Abstract
α4β7 integrin is a cell adhesion receptor that is crucial for the migration of hematopoietic progenitors and mature effector cells in the periphery, but its role in adult hematopoiesis is controversial. We identified a subset of hematopoietic stem cells (HSCs) in the bone marrow (BM) that expressed β7 integrin. These β7+ HSCs were capable of multilineage, long-term reconstitution and had an inherent competitive advantage over β7− HSCs. On the other hand, HSCs that lacked β7 integrin (β7KO) had reduced engraftment potential. Interestingly, quantitative RT-PCR and flow cytometry revealed that β7KO HSCs expressed lower levels of the chemokine receptor CXCR4. Accordingly, β7KO HSCs exhibited impaired migration abilities in vitro and BM homing capabilities in vivo. Lethal irradiation induced expression of the α4β7 integrin ligand—mucosal addressin cell adhesion molecule-1 (MAdCAM-1) on BM endothelial cells. Moreover, blocking MAdCAM-1 reduced the homing of HSCs and impaired the survival of recipient mice. Altogether, these data indicate that β7 integrin, when expressed by HSCs, interacted with its endothelial ligand MAdCAM-1 in the BM microenvironment, thereby promoting HSC homing and engraftment.
Introduction
Hematopoietic stem cells (HSCs) are blood-forming stem cells that are responsible for the continual regeneration of blood and immune cells throughout a person's life. The ability to self-renew and differentiate into all mature types of blood cells is unique to HSCs. Based on these functional properties, HSC transplants are routinely used to treat patients who have hematologic malignancies and other disorders of the blood and immune system. After exposure to high doses of chemotherapy and/or radiation to eradicate dysfunctional and malignant cells, myelosuppressed patients receive transplants of HSCs from healthy donors to reconstitute the patients' hematopoietic system.
After transplantation, the first step necessary for successful engraftment and repopulation of all the hematopoietic lineages is for donor HSCs to migrate and home to the recipient's bone marrow (BM) through the circulatory system. It is believed that the trafficking of HSCs follows a process that resembles leukocyte migration to lymph nodes and inflamed tissues. This multistep process is mediated by direct interactions between circulating cells and endothelial cells (ECs), and occurs through their binding to cell adhesion molecules expressed on the vascular endothelium [1]. The initial interactions between selectins/integrins and their endothelial ligands permit cell tethering and rolling. This is followed by the activation of chemokine receptors and integrins that results in high affinity binding to immunoglobulin superfamily ligands, and enables firm adhesion and transmigration through the endothelial barrier [2,3]. Thus far, studies investigating the molecular interactions that regulate the trafficking of HSCs have established the importance of the chemokine receptor CXCR4/CXCL12 ligand [4–7] and α4β1 integrin/vascular cell adhesion molecule-1 (VCAM-1) adhesion pathways [8–12] for HSCs to home to the BM microenvironment of irradiated recipients. However, despite the identification of a few molecules that have been linked to the HSC homing process, the molecular mechanisms underlying the homing of transplanted HSCs to the BM remain largely unknown.
During the homing process, the dynamic interactions regulating cell adhesion and transendothelial migration are mediated by integrins and their ligands. Integrins are transmembrane cell adhesion receptors composed of single α and β subunits, which bind at the dimeric interface to enable cell–cell and cell–extracellular matrix interactions. The α4 (CD49d) subunit can pair with β1 (CD29) (α4β1 integrin; also known as very late antigen-4 [VLA-4]) to bind VCAM-1 or β7 (α4β7 integrin; also known as lymphocyte Peyer's patch adhesion molecule-1 [LPAM-1]) to bind mucosal addressin cell adhesion molecule-1 (MAdCAM-1) [13]. The α4β1 integrin/VCAM-1 interaction plays an important role in HSC homing to the BM [8–12], but further studies are required to dissect the exact role of α4β7 integrin in HSC trafficking.
In this study, we identified a subset of HSCs that express β7 integrin and showed a competitive advantage in long-term engraftment. Using in vitro transwell migration assays, in vivo homing assays, and various transplantation assays, we provide evidence that the recognition of β7 integrin on HSC surface by its endothelial ligand MAdCAM-1 promotes HSCs to home to and engraft in the BM.
Materials and Methods
Animals
C57BL/Ka, C57BL/Ka-CD45.1, C57BL/Ka-Thy1.1-CD45.1xC57BL/Ka-Thy1.1-CD45.2 F1, and β7 integrin-deficient (β7KO, CD45.2) mice were maintained by the Animal Resource Center of City of Hope or Stanford University under specific pathogen-free conditions. All mice used in this study were matched for sex and age (6–12 weeks). Mouse care and experimental procedures were performed in accordance with the federal guidelines and protocols approved by the Institutional Animal Care and Use Committee at City of Hope and Stanford University Administrative Panel on Laboratory Animal Care.
Flow cytometry and cell sorting
BM cells were harvested by gently crushing femurs and tibias with a mortar and pestle in PBS buffer, and then filtering through a 40 μm strainer (BD Biosciences). For the isolation of HSCs, hematopoietic stem and progenitor cells (HSPCs) were enriched from collected BM cells using the c-kit MicroBeads Kit (Miltenyi Biotec) according to the manufacturer's instructions. The enriched HSPCs were then stained with labeled antibodies against Sca-1, c-kit, CD150, Flk-2, β7 integrin, CD34, CD48, CD3, CD4, CD8, B220, Gr-1, CD11b, and Ter119. To identify BM ECs and stromal cells, BM cells were stained with labeled antibodies specific for CD31, Ter119, CD45, CD105, and MAdCAM-1. Flow cytometry analysis and cell sorting was performed on a 4-laser, 15-detector FACSAria III sorter (BD Biosciences), and all flow cytometry data were analyzed with FlowJo software (TreeStar). All antibodies were conjugated in-house or purchased from BioLegend or eBiosciences, unless otherwise noted.
Transplantation assays
For HSC engraftment, 100 purified HSPCs were double sorted from WT mice and together with 2 × 105 helper whole BM (WBM) cells, were retro-orbitally injected into WT recipient mice irradiated with 900 rads. For competitive reconstitution assays that used β7− and β7+ HSCs, 50 purified β7− and β7+ HSCs (LSK CD150+) were isolated from different congenic WT mice by two rounds of sorting and then, together with 2 × 105 helper WBM cells, were retro-orbitally injected into F1 (CD45.1/CD45.2) recipient mice irradiated with 900 rads. For competitive reconstitution assays using WT and β7KO HSCs, 50 purified HSCs (LSK CD150+Flk2−CD48−) were double sorted from WT (CD45.1) and β7KO (CD45.2) mice and together with 2 × 105 helper WBM cells were retro-orbitally injected into F1 (CD45.1/CD45.2) recipient mice irradiated with 1100 rads. Transplanted mice were bled at 4, 8, 12, and 16 weeks after transplant and donor chimerism was analyzed by staining cells with labeled antibodies specific for B220, CD19, CD3, TCRβ, CD11b, Gr-1, CD45.1, and CD45.2. For transplantation of WT and β7KO HSCs alone, 500 purified HSCs (LSK CD150+Flk2−) were double sorted from WT or β7KO mice then retro-orbitally injected into WT recipient mice irradiated with 900 rads, and their survival was monitored and recorded. For MAdCAM-1-blocking experiments, 200 purified HSCs (LSK CD150+Flk2−) were double sorted from WT mice. WT recipients were irradiated with 900 rads, and after 18 h, recipient mice were treated with isotype control IgG or MAdCAM-1-blocking antibodies (20 mg/kg body weight). Four hours after treatment, HSCs together with 2 × 105 helper WBM cells were retro-orbitally injected, and the survival of transplanted recipients was monitored and recorded.
In vitro transwell migration assay
We purified 20,000 LSK BM cells from WT or β7KO mice, suspended them in Iscove's modified Dulbecco's medium (IMDM; Gibco, Life Technologies) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals), and 10 ng/mL each of SCF and TPO (Peprotech), and placed them in the upper chamber of a transwell insert (5 μm pore size; Costar; Corning). We added 100 ng/mL of CXCL12 (PeproTech) to the lower chamber and after 3 h at 37°C, cells that migrated to the lower chambers were harvested and analyzed by flow cytometry.
In vivo homing assay
We purified 50,000 LSK BM cells from WT or β7KO mice, labeled them with carboxyfluorescein succinimidyl ester (CFSE) and retro-orbitally injected them into WT recipient mice irradiated with 800 rads. Sixteen hours after transplantation, BM cells were harvested from the mice and the number of transplanted CFSE-labeled cells recovered was determined by flow cytometry. For MAdCAM-1-blocking experiments, WT recipients were irradiated with 800 rads, and after 18 h, recipient mice were treated with isotype control IgG or MAdCAM-1-blocking antibodies (20 mg/kg body weight). Four hours after treatment, irradiated recipients were retro-orbitally injected with 50,000 sorted CFSE-labeled WT LSK BM cells. Sixteen hours after transplantation, the number of transplanted CFSE-labeled cells recovered was determined by flow cytometry.
Colony-forming assay
A single-cell CFU-C assay was performed as previously described [14]. Briefly, HSPCs were sorted individually into 96-well round bottom plates containing IMDM (Gibco) supplemented with 10% FBS (Atlanta Biologicals) and 10 ng/mL each of IL-1α, IL-3, IL-5, IL-6, IL-7, IL-9, IL-10, IL-11, GM-CSF, TPO, EPO, SCF, and Flt3L (PeproTech). Cells were cultured for 7 days at 37°C and individual wells were analyzed by flow cytometry to distinguish the different hematopoietic lineages.
Quantitative RT-PCR
Total RNA was isolated from HSCs (LSK CD150+Flk2−CD48−) purified from WT and β7KO mice using the RNeasy Plus Micro Kit (Qiagen) and reverse transcribed using the Sensiscript RT Kit (Qiagen). Quantitative RT-PCR was performed using CXCR4 (Mm01292123_m1) and GAPDH (Mm9999915_g1) TaqMan Gene Expression Assays (Applied Biosystems) in a ViiA7 Real-Time PCR System (Applied Biosystems).
Irradiation experiments
WT mice were irradiated with 1200 rads and peripheral blood (PB), spleen, femurs, and tibias were collected at 6, 12, 18, and 24 h after total body irradiation (TBI). The cell surface expression of MAdCAM-1 on BM cells and splenocytes was analyzed by flow cytometry. BM supernatant was prepared by aspirating the marrow from tibias and femurs using 500 μL PBS and then collecting the supernatant. Aliquots of BM supernatant and PB plasma samples were stored at −80°C until used. The concentrations of cytokines/chemokines in the BM supernatant and PB plasma were analyzed in duplicate using the MILLIPLEX MAP Multiplex Assay (Millipore). The concentration was determined for these 31 mouse cytokines/chemokines: eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, interleukin (IL)-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IFN-γ-inducible protein 10 (IP-10), keratinocyte-derived chemokine (KC), leukemia inhibitory factor (LIF), lipopolysaccharide-inducible CXC chemokine (LIX), monocyte chemotactic protein-1 (MCP-1), macrophage colony-stimulating factor (M-CSF), monokine induced by IFN-γ (MIG), macrophage inflammatory proteins (MIP)-1α, MIP-1β, MIP-2, regulated on activation normal T-cell expressed and secreted (RANTES), and tumor necrosis factor (TNF)-α.
Statistical analyses
Statistically significant differences were calculated with paired or unpaired two-tailed Student's t-test or analysis of variance using GraphPad Prism software v.6. Survival curves were analyzed using the Kaplan–Meier analysis. A P value of at least <0.05 was considered significant. All pooled values are expressed as mean ± SEM.
Results
A subset of HSCs in the BM express β7 integrin
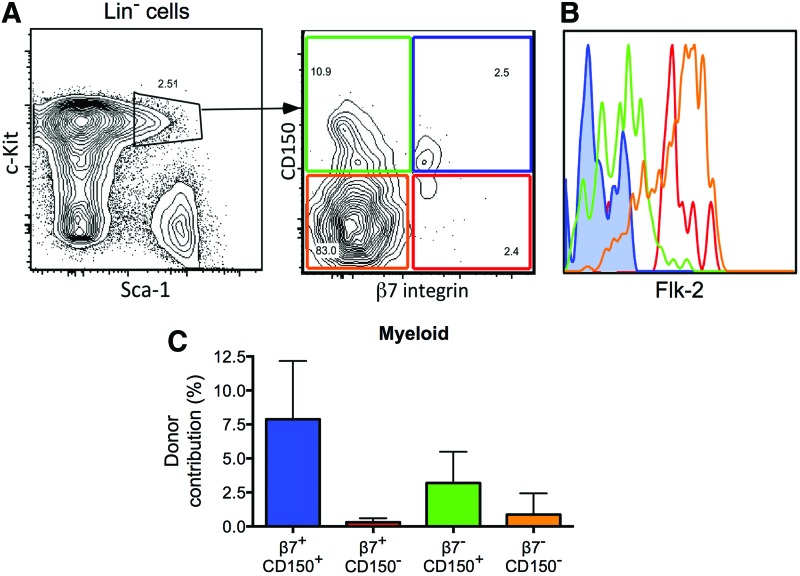
We used flow cytometry to determine whether HSCs express β7 integrin, and we detected a small subpopulation of HSCs (LSK CD150+) in the BM that expressed high levels of β7 integrin on their cell surface (β7+ HSCs; Fig. 1A). These β7+ HSCs expressed low levels of the differentiation marker Flk-2 (Fig. 1B). When we assessed the in vitro differentiation potential of these β7+ HSCs in single-cell colony-forming assays [14], using the chi-square test, we did not find any significant difference between β7+ and β7 negative to low (β7−) HSCs (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd). We then compared the long-term repopulating potential of the β7+ and β7− HSCs by transplanting 100 β7− or β7+ HSCs (LSK CD150+), together with 2 × 105 BM helper cells, into lethally irradiated mice. We found that both β7− and β7+ HSCs were able to repopulate the lymphoid and myeloid lineages 16 weeks after transplant (Fig. 1C and Supplementary Fig. S1B, C). Collectively, these data indicate that β7 integrin was highly expressed on a subset of HSCs in the BM, which, like β7− HSCs, were capable of multilineage, long-term reconstitution of irradiated recipients.
FIG. 1.
β7+ hematopoietic stem cells (HSCs) are capable of multilineage, long-term reconstitution. (A) Representative profile of flow cytometry analyses of CD150 and β7 integrin expression on Lin−Sca-1+c-kit+ (LSK) bone marrow (BM) cells. (B) Representative histogram of flow cytometry analyses of Flk-2 expression on cell populations specified in (A) (β7−CD150+ cells shown in green; β7+CD150+ cells shown in blue; β7−CD150− cells shown in orange; β7+CD150− cells shown in red). (C) Frequency of donor-derived myeloid cells in the peripheral blood of recipient mice at 16 weeks after transplantation with 100 test cells plus 2 × 105 competitor whole BM (WBM) cells. Data are mean ± SEM (n = 13 per group, from three independent experiments). Color images available online at www.liebertpub.com/scd
β7 integrin expression on HSCs enhances their engraftment in the BM
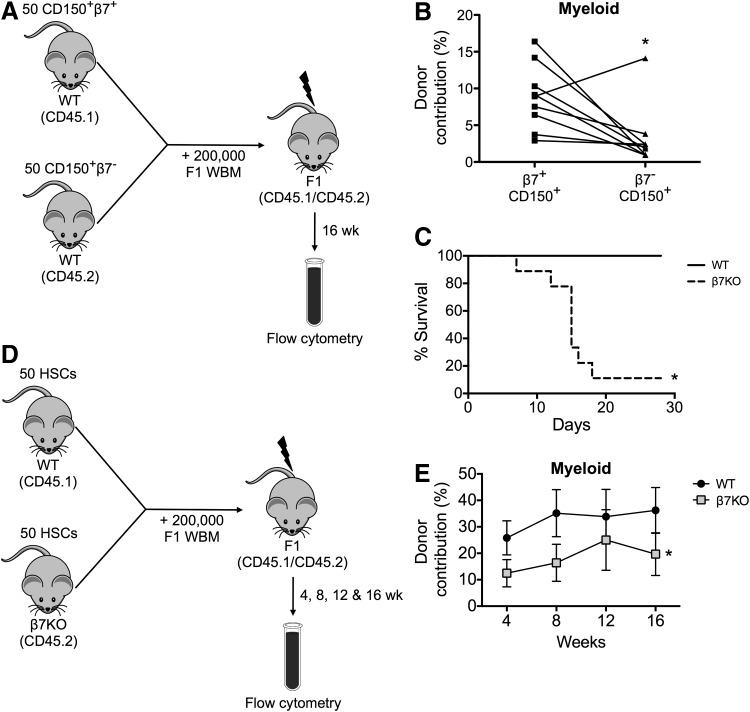
Since we observed a trend toward an increased engraftment ability of β7+ HSCs when compared to β7− HSCs in the standard transplantation assay, we next characterized the engraftment potential of β7+ HSCs in a more rigorous assay that involves competitive reconstitution. We sorted 50 β7− and β7+ HSCs (LSK CD150+) from different congenic donors and transplanted them together with 2 × 105 helper BM cells into lethally irradiated recipients (CD45.1/CD45.2 F1) (Fig. 2A). We detected significantly higher myeloid contributions from β7+ HSCs at 16 weeks after transplantation compared to the β7− HSCs that were simultaneously transplanted into the same lethally irradiated mice (Fig. 2B and Supplementary Fig. S2B). These data suggest that β7+ HSCs had an enhanced engraftment potential compared to β7− HSCs under transplant conditions. To extend these observations, we evaluated the engraftment potential of HSCs that lack β7 integrin. First, we sorted 500 HSCs (LSK CD150+Flk2−) from the BM of WT or β7 integrin-deficient (β7KO) mice, and transplanted them into lethally irradiated recipients. We observed reduced survival of recipient mice transplanted with β7KO HSCs compared to mice transplanted with WT HSCs (Fig. 2C), demonstrating that 500 β7KO HSCs could not rescue irradiated recipients. We next sorted 50 HSCs (LSK CD150+CD48−Flk2−) from the BM of WT (CD45.1) or β7KO (CD45.2) mice, and transplanted them together with 2 × 105 helper BM cells into lethally irradiated recipients (CD45.1/CD45.2 F1; Fig. 2D and Supplementary Fig. S2A). While CD45.2 hematopoietic cells have an inherent engraftment advantage [15,16], we found that the CD45.1 WT HSCs outcompeted the CD45.2 β7KO HSCs and thus, the β7KO HSCs had a significantly reduced ability to reconstitute the lymphoid and myeloid lineages as compared to competitor WT HSCs (Fig. 2E and Supplementary Fig. S2C). Consistent with our previous findings, these results demonstrated that the repopulating capacity of β7KO HSCs was reduced in direct competition with WT HSCs. Taken together, these data indicate that the expression of β7 integrin on HSCs conferred them with a competitive advantage during engraftment.
FIG. 2.
β7 expression on HSCs enhances engraftment potential. (A) Experimental design of competitive reconstitution assay using CD150+β7+ and CD150+β7− LSK BM cells. (B) Frequency of donor-derived myeloid cells in the peripheral blood of recipient mice at 16 weeks after transplantation (n = 9 per group, from three independent experiments, *P < 0.05). (C) Lethally irradiated mice were transplanted with 500 WT or β7KO HSCs and their survival was monitored (n = 8–9 per group, from two independent experiments, *P < 0.05). (D) Experimental design of competitive reconstitution assay using wild type (WT) and β7KO HSCs. (E) Frequency of donor-derived myeloid cells in the peripheral blood of recipient mice between 4 and 16 weeks after transplantation. Data are mean ± SEM (n = 11–17 per group, from four independent experiments, *P < 0.05).
β7 integrin deficiency impairs the homing of HSCs to the BM
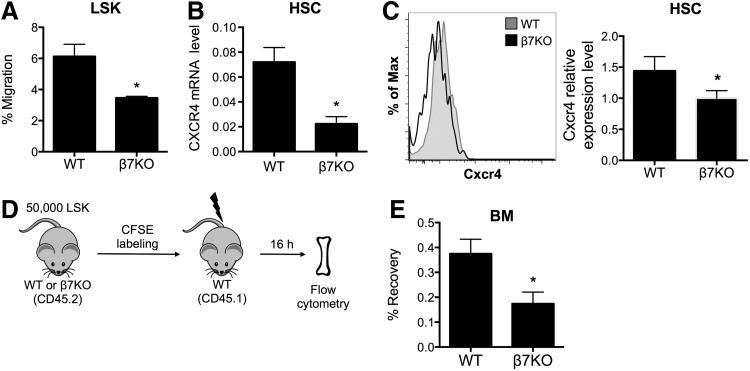
Our observation that the repopulating capacity of β7KO HSCs was reduced suggests that β7 integrin may play a role in HSC homing to the BM after transplantation into irradiated recipients. CXCL12/CXCR4 signaling plays an important role in the trafficking of HSCs, including homing, lodgment, and retention. To assess the homing abilities of β7KO HSCs, we performed an in vitro transwell migration assay using sorted WT and β7KO LSK cells. We observed that the migration of β7KO LSK cells toward CXCL12, a chemoattractant for HSPCs, was decreased compared to WT LSK cells (Fig. 3A). Because CXCL12 signaling is mediated through the chemokine receptor CXCR4, we next examined if CXCR4 was expressed by β7KO HSCs. We found that β7KO HSCs expressed a significantly lower level of CXCR4 mRNA (Fig. 3B) and cell surface protein (Fig. 3C) compared to WT HSCs. To then evaluate the in vivo homing potential of β7KO HSCs, we transplanted 50,000 CFSE-labeled WT or β7KO LSK cells into lethally irradiated mice and quantified the number of CFSE+ donor cells that were recovered from the recipient's BM 16 h after transplantation (Fig. 3D and Supplementary Fig. S3). Consistent with the transwell migration assay, significantly fewer β7KO LSK cells were recovered from the BM of lethally irradiated mice compared to WT LSK cells (Fig. 3E). These data indicate that the ability of β7KO HSCs to home to the BM is reduced after transplantation into lethally irradiated recipients. Altogether, the results support our hypothesis that β7 integrin enhances HSC homing to the BM during transplantation.
FIG. 3.
β7KO HSCs have impaired homing capabilities. (A) Frequency of WT and β7KO LSK cells that migrated toward CXCL12 in the transwell migration assay. Data are mean ± SEM (n = 8–11 per group, from three independent experiments, *P < 0.05). (B) Quantitative real-time PCR for CXCR4 mRNA levels in sorted WT and β7KO HSCs. Samples were run in triplicate and each value was normalized to GAPDH expression. Data are mean ± SEM (*P < 0.05). (C) Representative histogram of flow cytometry analysis (left) and CXCR4 relative expression level (right) on WT (gray) and β7KO (black) HSCs (n = 4 per group, from two independent experiments, *P < 0.05). (D) Experimental design of the in vivo homing assay. (E) Frequency of CFSE+-transplanted cells that were recovered in the BM of recipient mice at 16 h after transplantation. Data are mean ± SEM (n = 6–8 per group, from three independent experiments, *P < 0.05).
Blocking MAdCAM-1 reduces the homing of HSCs to the BM and survival of irradiated recipients
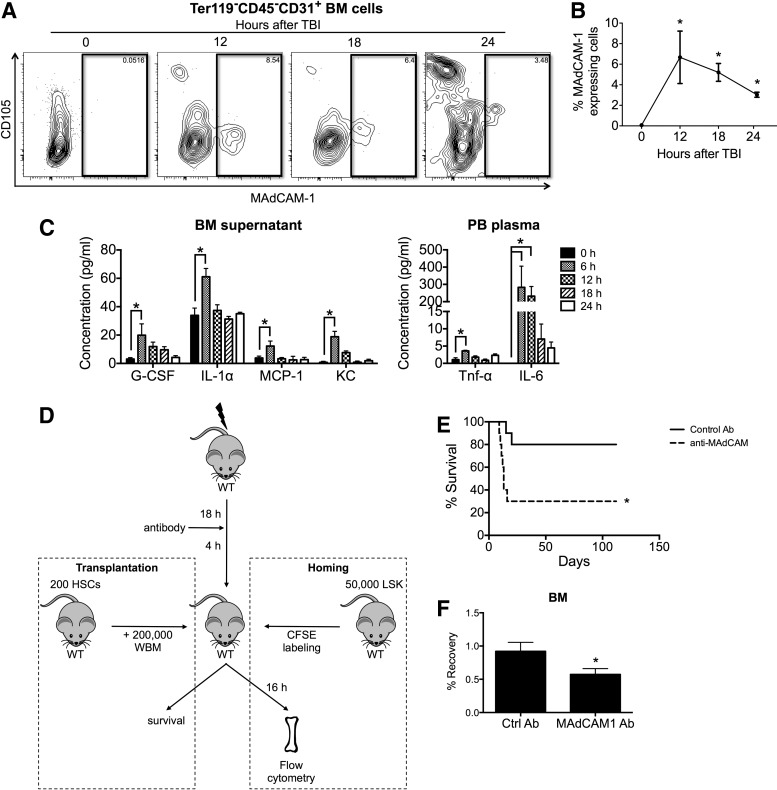
Myelosuppression induces an inflammatory environment within the BM that is associated with higher levels of proinflammatory cytokines [17]. Expression of MAdCAM-1 is regulated by the NF-κB and PI3K/AKT signaling pathways [18–21], both of which strongly correlate with the inflammatory response. Indeed, treatments with a variety of proinflammatory molecules, including lipopolysaccharide, TNF-α, IL-1β, IFN-γ, and CXCL12 [18,19,21–23], can enhance MAdCAM-1 expression in EC cell lines. However, it is unknown whether endothelial MAdCAM-1 expression within the BM microenvironment is regulated by similar molecular mechanisms. Therefore, we examined whether myelosuppression could trigger MAdCAM-1 expression within the BM microenvironment. Mice treated with TBI and MAdCAM-1 expression in the BM were analyzed by flow cytometry at specific times after irradiation. We observed a significant increase in the frequency of MAdCAM-1-expressing ECs (Ter119−CD45−CD31+ BM cells) in the BM after lethal irradiation (Fig. 4A, B and Supplementary Fig. S4). Furthermore, we found that the concentrations of G-CSF, IL-1α, MCP-1, and KC in the BM supernatant, and those of TNF-α and IL-6 in the PB plasma of lethally irradiated mice, were significantly increased after TBI (Fig. 4C). Collectively, the data indicate that irradiation triggered the release of proinflammatory cytokines and enhanced levels of MAdCAM-1 expression on BM and spleen ECs and stromal cells.
FIG. 4.
MAdCAM-1 expression on BM ECs increases in response to lethal irradiation and blocking MAdCAM-1 reduces the survival of irradiated recipients due to decreased BM homing. (A) Representative profile of flow cytometry analyses of MAdCAM-1 expression on Ter119−CD45−CD31+ BM endothelial cells (ECs) at the indicated times after total body irradiation (TBI). (B) Frequency of MAdCAM-1-expressing cells among BM ECs at the indicated times after TBI. Data are mean ± SEM (n = 4 per group, representative of three independent experiments, *P < 0.05). (C) Concentration of cytokines and chemokines in the BM supernatant (left) and peripheral blood plasma (right) at the indicated times after TBI. Samples were run in duplicate. Data are mean ± SEM (n = 2–4 per group, *P < 0.05). (D) Experimental design of the transplantation assay and in vivo homing assay using a control or MAdCAM-1 blocking antibody. (E) Survival of lethally irradiated recipients treated with control antibody or MAdCAM-1-blocking antibody before HSC transplantation (n = 10 per group, from two independent experiments, *P < 0.05). (F) Frequency of CFSE+-transplanted cells that were recovered in the BM of recipient mice at 16 h after transplantation. Data are mean ± SEM (n = 5–8 per group, from three independent experiments, *P < 0.05).
Since our data suggest that β7 integrin can promote the homing of HSCs and indicate that TBI induces MAdCAM-1 expression on BM ECs, we hypothesized that the ability of α4β7 integrin to influence the homing of HSCs can be mediated through a specific interaction with the MAdCAM-1 ligand in the BM microenvironment. To test this hypothesis, we used a MAdCAM-1 antibody to block its interaction with α4β7 integrin. We found that lethally irradiated mice treated with the MAdCAM-1 blocking antibody before HSC transplantation had a lower survival rate than those treated with an isotype control antibody (Fig. 4D, E). Moreover, significantly fewer WT LSK cells were recovered from the BM of irradiated mice that received the MAdCAM-1 blocking antibody compared to those that received an isotype control antibody (Fig. 4D–F). These data indicate that antibody blocking of the interactions between β7 integrin and MAdCAM-1 severely limited the ability of HSCs to home to the BM after transplantation. Together, these results support our hypothesis that β7 integrin binds to MAdCAM-1 to mediate an interaction between HSCs and the BM microenvironment that facilitates HSC homing.
Discussion
This study identified a subset of HSCs that express high levels of surface β7 integrin. Functionally, these β7+ HSCs exhibited a competitive advantage over the corresponding β7− HSCs for long-term engraftment in lethally irradiated mice. Conversely, either genetic β7 deficiency or inhibiting MAdCAM-1 through blocking antibody impaired the abilities of HSCs to home to and engraft in the BM after transplantation. Altogether, our data indicates that β7 integrin on HSC surface, by interacting with its endothelial ligand MAdCAM-1 in the BM, can enhance HSC homing and subsequent engraftment.
Recent evidence suggests that the primitive HSC pool is comprised of several distinct HSC subtypes that functionally differ in their lineage potential and self-renewal capacity [24,25]. According to the expression patterns of CD150 and β7 integrin in the LSK cell population, we identified a subset of β7+ HSCs (LSK CD150+) in the BM at steady state (Fig. 1A, B). Prior studies have demonstrated the expression of α4β7 integrin on a heterogeneous population of murine HSPCs [26]. It was previously reported that the expression of β7 appeared to be restricted to various mature hematopoietic cells and committed progenitors [27]. However, we showed that β7+ HSCs were capable of reconstituting myeloid and lymphoid populations in irradiated mice long term without obvious lineage bias (Fig. 1C and Supplementary Fig. S1B). These data provide functional evidence against the possibility that the surface expression of β7 on HSCs is a marker for a homogeneous but lineage-restricted progenitor population. While the repopulating potential of β7+ and β7− HSCs differed, no difference was observed in their lineage potential in vivo. This may suggest functional differences in HSCs, however, further studies are required.
Our finding that β7KO HSCs have reduced migration toward CXCL12 in vitro (Fig. 3A) prompted us to investigate CXCR4/CXCL12 signaling. We discovered that β7KO HSCs expressed significantly lower levels of CXCR4 mRNA (Fig. 3B) and cell surface protein (Fig. 3C) compared to WT HSCs. Therefore, it seems likely that the reduced migration of the β7KO LSK cells is caused by the lower cell surface expression of the CXCR4 receptor, which impairs the ability of the β7KO LSK cells to respond to its ligand CXCL12. Since both adhesion molecules and chemokines play crucial roles in BM homing, this led us to hypothesize that the engraftment defect of β7KO HSCs may primarily reflect inefficient homing to the BM after transplantation. Indeed, we found that the ability of β7KO HSCs to populate the BM in vivo was severely impaired following transplantation into irradiated recipients (Fig. 3E). This is in agreement with previous studies demonstrating that antibody blocking of α4β7 integrin on donor WBM cells impedes cell rolling on the BM vasculature [26] and subsequently BM homing after transplant [26,28]. Altogether, we conclude that the impaired ability of β7KO HSCs to home into the BM of irradiated hosts after transplantation accounted for their reduced engraftment potential.
Various factors such as Robo4 [29] and sphingophospholipids (S1P and C1P) [30] may influence HSC homing. Although some recent studies have questioned the role of CXCR4 in HSC homing in fetal liver hematopoiesis [31] and CXCR4-overexpression studies [32], it is commonly accepted that the CXCR4/CXCL12 axis plays a significant role in the BM homing of transplanted HSPCs [33,34]. Consistent with the role of CXCR4 in HSC homing, we observed that β7KO HSCs were impaired in their ability to migrate toward CXCL12 in vitro and home to BM in vivo (Fig. 3A–E). However, it is unclear how CXCR4 expression is mechanistically regulated by β7 integrin. One study has reported the integrin-mediated upregulation of CXCR4 expression on pancreatic cancer cells [35]. It is thus possible that β7 integrin expression on HSCs may directly regulate CXCR4 cell surface expression and/or reduce its internalization. Alternatively, several reports have shown that exposure to hypoxia led to upregulation of CXCR4 expression in a diverse range of cancer cells [36–42]. Thus, the surface expression of β7 integrin on HSCs may influence HSC locomotion within a specific BM niche, where low oxygen tension may favor the expression of CXCR4.
Although β7 deficiency reduced CXCR4 mRNA level in HSCs by two-third, the surface expression was only partly affected (1.5-fold) (Fig. 3B, C). Since CXCR4 internalization and degradation is dynamically regulated, the cell surface staining does not necessarily parallel CXCR4 protein levels. CXCR4 expression is influenced by a wide variety of physiological stimuli [43]. CXCL12 ligand binding also causes receptor activation and subsequent rapid internalization [44–46]. Following internalization, CXCR4 can be marked by ubiquitination for lysosomal degradation [47] or recycled back to the plasma membrane for further ligand binding [46]. It has been shown that in many different cell types, lack of CXC12 rapidly upregulated CXCR4 surface expression during short-term culture [34,48], suggesting that CXCR4 cell surface expression and internalization is tightly regulated posttranslationally.
Consistent with reports by Tada and colleagues, we observed that MAdCAM-1 expression was induced on BM ECs within 24 h after lethal irradiation (Fig. 4A, B) [28,49]. Moreover, we found that blocking MAdCAM-1 lowered survival rate (Fig. 4E) and reduced HSC homing to the BM (Fig. 4F) in the HSC-transplanted mice. These data suggest that the activity of α4β7 integrin on HSCs was mediated through its specific interaction with its endothelial ligand MAdCAM-1 in the BM microenvironment. Additionally, because lethal irradiation also induces the expression of fibronectin and VCAM-1 on BM ECs [28,49], we cannot exclude the possibility that α4β7 integrin binding to these low-affinity ligands may also be involved in HSC homing to the BM.
The role of α4β7 integrin in HSC trafficking has remained unclear. Previous studies have suggested a potential role for α4β7 integrin and MAdCAM-1 in HSC homing to the recipient's BM using cell lines and blocking antibodies. However, the use of a mixed population of HSPCs, not a purified HSC population, made it difficult to interpret their results [26,28]. Therefore, the homing of HSCs, committed progenitors and terminally differentiated mature hematopoietic cells cannot be distinguished from one another, and thus, whether or not the outcomes are the consequence of a direct effect on HSCs cannot be determined. Here, our KO mouse studies using highly purified cell populations clearly demonstrate the importance of α4β7 integrin for the BM homing of transplanted HSCs. We performed our in vivo homing assay by transplanting sorted β7KO LSK cells and then used the CFSE label to distinguish homed donor HSCs from the residual host cells in the recipient BM. Our findings are consistent with previous studies by Katayama et al. in which they transplanted whole BM cells that had been treated with the α4β7 blocking antibody and then used a CFU-C assay to quantify the number of transplanted cells that had homed to the BM.
In summary, our study indicates that β7 integrin on the HSC surface interacts with endothelial MAdCAM-1 in the BM to promote the homing of HSCs and thereby enhance their engraftment. A better understanding of how the differential expression of adhesion molecules influences the trafficking of individual subsets of circulating HSCs will support the development of novel strategies for controlling the fate of transplanted HSCs, thus, improving the engraftment efficiency of clinical HSC transplantation.
Supplementary Material
Acknowledgments
The authors thank the Analytical Cytometry Core and Animal Resource Center at City of Hope for their excellent technical support and Margaret Morgan for critical review and editorial assistance. This study was supported, in part, by grants from the Children Leukemia Research Association, the ThinkCure! Foundation, the Margaret E. Early Medical Research Trust, the Tim Nesvig Lymphoma Research Fund and the NIH grant HL087936 (to C.-C.C.), NIH grants AI23990, AI070813, and CA72074 (to S.J.G.), and CA086065 and HL058770 (to I.L.W.). J.L.M. was supported by a Parsons Foundation Fellowship and NIH fellowship 1F31HL114393-01A1. X.H. was supported by a California Institute for Regenerative Medicine (CIRM) training grant. Research reported in this publication included work performed in the Analytical Cytometry Core and Animal Resource Center supported by the National Cancer Institute of the National Institutes of Health under award number P30CA33572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Laird DJ, von Andrian UH. and Wagers AJ. (2008). Stem cell trafficking in tissue development, growth, and disease. Cell 132:612–630 [DOI] [PubMed] [Google Scholar]
- 2.Garrood T, Lee L, and Pitzalis C. (2006). Molecular mechanisms of cell recruitment to inflammatory sites: general and tissue-specific pathways. Rheumatology (Oxford) 45:250–260 [DOI] [PubMed] [Google Scholar]
- 3.Lapidot T, Dar A. and Kollet O. (2005). How do stem cells find their way home? Blood 106:1901–1910 [DOI] [PubMed] [Google Scholar]
- 4.Lapidot T. and Kollet O. (2002). The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 16:1992–2003 [DOI] [PubMed] [Google Scholar]
- 5.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, et al. (2000). The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 95:3289–3296 [PubMed] [Google Scholar]
- 6.Plett PA, Frankovitz SM, Wolber FM, Abonour R. and Orschell-Traycoff CM. (2002). Treatment of circulating CD34(+) cells with SDF-1alpha or anti-CXCR4 antibody enhances migration and NOD/SCID repopulating potential. Exp Hematol 30:1061–1069 [DOI] [PubMed] [Google Scholar]
- 7.Tzeng YS, Li H, Kang YL, Chen WC, Cheng WC. and Lai DM. (2011). Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood 117:429–439 [DOI] [PubMed] [Google Scholar]
- 8.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV. and Wolf NS. (1995). The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A 92:9647–9651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott LM, Priestley GV. and Papayannopoulou T. (2003). Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol 23:9349–9360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams DA, Rios M, Stephens C. and Patel VP. (1991). Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature 352:438–441 [DOI] [PubMed] [Google Scholar]
- 11.Frenette PS, Subbarao S, Mazo IB, von Andrian UH. and Wagner DD. (1998). Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci U S A 95:14423–14428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch E, Iglesias A, Potocnik AJ, Hartmann U. and Fassler R. (1996). Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature 380:171–175 [DOI] [PubMed] [Google Scholar]
- 13.Gorfu G, Rivera-Nieves J. and Ley K. (2009). Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med 9:836–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco CB, Chen CC, Drukker M, Weissman IL. and Galli SJ. (2010). Distinguishing mast cell and granulocyte differentiation at the single-cell level. Cell Stem Cell 6:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu S, Ray A. and Dittel BN. (2013). Differential representation of B cell subsets in mixed bone marrow chimera mice due to expression of allelic variants of CD45 (CD45.1/CD45.2). J Immunol Methods 396:163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waterstrat A, Liang Y, Swiderski CF, Shelton BJ. and Van Zant G. (2010). Congenic interval of CD45/Ly-5 congenic mice contains multiple genes that may influence hematopoietic stem cell engraftment. Blood 115:408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cachaco AS, Carvalho T, Santos AC, Igreja C, Fragoso R, Osorio C, Ferreira M, Serpa J, Correia S, Pinto-do OP. and Dias S. (2010). TNF-alpha regulates the effects of irradiation in the mouse bone marrow microenvironment. PloS One 5:e8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanida S, Mizoshita T, Mizushima T, Sasaki M, Shimura T, Kamiya T, Kataoka H. and Joh T. (2011). Involvement of oxidative stress and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in inflammatory bowel disease. J Clin Biochem Nutr 48:112–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizushima T, Sasaki M, Ando T, Wada T, Tanaka M, Okamoto Y, Ebi M, Hirata Y, Murakami K, et al. (2010). Blockage of angiotensin II type 1 receptor regulates TNF-alpha-induced MAdCAM-1 expression via inhibition of NF-kappaB translocation to the nucleus and ameliorates colitis. Am J Physiol Gastrointest Liver Physiol 298:G255–G266 [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi M. and Baichwal VR. (1995). Induction of the gene encoding mucosal vascular addressin cell adhesion molecule 1 by tumor necrosis factor alpha is mediated by NF-kappa B proteins. Proc Natl Acad Sci U S A 92:3561–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa H, Binion DG, Heidemann J, Theriot M, Fisher PJ, Johnson NA, Otterson MF. and Rafiee P. (2005). Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells. Am J Physiol Cell Physiol 288:C272–C281 [DOI] [PubMed] [Google Scholar]
- 22.Chaitanya GV, Franks SE, Cromer W, Wells SR, Bienkowska M, Jennings MH, Ruddell A, Ando T, Wang Y, et al. (2010). Differential cytokine responses in human and mouse lymphatic endothelial cells to cytokines in vitro. Lymphat Res Biol 8:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connor EM, Eppihimer MJ, Morise Z, Granger DN. and Grisham MB. (1999). Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol 65:349–355 [DOI] [PubMed] [Google Scholar]
- 24.Muller-Sieburg CE, Sieburg HB, Bernitz JM. and Cattarossi G. (2012). Stem cell heterogeneity: implications for aging and regenerative medicine. Blood 119:3900–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Challen GA, Boles NC, Chambers SM. and Goodell MA. (2010). Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell 6:265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katayama Y, Hidalgo A, Peired A. and Frenette PS. (2004). Integrin alpha4beta7 and its counterreceptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood 104:2020–2026 [DOI] [PubMed] [Google Scholar]
- 27.Voura EB, Billia F, Iscove NN. and Hawley RG. (1997). Expression mapping of adhesion receptor genes during differentiation of individual hematopoietic precursors. Exp Hematol 25:1172–1179 [PubMed] [Google Scholar]
- 28.Tada T, Inoue N, Widayati DT. and Fukuta K. (2008). Role of MAdCAM-1 and its ligand on the homing of transplanted hematopoietic cells in irradiated mice. Exp Anim 57:347–356 [DOI] [PubMed] [Google Scholar]
- 29.Smith-Berdan S, Nguyen A, Hassanein D, Zimmer M, Ugarte F, Ciriza J, Li D, Garcia-Ojeda ME, Hinck L. and Forsberg EC. (2011). Robo4 cooperates with CXCR4 to specify hematopoietic stem cell localization to bone marrow niches. Cell Stem Cell 8:72–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratajczak MZ, Kim CH, Abdel-Latif A, Schneider G, Kucia M, Morris AJ, Laughlin MJ. and Ratajczak J. (2012). A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia 26:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foudi A, Jarrier P, Zhang Y, Wittner M, Geay JF, Lecluse Y, Nagasawa T, Vainchenker W. and Louache F. (2006). Reduced retention of radioprotective hematopoietic cells within the bone marrow microenvironment in CXCR4-/- chimeric mice. Blood 107:2243–2251 [DOI] [PubMed] [Google Scholar]
- 32.Lai CY, Yamazaki S, Okabe M, Suzuki S, Maeyama Y, Iimura Y, Onodera M, Kakuta S, Iwakura Y, et al. (2014). Stage-specific roles for CXCR4 signaling in murine hematopoietic stem/progenitor cells in the process of bone marrow repopulation. Stem Cells 32:1929–1942 [DOI] [PubMed] [Google Scholar]
- 33.Sharma M, Afrin F, Satija N, Tripathi RP. and Gangenahalli GU. (2011). Stromal-derived factor-1/CXCR4 signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cells Dev 20:933–946 [DOI] [PubMed] [Google Scholar]
- 34.Nagasawa T. (2014). CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med 92:433–439 [DOI] [PubMed] [Google Scholar]
- 35.Grzesiak JJ, Smith KC, Burton DW, Deftos LJ. and Bouvet M. (2007). Integrin-mediated laminin-1 adhesion upregulates CXCR4 and IL-8 expression in pancreatic cancer cells. Surgery 141:804–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh YS, Kim HY, Song IC, Yun HJ, Jo DY, Kim S. and Lee HJ. (2012). Hypoxia induces CXCR4 expression and biological activity in gastric cancer cells through activation of hypoxia-inducible factor-1alpha. Oncol Rep 28:2239–2246 [DOI] [PubMed] [Google Scholar]
- 37.Cronin PA, Wang JH. and Redmond HP. (2010). Hypoxia increases the metastatic ability of breast cancer cells via upregulation of CXCR4. BMC Cancer 10:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, et al. (2003). Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med 198:1391–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azab AK, Weisberg E, Sahin I, Liu F, Awwad R, Azab F, Liu Q, Griffin JD. and Ghobrial IM. (2013). The influence of hypoxia on CML trafficking through modulation of CXCR4 and E-cadherin expression. Leukemia 27:961–964 [DOI] [PubMed] [Google Scholar]
- 40.Weisberg E, Azab AK, Manley PW, Kung AL, Christie AL, Bronson R, Ghobrial IM. and Griffin JD. (2012). Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia 26:985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo M, Cai C, Zhao G, Qiu X, Zhao H, Ma Q, Tian L, Li X, Hu Y, et al. (2014). Hypoxia promotes migration and induces CXCR4 expression via HIF-1alpha activation in human osteosarcoma. PloS One 9:e90518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubo M, Li TS, Kamota T, Ohshima M, Qin SL. and Hamano K. (2009). Increased expression of CXCR4 and integrin alphaM in hypoxia-preconditioned cells contributes to improved cell retention and angiogenic potency. J Cell Physiol 220:508–514 [DOI] [PubMed] [Google Scholar]
- 43.Busillo JM. and Benovic JL. (2007). Regulation of CXCR4 signaling. Biochim Biophys Acta 1768:952–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orsini MJ, Parent JL, Mundell SJ, Marchese A. and Benovic JL. (1999). Trafficking of the HIV coreceptor CXCR4. Role of arrestins and identification of residues in the c-terminal tail that mediate receptor internalization. J Biol Chem 274:31076–31086 [DOI] [PubMed] [Google Scholar]
- 45.Haribabu B, Richardson RM, Fisher I, Sozzani S, Peiper SC, Horuk R, Ali H. and Snyderman R. (1997). Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J Biol Chem 272:28726–28731 [DOI] [PubMed] [Google Scholar]
- 46.Signoret N, Oldridge J, Pelchen-Matthews A, Klasse PJ, Tran T, Brass LF, Rosenkilde MM, Schwartz TW, Holmes W, et al. (1997). Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol 139:651–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchese A. and Benovic JL. (2001). Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem 276:45509–45512 [DOI] [PubMed] [Google Scholar]
- 48.Shiba Y, Takahashi M, Hata T, Murayama H, Morimoto H, Ise H, Nagasawa T. and Ikeda U. (2009). Bone marrow CXCR4 induction by cultivation enhances therapeutic angiogenesis. Cardiovasc Res 81:169–177 [DOI] [PubMed] [Google Scholar]
- 49.Tada T. and Fukuta K. (2010). Expression of cell adhesion molecules at the collapse and recovery of haematopoiesis in bone marrow of mouse. Anat Histol Embryol 39:403–410 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.