073/pnas.92.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojman P, Brolin C, Gissel H, Brandt C, Zerahn B, Pedersen BK, Gehl J. Erythropoietin over-expression protects against diet-induced obesity in mice through increased fat oxidation in muscles. PLoS One. 2009;4:e5894. doi: 10.1371/journal.pone.0005894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz O, Stuible M, Golishevski N, Lifshitz L, Tremblay ML, Gassmann M, Mittelman M, Neumann D. Erythropoietin treatment leads to reduced blood glucose levels and body mass: insights from murine models. J Endocrinol. 2010;205:87–95. doi: 10.1677/JOE-09-0425. [DOI] [PubMed] [Google Scholar]
- Mancour LV, Daghestani HN, Dutta S, Westfield GH, Schilling J, Oleskie AN, Herbstman JF, Chou SZ, Skiniotis G. Ligand-induced architecture of the leptin receptor signaling complex. Mol Cell. 2012;48:655–661. doi: 10.1016/j.molcel.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengozzi M, Cervellini I, Villa P, Erbayraktar Z, Gokmen N, Yilmaz O, Erbayraktar S, Manohasandra M, Van Hummelen P, Vandenabeele P, et al. Erythropoietin-induced changes in brain gene expression reveal induction of synaptic plasticity genes in experimental stroke. Proc Natl Acad Sci U S A. 2012;109:9617–9622. doi: 10.1073/pnas.1200554109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K, O'Sullivan U, Harmer CJ. Erythropoietin enhances hippocampal response during memory retrieval in humans. J Neurosci. 2007;27:2788–2792. doi: 10.1523/JNEUROSCI.5013-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Noguchi CT, Wang L, Rogers HM, Teng R, Jia Y. Survival and proliferative roles of erythropoietin beyond the erythroid lineage. Expert Rev Mol Med. 2008;10:e36. doi: 10.1017/S1462399408000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang S, He F. Phylogeny of a growth hormone-like cytokine superfamily based upon 3D structure. J Mol Evol. 2003;56:131–136. doi: 10.1007/s00239-002-2385-2. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52:232–238. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Ohneda O, Takahashi S, Higuchi M, Mukai HY, Nakahata T, Imagawa S, Yamamoto M. Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood. 2002;100:2279–2288. doi: 10.1182/blood-2002-01-0124. [DOI] [PubMed] [Google Scholar]
- Teng R, Gavrilova O, Suzuki N, Chanturiya T, Schimel D, Hugendubler L, Mammen S, Yver DR, Cushman SW, Mueller E, et al. Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nat Commun. 2011;2:520. doi: 10.1038/ncomms1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19:634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chopp M, Gregg SR, Zhang RL, Teng H, Jiang A, Feng Y, Zhang ZG. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28:1361–1368. doi: 10.1038/jcbfm.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Yu X, Shacka JJ, Eells JB, Suarez-Quian C, Przygodzki RM, Beleslin-Cokic B, Lin CS, Nikodem VM, Hempstead B, Flanders KC, et al. Erythropoietin receptor signalling is required for normal brain development. Development. 2002;129:505–516. doi: 10.1242/dev.129.2.505. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang L, Dey S, Alnaeeli M, Suresh S, Rogers H, Teng R, Noguchi CT. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci. 2014;15:10296–10333. doi: 10.3390/ijms150610296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuure WA, Roberts AL, Quennell JH, Anderson GM. Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. J Neurosci. 2013;33:17874–17883. doi: 10.1523/JNEUROSCI.2278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]