Abstract
Obective
Glucagon-like peptide-1 (GLP-1) receptor agonists are indicated for treatment of type 2 diabetes since they mimic the actions of native GLP-1 on pancreatic islet cells, stimulating insulin release, while inhibiting glucagon release, in a glucose-dependent manner. The observation of weight loss has led to exploration of their potential as antiobesity agents, with liraglutide 3.0 mg day−1 approved for weight management in the US on December 23, 2014, and in the EU on March 23, 2015. This review examines the potential nonglycemic effects of GLP-1 receptor agonists.
Methods
A literature search was conducted to identify preclinical and clinical evidence on nonglycemic effects of GLP-1 receptor agonists.
Results
GLP-1 receptors are distributed widely in a number of tissues in humans, and their effects are not limited to the well-recognized effects on glycemia. Nonglycemic effects include weight loss, which is perhaps the most widely recognized nonglycemic effect. In addition, effects on the cardiovascular, neurologic, and renal systems and on taste perception may occur independently of weight loss.
Conclusions
GLP-1 receptor agonists may provide other nonglycemic clinical effects besides weight loss. Understanding these effects is important for prescribers in using GLP-1 receptor agonists for diabetic patients, but also if approved for chronic weight management.
Introduction
Glucagon-like peptide-1 (GLP-1) is a gut hormone that is secreted by the intestine in response to meal ingestion and potentiates glucose-dependent insulin secretion from the pancreatic beta-cells (1). In addition, GLP-1 suppresses glucagon secretion by alpha-cells, leading to a glucose-dependent reduction in hepatic glucose production (1). Glucagon may be regulated in a paracrine manner, by the secretion of somatostatin from the neighboring delta-cells (2).
GLP-1 receptor agonists mimic the pancreatic actions of native GLP-1, leading to glycosylated hemoglobin (HbA1c) reductions within the range of 1.0–1.5% (3), as well as decreased postprandial plasma glucose levels (4). Several GLP-1 receptor agonists are now approved for use as second-line therapy in the treatment of type 2 diabetes (Table 1) (13).
TABLE 1.
GLP-1 receptor agonists approved for treatment of type 2 diabetes
| Agent | Recommended dose and schedule | Amino acid sequence of GLP-1 component | Homology to naturally occurring GLP-1 |
|---|---|---|---|
| Naturally occurring human GLP-1(5) | N/A | His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Lys-Gly-Arg-Gly | N/A |
| Albiglutide (6) | 30 mg QW | His-Gly-Glu-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Lys-Gly-Arg | 97%; modified GLP-1 dimer fused in series to human albumin. Amino acid substitution at position 8 (glycine to alanine), dimer. |
| Dulaglutide (7,8) | 0.75 − 1.5 mg QW | His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Glu-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Lys-Gly-Gly-Gly | 90%; synthetic human GLP-1 dimer (8-glycine,22-glutamic acid,36-glycine) fusion protein with peptide (synthetic 16-amino acid linker) fusion protein with immunoglobulin G4 (synthetic human Fc fragment). |
| Exenatide (9,10) | 10 μg BID, ER formulation: 2 mg QW | His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2 | 53%; synthetic version of exendin-4. |
| Liraglutide (5,11) | 1.2 or 1.8 mg QD | His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly | 97%; glutamic acid and 16-C free fatty acid addition at position 26. Amino acid substitution at position 34 (lysine to arginine). |
| Lixisenatide* (12) | 20 μg QD | His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Ser-Lys-Lys-Lys-Lys-Lys-Lys-NH2 | Homology not published but, based on amino acid sequence, likely to be <50%. Synthetic version of exendin-4. |
Licensed in Europe. Not approved in the United States.
QW, once weekly; QD, once daily; BID, twice daily; ER, extended release.
In addition to improving glycemic control, studies in patients with diabetes show that GLP-1 receptor agonists produce weight loss (3,4). This is an advantage, given that most people with type 2 diabetes are overweight or obese and many also find it difficult to lose weight. Furthermore, there is evidence that weight loss improves glycemic control and has a positive impact on various comorbidities associated with the disease, including cardiovascular risk factors (14).
Accumulating evidence from preclinical and clinical studies indicates that the effects of GLP-1 receptor agonists go beyond glycemic control and weight reduction alone (15,16).
GLP-1 Receptor Agonists: Effects on Body Weight
In addition to their effects on glycemic control, GLP-1 receptor agonists have been shown to produce clinically relevant reductions in weight, body mass index (BMI), and waist circumference in overweight or obese individuals with or without diabetes (17,18). This finding has prompted consideration of GLP-1 receptor agonists as potential weight-loss agents (19). Indeed, liraglutide 3.0 mg day−1 was approved for weight management in the US on December 23, 2014 (20), and in the EU on March 23, 2015.
The mechanisms by which GLP-1 receptor agonists mediate weight loss are not yet fully understood. However, a study involving obese individuals without diabetes treated with liraglutide 3.0 mg provides some insight. Liraglutide was titrated from a starting dose of 0.6 mg day−1 to 3.0 mg day−1 at 5 weeks, to minimize nausea and vomiting (21). By 5 weeks, subjects’ mean weight was reduced by −2.5 kg, despite them being asked to maintain their usual diet and physical activity. The study demonstrated that liraglutide 3.0 mg day−1 increased mean postprandial satiety and fullness ratings, reduced hunger and prospective food consumption, and decreased ad libitum energy intake by approximately 16%. Liraglutide 3.0 mg, similarly to the 1.8 mg dose, also delayed gastric emptying. Conversely, energy expenditure in subjects treated with liraglutide 3.0 mg day−1 decreased, even when corrected for weight loss, which was probably reflective of metabolic adaptation. Thus, the evidence supports reduced appetite and food intake, without an increase in energy expenditure, as the mechanism underlying weight loss with liraglutide. Body composition studies in humans have shown that weight loss with liraglutide appears to correspond to a reduction in predominantly visceral and subcutaneous fat, rather than lean tissue mass (22,23).
Table 2 summarizes the clinical evidence for weight loss with GLP-1 receptor agonists in patients who are overweight or obese. Of these, liraglutide has been the most extensively studied, with the clinical data presented at scientific meetings (29–34) and in peer-reviewed journals (24,25). Liraglutide phase III studies included populations with overweight and obesity, including prediabetes, hypertension, dyslipidemia, type 2 diabetes, and/or moderate or severe obstructive sleep apnea. In these studies, liraglutide 3.0 mg and lifestyle intervention was associated with a greater weight loss of approximately −5%, compared with placebo and identical lifestyle intervention. Total mean weight loss from baseline in these studies is in the range of −6% to −8% with liraglutide 3.0 mg.
TABLE 2.
Effects of GLP-1 receptor agonists on weight in patients who are overweight or obese
| Study, author, and population | Study design | Sample size and retention | Dose and dosing regimen | Weight loss and time point |
|---|---|---|---|---|
| Astrup et al., 2009 (24) Adults with BMI 30-40 kg m−2 | All subjects on −500 kcal deficit diet and increased physical activity; 2-week placebo run-in, followed by randomization 4-week titration from 0.6 mg; 16-week constant dose treatment. | N = 564; completers: 85/95 (1.2 mg), 74/90 (1.8 mg), 73/93 (2.4 mg), 82/93 (3.0 mg), 79/98 (placebo), 79/95 (orlistat); total 472/564 completers = 84% | Liraglutide 1.2 mg once daily (n = 94) | Change from baseline at 20 weeks: Weight: −4.8 kg; weight loss >5%: 52.1%; weight loss >10%: 7.4% |
| Liraglutide 1.8 mg once daily (n = 90) | Weight: −5.5 kg; weight loss >5%: 53.3%; weight loss >10%: 18.9% | |||
| Liraglutide 2.4 mg once daily (n = 92) | Weight: −6.3 kg; weight loss >5%: 60.8%; weight loss >10%: 22.8% | |||
| Liraglutide 3.0 mg once daily (n = 92) | Weight: −7.2 kg; weight loss >5%: 76.1%; weight loss >10%: 28.3% | |||
| Placebo injection (n = 98) | Weight: −2.8 kg; weight loss >5%: 29.6%; weight loss >10%: 2.0% | |||
| Orlistat 120 mg TID (open-label) (n = 95) | Weight: −4.1 kg; weight loss >5%: 44.2%; weight loss >10%: 9.5% | |||
| Astrup et al., 2012 Adults with BMI 30-40 kg m−2 | 2-year extension of above study. | N = 398 entered the extension; completers: 46/68 (1.2 mg), 38/59 (1.8 mg), 45/65 (2.4 mg), 47/72 (3.0 mg), 47/67 (placebo), 45/67 (orlistat); total 268/398 completers = 67% | Liraglutide 2.4/3.0 mg once daily pooled group (n = 92) | Change from baseline at year 2 (completers): Weight: −7.8 kg; weight loss >5%: 69%; weight loss >10%: 43% |
| Orlistat 120 mg TID (open-label) (n = 45) | Weight: −5.4 kg; weight loss >5%: 49%; weight loss >10%: 31% | |||
| Wadden et al., 2013 (25) Adults with BMI ≥30 or ≥27 kg m−2 | Obese/overweight participants who lost ≥5% of initial weight during a low-calorie diet run-in were randomly assigned to liraglutide 3.0 mg per day or placebo (subcutaneous administration) for 56 weeks. Diet and exercise counseling were provided throughout the trial. | N = 422; completers: 159/212 (3.0 mg), 146/210 (placebo) | Liraglutide 3.0 mg once daily (n = 207) | Change from randomization to 56 weeks (full analysis set with last observation carried forward): Weight: −6.0 kg (−6.2%); weight loss >5%: 50.5%; weight loss >10%: 26.1% |
| Placebo injection (n = 206) | Weight: −0.1 kg (−0.2%); weight loss >5%: 21.8%; weight loss >10%: 6.3% | |||
| Rosenstock et al., 2010 (26) Adults with BMI ≥30 kg m−2 | Obese adults were randomized to exenatide or placebo, combined with lifestyle modification and decreased calorie intake, for 24 weeks. | N = 152; 102 completed the 24-week treatment period | Exenatide 10 μg twice daily (following a 4-week 5 μg dose-initiation period) | Weight from baseline compared with lifestyle modification alone: Exenatide −5.1 kg; placebo −1.6 kg; exenatide − placebo (P < 0.001); placebo-subtracted difference in percent weight loss −3.3 (P < 0.001) |
| Kelly et al., 2013 (27) Adolescents with BMI ≥1.2x the 95th percentile or >35 kg m−2 | 26 adolescents (aged 12-19 years) with severe obesity in double-blind, placebo-controlled study; randomized 3-month period, followed by 3-month open-label extension where all subjects received exenatide 10 mg subcutaneously twice daily. | N = 26; completers at 3 months: 22 total, 12 on exenatide, 10 on placebo; completers at 6 months: 19 | Dose titration from 5 μg twice daily for 1 month and increased to 10 μg twice daily for first 3 months; exenatide (n =13) | Change from randomization to 3 months for completers: BMI −2.90%; BMI −1.18 kg m−2; weight loss −3.26 kg; after open-label extension: BMI −4% from randomization |
| Placebo | BMI −0.15%; BMI −0.04 kg m−2; weight loss −0.32 kg; after open-label extension: BMI +0.25% from randomization | |||
| Dushay et al., 2012 (28) Obese women without type 2 diabetes | 41 obese women (age 48 ± 11 years), BMI 33.1 ± 4.1 kg m−2; double-blind, placebo-controlled cross-over study; two 16-week treatment periods separated by 3-week washout. No lifestyle intervention. | Exenatide 5 μg twice daily for 2 weeks, then 10 μg twice daily for 16 weeks | Overall dropout rate 35%; 17% dropped out before randomization and 18% dropped out after randomization | Change from randomization to week 16: Exenatide: Weight −2.77% |
| Placebo | Placebo: Weight +0.48% |
One study (25) tested the effect of liraglutide 3.0 mg vs. placebo following successful initial weight loss on a low calorie diet. Individuals who achieved ≥5% weight loss in the 4- to 12-week run-in period (77% of enrollees achieved this benchmark) were randomly assigned to either liraglutide 3.0 mg or placebo; both groups received diet and exercise counselling. Mean (± standard deviation) percentage weight loss in the run-in period was −6.0% (± 0.9) and, after 56 weeks of therapy postrandomization, subjects in the liraglutide group lost an additional −6.2% (± 7.3) compared with −0.2% (±7.0) in the placebo group (25).
Other GLP-1 receptor agonists also mediate weight loss. Although no systematic long-term clinical trial program has been conducted with exenatide in patients with obesity and it is not currently approved for weight management, a 24-week randomized, placebo-controlled trial involving nondiabetic obese subjects showed that exenatide 10 μg twice daily (BID), combined with lifestyle modification and decreased calorie intake, produced significant weight loss from baseline compared with lifestyle modification alone (−5.1 kg vs. −1.6 kg, P < 0.001); the placebo-subtracted difference in percent weight loss was −3.3 kg (P < 0.001) (Table 2) (26). There is also evidence, from two small, short-term studies, to indicate that exenatide results in weight loss in nondiabetic adolescents with severe obesity and in obese women (27,28). Furthermore, there is evidence from clinical studies of GLP-1 receptor agonists more recently approved for the treatment of type 2 diabetes, albiglutide, dulaglutide and lixisenatide, of favorable effects on weight loss in diabetes patients (35–37).
Potential pleiotropic effects of GLP-1 receptor agonists beyond glycemia and weight loss
GLP-1 receptors are widely expressed in many tissues beyond the pancreas, including the gastrointestinal system, cardiovascular system, central nervous system and kidneys (15,38,39). Therefore, it is logical to assume that GLP-1 receptor agonists mediate multiple physiological effects, independent of their actions of improving glycemic control and stimulating weight loss (Figure 1).
Figure 1.
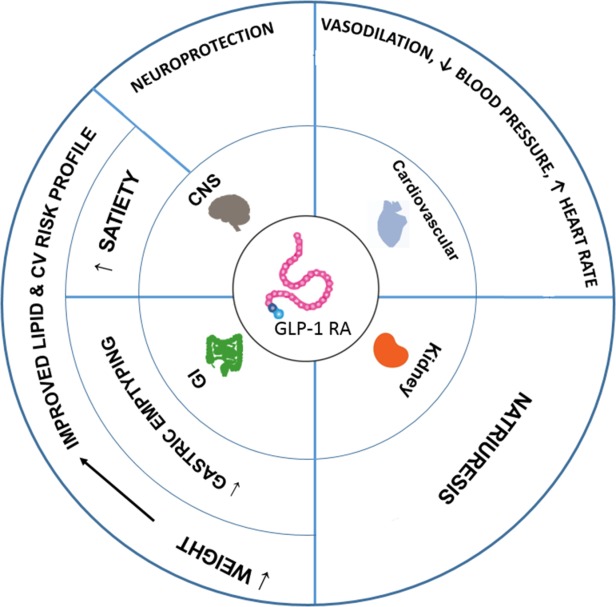
GLP-1RA: Actions beyond glycemic control (15,40,41). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Cardiovascular effects
Obesity is a substantial contributor to the risk of cardiovascular disease (CVD) (42), but the benefits of lifestyle interventions that reduce weight, although readily demonstrated to improve risk factors, have not been definitively proven to reduce the rate of cardiovascular events, with studies in different patient populations and different lengths of follow-up providing contrasting results (43,44). Certainly, a favorable effect on cardiovascular risk factors is desirable in medications used for weight loss, and there is a need for agents that have positive effects on cardiovascular risk reduction through mechanisms other than those induced by weight loss per se (44,45).
GLP-1 receptor agonists appear to positively influence the cardiovascular risk profile (with the exception of heart-rate elevation, as discussed below) by exerting a range of additional effects, both direct and indirect (16,39,40,46). A meta-analysis of clinical trials in type 2 diabetes showed that GLP-1 receptor agonists were associated with a significant reduction in the incidence of major cardiovascular events, compared with placebo and pioglitazone, and a similar effect as active comparators (sulfonylureas, insulin and dipetidyl-peptidase [DPP]-4 inhibitors) (47). A retrospective study of patients undergoing treatment for type 2 diabetes found that exenatide therapy was associated with a lower risk of cardiovascular events and hospitalizations (CVD-related and all-cause) than treatment with other glucose-lowering therapies, including metformin, alpha-glucosidase inhibitors, thiazolidinediones, sulfonylureas, DPP-4 inhibitors, and insulin (48). The authors speculate on the factors that may account for the beneficial impact of exenatide on such outcomes, including a reduction in hyperglycemia with a lower risk of hypoglycemia, and improvements in cardiovascular risk parameters (48).
Some of the cardiovascular effects observed with GLP-1 receptor agonists are likely to be related to the effects of weight loss and/or glycemic control. For example, several clinical trials have shown that GLP-1 receptor agonists improve lipid profiles in patients with type 2 diabetes through reductions in LDL cholesterol, total cholesterol, triglyceride, and free fatty acid levels (40,47).
Clinical studies have shown that GLP-1 receptor agonists reduce both systolic and diastolic blood pressure, in comparison to placebo and active controls (49,50). Although the mechanism of blood pressure reduction is unclear, it has been hypothesized that this could result from the natriuretic/diuretic effects of GLP-1 agonists on the kidney (51), their vasodilatory effect on the blood vessels (52), and/or interactions with the central nervous system (53).
Interestingly, a preclinical study in transgenic mice also suggested that the antihypertensive effects of GLP-1 receptor agonists may be linked to the release of atrial natriuretic peptide (ANP) (54). In this study, the authors demonstrated that GLP-1 receptor expression is mainly localized to the cardiac atria, and that receptor activation with liraglutide led to the secretion of ANP and reduction of blood pressure. However, a subsequent clinical study was unable to confirm the existence of this gut–heart GLP-1 receptor-dependent and ANP-dependent axis in human subjects (55).
In clinical studies, reduction in blood pressure with GLP-1 receptor agonists tends to occur early on during treatment (after 2 weeks), before significant weight loss is observed, which suggests that these agents have direct hypotensive effects, and that reduction of blood pressure is not due to weight loss alone (49,50).
GLP-1 receptor agonists have also been associated with a slight increase in heart rate, although the underlying mechanism and clinical significance of this effect remains to be determined (50). Evidence suggests that, for liraglutide, the heart-rate increases are not dose dependent, occur soon after drug administration and revert to baseline on drug cessation (16,56). The mechanism underlying the increase in resting heart rate with liraglutide, which is two to three beats per minute on average, is not currently understood, but does not appear to involve increased activation of the sympathetic nervous system, with no increase in urinary catecholamines (56). One proposed mechanism for the heart-rate elevation is that GLP-1 receptors on the sino-atrial node (38) produce the increase.
Preclinical studies suggest that GLP-1 receptor agonists can have a direct role in the prevention of atherogenesis through the modulation of vascular inflammation and improvement of endothelial dysfunction (57,58). One study in apolipoprotein E-deficient (apoE−/−) mice demonstrated that the GLP-1 receptor agonist exendin-4 suppressed the accumulation of monocyte and macrophages in the artery wall through the downregulation of various inflammatory and adhesion molecules on these cells (57). Another study in apoE−/− mice demonstrated that liraglutide inhibited atherogenesis in early-onset atherosclerotic disease, and also reduced progression of atherosclerotic plaque formation and enhanced plaque stability (58).
Vascular endothelial dysfunction has a major role in the development of atherosclerosis, usually preceding its development. A clinical study involving 28 subjects with recent-onset type 2 diabetes with impaired glucose tolerance demonstrated that administration of exenatide improved postprandial endothelial dysfunction following a high-fat meal (59). Although part of this improvement was due to the reduction of postprandial triglyceride levels, the authors concluded that exenatide may also improve endothelial function through other mechanisms (59).
There is evidence from preclinical and clinical studies that GLP-1 and GLP-1 receptor agonists have cardioprotective benefits. Several animal models and clinical studies have demonstrated that administration of GLP-1 improves outcomes following cardiac injury (60–62). In a canine model of pacing-induced advanced dilated cardiomyopathy, a 48-h infusion with recombinant GLP-1 dramatically improved left ventricular performance and systemic hemodynamics. These improvements were associated with increased myocardial glucose uptake (60). A small clinical study in patients with myocardial infarction and left ventricular dysfunction following angioplasty found that a 72-h infusion with GLP-1, in combination with standard therapy, significantly improved left ventricular function (61). Another clinical study involving patients with chronic heart failure found that a 5-week GLP-1 infusion, in combination with standard therapy, significantly improved left ventricular function, functional status and quality of life, compared with standard therapy alone (62).
In a mouse model of myocardial infarction, 7 days of liraglutide treatment (200 µg kg−1, twice daily) prior to myocardial infarction increased survival by 57% (P = 0.0001), reduced cardiac rupture and infarct size (20.9% ± 1.7% vs. 28.8% ± 3.3%; P = 0.02), and improved cardiac output (12.4 ± 0.6 vs. 9.7 ± 0.6 mL min−1; P < 0.05), compared with 7 days of treatment with saline (63). Administration of liraglutide was also found to induce expression of several cardioprotective proteins in the mouse heart. Another study conducted in rats showed that the exenatide analogue AC3174 also increased survival and improved cardiac function, postmyocardial infarction (64). Furthermore, rodent studies have demonstrated that lixisenatide reduces infarct size when used in the acute treatment setting, and also improves cardiac function when administered long-term, following ischemia-reperfusion injury (65).
Few clinical studies have evaluated the cardioprotective effects of GLP-1 receptor agonists in humans. One study, conducted in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention, found that administration of exenatide at the time of reperfusion increases the myocardial salvage index (a measure of cardioprotective effectiveness, calculated as the difference between the myocardial area at risk [AAR] and the final infarct size, divided by the AAR) by 15%, compared with placebo (0.71 ± 0.13 vs. 0.62 ± 0.16; P = 0.003) (66).
The mechanisms through which GLP-1 and GLP-1 receptor agonists provide cardioprotective effects are complex and not fully understood. There is evidence from animal studies that GLP-1 exerts direct effects on the myocardium, such as improvements in glucose uptake and metabolism (60), but it is not clear if their actions are mediated dependently or independently of the GLP-1 receptor. The precise localization of GLP-1 receptors in the human heart is a matter of debate, but evidence from recent studies suggest they are predominantly located in the sino-atrial node (38,54).
GLP-1 receptor agonist therapy is associated with improvements in levels of cardiovascular risk biomarkers (67,68). One study in patients with type 2 diabetes found that liraglutide was associated with significant reductions of serum plasminogen activator inhibitor-1 (PAI-1), brain natriuretic peptide (BNP), and a dose-dependent reduction in high-sensitivity C-reactive protein (hsCRP) (67). Liraglutide treatment did not significantly affect levels of adiponectin, leptin, interleukin-6, or tumor necrosis factor-alpha (67). Another study in patients with type 2 diabetes showed that exenatide improved the profile of circulating biomarkers of cardiovascular risk: 1 year of exenatide treatment was associated with a 12% increase in adiponectin levels and a 61% reduction in hsCRP, and these changes were reported to be independent of weight loss during the study (68).
While the results from studies showing cardiovascular benefits with GLP-1 receptor agonists are promising, they mainly derive from preclinical models and short clinical trials, and so require validation in longer-term clinical studies and outcome trials. Indeed, several ongoing studies are evaluating the longer-term effects of GLP-1 receptor agonists on cardiovascular outcomes (69), including lixisenatide (ELIXA study), liraglutide 1.8 mg (LEADER study), exenatide (EXSCEL study) and dulaglutide (REWIND study) (70).
Furthermore, many of the clinical studies were carried out in patients with type 2 diabetes and require confirmation in additional patient populations, including individuals with prediabetes, obesity, hypertension, or dyslipidemia.
Potential neuroprotective effects
There is growing evidence supporting a link between obesity and diabetes and neurodegeneration/cognitive impairment (71,72). The increased incidence of Alzheimer's disease and Parkinson's disease in patients with type 2 diabetes suggest that common mechanisms and/or pathways of cell death underlie these conditions (73).
GLP-1 receptors are expressed by neurons in several areas of the brain, including the pyramidal neurons in the hippocampus and neocortex, and the Purkinje cells in the cerebellum (74). Studies in mice models suggest that many of the new GLP-1 receptor agonists have the ability to cross the blood−brain barrier (75,76), making them attractive therapeutic options for the treatment of neurodegenerative central nervous system disorders (74).
Several studies have demonstrated the neuroprotective effects of GLP-1 receptor (75) agonists in animal models of Alzheimer's disease (41,75,77). For example, one study in the amyloid precursor protein/presenilin-1 (APP/PS1) mouse model showed that liraglutide prevented memory impairments in object recognition (75). Treatment with liraglutide restored the ability of APP/PS1, but not wild-type, mice to distinguish between novel and familiar objects, with the overall difference scores vs. saline-treated controls significantly increased (Student's t test, P = 0.0495) (75). In the same study, liraglutide prevented synapse loss and deterioration of synaptic plasticity in the hippocampus (75). Liraglutide also reduced the number of amyloid plaques in the brain, and decreased the inflammatory response by ∼50% (75). In the same mouse model, the GLP-1 receptor analog Val8-GLP-1 was shown to prevent or delay age-related synaptic neurodegenerative processes (77). Furthermore, exenatide has also demonstrated neuroprotective effects by reducing the amount of amyloid-beta peptide accumulation in the 3xTg-AD mice model (78).
A single-blind, randomized clinical study evaluating treatment with exenatide or usual medications (control group) in 45 patients with Parkinson's disease has also been published. Although this study was not placebo-controlled, blinded ratings showed clinical improvement in exenatide-treated patients, compared to controls (79). The potential clinical benefits for Alzheimer's and Parkinson's disease patients receiving GLP-1-based therapies is not yet known, although a few clinical studies are investigating this (74).
Effects on kidney function and disease
GLP-1 receptor agonists exert several effects on kidney function, some of which are potentially renoprotective (51,80). GLP-1 receptors have been found in kidney tissue from several types of animal, including rodents (81,82), pigs (83) and cattle (84). In rats and pigs, expression has been detected in the proximal tubular cells (82,83). GLP-1 receptors have also been detected in human and monkey kidney, although renal expression appears to be restricted to smooth muscle cells in the walls of arteries and arterioles (38).
Several studies have demonstrated that GLP-1 promotes natriuresis and diuresis in animal models (85) and humans (86). Moreover, rodent studies have confirmed that GLP-1 receptor agonists also exert these effects (54,87). Sodium excretion is most likely mediated via the inhibition of the Na+/H+ ion exchanger isoform 3 (NHE3) in the proximal tubule, and may contribute to the antihypertensive effects of GLP-1 receptor agonists (88).
It is well established that obesity is a risk factor for a range of comorbid conditions, including type 2 diabetes and hypertension, both of which contribute to the development of chronic kidney disease (CKD) (89). In the US, diabetic nephropathy is the leading cause of CKD (90). Preclinical studies indicate that the use of GLP-1 receptor agonists reduce risk factors of diabetic nephropathy, thereby offering renoprotection (80). Various diabetic rodent models have demonstrated that administration of GLP-1 receptor agonists inhibits the development of hypertension, reduces urine albumin levels, and leads to histological improvements in renal morphology (91–94).
Although some of these renoprotective benefits may have been the result of the improved glycemic control and/or weight loss, it is likely that GLP-1 receptor agonists also have direct effects on renal function. For example, GLP-1 appears to offer renoprotection through its actions on systemic blood pressure and kidney hemodynamics (80). One small clinical study demonstrated that a 3-h GLP-1 infusion decreased glomerular filtration rate (GFR) by 6% in obese, insulin-resistant men, but did not affect GFR in healthy men (86). The authors concluded that reduction of glomerular hyperfiltration was related, via tubuloglomerular feedback, to the direct effect of GLP-1 in promoting sodium excretion in the renal tubule. Obesity and type 2 diabetes are often associated with increased tubular sodium resorption, which may lead to hypertension (86). The findings of this study, together with data from rodent studies, suggest that the actions of GLP-1 and GLP-1 receptor agonists may be more prominent in individuals with obesity/diabetes than in healthy individuals, and protect the kidney damage from the effects of volume expansion, glomerular hyperfiltration, and systemic hypertension (86,88).
Rodent models have also demonstrated that administration of GLP-1 receptor agonists decreases inflammatory cytokines and pro-fibrotic factors associated with the development of nephropathy, including transforming growth factor beta 1 (TGF-β1) and fibronectin (91–93). Clinical studies in patients with type 2 diabetes have also reported reductions of inflammation markers with GLP-1 receptor agonists (95).
Not all the effects of GLP-1 agonists on renal function are beneficial, however, and there have been some case reports of acute kidney injury with these agents (51). Exenatide is primarily eliminated via the kidneys and is not recommended for use in patients with severe renal impairment or end-stage renal disease, while caution is advised during treatment initiation in patients with moderate renal disease (9).
Liraglutide is not eliminated via the kidneys or liver, but completely degraded within the body (most likely by DPP-4 and neutral endopeptidase), as evidenced by the lack of intact liraglutide excreted in the urine and feces (96). The US label for liraglutide use in diabetes advises that it be used with caution in patients with type 2 diabetes and renal impairment, due to limited therapeutic experience in this population (11). In Europe, no dose adjustment of liraglutide 1.2 or 1.8 mg is required in patients with type 2 diabetes with mild or moderate renal impairment (creatinine clearance [CrCl] 60–90 mL min−1 and 30–59 mL min−1, respectively) (97). There is no therapeutic experience of liraglutide 1.2 or 1.8 mg in patients with type 2 diabetes with severe (CrCl <30 mL min−1) renal impairment, and liraglutide cannot currently be recommended for such patients or those with end-stage renal disease (97).
There are limited data on albiglutide in severe renal impairment and, in Europe, it is not recommended for this condition (98). Also, the frequency of gastrointestinal events increases as renal function declines, and the albiglutide US label advises caution when using dose escalations in patients with renal function impairment (99). Lixisenatide is primarily cleared through the kidney (100). In Europe, lixisenatide is not recommended for use in subjects with severe renal impairment and caution is advised if subjects have moderate renal impairment (100).
Effects on nonalcoholic fatty liver disease
GLP-1 receptor agonists may have beneficial effects on nonalcoholic fatty liver disease (NAFLD), a condition that is increasing in parallel with the global obesity epidemic (101). While therapeutic experience in patients with hepatic impairment is currently too limited to recommend the use in such patients (97), studies are ongoing to evaluate the efficacy of liraglutide 1.8 mg in patients with nonalcoholic steatohepatitis (NASH) (102). In a small observational study, Japanese subjects who failed to reach HbA1c levels of less than 6.0% and/or alanine aminotransferase (ALT) levels lower than baseline levels following 24 weeks of lifestyle modifications (Stage 1) were treated with 0.9 mg day−1 liraglutide (the approved dose in Japan) for an additional 24 weeks (Stage 2) (103). Some patients completed a further 96 weeks treatment with liraglutide (Stage 3). At the end of Stage 2, liraglutide treatment was associated with significant improvements compared with the end of Stage 1 in BMI, visceral fat area, and liver function parameters. At the end of Stage 3, improvements in inflammation, fibrosis, and NAFLD activity score were indicated with liraglutide treatment (103). While the mechanisms by which liraglutide may improve NASH remain to be elucidated, the authors of this study reported correlations between the improvements and reductions in bodyweight and HbA1c (103). Hepatocytes affected by excess fat deposition are more susceptible to ischemic events, such as those induced during liver surgery, heart failure, and cardiogenic shock. Preclinical data suggest that GLP-1 receptor agonists may have a role in protecting both lean and fatty livers from ischemic injury (104).
Effects on saliva, taste, and taste perception
The first GLP-1 agonist, exendin-4, was discovered in the venom and saliva of the Gila monster (105). GLP-1 is not present in human saliva (106) but is expressed in taste buds, especially in type II and III taste cells in rodents and macaques (107). In type II taste cells, GLP-1-expressing taste cells also express α-gustducin and taste 1 receptor 3 (T1R3), whereas in type III taste cells serotonin is coexpressed (107). The GLP-1 cognate receptor is not expressed in taste cells but in the nerve endings innervating the taste bud (107). Interestingly, taste buds contain little or no DPP-IV (107).
The presence of GLP-1 and its receptor in taste buds suggests a role in taste preferences. GLP-1 receptor knockout mice display a significantly reduced response to natural (sucrose) and artificial (sucralose) sweeteners (107), and an increased response to umami (108), in comparison to wild-type controls. No significant differences in taste responses were seen for bitter, salt, or sour stimuli. These findings suggest that GLP-1 is essential to maintain or enhance the taste sensitivity to sweet stimuli and umami. Additionally, the GLP-1-mediated sweet attraction is reinforced when long-chain fatty acids stimulate the taste buds through the GPR120 lipid-sensor receptor (109).
It is interesting to note that enteroendocrine L cells have chemosensory machinery that is similar to taste cells, and stimulation of α-gustducin, T1R3 and GPR120 in enteroendocrine L cells stimulates the release of GLP-1 (110–113). Furthermore, taste chemosensors in the gut are essential for the regulation and secretion of gastrointestinal hormones, such as GLP-1, OXM, or PYY3-36. For example, in the α-gustducin knockout mouse model, GLP-1 secretion is attenuated after nutrient stimuli to the gut as well as after the rapid increase of GLP-1 following Roux-in-Y gastric bypass (RYGB) (114).
Additionally, bariatric surgery produces a rapid increase in GLP-1 and other gastrointestinal hormones (115), and recent studies have shown that RYGB changes taste preferences by decreasing the preference for sucrose, the perceived sweetness of sucrose and the cravings for sweets and fast foods. RYGB also shifts sweetness palatability from pleasant to unpleasant (116). The effect of bariatric surgery on taste preferences may be mediated by a rapid increase in GLP-1 (117); however, further studies are needed to understand these mechanisms.
The potential to exploit the presence of GLP-1 receptors on taste cells is currently unexplored, though oral GLP-1 therapy might be an avenue for therapeutic intervention to maximize diet efficacy.
Potentially unfavorable nonglycemic actions of glp-1 receptor agonists
No drug can be entirely safe and entirely effective, and this is true for GLP-1 receptor agonists. The potential for adverse renal effects with GLP-1 receptor agonists has been discussed above. Additionally, these compounds are proteins so there is the risk of antibody formation and allergic reactions: anaphylaxis and angioedema (although this is rare in this class of compounds). This topic has been reviewed recently (118), and antibody formation and injection-site reactions are reportedly more frequent for the exendin-4-based compounds (for example, exenatide and lixisenatide), compared with liraglutide. A pooled analysis of the LEAD trials (1, 2, 4, and 5) demonstrated that 8.7% and 8.3% patients had low-level antibodies to liraglutide 1.2 and 1.8 mg, respectively, following 26 weeks of therapy and levels remained low at the end of a 2-year open-label extension period (119). In LEAD-6, 61% patients treated with exenatide 10 μg for 26 weeks had antiexenatide antibodies (119). After switching from exenatide to liraglutide 1.8 mg, 50% and 17% of patients had persistent antiexenatide antibodies at weeks 40 and 78; in comparison, at week 79, only 2.6% and 3.0% of patients who continued on liraglutide or switched from liraglutide to exenatide, respectively, had antiliraglutide antibodies (119). These relatively high frequencies observed with exenatide compared with liraglutide treatment, may be explained by the difference in sequence homology versus native GLP-1, of which exenatide shares only 53% and liraglutide 97% (120).
Obesity, particularly central obesity, is a well-established risk factor for gallstone disease (121). Although infrequent, gallbladder-related adverse events (mainly cholelithiasis and cholecystitis) were observed at a higher frequency with liraglutide 3.0 mg versus placebo (56). Events occurred more frequently in female patients and those who experienced greater weight loss, both of which are associated with a higher risk of gallstone formation, and may explain why similar findings were not observed with lower doses of liraglutide in the type 2 diabetes trials (56). As the increased incidence of gallbladder-related adverse events with liraglutide 3.0 mg was seen across weight-loss categories, however, factors in addition to weight loss would appear to be involved (56).
Both type 2 diabetes and obesity, in particular abdominal obesity, has been associated with an increased risk of acute pancreatitis (122). A small number of cases of pancreatitis have been reported in patients with type 2 diabetes treated in clinical trials with GLP-1 receptor agonists (123). It has not, however, been possible to establish a cause for this relationship because type 2 diabetes, a common comorbidity in obese subjects, is associated with a three-fold increased risk of acute pancreatitis compared with controls without type 2 diabetes (124).
For several drugs in the GLP-1 receptor agonist class (albiglutide, dulaglutide, exenatide extended release, liraglutide), an FDA-approved Risk Evaluation and Mitigation Strategy (REMS) is ongoing in order to provide further information about acute pancreatitis and also medullary thyroid carcinoma. As pancreatitis is a risk factor for pancreatic cancer, concern about the potentially increased risk of such cancer has been raised. The regulatory agencies in the US and in Europe (FDA and EMA) recently issued a joint statement, which included the following paragraph:
“Both agencies agree that assertions concerning a causal association between incretin-based drugs (DPP4 inhibitors and GLP-1 agonists) and pancreatitis or pancreatic cancer, as expressed recently in the scientific literature and in the media, are inconsistent with the current data. The FDA and the EMA have not reached a final conclusion at this time regarding such a causal relationship. Although the totality of the data that have been reviewed provides reassurance, pancreatitis will continue to be considered a risk associated with these drugs until more data are available; both agencies continue to investigate this safety signal” (125).
Thus, it appears that prescribers of GLP-1 receptor agonists should be aware of the association of this class with pancreatitis, and should have a high degree of suspicion should the patient develop severe abdominal pain, with or without nausea and vomiting.
All drugs in this class carry a boxed warning on their package inserts regarding thyroid C-cell tumors. Such tumors have been observed in rodent studies with GLP-1 receptor agonists at clinically relevant exposures, although there has been no association of human C-cell tumors with these drugs. Nevertheless, the labels are required to specify a contraindication, in the case of patients with a personal or family history of medullary thyroid cancer, or with multiple endocrine neoplasia syndrome type 2.
Conclusion
GLP-1 receptors are located throughout the body, and thus are likely to mediate multiple physiological effects, beyond glycemic control and weight loss. There is increasing evidence from preclinical and clinical studies to suggest that these agents may have a spectrum of beneficial effects, some of which may occur independently of glycemic effects and weight loss. Conversely, there may be some unfavorable effects of GLP-1 receptor agonists, but prescribers should be able to manage these medications by monitoring their patients and by prescribing judiciously.
GLP-1 receptor agonists may reduce the risk of cardiovascular disease through their effects on blood-pressure reduction and the prevention of atherosclerosis, and they may also have a cardioprotective role. The heart-rate elevation may not be an adverse prognostic indicator, but further study is needed to determine the underlying mechanism driving this phenomenon. Cardiovascular outcome trials will, in any event, be the ultimate determinant of overall cardiovascular benefit or risk. There is evidence that GLP-1 receptor agonists may have neuroprotective and renoprotective effects, although consideration of the latter should be balanced with reports of potentially adverse effects in the kidney, especially with exenatide.
Given their potential benefits beyond glycemic control and weight loss, GLP-1 receptor agonists may enhance the treatment of type 2 diabetes and obesity in the future. However, additional clinical studies are needed to further and fully elucidate the pleiotropic effects and potential benefits of these agents. Finally, clinicians must prescribe carefully to avoid potentially harmful effects, while providing opportunity for health improvement in their patients with type 2 diabetes and other obesity-related morbidities.
References
- 1.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Holst JJ, Christensen M, Lund A, et al. Regulation of glucagon secretion by incretins. Diabet Obes Metab. 2011;13(Suppl 1):89–94. doi: 10.1111/j.1463-1326.2011.01452.x. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American diabetes association (ADA) and the European association for the study of diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 4.Gerich J. Pathogenesis and management of postprandial hyperglycemia: role of incretin-based therapies. Int J Gen Med. 2013;6:877–895. doi: 10.2147/IJGM.S51665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 6.Committee for Medicinal Products for Human Use (CHMP) EMA. Assessment Report: Eperzan (albiglutide) 23 January 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002735/WC500165119.pdf. Last accessed February 2015.
- 7.Highlights of Prescribing Information: Trulicity (dulaglutide) Available at: http://pi.lilly.com/us/trulicity-uspi.pdf ). Last accessed February 2015.
- 8.Glaesner W, Vick AM, Millican R, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue ly2189265, an fc fusion protein. Diabet Metabol Res Rev. 2010;26:287–296. doi: 10.1002/dmrr.1080. [DOI] [PubMed] [Google Scholar]
- 9.Highlights of Prescribing Information: BYDUREON (exenatide) Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022200s000lbl.pdf ). Last accessed February 2015.
- 10.Christensen M, Knop FK. Once-weekly GLP-1 agonists: how do they differ from exenatide and liraglutide? Curr Diabet Rep. 2010;10:124–132. doi: 10.1007/s11892-010-0102-x. [DOI] [PubMed] [Google Scholar]
- 11.Highlights of US prescribing information Victoza®. 2015. Available at: http://www.novo-pi.com/victoza.pdf. Last accessed February.
- 12.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes A. Standards of medical care in diabetes—2015. Diabet Care. 2015;38:S1–S93. [Google Scholar]
- 14.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabet Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilsboll T, Garber AJ. Non-glycaemic effects mediated via GLP-1 receptor agonists and the potential for exploiting these for therapeutic benefit: focus on liraglutide. Diabet Obes Metab. 2012;14(Suppl 2):41–49. doi: 10.1111/j.1463-1326.2012.01579.x. [DOI] [PubMed] [Google Scholar]
- 16.Seufert J, Gallwitz B. The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabet Obes Metab. 2014;16:673–688. doi: 10.1111/dom.12251. [DOI] [PubMed] [Google Scholar]
- 17.Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ (Clin Res Ed) 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F, Tong Y, Su N, et al. Weight loss effect of glucagon-like peptide-1 mimetics on obese/overweight adults without diabetes: a systematic review and meta-analysis of randomized controlled trials. J Diabet. 2014 doi: 10.1111/1753-0407.12198. Jul 15. doi: 10.1111/1753-0407.12198. [DOI] [PubMed] [Google Scholar]
- 19.Holst JJ, Deacon CF. Is there a place for incretin therapies in obesity and prediabetes? Trends Endocrinol Metab. 2013;24:145–152. doi: 10.1016/j.tem.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 20.FDA. FDA approves weight-management drug Saxenda. December 23, 2014.
- 21.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2014;38:784–793. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jendle J, Nauck MA, Matthews DR, et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabet Obes Metab. 2009;11:1163–1172. doi: 10.1111/j.1463-1326.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 23.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36:843–854. doi: 10.1038/ijo.2011.158. Erratum in Int J Obes (Lond). 2013 Feb;2037(2012):2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 25.Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes. 2013;37:1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 26.Rosenstock J, Klaff LJ, Schwartz S, et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabet Care. 2010;33:1173–1175. doi: 10.2337/dc09-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly AS, Rudser KD, Nathan BM, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167:355–360. doi: 10.1001/jamapediatrics.2013.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dushay J, Gao C, Gopalakrishnan GS, et al. Short-term exenatide treatment leads to significant weight loss in a subset of obese women without diabetes. Diabet Care. 2012;35:4–11. doi: 10.2337/dc11-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies M, Bode BW, Kushner RF, et al. Liraglutide 3.0 mg for weight management in obese/overweight adults with type 2 diabetes: results from the SCALE™ diabetes 56-week randomized, double-blind, placebo-controlled trial (abstract) Am Diabet Assoc 74th Sci Sessions. 2014 A26:97-OR. [Google Scholar]
- 30.DeFronzo RA, Bergenstal RM, Bode B, et al. Effects of liraglutide 3.0 mg and 1.8 mg on body weight and cardiometabolic risk factors in overweight and obese adults with type 2 diabetes mellitus (T2DM): the SCALE diabetes randomized, double-blind, placebo-controlled, 56-week trial (abstract). 16th International Congress of Endocrinology (ENDO 2014). SAT-0930.
- 31.Lau DC, Astrup A, Fujioka K, et al. Safety and tolerability of liraglutide 3.0 mg in overweight and obese adults: the SCALE obesity and prediabetes randomized, double-blind, placebo-controlled 56-week trial (abstract). 16th International Congress of Endocrinology (ENDO 2014). SAT-0929.
- 32.Pi-Sunyer FX, Astrup A, Fujioka K, et al. Liraglutide 3.0 mg reduces the prevalence of prediabetes and delays onset of type 2 diabetes in overweight and obese adults: results from SCALE obesity and prediabetes, a randomized, double-blind and placebo-controlled 56-week trial (abstract). 16th International Congress of Endocrinology (ENDO 2014). PP05-4. SAT-0925.
- 33.Roux CL, Astrup A, Fujioka K, et al. Liraglutide 3.0 mg improves body weight and cardiometabolic risk factors in overweight or obese adults without diabetes: the SCALE obesity and prediabetes randomized, double-blind, placebo-controlled 56-week trial (abstract). 16th International Congress of Endocrinology (ENDO 2014). SAT-0937.
- 34.Wilding J, Astrup A, Fujioka K, et al. Liraglutide 3.0 mg improves insulin secretion and action in overweight and obese adults without diabetes: results from SCALE obesity and prediabetes, a randomized, double-blind and placebo-controlled 56-week trial (abstract). 16th International Congress of Endocrinology (ENDO 2014). SAT-0931.
- 35.Ahren B, Johnson SL, Stewart M, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabet Care. 2014;37:2141–2148. doi: 10.2337/dc14-0024. [DOI] [PubMed] [Google Scholar]
- 36.Raccah D, Gourdy P, Sagnard L, Ceriello A. Lixisenatide as add-on to oral antidiabetic therapy: an effective treatment for glycemic control with body weight benefits in type 2 diabetes. Diabet Metab Res Rev. 2014;30:742–748. doi: 10.1002/dmrr.2548. [DOI] [PubMed] [Google Scholar]
- 37.Umpierrez GE, Blevins T, Rosenstock J, et al. The effects of LY2189265, a long-acting glucagon-like peptide-1 analogue, in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabet Obes Metab. 2011;13:418–425. doi: 10.1111/j.1463-1326.2011.01366.x. [DOI] [PubMed] [Google Scholar]
- 38.Pyke C, Heller RS, Kirk RK, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 39.Herzlinger S, Horton ES. Extraglycemic effects of glp-1-based therapeutics: addressing metabolic and cardiovascular risks associated with type 2 diabetes. Diabet Res Clin Practice. 2013;100:1–10. doi: 10.1016/j.diabres.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Burgmaier M, Heinrich C, Marx N. Cardiovascular effects of GLP-1 and GLP-1-based therapies: implications for the cardiovascular continuum in diabetes? Diabet Med J Br Diabet Assoc. 2013;30:289–299. doi: 10.1111/j.1464-5491.2012.03746.x. [DOI] [PubMed] [Google Scholar]
- 41.Xiong H, Zheng C, Wang J, et al. The neuroprotection of liraglutide on Alzheimer-like learning and memory impairment by modulating the hyperphosphorylation of tau and neurofilament proteins and insulin signaling pathways in mice. J Alzheimer's Dis. 2013;37:623–635. doi: 10.3233/JAD-130584. [DOI] [PubMed] [Google Scholar]
- 42.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 43.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the da qing diabetes prevention study: a 23-year follow-up study. Lancet Diabet Endocrinol. 2014;2:474–480. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 44.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA J Am Med Assoc. 2014;311:74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Genugten RE, Moller-Goede DL, van Raalte DH, Diamant M. Extra-pancreatic effects of incretin-based therapies: potential benefit for cardiovascular-risk management in type 2 diabetes. Diabet Obes Metab. 2013;15:593–606. doi: 10.1111/dom.12050. [DOI] [PubMed] [Google Scholar]
- 47.Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabet Obes Metab. 2014;16:38–47. doi: 10.1111/dom.12175. [DOI] [PubMed] [Google Scholar]
- 48.Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabet Care. 2011;34:90–95. doi: 10.2337/dc10-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang B, Zhong J, Lin H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabet Obes Metab. 2013;15:737–749. doi: 10.1111/dom.12085. [DOI] [PubMed] [Google Scholar]
- 50.Robinson LE, Holt TA, Rees K, Randeva HS, O'Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3:piie001986. doi: 10.1136/bmjopen-2012-001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Filippatos TD, Elisaf MS. Effects of glucagon-like peptide-1 receptor agonists on renal function. World J Diabet. 2013;4:190–201. doi: 10.4239/wjd.v4.i5.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nystrom T, Gonon AT, Sjoholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Sivertsen J, Rosenmeier J, Holst JJ, Vilsboll T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol. 2012;9:209–222. doi: 10.1038/nrcardio.2011.211. [DOI] [PubMed] [Google Scholar]
- 54.Kim M, Platt MJ, Shibasaki T, et al. GLP-1 receptor activation and epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 55.Skov J, Holst JJ, Gotze JP, Frokiaer J, Christiansen JS. Glucagon-like peptide-1: effect on pro-atrial natriuretic peptide in healthy males. Endocrine Connect. 2014;3:11–16. doi: 10.1530/EC-13-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liraglutide 3.0 mg for Weight Management (NDA 206-321). Briefing Document. Endocrinologic and Metabolic Drug Advisory Committee, September 11, 2014 [cited 2014 24 September]. Available at: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm413318.pdf. Last accessed February 2015.
- 57.Arakawa M, Mita T, Azuma K, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaspari T, Welungoda I, Widdop RE, Simpson RW, Dear AE. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE(-/-) mouse model. Diabet Vasc Dis Res Off J Int Soc Diabet Vasc Dis. 2013;10:353–360. doi: 10.1177/1479164113481817. [DOI] [PubMed] [Google Scholar]
- 59.Koska J, Schwartz EA, Mullin MP, Schwenke DC, Reaven PD. Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabet Care. 2010;33:1028–1030. doi: 10.2337/dc09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nikolaidis LA, Elahi D, Hentosz T, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 61.Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 62.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Cardiac Failure. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 63.Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Q, Anderson C, Broyde A, et al. Glucagon-like peptide-1 and the exenatide analogue ac3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovasc Diabetol. 2010;9:76. doi: 10.1186/1475-2840-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wohlfart P, Linz W, Hubschle T, et al. Cardioprotective effects of lixisenatide in rat myocardial ischemia-reperfusion injury studies. J Trans Med. 2013;11:84. doi: 10.1186/1479-5876-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lonborg J, Vejlstrup N, Kelbaek H, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 67.Courreges JP, Vilsboll T, Zdravkovic M, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med J Br Diabet Assoc. 2008;25:1129–1131. doi: 10.1111/j.1464-5491.2008.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bunck MC, Diamant M, Eliasson B, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabet Care. 2010;33:1734–1737. doi: 10.2337/dc09-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tibble CA, Cavaiola TS, Henry RR. Longer acting GLP-1 receptor agonists and the potential for improved cardiovascular outcomes: a review of current literature. Expert Rev Endocrinol Metab. 2013;8:247–259. doi: 10.1586/eem.13.20. [DOI] [PubMed] [Google Scholar]
- 70. Available at: http://ClinicalTrials.gov.
- 71.Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 72.de la Monte SM. Relationships between diabetes and cognitive impairment. Endocrinol Metab Clin North Am. 2014;43:245–267. doi: 10.1016/j.ecl.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holst JJ, Burcelin R, Nathanson E. Neuroprotective properties of GLP-1: theoretical and practical applications. Curr Med Res Opin. 2011;27:547–558. doi: 10.1185/03007995.2010.549466. [DOI] [PubMed] [Google Scholar]
- 74.Holscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol. 2014;221:T31–T41. doi: 10.1530/JOE-13-0221. [DOI] [PubMed] [Google Scholar]
- 75.McClean PL, Parthsarathy V, Faivre E, Holscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci Off J Soc Neurosci. 2011;31:6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gengler S, McClean PL, McCurtin R, Gault VA, Holscher C. Val(8)GLP-1 rescues synaptic plasticity and reduces dense core plaques in APP/ps1 mice. Neurobiol Aging. 2012;33:265–276. doi: 10.1016/j.neurobiolaging.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 78.Li Y, Duffy KB, Ottinger MA, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimer's Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson's disease. J Clin Investig. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muskiet MH, Smits MM, Morsink LM, Diamant M. The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol. 2014;10:88–103. doi: 10.1038/nrneph.2013.272. [DOI] [PubMed] [Google Scholar]
- 81.Campos RV, Lee YC, Drucker DJ. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134:2156–2164. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- 82.Crajoinas RO, Oricchio FT, Pessoa TD, et al. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol. 2011;301:F355–F363. doi: 10.1152/ajprenal.00729.2010. [DOI] [PubMed] [Google Scholar]
- 83.Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regulat Pept. 2007;141:120–128. doi: 10.1016/j.regpep.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 84.Pezeshki A, Muench GP, Chelikani PK. Short communication: expression of peptide YY, proglucagon, neuropeptide Y receptor y2, and glucagon-like peptide-1 receptor in bovine peripheral tissues. J Dairy Sci. 2012;95:5089–5094. doi: 10.3168/jds.2011-5311. [DOI] [PubMed] [Google Scholar]
- 85.Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–1135. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 86.Gutzwiller JP, Tschopp S, Bock A, et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89:3055–3061. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- 87.Rieg T, Gerasimova M, Murray F, et al. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol. 2012;303:F963–F971. doi: 10.1152/ajprenal.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Skov J. Effects of GLP-1 in the kidney. Rev Endocr Metab Disord. 2014;15:197–207. doi: 10.1007/s11154-014-9287-7. [DOI] [PubMed] [Google Scholar]
- 89.Hall ME, do Carmo JM, da Silva AA, et al. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75–88. doi: 10.2147/IJNRD.S39739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Buren PN, Toto R. Hypertension in diabetic nephropathy: epidemiology, mechanisms, and management. Adv Chronic Kidney Dis. 2011;18:28–41. doi: 10.1053/j.ackd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hendarto H, Inoguchi T, Maeda Y, et al. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metab Clin Exp. 2012;61:1422–1434. doi: 10.1016/j.metabol.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Kodera R, Shikata K, Kataoka HU, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965–978. doi: 10.1007/s00125-010-2028-x. [DOI] [PubMed] [Google Scholar]
- 93.Park CW, Kim HW, Ko SH, et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol. 2007;18:1227–1238. doi: 10.1681/ASN.2006070778. [DOI] [PubMed] [Google Scholar]
- 94.Mima A, Hiraoka-Yamomoto J, Li Q, et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCbeta activation in diabetes. Diabetes. 2012;61:2967–2979. doi: 10.2337/db11-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chaudhuri A, Ghanim H, Vora M, et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab. 2012;97:198–207. doi: 10.1210/jc.2011-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malm-Erjefalt M, Bjornsdottir I, Vanggaard J, et al. Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metab Disposit Biol Fate Chem. 2010;38:1944–1953. doi: 10.1124/dmd.110.034066. [DOI] [PubMed] [Google Scholar]
- 97.Summary of Product Characteristics Victoza®. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf ). Last accessed February 2015.
- 98.Summary of Product Characteristics Eperzan®. 2015. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002735/WC500165117.pdf. Last accessed February.
- 99.Highlights of US prescribing information TANZEUM™. 2015. Available at: https://www.gsksource.com/gskprm/htdocs/documents/TANZEUM-PI-MG-IFU-COMBINED.PDF ). Last accessed February.
- 100.Summary of Product Characteristics Lyxumia®. 2015. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf. Last accessed February.
- 101.Samson SL, Bajaj M. Potential of incretin-based therapies for non-alcoholic fatty liver disease. J Diabet Compl. 2013;27:401–406. doi: 10.1016/j.jdiacomp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 102.Armstrong MJ, Barton D, Gaunt P, et al. Liraglutide efficacy and action in non-alcoholic steatohepatitis (LEAN): study protocol for a phase II multicentre, double-blinded, randomised, controlled trial. BMJ Open. 2013;3:e003995. doi: 10.1136/bmjopen-2013-003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eguchi Y, Kitajima Y, Hyogo H, et al. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J) Hepatol Res Off J Jpn Soc Hepatol Res. 45(3):269–78. doi: 10.1111/hepr.12351. 2015 Mar; doi: 10.1111/hepr.12351. [DOI] [PubMed] [Google Scholar]
- 104.Gupta NA, Kolachala VL, Jiang R, et al. The glucagon-like peptide-1 receptor agonist exendin 4 has a protective role in ischemic injury of lean and steatotic liver by inhibiting cell death and stimulating lipolysis. Am J Pathol. 2012;181:1693–1701. doi: 10.1016/j.ajpath.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267:7402–7405. [PubMed] [Google Scholar]
- 106.Messenger B, Clifford MN, Morgan LM. Glucose-dependent insulinotropic polypeptide and insulin-like immunoreactivity in saliva following sham-fed and swallowed meals. J Endocrinol. 2003;177:407–412. doi: 10.1677/joe.0.1770407. [DOI] [PubMed] [Google Scholar]
- 107.Shin YK, Martin B, Golden E, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martin B, Dotson CD, Shin YK, et al. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann N Y Acad Sci. 2009;1170:98–101. doi: 10.1111/j.1749-6632.2009.03920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martin C, Passilly-Degrace P, Chevrot M, et al. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of gpr120 and impact on taste sensitivity. J Lipid Res. 2012;53:2256–2265. doi: 10.1194/jlr.M025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- 111.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr. 2009;90:822s–825s. doi: 10.3945/ajcn.2009.27462T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hirasawa A, Tsumaya K, Awaji T, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through gpr120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 114.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab. 2014;3:191–201. doi: 10.1016/j.molmet.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Acosta A, Abu Dayyeh BK, Port JD, Camilleri M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut. 2014;63:687–695. doi: 10.1136/gutjnl-2013-306235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, Klein S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring) 2014;22:E13–E20. doi: 10.1002/oby.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miras AD, le Roux CW. Bariatric surgery and taste: novel mechanisms of weight loss. Curr Opin Gastroenterol. 2010;26:140–145. doi: 10.1097/MOG.0b013e328333e94a. [DOI] [PubMed] [Google Scholar]
- 118.Jespersen MJ, Knop FK, Christensen M. GLP-1 agonists for type 2 diabetes: pharmacokinetic and toxicological considerations. Expert Opin Drug Metab Toxicol. 2013;9:17–29. doi: 10.1517/17425255.2013.731394. [DOI] [PubMed] [Google Scholar]
- 119.Buse JB, Garber A, Rosenstock J, et al. Liraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the liraglutide effect and action in diabetes (LEAD) trials. J Clin Endocrinol Metab. 2011;96:1695–1702. doi: 10.1210/jc.2010-2822. [DOI] [PubMed] [Google Scholar]
- 120.Unger JR, Parkin CG. Glucagon-like peptide-1 (GLP-1) receptor agonists: differentiating the new medications. Diabet Ther Res Treat Educ Diabet Related Disord. 2011;2:29–39. doi: 10.1007/s13300-010-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 122.Sadr-Azodi O, Orsini N, Andren-Sandberg A, Wolk A. Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. Am J Gastroenterol. 2013;108:133–139. doi: 10.1038/ajg.2012.381. [DOI] [PubMed] [Google Scholar]
- 123.Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Glucagon-like peptide-1 receptor agonists and pancreatitis: a meta-analysis of randomized clinical trials. Diabet Res Clin Pract. 2014;103:269–275. doi: 10.1016/j.diabres.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 124.Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabet Care. 2009;32:834–838. doi: 10.2337/dc08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs–FDA and EMA assessment. N Engl J Med. 2014;370:794–797. doi: 10.1056/NEJMp1314078. [DOI] [PubMed] [Google Scholar]


