Abstract
Background: Medical interventions are an important component of the illness experience in advanced cancer.
Objective: To describe the use of medical interventions between diagnosis and death in decedents with metastatic lung and colorectal cancer.
Design: Retrospective analysis of a prospective cohort study.
Setting/Subjects: We studied 1,840 decedents from the Cancer Care Outcomes Research and Surveillance (CanCORS) study. Subjects had been diagnosed with stage IV lung or colorectal cancer between 2003 and 2005.
Measurements: Hospitalizations, surgeries, radiation therapy treatments, chemotherapy treatments, and end-of-life care, reported by tertile of overall survival time.
Results: Median survival in the bottom, middle, and top tertiles of survival was 1.2, 5.3, and 15.3 months for lung cancer, and 3.0, 18.0, and 44.4 months for colorectal cancer. Hospitalizations, chemotherapy receipt, and hospice enrollment increased with increasing survival. The median duration of chemotherapy in the top survival tertile was 149 days for lung cancer and 498 days for colorectal cancer. A minority of decedents used any hospice services, and the median duration of hospice enrollment exceeded 30 days only for enrollees in the top survival tertile (lung cancer, 35 days; colorectal cancer, 66 days).
Conclusions: For patients with metastatic lung and colorectal cancer, longer survival is associated with increased intensity of medical care, characterized by greater use of chemotherapy and acute hospital care. Hospice utilization was uniformly low, and most hospice enrollees were referred to hospice in the last 30 days of life.
Introduction
Advanced-stage epithelial cancers are incurable, with rare exception.1,2 For this reason, anticancer treatments for most patients with advanced solid tumors should serve the goal of maximizing both the length and quality of a patient's remaining lifespan.3 These goals are often in conflict with each other—in seeking to prolong their lives, patients accept treatments that may diminish their quality of life.4 Ultimately, patients must rely on their physician-led care team to help them interpret the balance of risks, side effects, and potential benefits associated with anticancer treatments and interventions.5
For patients, medical interventions represent a substantial and sometimes burdensome component of living with advanced cancer. Medical interventions contribute to patient experience in at least three ways. First, medical treatments are intended to provide relief from cancer-related symptoms. Inescapably, however, medical interventions require large amounts of time that must be spent in hospital and clinic environments, instead of at home or in the workplace. Last, the side effects of cancer treatment often become a prominent component of living with advanced cancer, including “financial toxicity” associated with out-of-pocket treatment costs.6
While some studies have examined the burdens associated with cancer care received in the last days or weeks of life,7–9 there is a paucity of research describing the longitudinal experience of patients with advanced cancer between diagnosis and death. We studied the receipt of medical interventions as experienced by patients with stage IV lung and colorectal cancer in the Cancer Care Outcomes Research and Surveillance (CanCORS) study cohort10 and report detailed information regarding the timing and frequency of surgeries, chemotherapy, radiation treatments, hospital admissions, and other clinical events.
Patients and Methods
The CanCORS study enrolled approximately 10,000 patients diagnosed with lung or colorectal cancer between 2003 and 2005. Patients were enrolled from 5 geographic regions (Northern California, Los Angeles County, North Carolina, Iowa, or Alabama), 5 participating integrated delivery systems, and 15 Veterans Affairs Medical Centers (VA).10,11 Each site identified incident cancer cases using a comprehensive rapid case ascertainment protocol. The study was approved by the human subjects committees at all participating institutions. Analyses utilized CanCORS core data version 1.17, medical record abstraction data versions 1.12 (CanCORS I) and 1.2 (CanCORS II), and patient interview data versions 1.12 (CanCORS I) and 1.0 (CanCORS II).
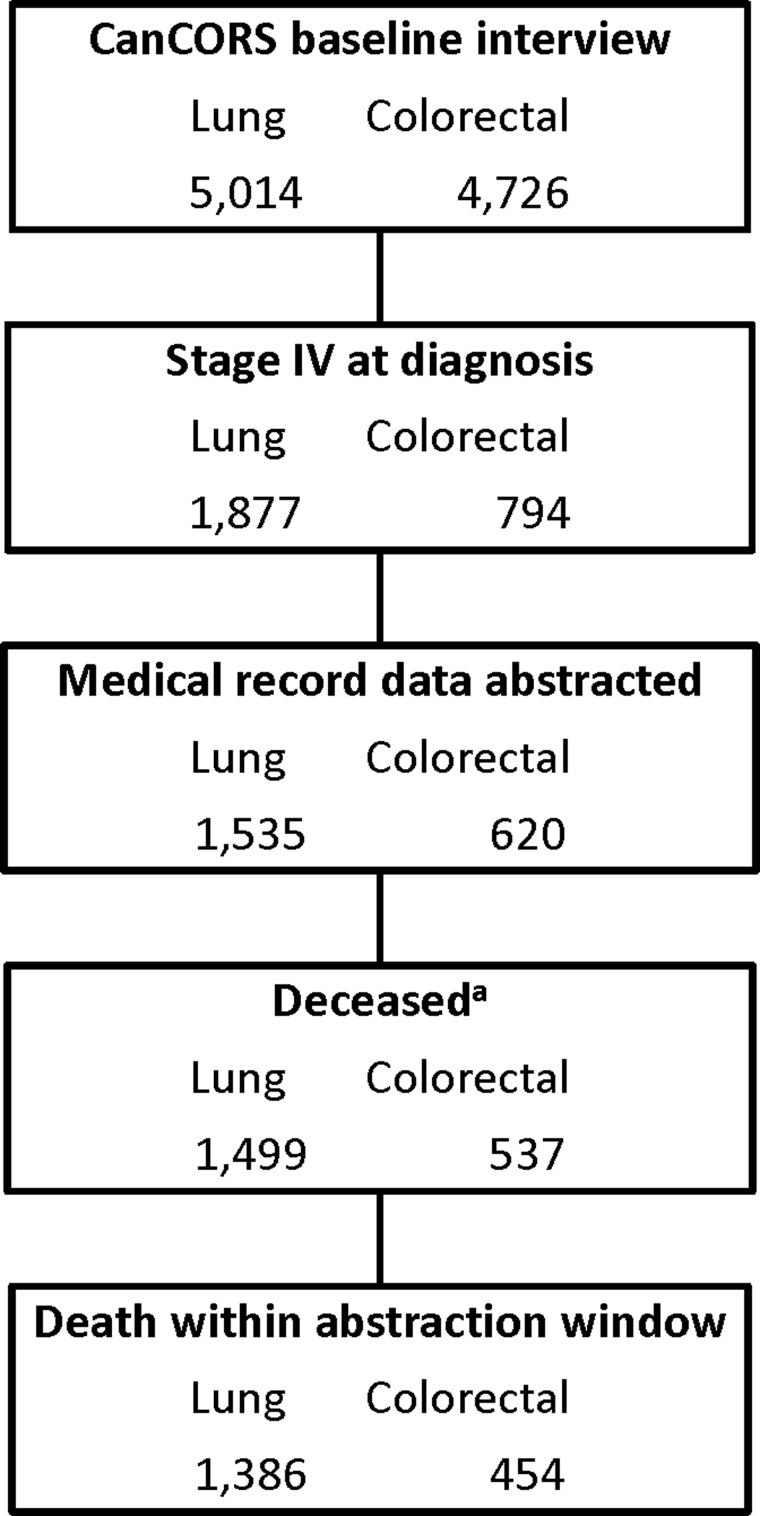
We studied patients with lung and colorectal cancer who had stage IV (metastatic) cancer at the time of initial diagnosis. Because we sought to describe medical care delivered between cancer diagnosis and death, we limited our analysis to patients who were known to have died within the medical record abstraction period; patients who survived beyond the abstraction period or who were lost to follow-up were excluded. Follow-up with medical record abstraction was complete until death or 2 years from diagnosis for 88% of patients in the parent cohort (92% of lung and 79% of colorectal cancer patients). Figure 1 shows details of cohort selection; the final cohort included 1,386 decedents with stage IV lung cancer and 454 decedents with stage IV colorectal cancer.
FIG. 1.
Cohort assembly diagram. aDeceased according to query of plan records for managed care sites (CRN and VA) or to linkage to national death records (all other sites). CanCORS, Cancer Care Outcomes Research and Surveillance; CRN, Cancer Research Network; VA, Veterans Affairs Medical Center.
Data collection
Outcomes collected include overall survival from diagnosis; frequency and timing of hospitalization, chemotherapy, radiation therapy, or primary cancer site-directed surgery; information on clinical trial participation; and the first incidence of any documented end-of-life (EOL) discussion,12 do-not-resuscitate (DNR) order, intensive care unit (ICU) admission, or hospice enrollment. All outcomes were determined from medical record abstraction.
Medical record abstraction in the original CanCORS protocol was carried out for the interval beginning 3 months before cancer diagnosis and continuing until death or 15 months after diagnosis, whichever came first. For patients surviving beyond 15 months after diagnosis, additional medical record abstraction was subsequently carried out under the CanCORS II protocol. Medical records from hospitals, radiation treatment facilities, and medical offices were abstracted using a standardized protocol. The length of the abstraction period varied between participating sites, but continued until at least October 2010. All outcomes reported here were assessable from both data collection rounds, and care was taken to exclude duplicate reporting of events that were represented in both data sets.
Medical record abstraction included start dates and end dates for chemotherapy treatments, radiation courses, and acute care episodes. Chemotherapy regimens were considered to be distinct when new chemotherapy agents were started or when there was a break of 6 weeks or longer between chemotherapy administrations. Continuation of a treatment regimen with reduction in intensity (e.g., by discontinuation of oxaliplatin from FOLFOX treatment) was not considered a distinct treatment regimen. The duration of a chemotherapy regimen was calculated as the time elapsed between the first and last days of treatment.
Statistical analyses
Results are presented separately by cancer site and are further stratified by tertile of overall survival time. Because our goal was to provide information that would reflect the range of experiences for patients diagnosed with advanced cancer, survival tertiles were calculated based on the survival trends observed in the parent cohort of all CanCORS patients diagnosed with advanced cancer who had any available medical record abstraction data (including both decedents and patients who survived beyond the 5-year medical record abstraction period). Assessment of the timing and frequency of medical interventions was limited to the primary analysis population of decedents with complete medical record abstraction from diagnosis to death (Fig. 1).
Associations between patient factors and survival tertile were assessed using χ2 tests. Statistical analyses were conducted using Stata version 11.2 (StataCorp LP, College Station, TX).
For chemotherapy receipt, radiation therapy, surgery, and hospital admission, we calculated the median time from diagnosis to intervention start and, where applicable, the median duration of time for the intervention (e.g., chemotherapy, hospitalizations). For radiation therapy, chemotherapy, and hospitalizations, data are reported for the initial intervention as well as for subsequent interventions (e.g., second-line chemotherapy). For the other outcomes (EOL discussions, DNR orders, ICU admissions, and hospice enrollment) comprehensive medical record abstraction data were available only on the first instance of the outcome. In general, the timing of an outcome is shown when at least 10% of the subset of interest experienced that outcome. For hospitalization, chemotherapy, radiation therapy, and hospice we also report the percentage of life (between cancer diagnosis and death) over which these interventions occurred. We performed a sensitivity analysis to assess the impact of our decision to include only decedents in the primary analysis population; in that analysis, we compared the timing and frequency of medical interventions in the primary analysis population with the findings from the parent cohort that included long-term survivors in addition to decedents.
Results
Demographic characteristics and median survival for decedents with stage IV lung and colorectal cancer are shown in Table 1, stratified by tertile of overall survival. Median survival from cancer diagnosis exceeded 1 year for patients with lung cancer in the top tertile of survival (15 months) and for patients with colorectal cancer in the middle and top tertiles of survival (18 and 44 months, respectively). Demographic factors associated with differences across survival tertiles included age (in both lung and colorectal cancer cohorts; younger age associated with longer survival), study site (both cohorts), and comorbidity (lung cancer cohort only; fewer comorbidities associated with longer survival). Gender, race/ethnicity, and HMO participation were not significantly associated with overall survival. We examined multivariable models using the characteristics in Table 1 as predictors for the tertile of survival, but the predictive ability of these models was poor (data not shown).
Table 1.
Demographic Characteristics of Decedents with Stage IV Lung and Colorectal Cancer, Stratified by Tertiles of Survival Time
| Lung cancer | Colorectal cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| Bottom tertile n = 489 | Middle tertile n = 485 | Top tertile n = 412 | p | Bottom tertile n = 196 | Middle tertile n = 163 | Top tertile n = 95 | p | |
| Survival (months)a | ||||||||
| Median | 1.2 | 5.3 | 15 | 3.0 | 18 | 44 | ||
| Range | 0.03, 2.7 | 2.7, 9.4 | 9.4, 99 | 0.03, 10 | 11, 31 | 31, 95 | ||
| Age at diagnosis | <0.001 | <0.001 | ||||||
| 21–54 | 47 (10) | 59 (12) | 59 (14) | 31 (16) | 44 (27) | 31 (33) | ||
| 55–64 | 106 (22) | 135 (28) | 115 (28) | 33 (17) | 46 (28) | 29 (31) | ||
| 65–74 | 146 (30) | 180 (37) | 137 (33) | 53 (27) | 43 (26) | 22 (23) | ||
| 75+ | 190 (39) | 111 (23) | 101 (25) | 79 (40) | 30 (18) | 13 (14) | ||
| Gender | 0.25 | 0.046 | ||||||
| Male | 298 (61) | 317 (65) | 250 (61) | 101 (52) | 100 (61) | 62 (65) | ||
| Female | 191 (39) | 168 (35) | 162 (39) | 95 (48) | 63 (39) | 33 (35) | ||
| Race/ethnicityb | 0.67 | 0.34 | ||||||
| Non-Hispanic white | 373 (76) | 379 (78) | 304 (74) | 127 (65) | 98 (60) | 54 (57) | ||
| Non-Hispanic black | 43 (9) | 49 (10) | 47 (11) | 35 (18) | 37 (23) | 17 (18) | ||
| Other | 73 (15) | 57 (12) | 61 (15) | 34 (17) | 28 (17) | 24 (25) | ||
| Comorbidity | 0.01 | 0.11 | ||||||
| None | 85 (17) | 87 (18) | 95 (23) | 50 (26) | 54 (33) | 33 (35) | ||
| Mild | 173 (35) | 182 (38) | 163 (40) | 75 (38) | 63 (39) | 34 (36) | ||
| Moderate | 105 (21) | 111 (23) | 90 (22) | 35 (18) | 28 (17) | <25 | ||
| Severe | 126 (26) | 105 (22) | 64 (16) | 36 (18) | 18 (11) | <10 | ||
| HMO | 0.24 | 0.14 | ||||||
| No | 351 (72) | 367 (76) | 293 (71) | 136 (69) | 126 (77) | 64 (67) | ||
| Yes | 138 (28) | 118 (24) | 119 (29) | 60 (31) | 37 (23) | 31 (33) | ||
| Study site | <0.001 | 0.001 | ||||||
| CRN | 90 (18) | 66 (14) | 65 (16) | 29 (15) | 24 (15) | 17 (18) | ||
| Northern California | 94 (19) | 103 (21) | 91 (22) | 62 (32) | 35 (21) | 24 (25) | ||
| Alabama | 50 (10) | 56 (12) | 64 (16) | 24 (12) | 38 (23) | 19 (20) | ||
| Los Angeles county | 93 (19) | 78 (16) | 41 (10) | 49 (25) | 23 (14) | 15 (16) | ||
| Iowa | 130 (27) | 126 (26) | 92 (22) | — | — | — | ||
| North Carolina | — | — | — | <25 | 20 (12) | <25 | ||
| VA | 32 (7) | 56 (12) | 59 (14) | <10 | 23 (14) | <10 | ||
Tertiles have unequal numbers of patients because tertile boundaries were calculated from the survival times of all patients with known vital status and any medical record abstraction data. Select cells frequencies are shown as <10 or <25 patients so that cells with <10 patients are masked.
Survival from diagnosis.
8% of lung and 5% of colorectal cancer patients were Hispanic; and 6% of lung and 5% of colorectal cancer patients were Asian.
Table 2 shows the number of days and the corresponding percentage of life (from cancer diagnosis to death) spent receiving inpatient hospital care, chemotherapy, radiation therapy, and hospice care. The median number of hospital days was least for decedents in the bottom tertile of survival, and was similar for the middle and top tertiles (for both lung and colorectal cancer). The percentage of life spent in the hospital was 3% or less for all strata. The median number of days on chemotherapy treatment increased with increasing survival. A minority of patients in the bottom tertile of survival received any chemotherapy (for both lung and colorectal cancer), while patients in the top tertile of survival were on active chemotherapy treatment for a median of 149 and 498 days in the lung and colorectal cancer cohorts, respectively. The percentage of life on chemotherapy treatment was similar for both the middle and top tertiles of survival (25% versus 32% of life after diagnosis for lung cancer, and 42% of life in both groups for colorectal cancer). Clinical trial participation rates increased with increasing survival in both cancer cohorts, ranging from 0.8% of patients with lung cancer in the bottom survival tertile to 18% in patients with colorectal cancer in the top survival tertile. A minority of decedents in each cohort received any hospice care (37% in the lung cancer cohort and 32% in the colorectal cancer cohort). Among those who did receive hospice care, the absolute number of days on hospice increased with increasing survival, but the percentage of life after diagnosis spent receiving hospice care declined across tertiles.
Table 2.
Total Days and Percentage of Life Spent in Hospital, Receiving Chemotherapy, Receiving Radiation Therapy, or Hospice for Decedents with Stage IV Lung and Colorectal Cancer
| Bottom tertile | Middle tertile | Top tertile | |
|---|---|---|---|
| Lung cancer | |||
| Hospitalization | |||
| - Median days (IQR) | 0 (0, 6) | 5 (0, 13) | 5 (0, 14) |
| - % of life, median (IQR) | 0 (0, 15) | 3 (0, 9) | 1 (0, 3) |
| Chemotherapy | |||
| - Median days on treatment (IQR) | 0 (0, 1) | 37 (0, 86) | 149 (59, 280) |
| - % of life on treatment, median (IQR) | 0 (0, 1) | 25 (0, 49) | 32 (10, 50) |
| - % participating in a clinical triala | 0.8 | 4 | 9 |
| Radiation therapy (to any site) | |||
| - Median days (IQR) | 0 (0, 12) | 13 (0, 27) | 15 (0, 38) |
| - % of life, median (IQR) | 0 (0, 25) | 7 (0, 19) | 3 (0, 7) |
| Hospice, all patients | |||
| - Median days (SD) | 0 (0, 4) | 0 (0, 9) | 0 (0, 31) |
| - % of life, median (IQR) | 0 (0, 13) | 0 (0, 6) | 0 (0, 6) |
| - % receiving any hospice care | 32 | 35 | 44 |
| Hospice, among patients receiving hospice | |||
| - Median days (SD) | 11 (4, 23) | 21 (7, 52) | 35 (16, 103) |
| - % of life, median (IQR) | 44 (15, 72) | 13 (5, 42) | 7 (3, 21) |
| Colorectal cancerb | |||
| Hospitalization | |||
| - Median days (IQR) | 3 (0, 9) | 12 (3, 23) | 12 (6, 27) |
| - % of life, median (IQR) | 2 (0, 9) | 2 (0, 4) | 1 (0, 2) |
| Chemotherapy | |||
| - Median days on treatment (IQR) | 0 (0, 36) | 233 (111, 342) | 498 (316, 756) |
| - % of life on treatment, median (IQR) | 0 (0, 26) | 42 (20, 63) | 42 (24, 55) |
| - % participating in a clinical triala | 3 | 11 | 18 |
| Hospice, all patients | |||
| - Median days (SD) | 0 (0, 0) | 0 (0, 13) | 0 (0, 39) |
| - % of life, median (IQR) | 0 (0, 0) | 0 (0, 2) | 0 (0, 3) |
| - % receiving any hospice care | 24 | 34 | 43 |
| Hospice, among patients receiving hospice | |||
| - Median days (IQR) | 16 (6, 29) | 28 (12, 73) | 66 (13, 103) |
| - % of life, median (IQR) | 21 (8, 41) | 5 (2, 13) | 5 (1, 9) |
Clinical trial participation during any line of chemotherapy.
Radiation therapy figures are omitted for colorectal cancer, due to low rates of radiation use in this group.
IQR, interquartile range; SD, standard deviation.
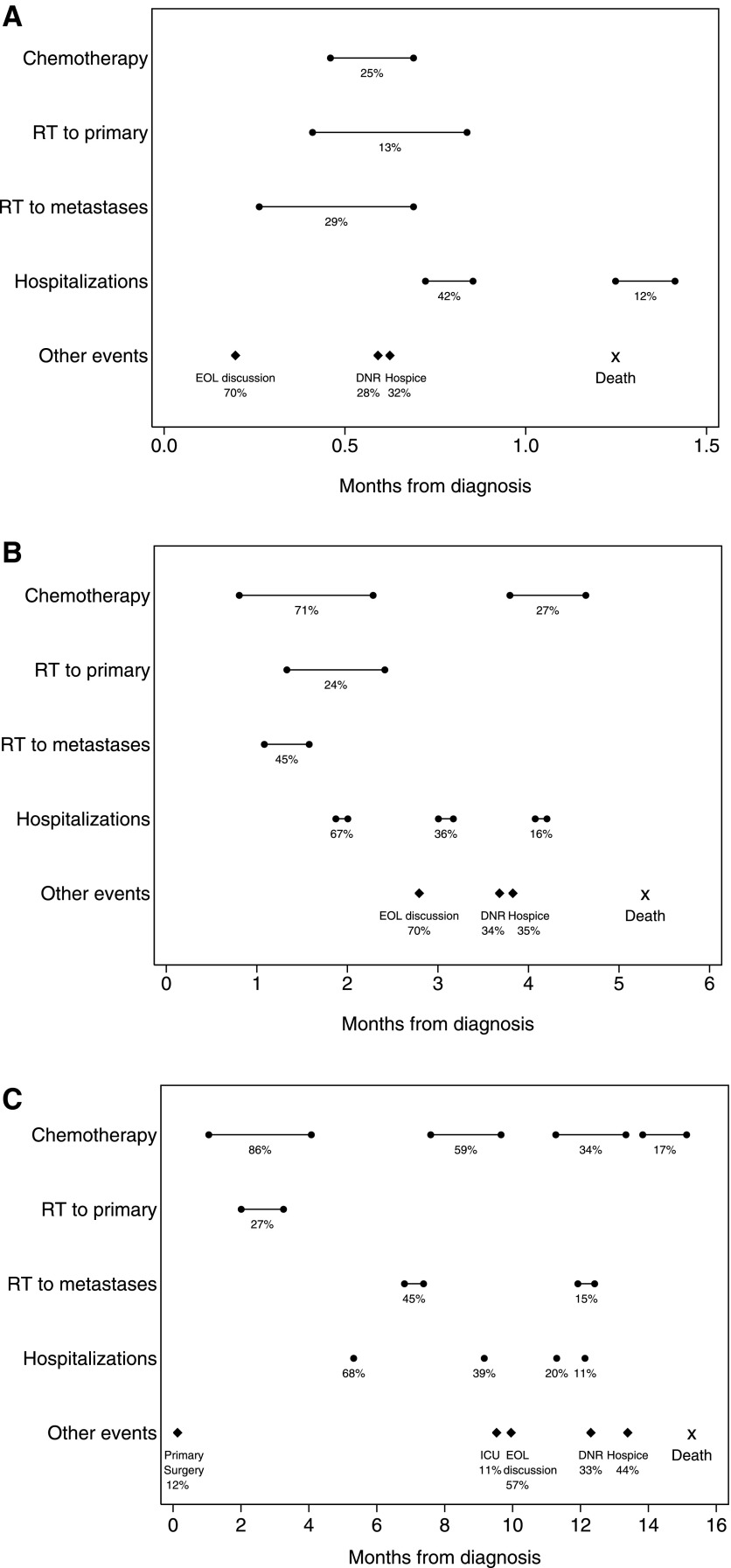
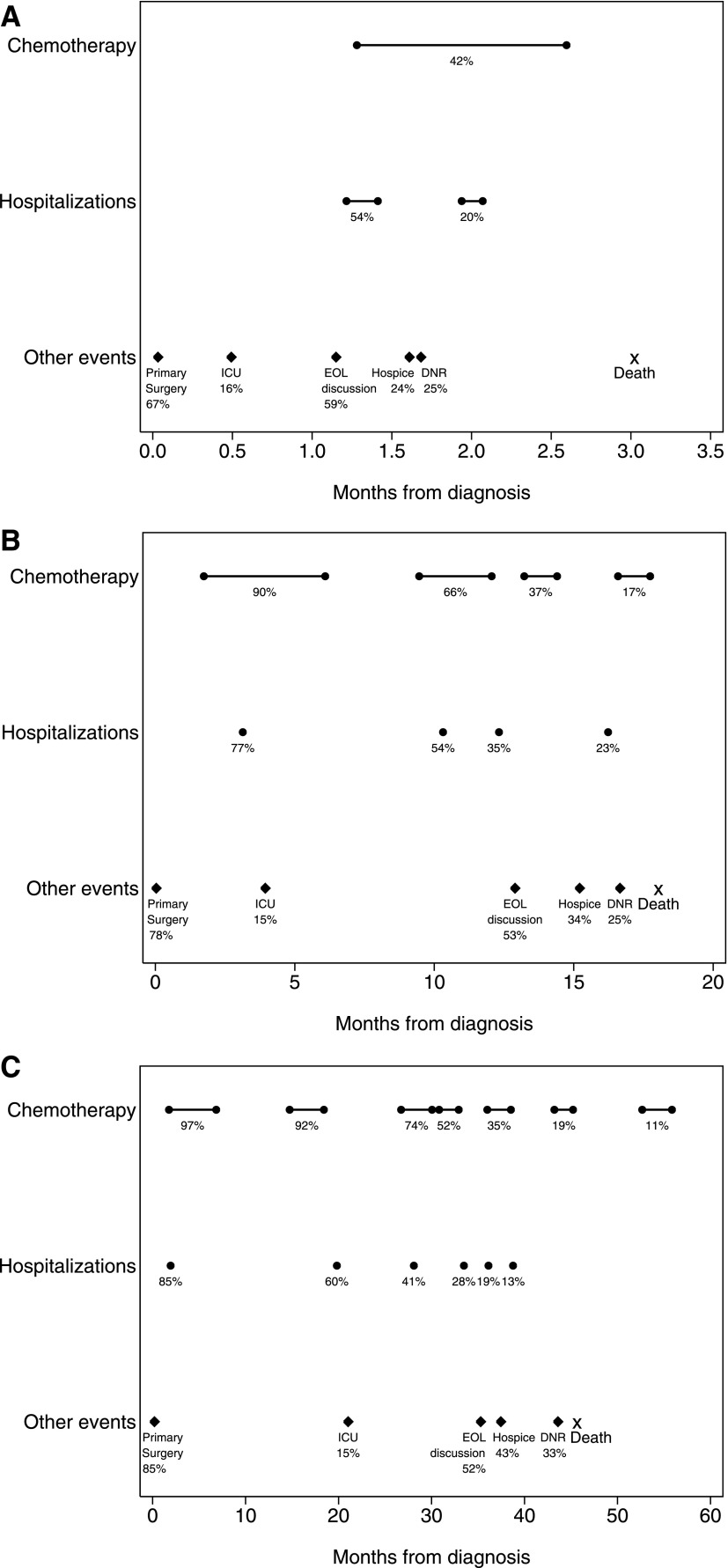
Figures 2 and 3 illustrate the sequence, timing, and duration of health care interventions in patients with advanced lung and colorectal cancer, stratified by survival group. Events occurring in at least 10% of patients within each survival stratum are shown, along with the median time of occurrence for those events.
FIG. 2.
Frequency and timing of medical interventions in patients with lung cancer. A–C: Outcomes for patients in the bottom, middle, and top tertile of survival, respectively, are shown. Timing (with respect to cancer diagnosis) is shown for events that occurred in at least 10% of patients. Percentages indicate the proportion of patients experiencing each event. Circles connected by bars indicate the median start date and median duration for chemotherapy treatment lines, radiation therapy courses, and hospitalizations. Diamonds indicate the median time to occurrence for other discrete events, and the median time to death is marked with an ‘X’. Due to scale, only the start date of hospitalization is indicated in (C). RT, radiation therapy; EOL, end-of-life; DNR, do-not-resuscitate order; ICU, intensive care unit admission.
FIG. 3.
Frequency and timing of medical interventions in patients with colorectal cancer. A–C: Outcomes for patients in the bottom, middle, and top tertile of survival, respectively, are shown. Timing (with respect to cancer diagnosis) is shown for events that occurred in at least 10% of patients. Percentages indicate the proportion of patients experiencing each event. Circles connected by bars indicate the median start date and median duration for chemotherapy treatment lines and hospitalizations. Diamonds indicate the median time to occurrence for other discrete events, and the median time to death is marked with an ‘X’. Due to scale, only the start date of hospitalization is indicated in (B) and (C). EOL, end-of-life; DNR, do-not-resuscitate order; ICU, intensive care unit admission.
For decedents in the lung cancer cohort, rates of thoracic surgery were low across all survival strata (less than 10% in the bottom and middle tertiles, and 12% in the top tertile). Rates of first-line chemotherapy receipt increased from 25% in the bottom tertile to 71% and 86% in the middle and top tertiles. The median duration of first-line chemotherapy also increased across tertiles, as did the usage of second and later lines of chemotherapy. Thirty-four percent of decedents in the top survival tertile received at least three separate courses of chemotherapy, but less than 4% of treatments received in third or subsequent lines of treatment were administered within a clinical trial (data not shown). The number of hospitalizations also increased across tertiles.
For decedents in the colorectal cancer cohort, rates of cancer-directed colorectal surgery were high, and increased with increasing survival (67%, 78%, and 85% in the bottom, middle, and top tertiles). Surgery dates were clustered around the time of cancer diagnosis. Forty-two percent of decedents in the bottom survival tertile received first-line chemotherapy, compared with 90% and 97% in the middle and top tertiles. Less than 10% of patients with colorectal cancer in the bottom survival tertile received second-line chemotherapy. The percentage of patients receiving second-line chemotherapy regimens was 66% and 92% in the middle and the top tertiles; corresponding figures for third-line chemotherapy were 37% and 74%. Across all survival tertiles, 9% of third and subsequent lines of treatment were administered within a clinical trial (data not shown). The median numbers of hospitalizations per patient in the colorectal cancer cohort were one, two, and two (for the lowest, middle, and top tertiles), and the percentage of patients with two or more hospitalizations increased with increasing survival.
In both cohorts and across all tertiles of survival, a majority of patients had discussions with physicians about EOL care planning (52%–70%). EOL discussions were followed in time by DNR orders and then hospice referral for a minority of patients in all strata. The sensitivity analysis—which included long-term survivors in addition to decedents—showed qualitatively similar patterns of medical interventions compared with the decedent-only analysis, with modest changes in estimates for rates of medical interventions among patients in the top tertile of survival (data not shown).
Discussion
We used data from the population- and health-system based CanCORS study to describe the medical care that is typical for patients with advanced lung and colorectal cancer between diagnosis and death. Survival outcomes in patients with advanced cancer are known to be heterogeneous,13 and our findings demonstrate that patients in different strata of advanced cancer survival also receive distinct patterns of medical care.
Our findings highlight several issues that deserve further consideration. First, use of any hospice care was infrequent, despite broad acceptance of the benefits of hospice care among U.S. specialty societies.14,15 EOL discussions and hospice use showed only modest variation across prognostic groups, with a greater prevalence of EOL discussions in patients with shorter survival, but greater hospice use among patients with longer survival. It is particularly striking that hospice use was not greater among patients with the shortest survival, of whom only 32% (lung cancer) and 24% (colorectal cancer) received any hospice care. Although there is no defined optimal rate of hospice utilization, use of hospice among less than one-third of patients in the lowest tertile of survival suggests an opportunity for improved supportive care in patients presenting with poor-prognosis cancer.
Second, our study findings confirm that patients who live longer receive more medical interventions, including more cancer treatment (especially chemotherapy) and more inpatient hospitalizations. It is likely that patients benefit substantially from many of these interventions. However, the number of chemotherapy regimens received by some patients suggests that that at least some of these regimens are not evidence-supported and are likely to be of limited or no benefit. Examples of treatments with uncertain benefit include the 17% of lung cancer patients in the top survival tertile who received four or more distinct chemotherapy regimens, and the 35% of colorectal cancer patients who received five or more chemotherapy regimens. Treatments received within a clinical trial represented a small proportion (<10%) of these late-line chemotherapy regimens.
Third, we found that clinical trial participation in any line of chemotherapy was infrequent and was skewed toward patients in the longer survival groups. In the lowest survival tertile, 0.8% and 3% of patients with lung and colorectal cancer participated in clinical trials at any point during their care, compared with 9% and 18% of patients in the highest survival tertile. Prior research has shown even lower rates of clinical trial participation in population-based cohorts,16 however, improving access to and participation in clinical trials is a critical goal for enhancing the efficacy of advanced cancer treatment.
Fourth, we found high rates of surgery in patients with metastatic colorectal cancer. More than 75% of patients with colorectal cancer had colorectal surgery, regardless of survival duration. Some of these surgeries were undoubtedly necessitated by tumor obstruction or perforation. Nevertheless, the proportion of patients receiving colorectal surgery is higher than had been previously reported from population-based sources.17 There are no prospective studies to support a benefit from colectomy in patients with uncomplicated primary colorectal tumors, and the role of surgery in patients with metastatic colorectal cancer is controversial,18–20 and clinical experience has shown that patients who do not receive upfront surgery infrequently require subsequent symptom-directed intervention.21
Our study's strengths include the large, population-based cohort of patients with advanced lung and colorectal cancer, who are representative of patients diagnosed with these cancers.11 Interpretation of our data is limited in a number of ways. Our primary analysis included only decedents, whose patterns of care could be observed over the entire trajectory between cancer diagnosis and death. Therefore, our data do not reflect medical interventions received by patients who were diagnosed with stage IV disease but remained alive beyond the time period for which data were available. Nevertheless, a sensitivity analysis that included long-term survivors with available data showed patterns of care that were similar to those observed in the primary analysis. While our study describes medical care that is typical in patients with advanced cancer, these observational data do not allow us to define optimal care. In addition, the benefits of cancer treatment for patients with incurable disease are measured not only by survival, but also by symptom palliation22,23 and the psychological benefit of efforts to treat the disease.24
Although others have evaluated advanced cancer care by focusing on interventions delivered at the end of life,7–9 patients and clinicians must make many critical decisions before those final days. Patients with advanced cancer may be profoundly affected by interventions provided months or even years before death. The benefits of early integration of palliative care in patients with stage IV lung cancer have been demonstrated in a randomized clinical trial,25 emphasizing the importance of prospective, patient-centered care. Measures to evaluate the quality of care for patients with incurable cancer should consider patient experiences throughout their disease trajectory, from diagnosis to death.
In summary, we sought to describe typical patterns of cancer-directed therapy received by patients with metastatic lung and colorectal cancer between diagnosis and death. This perspective is forward-looking and clinically relevant,26 providing a range of estimates of the burdens associated with advanced cancer treatment. These data can be used to help patients make more informed decisions about treatment of advanced cancer.
Acknowledgments
G.A.B. received research support from a Young Investigator Award from the Conquer Cancer Foundation and from a program grant from the National Cancer Institute of the National Institutes of Health (NCI, R25CA09220). N.L.K. was supported by 1R01CA164021-01A1 from the NCI. The work of the CanCORS Consortium was supported by grants from the NCI to the Statistical Coordinating Center (U01 CA093344) and the NCI-supported Primary Data Collection and Research Centers (Dana-Farber Cancer Institute/Cancer Research Network U01 CA093332, Harvard Medical School/Northern California Cancer Center U01 CA093324, RAND/UCLA U01 CA093348, University of Alabama at Birmingham U01 CA093329, University of Iowa U01 CA093339, University of North Carolina U01 CA093326) and by a Department of Veterans Affairs grant to the Durham VA Medical Center CRS 02-164. Contents of this publication are the sole responsibility of the authors, and do not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or the American Society of Clinical Oncology. The CanCORS initiative was funded as a cooperative agreement and hence involved significant participation from several scientific staff from the National Cancer Institute (NCI) and the Department of Veterans Affairs (VA). Specifically, investigators from the NCI and VA collaborated in the design and conduct of the study as well as data collection and management at some study sites but did not participate in analysis, interpretation, or preparation/review of this manuscript.
The authors would like to acknowledge the contributions of Jane C. Weeks, MD, MSc, who was integral to this work but could not see it to completion. Jane, we hope it sings.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Siegel R, Ma J, Zou Z, et al. : Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29 [DOI] [PubMed] [Google Scholar]
- 2.Kato I, Severson RK, Schwartz AG: Conditional median survival of patients with advanced carcinoma. Cancer 2001;92:2211–2219 [DOI] [PubMed] [Google Scholar]
- 3.Peppercorn JM, Smith TJ, Helft PR, et al. : American Society of Clinical Oncology Statement: Toward individualized care for patients with advanced cancer. J Clin Oncol 2011;29:755–760 [DOI] [PubMed] [Google Scholar]
- 4.Matsuyama R, Reddy S, Smith TJ: Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol 2006;24:3490–3496 [DOI] [PubMed] [Google Scholar]
- 5.Bruera E, Sweeney C, Calder K, et al. : Patient preferences versus physician perceptions of treatment decisions in cancer care. J Clin Oncol 2001;19:2883–2885 [DOI] [PubMed] [Google Scholar]
- 6.Zafar SY, Peppercorn JM, Schrag D, et al. : The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. Oncologist 2013;18:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emanuel EJ, Young-Xu Y, Levinsky NG, et al. : Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med 2003;138:639–643 [DOI] [PubMed] [Google Scholar]
- 8.Earle CC, Neville BA, Landrum MB, et al. : Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 2004;22:315–321 [DOI] [PubMed] [Google Scholar]
- 9.Morden NE, Chang CH, Jacobson JO, et al. : End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood) 2012;31:786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayanian JZ, Chrischilles EA, Wallace RB, et al. : Understanding cancer treatment and outcomes: the cancer care outcomes research and surveillance consortium. J Clin Oncol 2004;22:2992–2996 [DOI] [PubMed] [Google Scholar]
- 11.Catalano PJ, Ayanian JZ, Weeks JC, et al. : Representativeness of participants in the cancer care outcomes research and surveillance consortium relative to the surveillance, epidemiology, and end results program. Med Care 2013;51:e9–e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mack JW, Cronin A, Taback N, et al. : End-of-life care discussions among patients with advanced cancer: A cohort study. Ann Intern Med 2012;156:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiely BE, Soon YY, Tattersall MHN, et al. : How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: A systematic review of recent randomized trials. J Clin Oncol 2011;29:456–463 [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. Practice Guidelines in Oncology. Palliative care; www.nccn.org/professionals/physicians_gls/PDP/palliative.pdf (Last accessed November4, 2015) [Google Scholar]
- 15.National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. Pittsburgh, PA: www.nationalconsensusproject.org (Last accessed November4, 2015) [Google Scholar]
- 16.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 2004;291:2720–2726 [DOI] [PubMed] [Google Scholar]
- 17.Cook AD, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: An analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 2005;12:637–645 [DOI] [PubMed] [Google Scholar]
- 18.Ahmed S, Leis A, Fields A, et al. : Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: Results from a large population-based cohort study. Cancer 2014;120:683–691 [DOI] [PubMed] [Google Scholar]
- 19.Clancy C, Burke JP, Barry M, et al. : A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann Surg Oncol 2014;21:3900–3908 [DOI] [PubMed] [Google Scholar]
- 20.Hu CY, Bailey CE, You YN, et al. : Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surg 2015;150:245–251 [DOI] [PubMed] [Google Scholar]
- 21.Poultsides GA, Servais EL, Saltz LB, et al. : Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol 2009;27:3379–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belani CP, Pereira JR, von Pawel J, et al. : Effect of chemotherapy for advanced non-small cell lung cancer on patients' quality of life: A randomized controlled trial. Lung Cancer 2006;53:231–239 [DOI] [PubMed] [Google Scholar]
- 23.Langendijk J, Ten Velde G, Aaronson N, et al. : Quality of life after palliative radiotherapy in non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2000;47:149–55 [DOI] [PubMed] [Google Scholar]
- 24.Buiting HM, Terpstra W, Dalhuisen F, et al. : The facilitating role of chemotherapy in the palliative phase of cancer: Qualitative interviews with advanced cancer patients. PLoS One 2013;8:e77959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733–742 [DOI] [PubMed] [Google Scholar]
- 26.Bach PB, Schrag D, Begg CB: Resurrecting treatment histories of dead patients:A study design that should be laid to rest. JAMA 2004;292:2765–2770 [DOI] [PubMed] [Google Scholar]