Abstract
Identifying processes underlying the genetic and morphological differences among populations is a central question of evolutionary biology. Forest trees typically contain high levels of neutral genetic variation, and genetic differences are often correlated with geographic distance between populations [isolation by distance (IBD)] or are due to historic vicariance events [isolation by colonization (IBC)]. In contrast, morphological differences are largely due to local adaptation. Here, we examined genetic (microsatellite) and morphological (from a common garden experiment) variation in Populus nigra L., European black poplar, collected from 13 sites across western Europe and grown in a common garden in Belgium. Significant genetic differentiation was observed, with populations from France displaying greater admixture than the distinct Spanish and central European gene pools, consistent with previously described glacial refugia (IBC). Many quantitative traits displayed a bimodal distribution, approximately corresponding to small-leaf and large-leaf ecotypes. Examination of nine climatic variables revealed the sampling locations to have diverse climates, and although the correlation between morphological and climatic differences was significant, the pattern was not consistent with strict local adaptation. Partial Mantel tests based on multivariate summary statistics identified significant residual correlation in comparisons of small-leaf to large-leaf ecotypes, and within the small-leaf samples, but not within large-leaf ecotypes, indicating that variation within the small-leaf morphotype in particular may be adaptive. Some small-leaf populations experience climates very similar to those in large-leaf sites. We conclude that adaptive differentiation and persistent IBC acted in combination to produce the genetic and morphological patterns observed in P. nigra.
Keywords: biomass, common garden experiment, European black poplar, leaf size, microsatellites
Introduction
Identifying the evolutionary processes controlling genetic structure and morphological diversity is a central aim of evolutionary biology. The genetic structures of species have been examined using putatively neutral genetic markers for decades (Brown 1978; Loveless & Hamrick 1984; Hamrick & Godt 1996). Genetic differentiation at neutral markers is typically driven by gene flow between populations, genetic drift and mutation of novel alleles (Wright 1931). Natural selection is not expected to act on neutral markers; thus, genetic structure should reflect demographic histories, that is the level of inbreeding and migration within and among populations. In plant species, the mating system and dispersal mechanism have significant effects on the levels of genetic differentiation observed among populations (Loveless & Hamrick 1984; Duminil et al. 2007). Historic vicariance events may also affect the distribution of genetic variation, especially in longer-lived species (Nason et al. 2002; Petit et al. 2003; Savolainen & Pyhajarvi 2007).
Plant morphology, in contrast, is widely considered adaptive (Turesson 1922; Westoby & Wright 2006). Phenotypic differentiation among populations reflects a balance between natural selection in the local environment, migration of alleles via gene flow (Antonovics 1968) and, at a lower frequency, the acquisition of novel traits through mutation. Natural selection within a population must be strong enough to overcome gene flow from morphologically divergent populations in order to maintain phenotypic differentiation (Kremer et al. 2012). The ever-increasing literature comparing differentiation in morphological traits and that at neutral marker loci indicate that phenotypic differentiation is typically greater than neutral genetic differentiation, implying natural selection overcomes ongoing gene flow to maintain morphological differences (Merila & Crnokrak 2001; McKay & Latta 2002; Cavers et al. 2004; Steane et al. 2006; Hall et al. 2007; Leinonen et al. 2013).
The consequences of these adaptive and neutral processes are not mutually exclusive, and natural populations are expected to experience them in combination (Cavers et al. 2004; Orsini et al. 2013). Divergent phenotypic selection may drive genetic differentiation at neutral loci (Nosil et al. 2009). Significant structure is expected at neutral loci if selection is sufficient to reduce the fitness of maladapted migrants (LeCorre & Kremer 2003; Nosil et al. 2009). Over generations, multilocus genotypes at neutral loci may become differentiated as a result of selective pressures. While correlations between allele frequencies and environment have been reported in several plant species (e.g. Kelly et al. 2003; Mitton & Duran 2004; Sork et al. 2010), isolation by adaptation (IBA), the correlation between allele frequencies and morphology while controlling for geographic separation, is rarely considered in studies across larger geographic areas. Recent literature reviews identified up to 33 studies addressing IBA in genetic differentiation, with only two focusing on tree species (Nosil et al. 2009; Orsini et al. 2013).
The well-established and widely tested process of isolation by distance (IBD) results in genetic differentiation increasing as a function of the geographic distance between populations (Slatkin 1993; Rousset 1997). That is, populations that are more distant geographically will have lower rates of gene flow, and will differentiate even in the absence of divergent selection. This contemporary IBD has recently been called isolation-by-dispersal limitation (Orsini et al. 2013) Geographic structure may result in morphological divergence due to genetic drift alone (Dennison & Baker 1991; Eckert et al. 1996).
For many species, an extreme geographic isolation took place at the most recent glacial maximum (ending 15 000 years before present), which was sufficient to reduce gene flow among refugia across the Northern Hemisphere. This historic vicariance resulting from subsequent postglacial migration, called isolation by colonization (IBC), is distinct from contemporary IBD and may produce different patterns of genetic differentiation (Spurgin et al. 2014). If contemporary levels of gene flow and the time since colonization are sufficient, the genetic signatures of IBC may be eroded, leaving no relationship between geographic distance and genetic differentiation. Serial colonization, however, is expected to produce a persistent correlation between genetic and geographic distance at both neutral and adaptive loci (Orsini et al. 2013), even in the absence of local adaptation. Recolonization patterns following glacial melting have been well described in forest trees using chloroplast markers (Kremer et al. 2002; Petit et al. 2002; Palme et al. 2003; Cottrell et al. 2005). While historic genetic signatures are well studied, morphological differentiation among recolonized populations is rarely considered a consequence of historic phenotypes of the ancestral gene pools. Rather, even in long-lived forest trees, phenotype is expected to be a consequence of local adaptation (i.e. correlate to climatic differences and not geographic distance) (Gailing et al. 2012), and not reflect the historic differences (Kremer et al. 2002).
Populus nigra L., the European black poplar, is a riparian tree widely distributed across Europe and into Asia and northern Africa. Likely restricted to three glacial refugia in western Europe during the most recent glacial maximum (Cottrell et al. 2005), chloroplast haplotype patterns identified two major and one minor routes taken by P. nigra in recolonizing central Europe. Populations in France were most likely colonized from the Iberian refuge, while populations in Germany, the Lowlands, and eastward into central Europe probably arose from refugia in the Italian peninsula and Balkan region. Today, P. nigra is recognized as both ecologically and economically important in Europe. As a keystone species, P. nigra colonizes newly disturbed riparian sites and provides critical habitat in these human-impacted environments. Due to the modification of major rivers across Europe and its natural patchy preferred habitat, P. nigra is restricted to isolated populations across the landscape. As a fast-growing tree, P. nigra is the focus of breeding and management programmes for wood and biofuel production aimed at identifying genetic variants involved in growth (Fabbrini et al. 2012).
Across its range, P. nigra displays remarkable phenotypic variation. Trees from central Europe display high growth rates, large central stems and large leaf sizes, and consequently high biomass production. Black poplar trees from regions having hot and dry Mediterranean summers produce smaller leaves and a branching growth habit, likely partial adaptations to seasonal drought (Viger 2011). A better understanding of the evolution of morphological variation and genetic structure in P. nigra will assist breeding and conservation efforts.
This study tested for correspondence between genetic structure, morphological variation and climatic differences in P. nigra genotypes sampled from 13 populations from western Europe and grown in a common garden study. We tested for IBD and IBA by comparing neutral genetic markers, phenotypic traits and climate differences between sampling sites. Genetic differentiation was examined using a panel of nine microsatellite markers, and morphological variation was assessed for 12 traits in the common garden study. We then used multivariate analyses and full and partial Mantel tests to examine the relative roles of geographic and adaptive isolation in determining the genetic structure of a keystone forest tree.
Materials and methods
Study system and collections
Populus nigra L. is restricted to riparian habitat, resulting in a patchy occurrence across the broadest landscape scale. Unlike the canonical climax forest tree species, P. nigra is fast-growing, early successional, and dioecious, with male and female flowers occurring on separate plants. Samples were collected from 13 natural populations of P. nigra from France, Germany, Italy, the Netherlands and Spain (Rohde et al. 2011; Table 1, Fig.1). To describe the environmental differences experienced by each population, a variety of meteorological data was gathered for each collection site: four measures of temperature: mean annual temperature (MATemp, °C), temperature seasonality (standard deviation ×100, SDTemp), maximum temperature of the warmest month (MaxTemp, °C) and minimum temperature of the coldest month (MinTemp, °C); four measures of precipitation: mean total annual precipitation (MAPpt, mm), precipitation seasonality (a coefficient of variation; VarPpt), precipitation of the wettest month (MaxPpt, mm) and precipitation of the driest month (MinPpt, mm); and the maximum day length on the summer solstice (MaxDay, h). Temperature and precipitation data were taken from http://www.worldclim.org (Hijmans et al. 2005), a collection of data from the years 1950 to 2000. Maximum day length was from the U.S. Naval Observatory Astronomical Applications Department (http://aa.usno.navy.mil/data/docs/RS_OneDay.php) and served as a proxy for latitude.
Table 1.
Populus nigra populations sampled for phenotypic analysis in a common garden study
| Abbrev. | Country | Population | Latitude | Longitude | NM | NG |
|---|---|---|---|---|---|---|
| FR1 | France | Drôme 1 | 44.6833 | 5.4000 | 63 | 31 |
| FR2 | France | Drôme 6 | 44.7500 | 4.9167 | 63 | 45 |
| FR3 | France | Durance | 43.7847 | 5.5569 | 12 | 9 |
| FR4 | France | Loire East | 47.3295 | 2.9200 | 26 | 6 |
| FR5 | France | Loire West | 47.2571 | −0.5870 | 21 | 13 |
| GE | Germany | Kuhkopf | 49.8167 | 8.5000 | 56 | 44 |
| IT1 | Italy | La Zelata | 45.2667 | 8.9833 | 63 | 41 |
| IT2 | Italy | Siro Negri wood | 45.2000 | 9.0667 | 44 | 32 |
| NE1 | The Netherlands | Ijssel, Rhine | 52.2250 | 5.9600 | 31 | 23 |
| NE2 | The Netherlands | Individual clones | 51.3583 | 4.5500 | 7 | 5 |
| NE3 | The Netherlands | Waal, Maas | 51.8194 | 5.0900 | 12 | 9 |
| SP1 | Spain | Ebro 1 | 41.9333 | −1.3833 | 54 | 20 |
| SP2 | Spain | Ebro 2 | 41.5833 | −1.000 | 60 | 30 |
Latitude and longitude provided in dd.dddd.
NM = number of genets analysed for morphological traits, 512 in total.
NG = number analysed for genetic markers, 308 in total.
Fig. 1.
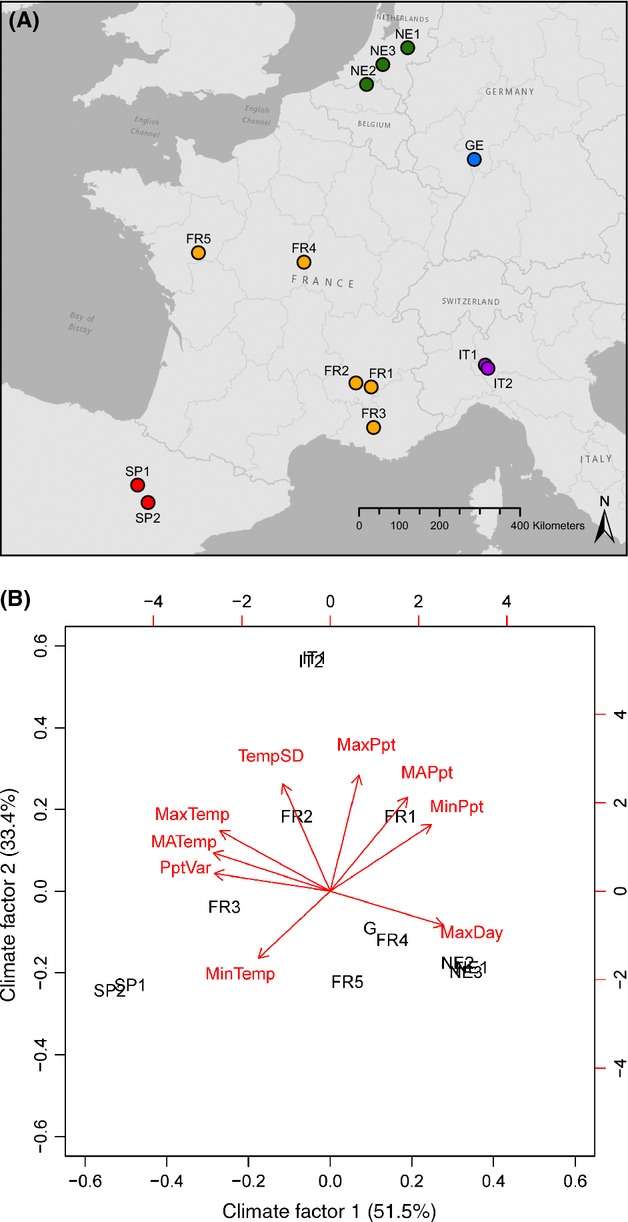
(A) Locations of Populus nigra populations sampled in Spain (red), France (orange), the Netherlands (green), Germany (blue) and Italy (purple) for the common garden study. Abbreviations follow Table 1. (B) Principal components analysis of nine climatic variables (following Table 2) reveal heterogeneous environments at the 13 sampling locations. Red arrows and text indicate the loadings of each climate variable, while black text indicates the relative climate of each sampling site.
To identify the most informative but least correlated variables from the climate data set, a principal component analysis (PCA) was conducted as a means of data reduction as implemented in the r programing language (version 3.0.2) using the package stats. The first two climate components were compared to morphological (discriminant factors) and genetic (principal coordinates) differences among populations. Correlation between climatic differences and geographic distances between populations was examined using Mantel tests as implemented in genalex v. 6 (Peakall & Smouse 2006).
Microsatellite analyses: resolving neutral genetic structure
A subset of genotypes from each population (N = 308 in total) were analysed for neutral nuclear markers (Table 1). Leaves from each genotype in the common garden experiment were sampled, flash frozen in liquid nitrogen and stored at −80 °C until processed. DNA extraction and microsatellite analyses followed standard procedures as detailed in the Methods S1 (Supporting information). A panel of nine biparentally inherited, codominant microsatellite markers was assayed for each sample: PMGC_14, PMGC_486, PMGC_2088, PMGC_2163, PMGC_2818 and PMGC_2879, from the Populus Molecular Genetics Cooperative (http://www.ornl.gov/sci/ipgc/ssr_resource.htm); and WPMS_14, WPMS_18 and WPMS_20 (Smulders et al. 2001).
Six standard measures of genetic diversity were assessed for each population: percentage of polymorphic loci (P), mean alleles per locus (A), effective number of alleles (Ae), observed (Ho) and expected (He) heterozygosity and the fixation index over loci (F), as implemented by GenAlEx v6 (Peakall & Smouse 2006). The presence of null alleles was assessed using the program microchecker (van Oosterhout et al. 2004) as described by DeWoody et al. (2006). Evidence of a recent genetic bottleneck was assessed for each population using the Wilcoxon sign rank test for heterozygosity excess under the two-phased model of mutation as implemented by the program bottleneck (Luikart & Cornuet 1998; Luikart et al. 1998). Due to insufficient sample sizes, populations FR3, NE2 and SP1 were omitted from the microchecker analysis and populations FR3, FR4 and NE2 from the bottleneck analysis. Overall genetic differentiation was quantified as ΦPT through the analysis of molecular variance (amova), and as individual locus FST, as implemented by genalex v. 6 (Peakall & Smouse 2006).
Admixture was assessed using structure v2.3.3 (Pritchard et al. 2000; Falush et al. 2003). The likelihood of the observed data fitting a model of K genetic groups for the set K = {1:13} was estimated over five simulation runs, using a burn-in of 50 000 followed by 500 000 replications. Correlation in allele frequencies was allowed, and the remaining parameters were set to the default values. Two methods were used to infer the most likely value of K. First, the delta-K method of (Evanno et al. 2005) was applied using the Web-based tool structure harvester (Earl & vonHoldt 2012). Second, the distribution of the log-likelihoods was examined for the value with the highest probability and lowest variance.
To summarize the genetic differences over multilocus microsatellite genotypes, the pairwise genetic distances among individuals (Peakall et al. 1995; Smouse & Peakall 1999) were subjected to a principal coordinates analysis (PCoA) based on the covariance matrix with data standardization as implemented by genalex v6 (Peakall & Smouse 2006).
Phenotypic measures: quantifying differences in morphology
In the spring of 2004, a common garden was established consisting of 512 genets planted as hardwood cuttings in six replicate, randomized blocks in a plantation located near the Institute of Forestry and Game Management near Geraardsbergen, Belgium (50.77N, 3.87E). Plantings were established on a grid with 0.75 × 2.0 m spacing, surrounded by a double row of the Populus cultivar ‘Muur’ to minimize edge effects. Once established, trees were cut back in early 2005 and regrowth pruned to a single dominant stem in June 2005. Site management, carried out by the local collaborators, included mechanical weed removal and fungicide application (Rohde et al. 2011). No irrigation or fertilizer was provided during the experiment. The number of replicates was reduced to three or four for the Spanish samples due to ramet mortality and labelling inconsistencies which were resolved using microsatellite data.
In the third year of growth (2006), each ramet of P. nigra in the common garden was examined for 12 morphological characteristics: five leaf traits, two biomass traits and five cell traits. Leaf measures included leaf area, leaf length, leaf width, leaf length:width ratio (LL_LW) and specific leaf area (SLA). Biomass traits were measured as stem height and circumference at the start of the 2006 growing season. Cellular traits were measured from epidermal imprints and included epidermal cell area (mm2), stomatal number, stomatal density (SD), stomatal index (SI) and cell number per leaf (CN). Detailed methods are provided in the Methods S1 (Supporting information).
To explore the variation in individual morphological traits, differences among population means were tested for each trait (y) using the model y = ax + by + ɛ, where ax is the population mean, by is the genotype nested within each population, and ε is the error. Scores for LL_LW were arcsine-transformed, and SD were log-transformed prior to analyses. This linear mixed model was implemented over genotype means using the lme function provided in the nlme package in r (R Project for Statistical Computing, Inc.) In addition, phenotypic correlations were estimated for each pair of traits using spss (v. 15.0.0; SPSS, Inc., Chicago, IL, USA).
To assess overall morphological differentiation of P. nigra populations for use in the IBA analyses, a discriminant analysis was performed on the genotype means of each trait measure. Those factors significantly contributing to differences between populations were used to visualize overall morphological differences between individuals and populations. Discriminant analyses were implemented using the Factor Analysis and Classification Analysis functions in spss (v. 15.0.0; SPSS, Inc.).
Tests for IBD and IBA
Four matrices were built to describe differences between populations of P. nigra: (i) genetic differences calculated as the Euclidian distance between the population means for the first two coordinates of the PCoA, (ii) morphological differences assessed as the Euclidian distance between the population means for the first two factors of the discriminant classification analysis, (iii) climate differences calculated as the Euclidian distance between the population means for the two climatic variables from the PCA and (iv) geographic distance from the latitude and longitude of the site of sample collection. To assess IBD, IBA and infer IBC, each response matrix (genetic and morphological differences) were compared to the two predictor matrices (climate differences and geographic distance) using simple Mantel tests (Smouse et al. 1986; Manly 1997). The simple Mantel tests were performed in genalex v. 6 (Peakall & Smouse 2006), with significance determined via permutation tests.
To test for IBA directly, correspondence between pairwise genetic, morphological and geographic distances was assessed using partial Mantel tests The first tested for IBD as the correlation between genetic and geographic differences when controlling for morphological differences (Gen, Geo ¦ Morpho). The second tested for IBA as the correlation between morphological and genetic differences when controlling for geographic distances (Gen, Morpho ¦ Geo). Partial Mantel tests were implemented using the Isolation by Distance Web Service v. 3.15 (Jensen et al. 2005), with significance determined via permutation tests. Where significant IBA was observed, regression analysis was used to describe the relationship of the residual morphological values vs. the residual genetic values (the partial regression plots) for all pairs of populations, comparisons of small-leaf to small-leaf populations, large-leaf to large-leaf populations, and small-leaf to large-leaf populations (Moya-Larano & Corcobado 2008), with significance determined using anova. All regression analyses were performed in spss v17 (SPSS, Inc.).
Results
Climate differences among sampling sites
The eight climate variables and day length varied across the sampling sites (Table 2). The principal components analysis revealed distinct climates based on the minimum temperature of the coldest month (MinTemp), the seasonality of temperature (SDTemp), mean precipitation of the wettest month (MaxPpt), and the maximum day length (MaxDay) (Fig.1). The first two components accounted for 84% of the variance in the data. These measures distinguished the sites in Spain, in Italy and in the Netherlands, with sites in France and Germany intermediate.
Table 2.
Climatic variables at 13 sites sampled for Populus nigra
| Pop. | MAPpt | VarPpt | MaxPpt | MinPpt | MATemp | SDTemp | MaxTemp | MinTemp | MaxDay |
|---|---|---|---|---|---|---|---|---|---|
| FR1 | 890 | 15 | 83 | 48 | 10.3 | 6259 | 25.7 | −2.4 | 15.6 |
| FR2 | 840 | 20 | 95 | 41 | 12.4 | 6357 | 28.1 | 0 | 15.6 |
| FR3 | 688 | 26.4 | 85 | 23.6 | 12.8 | 5945 | 27.4 | 0.6 | 15.5 |
| FR4 | 707 | 11.3 | 72 | 47.7 | 11 | 5708 | 24.8 | −0.1 | 16 |
| FR5 | 711 | 17.5 | 77 | 44.3 | 11.6 | 5145 | 24.4 | 1.6 | 16 |
| GE | 590 | 19 | 65 | 36.1 | 9.9 | 6525 | 24.5 | −1.8 | 16.3 |
| IT1 | 982 | 23 | 122 | 55 | 13 | 7248 | 29 | −1 | 15.7 |
| IT2 | 966 | 23 | 121 | 55 | 13 | 7296 | 29 | −0.9 | 15.7 |
| NE1 | 774 | 15.3 | 77 | 47.3 | 9.3 | 5451 | 21.4 | −0.7 | 16.8 |
| NE2 | 802 | 14.8 | 81 | 50.3 | 9.7 | 5359 | 21.1 | 0.2 | 16.5 |
| NE3 | 791 | 14.3 | 77 | 46.9 | 9.5 | 5383 | 21.4 | −0.3 | 16.7 |
| SP1 | 439 | 26 | 56 | 20 | 14.1 | 6097 | 29.7 | 1.8 | 15.2 |
| SP2 | 365 | 31 | 53 | 17 | 13.7 | 6243 | 29.5 | 1.3 | 15.2 |
MAPpt, mean annual precipitation (mm); VarPpt, precipitation seasonality; MaxPpt, mean precipitation of the wettest month (mm); MinPpt, mean precipitation of the driest month (mm); MATemp, mean annual temperature (°C); SDTemp, seasonality of temperature; MaxTemp, maximum temperature of the warmest month (°C); MinTemp, minimum temperature of the coldest month (°C); MaxDay, maximum day length (h).
Patterns of neutral genetic structure in Populus nigra
Microsatellite variation revealed high levels of polymorphism and moderate levels of allelic diversity and heterozygosity, with no evidence of allele fixation (Table 3). Null alleles were detected in three populations at locus PMGC_2088 (IT1, freq. 0.09; NE1, freq. 0.11, NE3, freq. 0.27) and at low frequency in a single population at WPMS_14 (FR5, freq. 0.15) and WPMS_20 (IT1, freq. 0.09). Low rates of mismatches between repeated samples resulted in low error rates: 3.3% per allele or 4.6% per reaction.
Table 3.
Genetic diversity (mean number of samples, mean alleles per locus, effective alleles per locus, observed and expected heterozygosity, and fixation) observed at nine microsatellite loci in 13 populations of Populus nigra
| Population | N | A | Ae | Ho | He | F |
|---|---|---|---|---|---|---|
| FR1 | 30.2 | 9.2 | 4.9 | 0.774 | 0.770 | −0.006 |
| FR2 | 42.4 | 11.2 | 6.0 | 0.799 | 0.808 | 0.012 |
| FR3 | 5.9 | 5.4 | 4.0 | 0.727 | 0.708 | −0.057 |
| FR4 | 6.0 | 5.9 | 4.2 | 0.796 | 0.736 | −0.084 |
| FR5 | 12.1 | 7.0 | 5.0 | 0.755 | 0.751 | −0.012 |
| GE | 42.0 | 9.0 | 4.3 | 0.767 | 0.743 | −0.026 |
| IT1 | 39.0 | 10.7 | 5.6 | 0.787 | 0.797 | 0.019 |
| IT2 | 30.4 | 9.6 | 6.1 | 0.817 | 0.802 | −0.021 |
| NE1 | 22.4 | 8.2 | 4.8 | 0.813 | 0.777 | −0.049 |
| NE2 | 5.0 | 5.2 | 4.2 | 0.867 | 0.749 | −0.168 |
| NE3 | 8.7 | 5.0 | 3.5 | 0.735 | 0.693 | −0.054 |
| SP1 | 15.3 | 6.1 | 4.2 | 0.640 | 0.719 | 0.071 |
| SP2 | 27.6 | 8.4 | 4.8 | 0.734 | 0.738 | 0.005 |
| Overall | 22.1 | 7.8 | 4.7 | 0.770 | 0.753 | −0.029 |
Sign tests for heterozygosity excess at Hardy–Weinberg equilibrium compared to that expected at mutation–drift equilibrium was observed for populations IT1 (P < 0.01), NE3 and SP1 (P < 0.05), indicating these populations have undergone a recent population bottleneck. Results for populations NE3 and SP1 may be influenced by the small sample size in this analysis. The bottleneck in population IT1 may have contributed to increased inbreeding and homozygosity identified as possible null alleles (above).
Overall genetic differentiation was significant (ΦPT = 0.120, P < 0.001), indicating gene flow among populations of P. nigra is restricted across western Europe. Per-locus estimates ranged from 0.044 to 0.118 (mean = 0.072, standard deviation = 0.021) (Supporting Information).
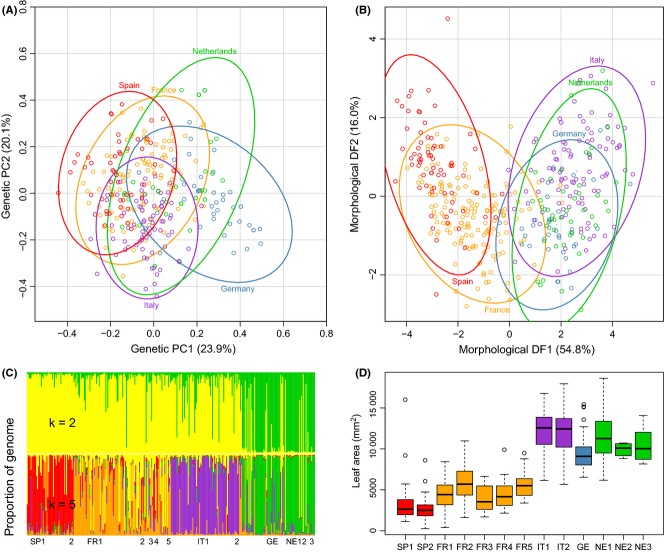
The number of genetic clusters (K) identified by the admixture analyses varied with the method of inference used. The structure harvester method of Evanno et al. (2005) identified K = 2 as most likely. Individual assignment tests over two genetic groups indicated the collections from Spain, France and Italy were distinct from trees from Germany and the Netherlands (Fig.2C). As experience indicates the Evanno method may be overly conservative in species with significant gene flow, to identify the mostly likely biologically meaningful value of K, direct examination of likelihood ratios indicated the tests for K = 5 genetic clusters resulted in the highest likelihood without an increase in variance (Fig. S1, Supporting information). Individual assignment tests to five genetic groups roughly correspond to country of origin (Fig.2C), with the samples from Germany and the Netherlands assigned to the same genetic cluster and samples from France displaying the greatest admixture.
Fig. 2.
(A) Principal coordinates analysis of microsatellite data reveals patterns of differentiation among populations of Populus nigra. (B) Discriminant classification analysis of 12 morphological traits reveals significant geographic structuring to phenotypic variation in Populus nigra. Each point represents a single genet, with colour corresponding to country of origin. Ellipses represent the 95% confidence intervals for the population means. Population means for each data type were used for pairwise comparisons in full and partial Mantel tests. (C) Admixture analysis of microsatellite data identified two (top) or five (bottom) genetic groups. Assignment of individual trees (vertical bars) roughly corresponded to geographic origin, with samples from France displaying greater admixture. Position of abbreviations below indicates the order and extent of each population. (D) Morphological differentiation among populations is typified by variation in leaf area quantified in a common garden study. Each box-and-whisker plot represents the observed measures for each population, with the centre bar indicating the median value.
The PCoA revealed overall differences to be of a smaller magnitude among individual trees than populations (Fig.2A). The first two components explained a cumulative total of 44.0% of the variance among individuals. Significant overlap was observed between populations.
Phenotypic differentiation among populations
All measures of phenotype varied significantly among populations (Table 4). Several traits displayed bimodal distributions across the collection. For instance, leaf size was strongly bimodal, with samples from Spain and France having smaller leaves than those from Italy, Germany or the Netherlands (Fig.2D, Tables S1–S5, Supporting information).
Table 4.
Morphological variation in leaf, cell and biomass traits measured in a common garden study of Populus nigra from 13 natural stands in western Europe revealed significant phenotypic variation. Discriminant factor analyses were used to reduce the multivariate data into two components for isolation by adaptation comparisons
| Trait | F-ratio | P-value | Component 1 | Component 2 |
|---|---|---|---|---|
| Leaf area | F12,499 = 135.1 | <0.0001 | 0.948 | −0.179 |
| Leaf length | F12,499 = 133.7 | <0.0001 | 0.935 | −0.178 |
| Leaf width | F12,499 = 111.4 | <0.0001 | 0.938 | −0.218 |
| Leaf length:width | F12,493 = 3.265 | <0.0001 | 0.931 | −0.168 |
| Specific leaf area | F12,467 = 160.0 | <0.0001 | 0.520 | 0.697 |
| Cell area | F12,453 = 3.36 | <0.0001 | 0.487 | 0.742 |
| Cell number per leaf | F12,451 = 67.8 | <0.0001 | 0.248 | 0.660 |
| Number of stomata (abaxial) | F12,498 = 10.5 | <0.0001 | 0.923 | 0.044 |
| Stomatal density (abaxial) | F12,489 = 6.27 | <0.0001 | 0.832 | −0.205 |
| Stomatal index (abaxial) | F12,443 = 2.659 | 0.0019 | 0.878 | −0.198 |
| Height (second year) | F12,449 = 44.7 | <0.0001 | −0.288 | 0.108 |
| Circumference (third year) | F12,499 = 78.4 | <0.0001 | −0.307 | −0.467 |
| % variation | 54.8 | 16.0 |
Correlations between morphological measures were highly significant but varied within and among trait classes (leaf traits, cell traits and biomass traits; Supporting information). The discriminant factor analysis (DFA) was required to reduce the variation of the highly correlated phenotypic measures to a smaller number of orthogonal factors (Table 4). The first two components of the DFA described cumulative total of 71% of the variation and revealed significant geographic patterning in morphology. Samples from Spain were morphologically distinct from those from Italy, Germany and the Netherlands, with the French populations intermediate (Fig.2B). Leaf area was correlated with the first factor (Table 4), indicating that identifying collections as small leaf (Spain and France) or large leaf (Germany, Italy and the Netherlands) may represent the two general morphotypes (Viger 2011).
Tests for IBD, by colonization and by adaptation
Simple Mantel test identified significant correlations between the genetic, morphological, geographic and climatic matrices distance matrices (Table 5). Correlations were strongest between the morphological and geographic matrices (r = 0.582, P < 0.001), genetic and geographic matrices (r = 0.577, P < 0.001) and morphological and genetic matrices (r = 0.522, P < 0.001). Weaker but significant correlations were observed between the morphological and climate matrices (r = 0.406, P < 0.001) and the genetic and climate matrices (r = 0.399, P = 0.004).
Table 5.
Correlations between genetic (from PCoA), morphological (from discriminant factor analysis), climatic (from principal component analysis) and geographic (km) differences among 13 populations of Populus nigra tested with simple and partial Mantel tests
| Comparison | r | P-value |
|---|---|---|
| Simple Mantel tests | ||
| Genetic, Geographic | 0.633 | <0.001 |
| Genetic, Climate | 0.551 | <0.001 |
| Morphological, Geographic | 0.582 | <0.001 |
| Morphological, Climate | 0.416 | 0.002 |
| Morphological, Genetic | 0.622 | <0.001 |
| Partial Mantel tests | ||
| Genetic, Geographic | Morphological | 0.426 | <0.001 |
| Genetic, Climate | Morphological | 0.418 | 0.004 |
| Morphological | Genetic, Geographic | 0.403 | 0.004 |
| Morphological | Genetic, Climate | 0.522 | <0.001 |
Significant correlation between genetic and geographic distance, independent of morphological divergence (Gen, Geo¦Morpho), indicates that IBD has influenced the genetic structure of P. nigra in western Europe (Table 5). Genetic differences increased as a function of distance, consistent with the genetic pattern resolved by the admixture analyses. Tests using pairwise FST (Rousset 1997) measures and log-transformed data were significant and concordant (Tables S1–S5, Supporting information).
Isolation by adaptation also contributed to the genetic structure of populations, as genetic similarity increased with phenotypic similarity, even when controlling for geographic structure among populations (Gen, Morpho¦Geo). Analyses of log-transformed data were consistent (data not presented). This result indicates that genetic differentiation at microsatellite loci may be influenced by reduced gene flow between morphologically distinct populations or reflect historic vicariance due to IBC.
Examination of partial regression plots of the genetic and morphological differences between populations further revealed the putative source of IBA (Fig. 4). IBA was significant among comparisons of all pairs of populations (F1,76 = 28.1, P < 0.001; slope = 0.038, 95% CI 0.023–0.052). Categorical comparisons of small-leaf and large-leaf pairs revealed the trend to be driven by differences among the small-leaf populations. Significant positive relationships were observed in small-leaf to small-leaf comparisons (F1,19 = 17.7, P < 0.001; slope = 0.07, 95% CI 0.035–0.105), and small-leaf to large-leaf comparisons (F1,40 = 26.5, P < 0.001; slope = 0.053, 95% CI 0.032–0.074), but not large-leaf to large-leaf comparisons (F1,13 = 3.52, P = 0.083; slope = 0.02, 95% CI −0.003–0.043). Thus, the genetic and morphological variation observed in the large-leaf populations was fully explained by IBD, with gene flow decreasing as a function of distance. However, when comparing small-leaf to small-leaf, or small-leaf to large-leaf populations, the genetic differentiation between populations resulted from IBA as well, and populations are more genetically dissimilar than their geographic distance would predict.
Fig. 4.
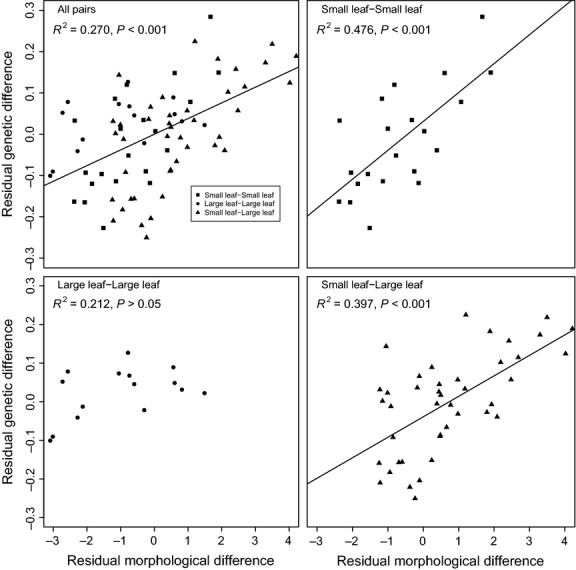
Partial regression plots illustrating correlated genetic and morphological differences between populations of Populus nigra after controlling for the effects of geographic distance. Points represent pairwise comparisons between two small-leaf populations (squares), two large-leaf populations (circles) or populations with differing morphology (triangles). Lines represent significant regressions of the residuals, with significance determined using anova. Comparisons of large-leaf to large-leaf populations revealed no significant relationship. Significance did not change after Bonferroni corrections.
Discussion
In the strictest terms, evidence of local adaptation requires that a species displays multiple morphotypes, each having higher fitness in its native habitat than the others, as confirmed through reciprocal transplant experiments (Kawecki & Evert 2004; Savolainen et al. 2013). Adaptive differentiation, however, can be described by examining patterns of genetic differences and climatic variation (Sork et al. 2010; Salmela 2014). Our study of P. nigra revealed distinct morphological and genetic variation in trees from across western Europe and related these patterns to differences in local climate at each site. We conclude that adaptive differentiation and persistent IBC acted in combination to produce the observed genetic and morphological patterns.
Leaf size, branching architecture and growth rate are all considered adaptive traits in trees and, in particular, may be linked to water availability (Dudley 1996a,b; Picotte et al. 2007; Yang et al. 2014). Species or morphotypes adapted to lower or seasonal precipitation tend to display smaller, thicker leaves, greater branching and slower growth rate in response to the environmental stress (Poorter et al. 2009; De Kort et al. 2014). In our common garden experiment, trees from Spain and France displayed small leaves, a branching architecture and smaller circumference, while trees from northern Italy, Germany and the Netherlands displayed large leaves, a straight architecture and large circumference. If these morphotypes are strictly adaptive, we would expect small-leaf trees to inhabit similar environments, and those would be distinct from climates experienced by large-leaf trees. Although the correlation between morphological difference and climatic difference was significant in P. nigra (Fig.3), the pattern did not reveal that different morphotypes inhabit different environments. Examination of nine climatic variables revealed the sampling locations to have diverse climates, with some climates in France (small-leaf populations) similar to the site in Germany (large-leaf), although climates in Spain (small-leaf), Italy (large-leaf) and the Netherlands (large-leaf) were all distinct (Fig.1).
Fig. 3.
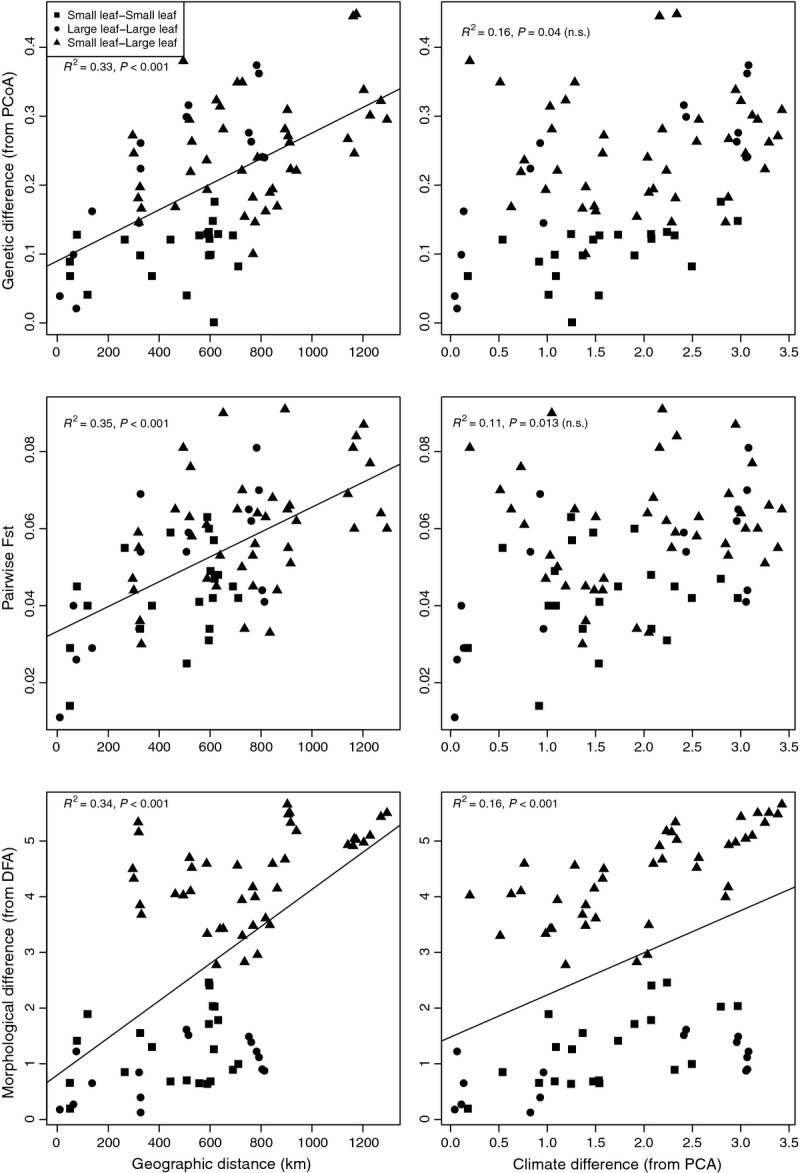
Genetic differences between populations (from PCoA, top row), allele frequency variance (FST, middle row) and morphological differences between populations (from discriminant factor analysis, bottom row) all correlate with geographic distance (left panels) more than climatic differences (right panels) between sampling sites. Note that high levels of morphological differences are observed between sites with similar climates (triangle symbols, bottom right pane). Correlations from simple Mantel tests, with significance determined using permutation tests. n.s. = P-values are nonsignificant after sequential Bonferroni corrections.
Further, if selection was strong enough to reduce successful gene flow between morphotypes, we would expect residuals from the partial correlations (i.e. IBA; Nosil et al. 2009), to be driven by comparisons of different morphotypes (e.g. small-leaf to large-leaf). The significant residual correlation in P. nigra was observed in small-leaf to large-leaf comparisons, but also in small-leaf to small-leaf comparisons, indicating that variation within the small-leaf morphotype in particular may be adaptive (Fig.4). For comparison, in a similar study of adaptive differentiation in Alnus, correlation between phenotypic and neutral genetic structure was significant but weak (adjR2cum = 0.05, P < 0.001), and no evidence was observed for IBD (De Kort et al. 2014).
Isolation by distance and IBC due to serial recolonization have also contributed to the morphological and genetic structure of P. nigra and may explain the inconsistency between morphology and climate in the populations from France. Several tree species maintain signatures of postglacial range expansions in their extant genetic structures (Vendramin et al. 2000; Petit et al. 2002; Palme et al. 2003; Tzedakis et al. 2013), although local adaptation is expected to remove these patterns from morphological traits (Cavers et al. 2004). For species existing as a metapopulation (P. nigra better fits a model of isolated populations requiring regular disturbance than the canonical climax forest tree), current genetic structure may reflect historic rather than extant demographic patterns (Orsini et al. 2008), although differences between IBD and IBC are difficult to distinguish using neutral markers (Orsini et al. 2013). If gene flow is insufficient to overcome the historic differentiation, a signature of historic vicariance may persist in the morphological differences between populations. The strongest evidence of IBC would come from comparing differences at slow-evolving or maternally inherited loci (e.g. chloroplast haplotypes), morphological differences and geographic distance. As the sole process influencing a species, IBC is expected to result in a lack of correlation between genetic differentiation and distance (IBD) or environment (IBA; Orsini et al. 2013). Although our study lacks direct tests of postglacial migration from plastid sequences, the pattern of postglacial recolonization in P. nigra has been previously described. Cottrell et al. (2005) identified up to three putative refugia and demonstrated that trees in France displayed haplotypes more similar to Iberian populations than those from central Europe. These patterns of plastid variation are generally similar to the pattern of morphological variation observed in the common garden study, where small-leaf trees from Spain and France overlapped in morphology, but were generally distinct from large-leaf trees from central Europe. There are three possible explanations for the similarities in the plastid and morphological patterns: (i) the plastid variation reflects adaptive differences and thus IBA, (ii) the morphological differences maintain a residual signature of the historic vicariance from the last glacial maximum, and (iii) the patterns are similar due to the influence of a third untested process. Given the growing literature demonstrating that plastid genomes are not strictly neutral (Bock et al. 2014), the congruence between the morphological differences and signature of recolonization described by plastid variation (Cottrell et al. 2005) may be adaptive. As with correlations between morphology and climate, if the plastid (historic) variation mirrors the selective differentiation, we would expect plants with similar morphology to inhabit similar environments, yet the diversity of climates inhabited by small-leaf populations in France (Fig.1) is inconsistent with this expectation. The morphological variation in P. nigra is consistent with the patterns of postglacial recolonization reported in a number of tree species, indicating morphology may reflect, in part, IBD due to serial colonization. However, we cannot rule out the third possibility that we have failed to identify the causative process underlying these correlations. Historic and extant population structure is gaining recognition as a potential force shaping morphological variation in forest trees.
Efforts to correlate phenotypic variation and specific climatic variables have met with mixed success. Phenological traits tend to vary with latitude or maximum day length of the origin site, consistent with strong selective pressure from growing season length on tree species (Hall et al. 2007; Rohde et al. 2011; De Kort et al. 2014; McKown et al. 2014). Evidence of phenotypic variation, especially biomass or ecophysiological traits, correlating with climatic variables related to precipitation or temperature is less common, but has been reported (Kelly et al. 2003; Royer et al. 2008; De Kort et al. 2014). Overall, these association studies demonstrate the difficulty of linking morphological variation to a single correlating (putatively causative) climate variable. Our multivariate approach allows a landscape-level comparison of population differences, providing evidence of adaptive differentiation at a broad scale (Sork et al. 2010; McKown et al. 2014).
The use of structure to infer the number of genetic clusters in a collection is an ad hoc application of the software commonly applied in population genetic studies (Pritchard et al. 2000). Multiple methods have been proposed to interpret the structure results, yet those methods often appear overly conservative (Earl & vonHoldt 2012) or liberal (Falush et al. 2003) in assigning the number of genetic clusters. Here, the Earl & vonHoldt (2012) method indicated K = 2, with the Italian samples clustering with those from France and Spain. This pattern varies from those reported in previous studies, where populations from Italy are more similar to the central European trees, and is likely a consequence of the sampling design and limited number of microsatellite data. The Italian trees formed a distinct cluster at K = 3 (data not presented). Attempting to identify biologically meaningful differences, we considered the k of the highest probability maintaining a small variance, K = 5, in which samples clustered according to country of origin, except those from Germany and the Netherlands clustered together. Samples from France were admixed between two genetic clusters. This pattern of admixture and differentiation was more consistent with previous AFLP and microsatellite data (Smulders et al. 2008).
In addition, anthropogenic factors may have contributed to the extant genetic structure of P. nigra. Tests of genetic bottlenecks identified three populations that may have recently undergone a population bottleneck. The heterozygosity excess indicated in three populations refers to the levels expected a mutation–drift equilibrium (not Hardy–Weinberg equilibrium), as estimated by the number of alleles in each sample. Human actions may have particularly affected population IT1, sampled along the Ticino River. A previous study reported the depletion of the natural poplar stands in the Ticino River region in northern Italy during the Second World War (Fossati et al. 2003), likely contributing to the genetic bottleneck identified from the microsatellite data and demonstrating the potential for management events to affect standing genetic variation in forest trees.
Understanding how IBD, IBA and IBC individually and jointly affect the genetic structure of a keystone species is critical for conservation and management, especially in context of a changing climate. Numerous models predicting climate changes for the next century have been published, and while results are variable (Blenkinsop & Fowler 2007), some consistent trends have emerged. In Europe, the mean temperature is expected to increase, following a large cline across latitudes, with the greatest increases in the northern areas (Raible et al. 2006; Gessler et al. 2007). In addition, rates and distribution of precipitation are also expected to change. Models have predicted that summer precipitation will decrease over much of Europe, with levels increasing only in the most northern latitudes (Blenkinsop & Fowler 2007). Drought events are predicted to increase in frequency (Blenkinsop & Fowler 2007; Penuelas et al. 2007), especially in southern, Mediterranean areas (Blenkinsop & Fowler 2007). These changes in temperature and precipitation will change the distribution of climate types across Europe. The distribution and abundance of climates analogous to those observed in 1945 (i.e. prewarming) are predicted to decrease in size, increase in fragmentation and generally shift to the northeast, with novel climatic conditions developing (Ohlemuller et al. 2006).
If morphological differentiation strictly tracks differences in environment, then comparing the current distribution of morphotypes and predicted distribution of environments may provide insight for conservation efforts (Sork et al. 2010; Kremer et al. 2012). However, if the morphological differentiation reflects a combination of adaptive and nonadaptive processes (such as IBC), predicting species response to climate change is further complicated, as the standing genetic variation may not match the most adaptive phenotype for an environment. For example, the P. nigra populations from central France (FR4 and FR5) experience a climate more similar to the site in Germany than Spain, but display morphology more similar to the Iberian collections (Fig.1). If the small-leaf morphotype is adapted to lower or more seasonal precipitation due to their Iberian ancestry (IBC), rather than the current climate, these populations may be ‘pre-adapted’ to higher drought frequency over the next century (Hu & He 2006; Kremer et al. 2012). Further, we predict the admixed nature of the populations in France may result in greater plasticity in response to varying environments. An ongoing study of two common garden experiments in contrasting environments (England and Italy) is expected to provide additional insight into the genetic basis of phenotypic differentiation in this important forest tree.
Together, these patterns of morphological, genetic, geographic and climate differences indicate that IBC due to serial colonization events likely continues to influence the morphological and genetic structure in a forest tree. Genetic structure was significantly correlated with geographic distance but not climatic differences, consistent with models of IBD or isolation by serial colonization. Morphological variation was correlated with both geographic distance and climatic differences. These patterns confirm that multiple evolutionary processes influence the morphological and genetic structure of long-lived species and indicate that the response to climate change may be influenced by historic factors.
Acknowledgments
The authors thank N.R. Street, S. Milner, M. Viger, M. Nelson, M.J. Tallis and M. Goolsby for field, laboratory and ArcGIS assistance. The common garden experiment in Belgium was planted and maintained by M. Steenackers and colleagues. This work was funded by the European Commission 5th Framework Programme for Research project POPYOMICS, the 6th Framework Programme for Research Network of Excellence EVOLTREE and an Overseas Research Student Award Scheme (to JD) and NERC (NER/S/2001/106361). This study does not necessarily reflect the Commission's views and in no way anticipates the Commission's future policy in this area.
Data accessibility
Morphological measures, microsatellite data and climate details are available in the Dryad Digital Repository under doi: 10.5061/dryad.kq0n5.
Supporting information
Additional supporting information may be found in the online version of this article.
Fig. S1 Results of the structure and structure harvester analyses of microsatellite data.
Methods S1 Details of microsatellite analyses and morphological data collection, with associated references.
Tables S1–S5 Correlation among climatic variables; Per-locus FST values; Phenotypic variation measured at 12 traits in trees from 13 populations grown in a common garden study; Correlation among population means for 12 morphological traits.
References
- Antonovics J. Evolution in closely adjacent plant populations VI. Manifold effects of gene flow. Heredity. 1968;23:508–524. [Google Scholar]
- Blenkinsop S, Fowler HJ. Changes in European drought characteristics projected by the PRUDENCE regional climate models. International Journal of Climatology. 2007;27:1595–1610. [Google Scholar]
- Bock DG, Andrew RL, Rieseberg L. On the adaptive value of cytoplasmic genomes in plants. Molecular Ecology. 2014;23:4899–4911. doi: 10.1111/mec.12920. [DOI] [PubMed] [Google Scholar]
- Brown AHD. Isozymes, plant population genetic structure and genetic conservation. Theoretical and Applied Genetics. 1978;52:145–157. doi: 10.1007/BF00282571. [DOI] [PubMed] [Google Scholar]
- Cavers S, Navarro C, Lowe AJ. Targeting genetic resource conservation in widespread species: a case study of Cedrela odorata L. Forest Ecology and Management. 2004;197:285–294. [Google Scholar]
- Cottrell JE, Krystufek V, Tabbener HE, et al. Postglacial migration of Populus nigra L.: lessons learnt from chloroplast DNA. Forest Ecology and Management. 2005;206:71–90. [Google Scholar]
- De Kort H, Vandepitte K, Bruun HH, et al. Landscape genomics and a common garden trial reveal adaptive differentiation to temperature across Europe in the tree species Alnus glutinosa. Molecular Ecology. 2014;23:4709–4721. doi: 10.1111/mec.12813. [DOI] [PubMed] [Google Scholar]
- Dennison MD, Baker AJ. Morphometric variability in Continental and Atlantic Island Populations of Chaffinches (Fringilla coelebs. Evolution. 1991;45:29–39. doi: 10.1111/j.1558-5646.1991.tb05263.x. [DOI] [PubMed] [Google Scholar]
- DeWoody J, Nason JD, Hipkins VD. Mitigating scoring errors in microsatellite data from wild populations. Molecular Ecology Notes. 2006;6:951–957. [Google Scholar]
- Dudley SA. Differing selection on plant physiological traits in response to environmental water availability: a test of adaptive hypotheses. Evolution. 1996a;50:92–102. doi: 10.1111/j.1558-5646.1996.tb04475.x. [DOI] [PubMed] [Google Scholar]
- Dudley SA. The response to differing selection on plant physiological traits: evidence for local adaptation. Evolution. 1996b;50:103–110. doi: 10.1111/j.1558-5646.1996.tb04476.x. [DOI] [PubMed] [Google Scholar]
- Duminil J, Fineschi S, Hampe A, et al. Can population genetic structure be predicted from life-history traits? American Naturalist. 2007;169:662–672. doi: 10.1086/513490. [DOI] [PubMed] [Google Scholar]
- Earl DA, vonHoldt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources. 2012;4:359–361. [Google Scholar]
- Eckert CG, Manicacci D, Barrett SCH. Genetic drift and founder effect in native versus introduced populations of an invading plant, Lythrum salicaria (Lythraceae) Evolution. 1996;50:1512–1519. doi: 10.1111/j.1558-5646.1996.tb03924.x. [DOI] [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software structure: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Fabbrini F, Gaudet M, Bastien C, et al. Phenotypic plasticity, QTL mapping and genomic characterization of bud set in black poplar. BMC Plant Biology. 2012;12:47. doi: 10.1186/1471-2229-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure: extensions to linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati T, Grassi F, Sala F, Castiglione S. Molecular analysis of natural populations of Populus nigra L. intermingled with cultivated hybrids. Molecular Ecology. 2003;12:2033–2043. doi: 10.1046/j.1365-294x.2003.01885.x. [DOI] [PubMed] [Google Scholar]
- Gailing O, Lind J, Lilleskov E. Leaf morphological and genetic differentiation between Quercus rubra L. and Q. ellipsoidalis E.J. Hill populations in contrasting environments. Plant Systematics and Evolution. 2012;298:1533–1545. [Google Scholar]
- Gessler A, Keitel C, Kreuzwieser J, et al. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees-Structure and Function. 2007;21:1–11. [Google Scholar]
- Hall D, Luquez V, Garcia VM, et al. Adaptive population differentiation in phenology across a latitudinal gradient in European aspen (Populus tremula, L.): a comparison of neutral markers, candidate genes and phenotypic traits. Evolution. 2007;61:2849–2860. doi: 10.1111/j.1558-5646.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- Hamrick JL, Godt MJW. Effects of life history traits on genetic diversity in plant species. Philosophical Transactions of the Royal Society B-Biological Sciences. 1996;351:1291–1298. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Hu X-S, He F. Seed and pollen flow in expanding a species' range. Journal of Theoretical Biology. 2006;240:662–672. doi: 10.1016/j.jtbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Jensen JL, Bohonak AJ, Kelley ST. Isolation by distance, web service. BMC Genetics. 2005;6:13. doi: 10.1186/1471-2156-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawecki TJ, Evert D. Conceptual issues in local adaptation. Ecology Letters. 2004;7:1225–1241. [Google Scholar]
- Kelly CK, Chase MW, De Bruijn A, Fay MF, Woodward FI. Temperature-based population segregation in birch. Ecology Letters. 2003;6:87–89. [Google Scholar]
- Kremer A, Kleinschmit J, Cottrell J, et al. Is there a correlation between chloroplastic and nuclear divergence, or what are the roles of history and selection on genetic diversity in European oaks? Forest Ecology and Management. 2002;156:75–87. [Google Scholar]
- Kremer A, Ronce O, Robledo-Arnuncio JJ, et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecology Letters. 2012;15:378–392. doi: 10.1111/j.1461-0248.2012.01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCorre V, Kremer A. Genetic variability at neutral markers, quantitative trait loci and trait in a subdivided population under selection. Genetics. 2003;164:1205–1219. doi: 10.1093/genetics/164.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen T, McCairns RJS, O'Hara RB, Merila J. Qst-Fst comparisons: evolutionary and ecological insights from genomic heterogeneity. Nature Reviews Genetics. 2013;14:179–190. doi: 10.1038/nrg3395. [DOI] [PubMed] [Google Scholar]
- Loveless MD, Hamrick JL. Ecological determinants of genetic structure in plant populations. Annual Review of Ecology and Systematics. 1984;15:65–95. [Google Scholar]
- Luikart G, Cornuet J-M. Empirical evaluation of a test for identifying recently bottlenecked populations from allele frequency data. Conservation Biology. 1998;12:228–237. [Google Scholar]
- Luikart G, Sherwin WB, Steele BM, Allendorf FW. Usefulness of molecular markers for detecting population bottlenecks via monitoring genetic change. Molecular Ecology. 1998;7:963–974. doi: 10.1046/j.1365-294x.1998.00414.x. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. Randomization, Bootstrap and Monte Carlo Methods in Biology. 2nd edn. London: Chapman & Hall; 1997. [Google Scholar]
- McKay JK, Latta RG. Adaptive population divergence: markers, QTL and traits. Trends in Ecology & Evolution. 2002;17:285–291. [Google Scholar]
- McKown AD, Guy RD, Klapste J, et al. Geographical and environmental gradients shape phenotypic trait variation and genetic structure in Populus trichocarpa. New Phytologist. 2014;201:1263–1276. doi: 10.1111/nph.12601. [DOI] [PubMed] [Google Scholar]
- Merila J, Crnokrak P. Comparison of genetic differentiation at marker loci and quantitative traits. Journal of Evolutionary Biology. 2001;14:892–903. [Google Scholar]
- Mitton JB, Duran KL. Genetic variation in piñon pine, Pinus edulis, associated with summer precipitation. Molecular Ecology. 2004;13:1259–1264. doi: 10.1111/j.1365-294X.2004.02122.x. [DOI] [PubMed] [Google Scholar]
- Moya-Larano J, Corcobado G. Plotting partial correlation and regression in ecological studies. Web Ecology. 2008;8:35–46. [Google Scholar]
- Nason JD, Hamrick JL, Fleming TH. Historical vicariance and postglacial colonization effects on the evolution of genetic structure in Lophocereus, a Sonoran desert columnar cactus. Evolution. 2002;56:2214–2226. doi: 10.1111/j.0014-3820.2002.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Nosil P, Funk DJ, Ortiz-Barrientos D. Divergent selection and heterogeneous genomic divergence. Molecular Ecology. 2009;18:375–402. doi: 10.1111/j.1365-294X.2008.03946.x. [DOI] [PubMed] [Google Scholar]
- Ohlemuller R, Gritti ES, Sykes MT, Thomas CD. Towards European climate risk surfaces: the extent and distribution of analogous and non-analogous climates 1931-2100. Global Ecology and Biogeography. 2006;15:395–405. [Google Scholar]
- van Oosterhout C, Hutchinson WF, Willis DPM, Shipley P. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes. 2004;4:535–538. [Google Scholar]
- Orsini L, Corander J, Alasentie A, Hanski I. Genetic spatial structure in a butterfly metapopulation correlates better with past than present demographic structure. Molecular Ecology. 2008;17:2629–2642. doi: 10.1111/j.1365-294X.2008.03782.x. [DOI] [PubMed] [Google Scholar]
- Orsini L, Vanoverbeke J, Swillen I, Mergeay J, De Meester L. Drivers of population genetic differentiation in the wild: isolation by dispersal limitation, isolation by adaptation and isolation by colonization. Molecular Ecology. 2013;22:5983–5999. doi: 10.1111/mec.12561. [DOI] [PubMed] [Google Scholar]
- Palme AE, Su Q, Rautenberg A, Manni F, Lascoux M. Postglacial recolonization and cpDNA variation of silver birch, Betula pendula. Molecular Ecology. 2003;12:201–212. doi: 10.1046/j.1365-294x.2003.01724.x. [DOI] [PubMed] [Google Scholar]
- Peakall R, Smouse PE. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes. 2006;6:288–295. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Smouse PE, Huff DR. Evolutionary implications of allozyme and RAPD variation in diploid populations of dioecious buffalograss Buchloë dactyloides. Molecular Ecology. 1995;4:135–147. [Google Scholar]
- Penuelas J, Prieto P, Beier C, et al. Response of plant species richness and primary productivity in shrublands along a north-south gradient in Europe to seven years of experimental warming and drought: reductions in primary productivity in the heat and drought year of 2003. Global Change Biology. 2007;13:2563–2581. [Google Scholar]
- Petit RJ, Csaikl UM, Bordacs S, et al. Chloroplast DNA variation in European white oaks – Phylogeography and patterns of diversity based on data from over 2600 populations. Forest Ecology and Management. 2002;156:5–26. [Google Scholar]
- Petit RJ, Aguinagalde I, de Beaulieu JL, et al. Glacial refugia: hotspots but not melting pots of genetic diversity. Science. 2003;300:1563–1565. doi: 10.1126/science.1083264. [DOI] [PubMed] [Google Scholar]
- Picotte J, Rosenthal D, Rhode J, Cruzan M. Plastic responses to temporal variation in moisture availability: consequences for water use efficiency and plant performance. Oecologia. 2007;153:821–832. doi: 10.1007/s00442-007-0794-z. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelley P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible CC, Casty C, Luterbacher J, et al. Climate variability-observations, reconstructions, and model simulations for the Atlantic-European and Alpine region from 1500-2100 AD. Climatic Change. 2006;79:9–29. [Google Scholar]
- Rohde A, Storme V, Jorge V, et al. Bud set in poplar – genetic dissection of a complex trait in natural and hybrid populations. New Phytologist. 2011;189:106–121. doi: 10.1111/j.1469-8137.2010.03469.x. [DOI] [PubMed] [Google Scholar]
- Rousset F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics. 1997;145:1219–1228. doi: 10.1093/genetics/145.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer DL, McElwain JC, Adams JM, Wilf P. Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii. New Phytologist. 2008;179:808–817. doi: 10.1111/j.1469-8137.2008.02496.x. [DOI] [PubMed] [Google Scholar]
- Salmela MJ. Rethinking local adaptaiton: mind the environment! Forest Ecology and Management. 2014;312:271–281. [Google Scholar]
- Savolainen O, Pyhajarvi T. Genomic diversity in forest trees. Current Opinion in Plant Biology. 2007;10:162–167. doi: 10.1016/j.pbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Savolainen O, Lascoux M, Merlia J. Ecological genomics of local adaptation. Nature Reviews Genetics. 2013;14:807–820. doi: 10.1038/nrg3522. [DOI] [PubMed] [Google Scholar]
- Slatkin M. Isolation by distance in equilibrium and non-equilibrium populations. Evolution. 1993;47:264–279. doi: 10.1111/j.1558-5646.1993.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Smouse PE, Peakall R. Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity. 1999;82:561–573. doi: 10.1038/sj.hdy.6885180. [DOI] [PubMed] [Google Scholar]
- Smouse PE, Long JC, Sokal RR. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Systematic Zoology. 1986;35:627–632. [Google Scholar]
- Smulders MJM, van der Schoot J, Arens P, Vosman B. Trinucleotide repeat microsatellite markers for black poplar (Populus nigra L.) Molecular Ecology Notes. 2001;1:188–190. [Google Scholar]
- Smulders MJM, Cottrell JE, Lefevre F, et al. Structure of the genetic diversity in black poplar (Populus nigra L.) populations across European river systems: consequences for conservation and restoration. Forest Ecology and Management. 2008;255:1388–1399. [Google Scholar]
- Sork VL, Davis FW, Westfall R, et al. Gene movement and genetic association with regional climate gradients in California valley oak (Quercus lobata Née) in the face of climate change. Molecular Ecology. 2010;19:3806–3823. doi: 10.1111/j.1365-294X.2010.04726.x. [DOI] [PubMed] [Google Scholar]
- Spurgin LG, Illera JC, Jorgensen TH, Dawson DA, Richardson DS. Genetic and phenotypic divergence in an island bird: isolation by distance, by colonization, or by adaptation? Molecular Ecology. 2014;23:1028–1039. doi: 10.1111/mec.12672. [DOI] [PubMed] [Google Scholar]
- Steane D, Conod N, Jones R, Vaillancourt R, Potts B. A comparative analysis of population structure of a forest tree, Eucalyptus globulus (Myrtaceae), using microsatellite markers and quantitative traits. Tree Genetics & Genomes. 2006;2:30–38. [Google Scholar]
- Turesson G. The genotypical response of the plant species to the habitat. Hereditas. 1922;3:211–350. [Google Scholar]
- Tzedakis PC, Emerson BC, Hewitt GM. Cryptic or mystic? Glacial tree refugia in northern Europe. Trends in Ecology & Evolution. 2013;28:696–704. doi: 10.1016/j.tree.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Vendramin GG, Anzidei M, Madaghiele A, Sperisen C, Bucci G. Chloroplast microsatellite analysis reveals the presence of population subdivision in Norway spruce (Picea abies K.) Genome. 2000;43:68–78. [PubMed] [Google Scholar]
- Viger M. 2011. p. 258. Physiology, genetics and genomics of drought adaptation in Populus. Doctoral Thesis, University of Southampton, Centre for Biological Sciences, Southampton, UK.
- Westoby M, Wright IJ. Land-plant ecology on the basis of functional traits. Trends in Ecology & Evolution. 2006;21:261–268. doi: 10.1016/j.tree.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Wright S. Evolution in Mendelian populations. Genetics. 1931;16:97–159. doi: 10.1093/genetics/16.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-D, Yan E-R, Chang SX, et al. Twig-leaf size relationships in woody plants vary intraspecifically along a soil moisture gradient. Acta Oecologica. 2014;60:17–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Results of the structure and structure harvester analyses of microsatellite data.
Methods S1 Details of microsatellite analyses and morphological data collection, with associated references.
Tables S1–S5 Correlation among climatic variables; Per-locus FST values; Phenotypic variation measured at 12 traits in trees from 13 populations grown in a common garden study; Correlation among population means for 12 morphological traits.
Data Availability Statement
Morphological measures, microsatellite data and climate details are available in the Dryad Digital Repository under doi: 10.5061/dryad.kq0n5.