Abstract
The role of PD-1 expression on CD4 T cells during HIV infection is not well understood. Here, we describe the differential expression of PD-1 in CD127high CD4 T cells within the early/intermediate differentiated (EI) (CD27highCD45RAlow) T cell population among uninfected and HIV-infected subjects, with higher expression associated with decreased viral replication (HIV-1 viral load). A significant loss of circulating PD-1highCTLA-4low CD4 T cells was found specifically in the CD127highCD27highCD45RAlow compartment, while initiation of antiretroviral treatment, particularly in subjects with advanced disease, reversed these dynamics. Increased HIV-1 Gag DNA was also found in PD-1high compared to PD-1low ED CD4 T cells. In line with an increased susceptibility to HIV infection, PD-1 expression in this CD4 T cell subset was associated with increased activation and expression of the HIV co-receptor, CCR5. Rather than exhaustion, this population produced more IFN-g, MIP1-a, IL-4, IL-10, and IL-17a compared to PD-1low EI CD4 T cells. In line with our previous findings, PD-1high EI CD4 T cells were also characterized by a high expression of CCR7, CXCR5 and CCR6, a phenotype associated with increased in vitro B cell help. Our data show that expression of PD-1 on early-differentiated CD4 T cells may represent a population that is highly functional, more susceptible to HIV infection and selectively lost in chronic HIV infection.
Introduction
PD-1 is expressed on the surface of T-cells, macrophages, and B cells and functions as an inhibitory co-receptor in the B7:CD28 family, specifically in the regulation of immune activation, inflammation and tolerance [1,2]. Studies of chronic viral infection have demonstrated the importance of PD-1 in the regulation of immune exhaustion in CD8 T cells, and to a lesser extent, CD4 T cells. Exhausted T cells are defined by the gradual loss of effector function, typically by decreased secretion of IFN-g, TNF-a, IL-2 cytokines, and terminal differentiation, and have been described in chronic viral infections in mice, rhesus macaques, and humans [3–6]. Interfering or blocking the PD-1 pathway can improve or restore functional CD8 T cells during chronic LCMV or SIV infection [5,7]. Recently it was also shown that blocking the PD-1/PD-L1 pathway resulted in clearance of parasitemia in a mouse model of blood-stage malaria with an increase in both CD4 T cell function and expansion of T follicular helper (TFH) cells and plasmablasts, indicating that this interaction is important for the development of pathogen-specific adaptive immune responses [8].
Multiple lines of evidence suggest that T cells, even those with an exhausted phenotype, may retain some functional and proliferative capacity during a chronic viral infection [9–11]. Specifically, recent evidence from adoptive transfer studies in mice show that antigen-specific CD8 T cells retain proliferative capacity, though with reduced effector function, despite an exhausted phenotype [12,13]. Another study of PD-1 expression during chronic SIV infection in Rhesus macaques demonstrated that PD-1 expression on CD4 T cells is associated with retained proliferative capacity based on ex vivo Ki-67 expression [14]. Taken together, these studies suggest that PD-1 expression by itself may not solely be a phenotypic marker of immune exhaustion, but may regulate subsets of T cells with a specific differentiation state and effector function, thereby limiting the inflammatory response and tissue damage during chronic infection [15].
Here, we show that in the EI CD4 T cell population there is increased expression of PD-1 relative to CTLA-4 within the subset that is CD127high¸ and this population is initially increased in HIV-infected compared to uninfected individuals, but then decreases concomitant with the expansion of PD-1highCTLA-4highCD127high EI CD4 T cells. HIV-infected subjects with higher plasma HIV RNA had a reduced frequency of PD-1high CD127high EI CD4 T cells along with increased cell-associated HIV gag DNA in this population. Further, we demonstrate that this population with increased PD-1 expression is also associated with increased in vitro cytokine production, suggesting PD-1 is expressed earlier in the differentiation of CD4 compared to CD8 T cells.
Materials and Methods
Study subjects
HIV uninfected peripheral blood mononuclear cells (PBMC) were obtained from individuals participating in the NIH research apheresis program. Cryopreserved, HIV-infected PBMCs were obtained from three different study populations. For untreated HIV infection, cells were obtained from volunteers who participated in a therapeutic vaccination trial (no efficacy was observed) prior to receiving anti-retroviral therapy [16], who had relatively preserved CD4 counts (median 525, interquartile range [IQR] 390–879). We also obtained PBMC from HIV-infected donors with more advanced HIV (median CD4 count 148 cells/μL, IQR 59–274) participating in AIDS Clinical Trials Group study A5142 prior to initiation of combination antiretroviral therapy (cART) and at 48 weeks of therapy [17,18]. The third study population consisted of donors obtained from a cohort used to identify individuals with HIV broadly neutralizing antibodies as previously described [19]. Characteristics of these populations are provided in Table 1. All studies involving human subjects were reviewed and approved by their respective institutional review boards to include the IRB's of the National Institute of Health, National Institute of Allergy and Infectious Diseases, the University of California San Diego, and the Walter Reed Army Institute of Research. Data and stored specimens were utilized from prior multi-center clinical studies under which written informed consent was obtained for all study volunteers to store samples for future use. The use of stored samples for this study was approved the Walter Reed Army Institute of Research Institutional Review Board and the NIH/NIAID. Regarding the ACTG samples, the use of the samples and the submitted manuscript was approved from the ACTG appropriate reviewing committee. ACTG, a multi-center network, samples were collected from different sites and analyzed based on their recovery and survival. This makes impossible to identify specific IRB protocol for the used ACTG samples.
Table 1. Characteristics of HIV-infected subjects.
| HIV-infected population | log10 HIV-1 RNAMean (95% CI) | CD4 countMedian (IQR) | |
|---|---|---|---|
| Cohort 1 (untreated,n = 31) | 3.99 (3.72–4.25) | 525 (390–879) | |
| Cohort 2 (n = 14) | |||
| Pre-cART | 4.82 (4.45–5.18) | 148 (59–274) | |
| 48 weeks Post-cART | <1.69 1 | 289 (200–743) | |
| Cohort 3 (untreated, n = 9)) | 3.53 (3.23–3.78) | 605 (550–821) |
Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; IQR, interquartile range;
1 all below detection limit of 50 copies/mL
Laboratory studies
Antibodies
Flow cytometry was performed using the following directly conjugated antibodies: (1) BD Biosciences: CD3-H7APC (SK7), CD45RA-Cy7PE (L48), CTLA-4-APC (BNI3), IFN-g-FITC (B27), CCR7-Alexa700 (150503), CCR5-FITC (2D7/CCR5), CCR4-PE (1G1) and IL-2-PE (MQ1-17H12), Ki67-FITC (B56); (2) Beckman Coulter: CD27-Alexa680 (IA4CD27), CD127-Cy5PE (R34.34), CD160-PE (BY55), BTLA-PE (J168-540); (3) BioLegend: PD-1-BV421 (EH12.2H7), 2B4-FITC (CD244, C1.7), IL-17a-Cy5.5PerCP (BL168), CCR6-Alexa647 (or PE, TG7/CCR6), CD27 Alexa647 (O323), CCR7 BV605 (G043H7), CXCR5 BV421 (J252D4), and CD154-Cy5PE (24–31); (4) Invitrogen: CD4-Cy5.5PE (S3.5), CD27-QD605 (CLB-27/1), CD8-QD800 (3B5). A biotinylated anti-PD-1 antibody was obtained from R&D (BAF 1086) and streptavidin-Qdot 655 was obtained from Molecular Probes. Quantum dots and Aqua amine viability dye were obtained from Invitrogen. CD27-Alexa 594, TNF-a Alexa 594, HLA-DR BV650, and CD38-Alexa 680 were conjugated in-house.
Polychromatic flow cytometry
For phenotypic analyses PBMCs were cultured in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum, 2mM L-glutamine, 100U/mL penicillin and 100 ug /mL streptomycin (Invitrogen). 1–2 x 106 PBMCs were incubated with Aqua viability dye and surface stained with titrated amounts of antibodies to panel (1): CD3, CD4, CD8, CD27, CD45RA, CD127, PD-1, 2B4, CD160 followed by intracellular staining for CTLA-4; (2) CD3, CD4, CD8, CD27, CD45RA, CD127, PD-1, CCR4, CCR5, CCR6, and CCR7; or (3) CD3, CD4, CD8, CD27, CD45RA, CD127, PD-1, BTLA, HLA-DR, and CD38; (4) CD3, CD4, CD27, CD45RA, CD127, CCR7, PD-1, CXCR5, CCR6 followed by intracellular staining for CTLA-4. Cells were then washed and fixed with 1% paraformaldehyde prior to event collection. In some experiments Ki67 was also used during the ICS staining. Given the relatively small size of the described parental populations, i.e. the PD-1high CTLA-4high CD4 T cells, analysis of the proliferation profile is presented only for samples whith reasonable anticipated populations. For intracellular cytokine staining (ICS) 3 x 106 PBMCs were rested for 2h and incubated in 1mL of medium containing brefeldin A (10ug/mL) in the absence or presence of HIV-1 Gag-peptide pools (15mers overlapping by 11 residues; National Institutes of Health AIDS Research and Reference Reagent Program), or 1 ug/mL SEB (Sigma) for 6 hours. After washing, cells were surface stained with Aqua, CD4, CD8, CD27, CD45RA, CD127, and PD-1, washed and incubated with fluorescent-conjugated streptavidin (for biotinylated PD-1). Cells were then washed again, permeabilized (Cytofix/Cytoperm kit; BD Biosciences), and stained with antibodies to CD3, IFN-g, IL-2, IL-17a or TNF-a, IL-2 and CTLA-4. After fixation with 1% paraformaldehyde, events were collected on a modified LSRII flow cytometer (BD Immunocytometry Systems). Electronic compensation was performed with antibody capture beads (BD Biosciences) stained separately with antibodies used in the test samples. Data were analyzed using FlowJo Version 9.6 (TreeStar, Ashland, OR).
Measurement of in vitro cytokine production
Fresh PBMCs (2 x 108) obtained from HIV-uninfected leukapheresis subjects were sorted after Ficoll separation. After incubation with Aqua viability dye, cells were stained with antibodies to CD4, CD8, CD19, CD14, CD27, CD45RA, PD-1, BTLA and CD127. CTLA-4 was not included due to the requirement for cell permeabilization. Four populations (CD27highCD45RAhigh, CD27lowCD45RAhigh/low, PD-1highCD127highCD27highCD45RAlow and PD-1lowCD127highCD27highCD45RAlow) of 0.5x106 PBMCs were sorted and stimulated with plate-bound anti-CD3 stimulation (10 mcg/mL, BD Pharmingen, clone UCHT1) and co-stimulatory anti-CD28/49d (1.3 mcg/mL, BD Fastimmune). Supernatants were harvested after overnight stimulation for subsequent cytokine (IFN-g, TNF-a, IL-2, IL-4, IL-5, IL-10, and IL-17) quantification using Luminex technology according to the manufacturer’s instructions (Milliplex MAP Kit, Cat. No. HCYTOMAG-60K, Millipore).
Telomerase activity
From the same sorting experiment and populations used for in vitro cytokine production, 30,000 cells each were sorted and lysed using the Quantitative Telomerase Detection (QTD) lysis buffer for telomerase activity, which was performed using the QTD Kit according to manufacturer’s instructions (Allied Biotech, Vallejo, CA).
HIV-1 Gag DNA PCR
Similarly, approximately 5000 cells were sorted from cryopreserved, HIV-infected PBMC’s directly into lysis buffer and quantification of HIV gag DNA was performed by quantitative PCR (qPCR) by means of a 5′ nuclease (TaqMan) assay with an ABI 7700 system (Perkin Elmer, Norwalk, CT) as previously described [20,21]. Standards were constructed for absolute quantification of gag and albumin copy number and were validated with sequential dilution of 8E5 cell lysates that contain one copy of gag per cell. Duplicate reactions were run and template copies calculated using ABI7700 software.
In vitro HIV infection
Sorted memory CD4 T cells from two healthy donors were subjected to in vitro infection with a R5-tropic EGFP HIV-1 AD8 at multiplicity of infection (MOI) of 0.01 for 5 days. Cells were harvested on day 5 and PD-1 levels in non-infected (GFP-) and cells harboring virus (EGFP+) were analyzed by flow cytometry.
Statistical analysis
Experimental variables were analyzed using nonparametric statistical tests of inference: Mann-Whitney U test, the Wilcoxon matched-pairs signed rank test, or the Kruskal-Wallis test with Dunn’s multiple comparison post-test as appropriate. Correlation analysis was performed using the nonparametric Spearman test. The Generalized Estimating Equations regression analysis was utilized to model longitudinal measurements of HIV-1 viral RNA or CD4 count to account for the non-independence of intra-individual repeated measures. Statistical analyses were performed with GraphPad Prism (GraphPad Software, version 5.0) or Stata Statistical Software, Release 11 (StataCorp, College Station, TX).
Results
Loss of PD-1highCTLA-4low early-differentiated CD4 T cells in advanced HIV infection
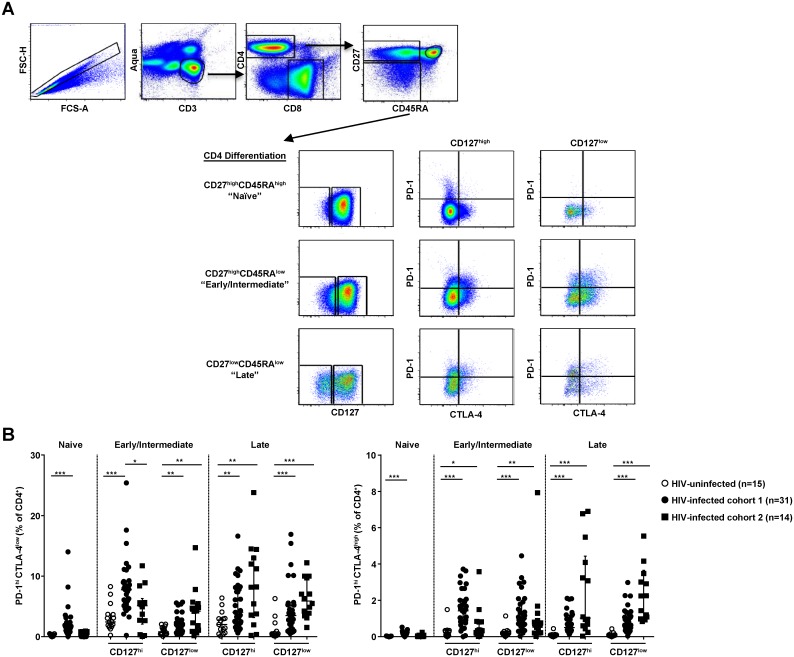
First, we determined the expression patterns for two major co-inhibitory receptors for CD4 T cells from HIV uninfected (n = 15) and untreated HIV-infected subjects from a cohort with earlier HIV infection (Cohort 1, n = 31, median CD4 count 525 cells/μl) or with more advanced disease prior to treatment (Cohort 2, n = 14, median CD4 count 148 cells/ μl) (Fig 1A and Table 1). Skewing of maturation subsets was evident by a significant lower frequency of CD27highCD45RAhigh (naïve) and CD27highCD45RAlow CD4 T cells associated with an increased frequency of CD27lowCD45RAlow (late differentiated, LD) CD4 T cells in Cohort 2 with lower median CD4 count, consistent with more advanced disease (S1A Fig). Although several markers have been used to define memory phenotypes, we found distinct patterns of PD-1 and CTLA-4 (two major regulators of T cell activation and function) expression in T cell populations dependent on CD127, the IL-7 receptor (Fig 1A and 1B). Overall, the relative frequency of cells expressing a PD-1highCTLA-4low phenotype was increased in HIV-infected compared to uninfected individuals, in all CD4 T cell memory subsets tested (Fig 1B). A strong association was found between the expression of PD-1 and differentiation of CD4 T cells in donors with less advanced disease (Cohort 1): the CD127high EI compartment had the highest frequency of PD-1high CD4 T cells (Fig 1B) (p < 0.0001, for PD-1highCTLA-4low in CD127high vs. CD127low EI and p = 0.268 for PD-1highCTLA-4low CD127high vs. CD127low in the LD compartments respectively). A significantly lower frequency of PD-1highCTLA-4lowCD127high EI CD4 T cells was found in the cohort with more advanced disease (Cohort 2), a pattern that was not seen in the other memory populations. On the other hand, the frequency of PD-1highCTLA-4high cells was decreased, although not significantly, in both CD127high and CD127low EI CD4 T cells from HIV-infected individuals with more advanced disease (Fig 1B).
Fig 1. The frequency of less differentiated PD-1high CD127high CD4 T cells is reduced compared with more differentiated subsets in advanced HIV infection.
(A) Gating strategy to define differentiation status of CD127, PD-1 and CTLA-4 expression by CD4 T cells. Differentiation was defined by gating on CD27 and CD45RA with CD27highCD45RAhigh (referred to as Naïve), CD27highCD45RAlow (Early/Intermediate), and CD27lowCD45RAlow (Late). (B) Distribution plots from HIV- infected subjects compared to HIV-uninfected (open circles, n = 15) from two cohorts with HIV infection: Cohort 1 (median CD4 count 525 cells/μl, filled circles, n = 31); and Cohort 2 with more advanced infection (median CD4 count 148 cells/μl, filled squares, n = 14) of PD-1 and PD-1/CTLA-4 expression by differentiation status and CD127 (IL-7Ra) staining demonstrating an altered/reduced frequency of PD-1high CTLA-4high/low CD127high CD4 T cells of early/intermediate differentiation compared to more differentiated subsets which show increased PD-1 expression with more advanced HIV infection. Plots include median and interquartile range, *p< 0.05, **p< 0.001, ***p< 0.0001 by Kruskal-Wallis or Mann-Whitney test.
The expression pattern of these receptors appeared to differ from CD8 T cells from HIV-infected and uninfected donors (S1C Fig), with differences in PD-1highCTLA-4low frequencies noted for more differentiated CD8 T cells (CD127low). Interestingly, the comparison between PD-1high and PD-1high CTLA-4high expression profiles on CD27highCD45RAlow CD4 T cells from HIV negative individuals indicates that PD-1 is up-regulated prior to CTLA-4 during CD4 T cell differentiation. Minimal expression of other negative co-stimulatory molecules (2B4, CD160) on CD4 T as compared to CD8 T cells was found (data not shown). These observations indicate that the regulation of both PD-1 and CTLA-4 differs between CD4 and CD8 T cells, particularly in early-differentiated CD4 (CD8) T cell populations.
To further investigate the impact of HIV infection on the described CD4 T cell populations, we combined data for HIV-infected subjects from Cohorts 1 and 2, before treatment (n = 45). Consistent with previous studies, we found increased frequencies of PD-1high cells in total (naïve and memory) CD4 and CD8 T cell compartments with higher viral load (Fig 2A). However, this association was stronger in the case of CD8 T cells (Spearman r = 0.45, p = 0.002) compared with CD4 T cells (r = 0.31, p = 0.044) (Fig 2A), where higher viral load was correlated with higher PD-1 expression on more differentiated, Late (CD127low CD27lowCD45RAlow) CD4 T cells (Spearman r = 0.341, p = 0.042) (Fig 2A). However, PD1highCD127high EI subset was negatively associated with viral load, independent of the expression of CTLA4 (Fig 2A).
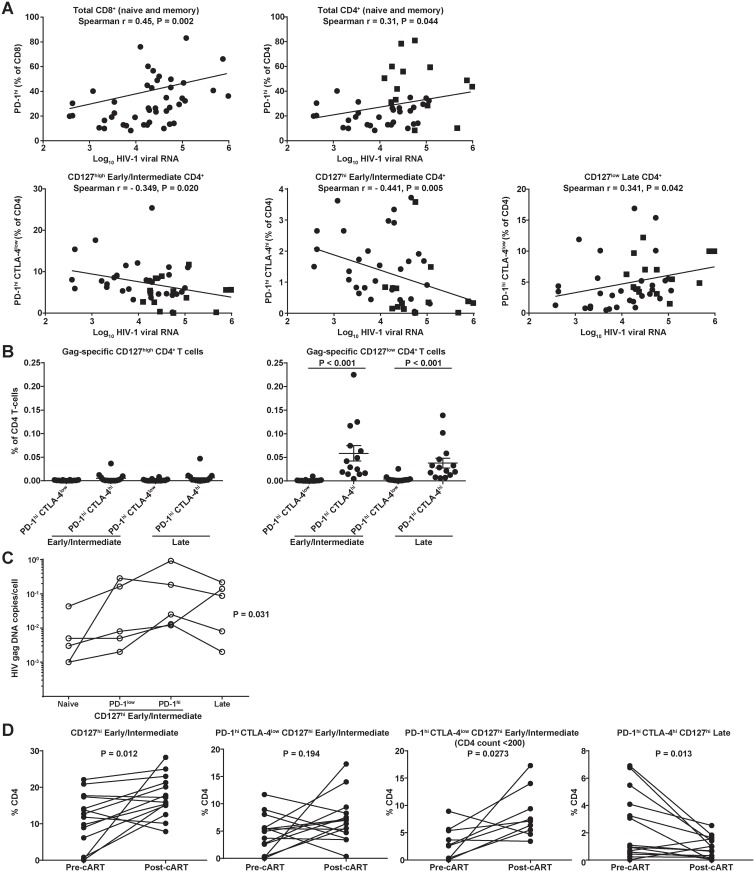
Fig 2. Seletctive loss of PD-1highCTLA-4low/highCD127high Early/Intermediate CD4 T cells occurs with higher plasma HIV-1 viral RNA levels and higher cell-associated viral DNA.
(A) Scatter plots of HIV-1 viral RNA and fitted regression lines for total (naïve and memory) CD8 and CD4 T cells demonstrating increased PD-1 expression with higher viral replication. However, for CD4 T cells of Early/Intermediate differentiation expressing CD127 and PD-1 or PD-1/CTLA-4 there is a negative association compared with more differentated (CD127low) CD4 T cells. Spearman rank correlation coefficients and associated p-values are shown. (B) Donors (n = 14, five from Cohort 1 and nine from Cohort 3) with HIV Gag-specific CD4 T-cell responses are more differentiated (CD127low) and co-express both PD-1 and CTLA-4. (C) Cell-associated HIV-1 gag DNA (no. copies/cell) for sorted T cell populations (see S3 Fig for gating strategy). Individual differences between differentiation subsets (shown for each individual by a connecting line) are statistically significant (p = 0.031 by Friedman test). (D) PD-1highCTLA-4lowCD127high Early/Intermediate CD4 T cells are increased after antiretroviral therapy. Relative frequencies of bulk CD4 populations before and after initiation of combination antiretroviral therapy (cART). Connected symbols represent pre-cART and 48 weeks post-cART (Cohort 2, n = 14, Wilcoxon matched-pairs test, p-values shown in figure). The PD-1highCTLA-4low CD127high group is analyzed separately for subjects who started cART with a CD4 count less than 200.
To extend these findings, we assessed the association of the EI CD4 T cell phenotype with longitudinal viral load measurements in a GEE regression analysis in which repeated measurements HIV-1 viral RNA were modeled as the dependent variable. We observed a slight decline in HIV-1 viral load over time associated with the PD-1high CTLA-4low CD127high EI CD4 T cell phenotype (regression coefficient = -0.062, p = 0.023), which was the only statistically significant phenotypic association with longitudinal viral load (other data not shown). We further examined untreated subjects for HIV Gag-specific responses (S2 Fig) as HIV-specific CD4 T cells have been shown to be preferentially infected [21]. HIV-specific CD4 T cells expressed an IFNg+IL-2- profile (S2 Fig). Among 14 subjects with a detectable HIV Gag-specific CD4 response, we found that the majority of Gag-specific CD4 T cells (median 65.0%, range 18.4 to 94.4%) were more differentiated (CD127low) and co-expressed PD-1 and CTLA-4 (Fig 2B), which is consistent with prior studies [22,23].
No down-regulation of PD-1 was observed with in vitro HIV infection of sorted, memory CD4 T cells in infected compared to uninfected cells (S3A Fig). Our data indicate that PD-1highCD127high EI CD4 T cells may be preferentially lost during chronic HIV infection. Furthermore, the very low frequency of HIV-specific CD4 T cells challenges their importance for CD4 dynamics within this early-differentiated compartment.
Restoration of PD-1highCTLA-4low early-differentiated CD4 T cells after antiretroviral therapy
Our data imply that the progressive loss of PD-1high CD4 T cells in an early differentiation state could be mediated, at least in part, by increased infection by HIV. To confirm this, we evaluated sorted CD4 T cell populations (Fig 2C) and observed an increase in the frequency of HIV-1 gag DNA (n = 5 donors) in the PD-1high compared to PD-1low populations. Since cell-associated DNA content will vary with plasma viral RNA, we compared paired differences in HIV-1 gag DNA across differentiation subsets which were statistically significant (p = 0.031, Friedman test), consistent with the interpretation that early differentiation is associated with increased HIV infection. Only the difference between the PD-1high CD127high EI and naïve compartments was statistically significant after Dunn’s multiple comparisons correction (p < 0.05). Interestingly, a comparable frequency of HIV-1 gag DNA copies was observed between the PD-1highCD127highEI and LD CD4 T cell compartments (Fig 2C).
In the HIV treatment cohort (Cohort 2, Table 1) in which PBMC were obtained before and 48 weeks after initiation of combination antiretroviral therapy (cART), we observed an increase of the relative frequency of CD4 T cells expressing high levels of CD127 with therapy as previously described [24] (Fig 2D). Consistent with their increased loss during advanced disease (Fig 1C), a significant expansion (p = 0.0273, Wilcoxon matched-pairs test) of the PD-1highCTLA-4lowCD127high EI CD4 T cell subset was found in subjects with lower CD4 counts (<200 cells/μL) at treatment initiation (Fig 2D). In contrast, cART led to a decreased frequency of PD-1highCTLA-4high cells, especially in theCD127high LD CD4 T cell compartment (p = 0.013), as well as within the respective CD127low populations (data not shown). Although the data shown are relative frequencies and not absolute counts, there is the possibility of redistribution of memory subsets following cART initiation. Taken together, our data indicate that the dynamics of early-differentiated CD4 T cells could be regulated by infection-depletion along with other mechanisms that could promote their differentiation towards more mature CD4 T cell phenotypes.
PD-1 up-regulation in early-differentiated CD4 T cells is associated with increased activation and expression of the HIV co-receptor CCR5
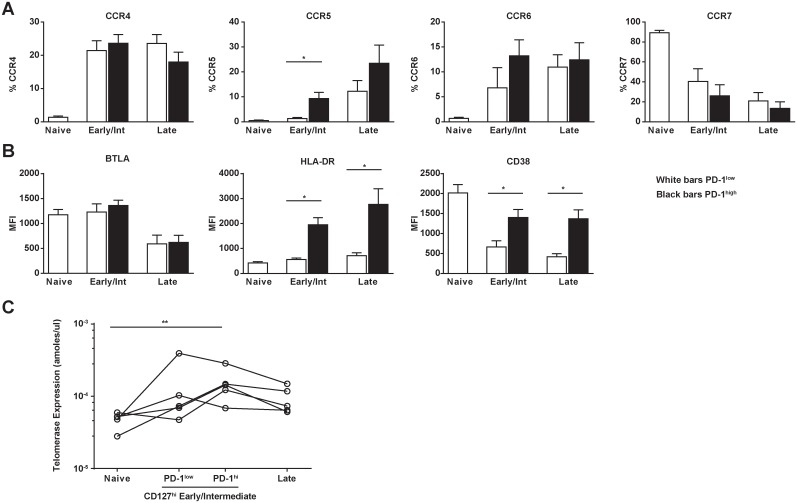
Next, we investigated whether expression of PD-1 in CD127high EI T cells was associated with differential expression of chemokine receptors, particularly CCR5 or activation markers, parameters directly associated to HIV infectivity. We performed further CD4 T cell phenotypic analysis for the chemokine receptors CCR4, CCR5, CCR6, and CCR7 as well as the activation markers CD38, HLA-DR, and BTLA, a co-inhibitory receptor with functional characteristics similar to PD-1 and CTLA-4, which is increased among less differentiated T cells [25]. PD-1high CD127high EI CD4 T cells had significantly higher CCR5 expression Compared to PD-1low CD127high EI CD4 T cells (Fig 3A), but did not show any other significant differences in chemokine receptor expression (Fig 3A). Increased expression of activation markers per cell (judged by Mean Fluorescence Intensity-MFI) was observed with increased differentiation. However, both HLA-DR and CD38 expression was significantly up-regulated in the PD-1high cells in the early differentiated CD27highCD45RAlow compartment and CD27lowCD45RAlowCD4 T cells (Fig 3B). We observed similar patterns of chemokine and activation marker expression in the CD127low compartment with increasing differentiation (data not shown) where there were significant differences in expression of CCR5, HLA-DR, and BTLA between PD-1 high and low in the EI CD4 T cell population. To assess whether this represents actual T cell activation rather than up-regulation of PD-1 by other bystander mechanisms [26], we characterized several sorted populations for telomerase activity. We found an overall increased telomerase activity with differentiation (p = 0.02), but the PD-1highCD127high EI population was the only differentiation phenotype significantly different from the naïve population (p < 0.05 after Dunn’s multiple comparisons correction, Fig 3C). This suggests that PD-1highCD127high EI CD4 T cells represent an early state of CD4 T cell differentiation that have received activation signals by TCR engagement [27,28]. Our data suggest that increased expression of CCR5 on an activated background could result in the increased susceptibility of PD-1highCD127high EI CD4 T cells to HIV infection.
Fig 3. PD-1highCD127high Early/Intermediate CD4 T cells express the HIV coreceptor CCR5, activation markers HLA-DR and CD38, and demonstrate evidence of TCR stimulation.
(A) Bar graphs showing the relative frequency of CD4 T cell populations expressing several chemokine receptors (CCR4, CCR5, CCR6, and CCR7) and (B) markers of activation/differentiation (BTLA, HLA-DR, and CD38) (n = 7 HIV-infected donors). All populations are CD127high. MFI, mean fluorescence intensity; bars represent mean and SEM, *p< 0.05, after correction by Dunn’s multiple comparisons test. (C) Evidence of recent TCR stimulation was assessed based on telomerase expression by qRT-PCR assay of sorted populations (see S3 Fig for gating strategy). Individual differences between differentiation subsets (shown for each individual by a connecting line) were statistically significant (p = 0.02, Friedman test).
PD-1high early-differentiated CD4 T cells are characterized by increased functionality
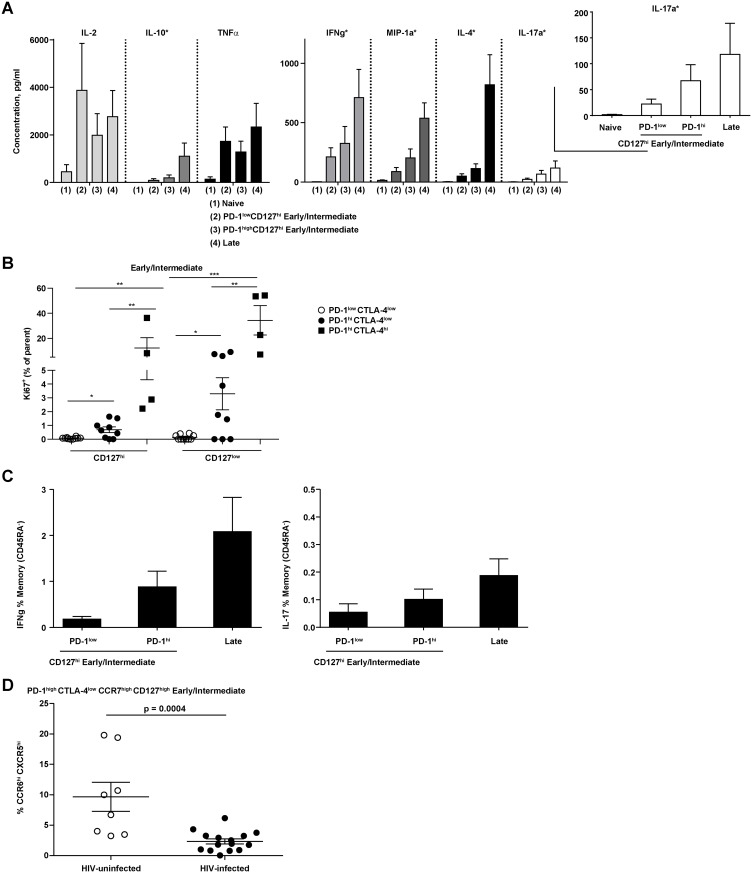
Given the role of PD-1 in CD8 T cell exhaustion during chronic viral infections [29] we sought to investigate whether PD-1highCD127high EI CD4 T cells were characterized by impaired functionality, a hallmark of the exhaustion phenotype. To this end, we used sorted CD4 (S3B Fig) T cell populations from uninfected individuals and examined the effect of in vitro TCR stimulation using a functional plate-bound anti-CD3 antibody and measuring cytokine production in the supernatants. We observed increased cytokine production (IFN-g, MIP1-a, IL-4, IL-10, and IL-17) from naïve to PD-1low, PD-1highCD127high EI and LD CD4 T cells (p = 0.0026, Kruskal-Wallis test, Fig 4A). Interestingly, a considerable production of IL-17 between PD-1highCD127high EI and LD CD4 T cells was found (Fig 4A) underlining the possible impact of the loss of this particular early-differentiated CD4 T cell population in the overall compromised of IL-17-mediated defense mechanisms in chronic HIV [30]. In addition, there was evidence of proliferative capacity with increased Ki-67 expression in the CD127high and CD127low EI populations with expression of PD-1 and CTLA-4 (Fig 4B, S3C Fig). We then used staphylococcal enterotoxin B (SEB) for polyclonal stimulation to assess, using a flow cytometry assay, ex vivo production of IFN-g and IL-17 in untreated HIV-infected individuals (n = 5) (Fig 4B, S3C Fig). We observed a similar pattern of cytokine secretion compared with anti-CD3 stimulation (Fig 4B). These results indicate the possible impact of the loss of EI CD4 T cell population in the overall compromised defense mechanisms of chronic HIV patients [30].
Fig 4. PD-1highCD127high Early/Intermediate CD4T cells maintain broad cytokine production.
(A) Cytokine production after polyclonal stimulation (anti-CD3 with anti-CD28 and anti-CD49d co-stimulation) measured by bead-based Luminex technology of fresh, sorted CD4 T cells from HIV-uninfected donors (n = 5, *p< 0.05 by Friedman test for each cytokine across cell populations). (B) Percent Ki67+ staining cells for CD127high and CD127low early/intermediate CD4 T cells from HIV-infected Cohort 1 (n = 11). (C) Differentiation phenotype of IFN-g or IL-17a positive cells detected after (6h) ex vivo SEB stimulation for HIV-infected donors (n = 5). No differences were statistically significant (Mann-Whitney test) (D) The relative frequency of the CCR6highCXCR5high population within the CCR7highPD-1highCD127high Intermediate CD4 T cell population is decreased in HIV-infected (n = 15) compared to uninfected (n = 8) individuals (p = 0.0004, Mann-Whitney test).
We have previously shown that CD4 T cells expressing a CCR7highCXCR5highCCR6highPD-1high phenotype can provide increased in vitro B cell help and is decreased in HIV infection [31]. Here, we further examined this “peripheral TFH ” phenotype and found that within the PD-1highCD127highCCR7high EI CD4 T compartment, a subset of CD4 T cells with increased HIV gag DNA content (Fig 2C), there is a significant loss of CXCR5highCCR6high cells (Fig 4C, S3D Fig). Taken together, these data indicate that PD-1 expression in early-differentiated CD4 T cells may not be associated with functional defects and exhaustion of this less differentiated population.
Discussion
PD-1 is considered to play an important role in the regulation of the CD-4 T cell response due to its increased expression in virus-specific CD4 T cells [22]. A large body of evidence has established that PD-1 is a critical mediator of CD8 T cell exhaustion. Up-regulation of PD-1 in antigen-specific CD8 T cells results in decreased proliferative and effector capacities in chronic viral infection [4,5,32] and cancer [33]. However, the role of PD-1 as a regulator of CD4 T cell function is not well understood. Here, we demonstrate that the pattern of PD-1 expression differs between bulk CD4 and CD8 T cells for both HIV-infected and uninfected individuals indicating a differential regulation of the receptor on CD8 T cells compared to CD4 T cells either at the transcription level or due to the sensitivity of extracellular/intracellular signals regulating the surface expression of PD-1. Analysis of the co-expression of PD-1 and CTLA-4 in early-differentiated (CD127highCD27highCD45RAlow) CD4 T cells from uninfected subjects revealed that PD-1 is expressed earlier than CTLA-4 during the differentiation of CD4 T cells (p < 0.0001). In agreement with previous studies [22,23], the majority of HIV Gag-specific cells express a more differentiated phenotype (CD127low) skewed towards PD-1highCTLA-4high, especially in the late-differentiated “effector memory” compartment (CD27lowCD45RAlow) where a minority of bulk CD4 T cells is characterized by a PD-1highCTLA-4low phenotype. However, the inclusion of CTLA-4 in our analysis revealed an altered dynamic between PD-1highCTLA-4low and PD-1highCTLA-4high early during the differentiation of CD4 T cells and with respect to HIV progression.
We further analyzed the phenotype and function of PD-1highCTLA-4lowCD127high EI CD4 T cells and found that PD-1 expression was associated with increased activation, measured by HLA-DR and CD38 expression and increased susceptibility to HIV infection, based on the expression of the HIV co-receptor CCR5. These cells are also characterized by increased telomerase activity (Fig 3C), suggesting that this population represents an early state of CD4 T cell differentiation that have been preferentially activated by TCR engagement [27,28]. Furthermore, PD-1high expression marks higher sensitivity to in vitro spontaneous and CD95/Fas-induced apoptosis in less differentiated CD4 T cells, although in significantly lower levels compared to PD-1high “effector” CD4 T cells (data not shown). Accordingly, a decreased frequency of PD-1highCTLA-4lowCD127high EI CD4 T cells was associated with increased HIV-1 viral load over time. This observation and the demonstration of increased HIV gag DNA in this compartment suggest increased susceptibility to HIV infection which is in line with previous studies, where HIV infection was analyzed in CD127high CD4 T cells [23]. We should emphasize that in vitro infection of CD4 T cells was not associated with any down-regulation of PD-1 in infected compared to uninfected cells. Hence, we hypothesize that increased infection and depletion could affect the dynamics of early-differentiated CD4 T cells along with other mechanisms that promote their differentiation towards mature CD4 T cell phenotypes. More importantly, the contribution of PD-1highCTLA-4lowCD127high EI CD4 T cells to the establishment of a latent HIV-1 reservoir compared to highly differentiated CD4 T cell populations (for example, effector PD-1high cells) which are more susceptible to cell death should be investigated further. Collectively, our data suggest that these early-differentiated CD4 T cells may be more susceptible to HIV infection due to increased activation and increased co-expression of CCR5 and PD-1. We propose that the dynamics of CD4 T cells may be altered by their susceptibility to HIV infection (PD-1highCD127highCD4 T cells) and the skewed maturation of HIV-specific CD4 T cells (PD-1highCD127low), which are preferentially infected and highly sensitive to viral load changes and TCR stimulation.
Interestingly, we found that PD-1high early-differentiated T cells were capable of producing a wide range of cytokines with overall cytokine production higher in PD-1high compared to PD-1low cells from the CD127high EI CD4 T cell compartment among HIV-uninfected donors. This is consistent with the observation in the Rhesus macaque SIV model in which PD-1 expression on CD4 T cells, although not defined by differentiation phenotype, had retained proliferative capacity [14]. Hence, PD-1 signaling in the CD4 T cell compartment does not necessarily appear to confer an “exhaustion” status [14]. A loss of CD4 T cells producing IL-17 in HIV infected individuals has been previously described [34,35]. Our data indicate that the decline of PD-1highCTLA-4lowCD127high EI CD4 T cells, mediated, at least in part, by increased susceptibility to HIV infection, could contribute to the loss of IL-17+ CD4 T cells even at a very early step of CD4 T cell differentiation. Similarly, we observed that this phenotype overlaps with a circulating “TFH” phenotype, which was decreased in HIV-infected subjects, consistent with our previous study [31]. Previous studies have shown that CD4 TFH cells within lymph nodes may be the major reservoir for HIV infection and replication [36]. Whether increased HIV gag DNA in circulating PD-1highCTLA-4lowCD127high EI CD4 T cells reflects increased infection of a particular follicular CD4 T cell population within the lymph node needs further investigation. Together, our data show an accelerated expression of PD-1 in the early differentiation of CD4 T cells that is associated with increased cytokine production as opposed to an expected decrease in cytokine response observed with PD-1 expression [4].
Overall, our data indicate that PD-1 and CTLA-4 could serve as a very early marker of differentiation of CD4 T cells during HIV infection marking cells with increased sensitivity to infection. In contrast to CD8 T cells, our data suggest that a functional restoration of CD4 T cells in HIV possibly requires the manipulation of PD-1 as well as other co-inhibitory receptors, like CTLA-4.
Supporting Information
(A) Distribution plots showing skewed CD4 differentiation of HIV- infected subjects compared to HIV-uninfected (open circles, n = 15) from two cohorts with HIV infection: Cohort 1 (median CD4 count 525 cells/μl, filled circles, n = 31); and Cohort 2 with more advanced infection (median CD4 count 148 cells/μl, filled squares, n = 14). (B) Representative flow cytometry plots and the gating strategy used to characterize CD4 and CD8 T cell populations. (C) Comparative plots of PD-1 and PD-1/CTLA-4 expression by differentiation status and CD127 staining for populations of Naive (CD27high CD45RAhigh), Early/Intermediate (CD27highCD45RAlow) and Late (CD27low CD45RAlow) CD8 T cells from HIV-uninfected (open circles, n = 9) and HIV-infected (filled circles, n = 31) subjects. *p< 0.05, **p< 0.001, ***p< 0.0001 by Mann-Whitney test.
(TIFF)
Representative flow cytometry, gating strategy and overlay plots of Gag-specific, IFN-g-producing CD4 and CD8 T-cells for specific populations is shown.
(TIFF)
(A) Sorted memory (Early/Intermediate, CD27high CD45RAhigh) CD4 T cells from two healthy donors were subjected in vitro HIV infection. PD-1 levels in non-infected (EGFP-) and cells harboring virus (EGFP+) were analyzed by flow cytometry. (B) Gating strategy for sorting PD-1highCD127high Early/Intermediate and other CD4 T cell populations. Due to the requirement for surface staining, intracellular anti-CTLA-4 was not included as sorting parameter. (C) Percent Ki67+ staining cells for CD127high and CD127low naïve and late CD4 T cells from HIV-infected Cohort 1 (n = 11). Not all populations for all donors are plotted due to the small population size.. (D) Representative flow cytometry, gating strategy and overlay plots after polyclonal stimulation with SEB for IFN-g or IL-17 (shown) producing CD4 T-cells for specific populations is shown. (E) Representative flow cytometry plot and gating strategy demonstrating loss of CD127highCCR7highPD-1highCTLA-4low CXCR5highCCR6high Early/Intermediate CD4 T cells with HIV infection.
(TIFF)
Acknowledgments
The authors would like to thank Dr. Mario Roederer for providing in-house conjugated antibodies; Dr. Mythreyi D. Shastri for her assistance with the manuscript preparation. This research was supported by the Intramural Research Program of the Vaccine Research Center, National Institutes of Allergy and Infectious Diseases, National Institutes of Health (AI064086; R.H.), the University of California San Diego Center for AIDS Research (AI36214; R.H.), and the San Diego AIDS Clinical Trial Group (CTU AI69432; R.H.). The work was also supported in part by an Interagency Agreement Y1-AI-2642-12 between the U.S. Army Medical Research and Material Command (USAMRMC) and the National Institutes of Allergy and Infectious Diseases and by a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD).
Disclaimer
The opinions herein are those of the authors and should not be construed as official or representing the views of the U.S. Department of Health and Human Services, National Institute for Allergy and Infectious Diseases, the Department of Defense, or the Department of the Army.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Intramural Research Program of the Vaccine Research Center, National Institutes of Allergy and Infectious Diseases, National Institutes of Health (AI064086; RH), the University of California San Diego Center for AIDS Research (AI36214; RH), and the San Diego AIDS Clinical Trial Group (CTU AI69432; RH). The work was also supported in part by an Interagency Agreement Y1-AI-2642-12 between the U.S. Army Medical Research and Material Command (USAMRMC) and the National Institutes of Allergy and Infectious Diseases and by a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD).
References
- 1. Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ (2007) The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8: 239–245. [DOI] [PubMed] [Google Scholar]
- 2. Kulpa DA, Lawani M, Cooper A, Peretz Y, Ahlers J, et al. (2013) PD-1 coinhibitory signals: the link between pathogenesis and protection. Semin Immunol 25: 219–227. 10.1016/j.smim.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, et al. (2006) PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 203: 2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, et al. (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443: 350–354. [DOI] [PubMed] [Google Scholar]
- 5. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, et al. (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439: 682–687. [DOI] [PubMed] [Google Scholar]
- 6. Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, et al. (2007) Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27: 670–684. [DOI] [PubMed] [Google Scholar]
- 7. Velu V, Titanji K, Zhu B, Husain S, Pladevega A, et al. (2009) Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458: 206–210. 10.1038/nature07662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, et al. (2012) Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, et al. (1995) CD8 naive T cell counts decrease progressively in HIV-infected adults. The Journal of Clinical Investigation 95: 2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, et al. (1999) Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283: 857–860. [DOI] [PubMed] [Google Scholar]
- 11. Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, et al. (1999) Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med 189: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, et al. (2012) Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338: 1220–1225. 10.1126/science.1229620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Utzschneider DT, Legat A, Fuertes Marraco SA, Carrie L, Luescher I, et al. (2013) T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol 14: 603–610. 10.1038/ni.2606 [DOI] [PubMed] [Google Scholar]
- 14. Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F (2013) Re-Evaluation of PD-1 Expression by T Cells as a Marker for Immune Exhaustion during SIV Infection. PLoS ONE 8: e60186 10.1371/journal.pone.0060186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salek-Ardakani S, Schoenberger SP (2013) T cell exhaustion: a means or an end? Nat Immunol 14: 531–533. 10.1038/ni.2619 [DOI] [PubMed] [Google Scholar]
- 16. Birx DL, Loomis-Price LD, Aronson N, Brundage J, Davis C, et al. (2000) Efficacy testing of recombinant human immunodeficiency virus (HIV) gp160 as a therapeutic vaccine in early-stage HIV-1-infected volunteers. rgp160 Phase II Vaccine Investigators. J Infect Dis 181: 881–889. [DOI] [PubMed] [Google Scholar]
- 17. Haubrich RH, Riddler SA, DiRienzo AG, Komarow L, Powderly WG, et al. (2009) Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS 23: 1109–1118. 10.1097/QAD.0b013e32832b4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riddler SA, Haubrich R, DiRienzo AG, Peeples L, Powderly WG, et al. (2008) Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 358: 2095–2106. 10.1056/NEJMoa074609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doria-Rose NA, Klein RM, Daniels MG, O'Dell S, Nason M, et al. (2010) Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol 84: 1631–1636. 10.1128/JVI.01482-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, et al. (2004) T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol 78: 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, et al. (2002) HIV preferentially infects HIV-specific CD4+ T cells. Nature 417: 95–98. [DOI] [PubMed] [Google Scholar]
- 22. Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, et al. (2007) Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 8: 1246–1254. [DOI] [PubMed] [Google Scholar]
- 23. Zaunders JJ, Ip S, Munier ML, Kaufmann DE, Suzuki K, et al. (2006) Infection of CD127+ (interleukin-7 receptor+) CD4+ cells and overexpression of CTLA-4 are linked to loss of antigen-specific CD4 T cells during primary human immunodeficiency virus type 1 infection. J Virol 80: 10162–10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colle JH, Moreau JL, Fontanet A, Lambotte O, Joussemet M, et al. (2006) Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients—effects of antiretroviral therapy. J Acquir Immune Defic Syndr 42: 277–285. [DOI] [PubMed] [Google Scholar]
- 25. Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, et al. (2003) BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 4: 670–679. [DOI] [PubMed] [Google Scholar]
- 26. Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, et al. (2008) The Common γ-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Induce the Expression of Programmed Death-1 and Its Ligands. The Journal of Immunology 181: 6738–6746. [DOI] [PubMed] [Google Scholar]
- 27. Hathcock KS, Kaech SM, Ahmed R, Hodes RJ (2003) Induction of telomerase activity and maintenance of telomere length in virus-specific effector and memory CD8+ T cells. J Immunol 170: 147–152. [DOI] [PubMed] [Google Scholar]
- 28. Weng NP, Hathcock KS, Hodes RJ (1998) Regulation of telomere length and telomerase in T and B cells: a mechanism for maintaining replicative potential. Immunity 9: 151–157. [DOI] [PubMed] [Google Scholar]
- 29. Wherry EJ (2011) T cell exhaustion. Nat Immunol 12: 492–499. [DOI] [PubMed] [Google Scholar]
- 30. Klatt NR, Brenchley JM (2010) Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS 5: 135–140. 10.1097/COH.0b013e3283364846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boswell KL, Paris R, Boritz E, Ambrozak D, Yamamoto T, et al. (2014) Loss of Circulating CD4 T Cells with B Cell Helper Function during Chronic HIV Infection. PLoS Pathog 10: e1003853 10.1371/journal.ppat.1003853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, et al. (2010) Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med 16: 1147–1151. 10.1038/nm.2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, et al. (2012) Safety, Activity, and Immune Correlates of Anti—PD-1 Antibody in Cancer. New England Journal of Medicine 366: 2443–2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, et al. (2008) Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112: 2826–2835. 10.1182/blood-2008-05-159301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gosselin A, Monteiro P, Chomont N, Diaz-Griffero F, Said EA, et al. (2010) Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J Immunol 184: 1604–1616. 10.4049/jimmunol.0903058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, et al. (2012) Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Distribution plots showing skewed CD4 differentiation of HIV- infected subjects compared to HIV-uninfected (open circles, n = 15) from two cohorts with HIV infection: Cohort 1 (median CD4 count 525 cells/μl, filled circles, n = 31); and Cohort 2 with more advanced infection (median CD4 count 148 cells/μl, filled squares, n = 14). (B) Representative flow cytometry plots and the gating strategy used to characterize CD4 and CD8 T cell populations. (C) Comparative plots of PD-1 and PD-1/CTLA-4 expression by differentiation status and CD127 staining for populations of Naive (CD27high CD45RAhigh), Early/Intermediate (CD27highCD45RAlow) and Late (CD27low CD45RAlow) CD8 T cells from HIV-uninfected (open circles, n = 9) and HIV-infected (filled circles, n = 31) subjects. *p< 0.05, **p< 0.001, ***p< 0.0001 by Mann-Whitney test.
(TIFF)
Representative flow cytometry, gating strategy and overlay plots of Gag-specific, IFN-g-producing CD4 and CD8 T-cells for specific populations is shown.
(TIFF)
(A) Sorted memory (Early/Intermediate, CD27high CD45RAhigh) CD4 T cells from two healthy donors were subjected in vitro HIV infection. PD-1 levels in non-infected (EGFP-) and cells harboring virus (EGFP+) were analyzed by flow cytometry. (B) Gating strategy for sorting PD-1highCD127high Early/Intermediate and other CD4 T cell populations. Due to the requirement for surface staining, intracellular anti-CTLA-4 was not included as sorting parameter. (C) Percent Ki67+ staining cells for CD127high and CD127low naïve and late CD4 T cells from HIV-infected Cohort 1 (n = 11). Not all populations for all donors are plotted due to the small population size.. (D) Representative flow cytometry, gating strategy and overlay plots after polyclonal stimulation with SEB for IFN-g or IL-17 (shown) producing CD4 T-cells for specific populations is shown. (E) Representative flow cytometry plot and gating strategy demonstrating loss of CD127highCCR7highPD-1highCTLA-4low CXCR5highCCR6high Early/Intermediate CD4 T cells with HIV infection.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.