Abstract
Oral preexposure prophylaxis (PrEP) trials report disparate efficacy attributed to variable adherence. HPTN 066 was conducted to establish objective, quantitative benchmarks for discrete, regular levels of adherence using directly observed dosing of tenofovir (TFV) disoproxil fumarate (TDF)/emtricitabine (FTC). Healthy, HIV-uninfected men and women were randomized to one of four oral regimens of fixed-dose TDF 300 mg/FTC 200 mg tablet for 5 weeks with all doses observed: one tablet weekly (one/week), one tablet twice weekly (two/week), two tablets twice weekly (four/week), or one tablet daily (seven/week). Trough serum TFV and FTC, peripheral blood mononuclear cell (PBMC), and CD4+ TFV-diphosphate (TFV-DP) and FTC-triphosphate (FTC-TP) concentrations were determined throughout dosing and 2 weeks after the last dose. Rectosigmoidal, semen, and cervicovaginal samples were collected for drug assessment at end of dosing and 2 weeks later in a subset of participants. The 49 enrolled participants tolerated the regimens well. All regimens achieved steady-state concentrations by the second dose for serum TFV/FTC and by 7 days for PBMC TFV-DP/FTC-TP. Steady-state median TFV-DP predose concentrations demonstrated dose proportionality: one/week 1.6 fmol/106 PBMCs, two/week 9.1, four/week 18.8, seven/week, 36.3. Further, TFV-DP was consistently quantifiable 2 weeks after the last dose for the ≥4/week regimens. Adherence benchmarks were identified using receiver operating characteristic curves, which had areas under the curve ≥0.93 for all analytes in serum and PBMCs. Intersubject and intrasubject coefficients of variation (%CV) ranged from 33% to 63% and 14% to 34%, respectively, for all analytes in serum and PBMCs. Steady-state PBMC TFV-DP was established earlier and at lower concentrations than predicted and was the only analyte demonstrating predose concentration dose proportionality. Steady-state daily dosing serum TFV and PBMC TFV-DP was consistent with highly effective PrEP clinical trials. HPTN 066 provides adherence benchmarks for oral TFV/FTC regimens to assist interpreting study outcomes.
Introduction
Randomized clinical trials of HIV preexposure prophylaxis (PrEP) have demonstrated the efficacy of daily oral tenofovir disoproxil fumarate (TDF)-containing regimens in high-risk individuals.1–4 While proven, PrEP efficacy has been highly variable, showing a relative risk reduction of HIV acquisition of 44% in men who have sex with men (MSM) and transgender women (TGW) (iPrEx),2 62% in heterosexual men and women (TDF2),4 67–75% in heterosexual serodiscordant men and women (Partners-PrEP),1 and 49% in people who inject drugs (Bangkok TDF).3 In contrast, two other PrEP studies (FEM-PrEP and VOICE) showed a lack of efficacy of PrEP in women.5,6 Notably, HIV seroconversion variability within and among randomized controlled trials of PrEP has largely been attributed to medication adherence.1,2,5,7–10 With the impact of adherence on efficacy and the emerging investigation of less than daily dosing regimens, quantitative measures that accurately assess adherence in a variety of PrEP dosing scenarios are essential.
PrEP trials have employed a variety of adherence measures, each with limitations.11 Self-report is limited by recall, overreporting, and social desirability biases. Pill counts, pharmacy records, and medication event monitoring systems may improve adherence data accuracy as compared to self-report, but do not record the actual dose-taking event. Using drug concentrations to assess adherence circumvents the research participant's ability to manipulate these other measures and provides evidence of dose taking; however, it requires differentiating the influence of variable adherence from variable pharmacokinetic influences, e.g., by drug half-life, food and drug interactions, genetic variations, and dosing regimen.12 These variables provide substantial challenges in establishing and interpreting quantitative adherence assessments. Therefore, understanding interindividual and intraindividual variability is important to adherence assessments using drug concentration.
The use of drug concentrations as a quantitative adherence measurement requires understanding the relationship between the dosing history and resulting drug concentrations. Establishing dose-concentration proportionality for a given pharmacologic measure makes it possible to make simple proportional assessments of adherence from drug concentrations. However, dose proportionality is traditionally assessed using peak concentration (Cmax) and area under the concentration-time curve (AUC) and these parameters are a challenge to capture in a randomized controlled trial. However, a drug with a long half-life relative to its dosing interval varies little in concentration within a dosing interval. In this special case, the timing of sample collection has little impact on drug concentration and the recent pattern of actual dose taking remains the most influential modifier of random drug concentration.
The dose proportionalities of Cmax and AUC plasma tenofovir (TFV) and emtricitabine (FTC) are well-established,13,14 but not practical in randomized controlled trials. Furthermore, because plasma TFV and FTC have half-lives shorter than a daily dosing interval, random drug concentrations are not expected to be dose proportional and they poorly reflect recent dose-taking patterns quantitatively. Conversely, the dose proportionality of the active phosphorylated drug form of emtricitabine, emtricitabine-triphosphate (FTC-TP), has yet to be determined in a healthy PrEP population. The half-lives of these phosphorylated drug forms may be long enough that drug concentration throughout a daily dosing interval may be sufficiently stable to demonstrate proportionality between dose frequency and drug concentration, therefore, increasing their utility in assessing adherence15 in a randomized clinical trial setting.
We performed a pharmacokinetic (PK) study of four dosing regimens of the fixed dose tablet of oral TDF/FTC under direct observation to remove adherence as an experimental variable. Our objective was to describe 100% adherence benchmarks for a range of dose-taking frequencies of oral TDF/FTC in healthy volunteers, evaluate dose proportionality, and describe drug concentration variability at steady state to interpret drug concentration as a quantitative adherence measure in a PrEP trial setting. We also characterized the safety profiles of the four different dosing regimens.
Materials and Methods
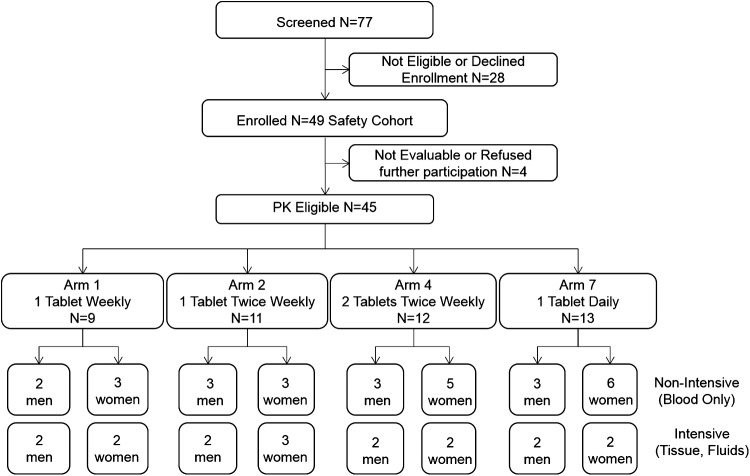
HPTN 066 was a two-site, open label, randomized, four-arm PK study of TFV, FTC, and their active phosphorylated metabolites in various body compartments following directly observed dosing of fixed-dose, combination tablets of TDF/FTC (Truvada® Gilead Sciences, Foster City, CA). All participants provided written informed consent prior to any study procedures. The study protocol was approved by the Institutional Review Boards at the University of North Carolina, Chapel Hill and The Johns Hopkins University (ClinicalTrials.gov Identifier: NCT01276600). The consort flow diagram is provided in Fig. 1.
FIG. 1.
Research participant disposition
Study participants and procedures
Eligible participants were sexually active HIV-negative healthy men and nonpregnant, nonbreast-feeding, and premenopausal women aged 18–44 years. Volunteers were recruited from the community surrounding the University of North Carolina, Chapel Hill and The Johns Hopkins Hospital in Baltimore, MD. Racial/ethnic categories were based on self-identification. Participants were screened for protocol eligibility based on history and physical, hematology, serum chemistry, urinalysis for proteinuria and glycosuria, coagulation panel, HIV and other sexually transmitted diseases, and hepatitis B surface antigen testing.
By protocol, 32 research participants were to be randomized 1:1:1:1 to one of four dosing regimens of oral Truvada (FTC 200 mg-TDF 300 mg) for nearly 5 weeks (35 days): Arm 1, one tablet orally once weekly (one/week); Arm 2, one tablet orally twice weekly (two/week); Arm 4, two tablets orally twice weekly (Monday and Thursday, four/week); and Arm 7, one tablet once daily (seven/week) (Fig. 2). Regardless of regimen, all doses, including weekends, were taken under direct face-to-face observation and documented by study personnel. Doses were taken without regard to food. By design, within each dosing regimen, four participants agreed to participate in the tissue-fluid sampling cohort and four were assigned to the blood only cohort. Blood only cohort participants had PK sampling only for blood; tissue-fluid cohort participants had additional biopsies and body fluid collections sampled on day 35 and day 49. Men and women were apportioned evenly in all regimens and in both the tissue-fluid and blood only cohorts. Evaluability for PK analysis required complete collection of all planned matrices at days 28 and 35 (a priori assumed to represent a steady-state defining concentration pair) and day 49 (multiple half-life TFV-DP decay time). These criteria were revised post hoc to be more inclusive (see Results).
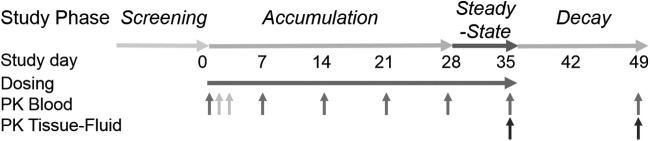
FIG. 2.
Study sampling schema. Study phases are indicated across study days from 0 to 49 as planned: Accumulation (day 0–28), Steady-State (day 28–35), and Decay (day 35–49). Up pointing gray arrows indicate all sampling is predose except for terminal day 49 sampling, which occurs 2 weeks after the last dose (day 35). Pharmacokinetic (PK) sampling of blood [serum, peripheral blood mononuclear cells (PBMCs), CD4+ cells)] occurs similarly for all subjects except for the presecond dose sample that occurs uniquely for more than weekly frequency regimens (light gray arrows) on day 2 (daily regimen) and day 3 (twice weekly regimens). Tissue-fluid cohort sampling is indicated by black arrows (days 35 and 49 only).
PK sampling
PK sampling of blood for serum and peripheral blood mononuclear cells (PBMCs) occurred at enrollment, prior to the second dose, prior to the scheduled dose on days 7, 14, 21, and 28, 24 h after the last dose (day 34) on day 35, and 2 weeks after the last dose on day 49. Per protocol, half of the participants in each treatment group were to undergo additional tissue-fluid sampling on days 35 and 49. Samples from men in the tissue-fluid cohort included rectal tissue (biopsy) and fluid (sponge via anoscopy) and self-collected semen (ejaculation), while samples from women were composed of vaginal tissue (biopsy) and cervicovaginal fluid (direct aspirate), followed by rectal tissue and fluid as in the men. Dosing and sampling for 5 weeks were selected to ensure achievement of at least two observations at steady state for PBMC TFV-DP estimated a priori to be day 28 and day 35 based on a TFV-DP half-life in HIV-infected patients of ∼150 h based on prior reports.15–17
PK sample processing
For blood processing in all participants, serum was prepared by centrifugation of coagulated blood in serum separator tubes at 1,500 × g for 10 min at 4°C, aliquoted into cryovials, and stored at −80°C until analysis. PBMCs were isolated via centrifugation of a cell preparation tube (CPT) at 1,800 × g for 20 min at ambient temperature (20–25°C), collected from the buffy coat, washed once with phosphate-buffered saline (PBS), and centrifuged at 400 × g for 15 min at 4°C. Cells were resuspended in 10 ml PBS for cell counting. The cells were centrifuged again at 400 × g for 15 min at 4°C. Cell pellets were lysed with 2 ml of 70% ice cold methanol in water and stored at −80°C until analysis.
For tissue-fluid cohort sample processing, rectal fluid was collected in a preweighed container using a sponge (Merocel eye-wick spears, Fisher Scientific #NC0093269) via a 4-cm anoscope, reweighed after sample collection, and stored at −80°C until analysis. Vaginal fluid was collected via direct aspiration using a specialized syringe (UNC CFAR Vaginal specimen aspirator) as previously published following which the sample was placed in a preweighed cryovial and subsequently reweighed and stored at −80°C.18,19 After liquefaction (within 30 min), semen was centrifuged at 600–800 × g for 10 min at 4°C, and supernatants were aliquoted and stored −80°C. Colon biopsies were collected via flexible sigmoidoscopy using 3.7-mm pinch biopsy forceps (Microvasive no. 1599; Boston Scientific Corp., Natick, MA) 10–20 cm from the anus. Vaginal biopsies were collected by direct visualization following speculum insertion. Up to five vaginal biopsies were taken with 2.3 × 4.2-mm Tischler gold-plated gynecological forceps.
Biopsies were placed in RPMI medium with l-glutamine and 10% fetal bovine serum (R10 media) until processing. Biopsies for homogenate were weighed and homogenized at room temperature with 0.5 ml of 70% ice cold methanol using the disposable pestle and cordless motor (VWR) for 2 min. Samples were spun in a microcentrifuge, 4°C, 14,000 × g for 10 min. Samples were immediately frozen at −80°C. To release colon and vaginal tissue cells for intracellular drug analysis, biopsies were incubated with an enzyme cocktail (collagenase type II, DNase I, elastase, and hyaluronidase) in RPMI containing l-glutamine, HEPES, and 7.5% fetal bovine serum (FBS) in 50-ml conical tubes at 37°C with agitation (Invitrogen, Carlsbad, CA), as previously described.20 Cells were counted via the Guava/Millipore EasyCyte Plus (Millipore, Billerica, MA). CD4+ cells were isolated via positive selection with CD4+ microbeads using magnetic affinity column separation according to the manufacturer's recommended protocol (Miltenyi Biotec, Auburn, CA). Thereafter, cells were processed similarly to the PBMCs as described above.
Drug concentration analysis
TFV, FTC, TFV-DP, and FTC-TP concentrations were determined by previously described liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) methods validated for each biological matrix of origin by the Johns Hopkins Clinical Pharmacology Analytical Laboratory (CPAL) and the Colorado Antiviral Pharmacology Laboratory (CAVP).21–23 Both laboratories participate in the National Institutes of Health-supported Clinical Pharmacology Quality Assurance (CPQA) program of assay method external review and approval and periodic proficiency testing for TFV and FTC in plasma.24 All assays were validated based on the recommendations of the Food and Drug Administration Guidance for Industry, Bioanalytical Method Validation and met all acceptability criteria. Validation metrics included precision, accuracy, stability, and matrix effects.
Briefly, thawed aliquots of serum and tissue homogenate, with 13C5-TFV and 15N213C – FTC internal standards (IS), were protein precipitated with methanol. Analytes of interest from CVF, rectal fluid, and semen aliquots were isolated using Oasis MCX 30-μm solid phase extraction plates (Waters Corporation, Milford, MA). Extracted eluants were collected, dried, and reconstituted in 0.5% acetic acid in water for analysis. Samples underwent chromatographic separation using gradient elution with a reversed phase C18 column on an Acquity UPLC system (Waters), and the dual detection of TFV and FTC was performed using an API 4000 tandem mass spectrometer (AB SCIEX, Foster City, CA), with an ESI source operated in positive ionization mode. Analytes were monitored in selective reaction monitoring mode. The analytical measuring ranges (primary linearity) for the assays are as follows: serum TFV: 0.31–1,000 ng/ml, FTC: 0.31–5,000 ng/ml, CVF TFV: 5–1,280 ng/ml, FTC: 20–5,120 ng/ml; rectal fluid TFV: 1.25–320 ng/sponge, FTC: 5–1,280 ng/sponge; seminal plasma TFV: 25–1,000 ng/ml, FTC:5–5,000 ng/ml; tissue TFV: 0.05–50 ng/sample (median LLOQ based on biopsy weights, colon 0.01 ng/mg, vagina 0.02 ng/mg), FTC: 0.25–250 ng/sample (median colon 0.04 ng/mg, vagina 0.08 ng/mg).
Tissue cell lysates and homogenates for TFV-DP quantitation were analyzed using an indirect assay measuring TFV in the sample after isolation of TFV-DP and enzymatic conversion to TFV, as previously described.22 TFV-DP was isolated from cell lysates on a Waters QMA cartridge (Waters Corporation, Milford, MA) over a KCl gradient, enzymatically dephosphorylated to TFV via sweet potato phosphatase digestion with 13C5-TFV internal standard. Desalted sample eluants were dried and reconstituted in 0.5% acetic acid in water for analysis. Samples were analyzed using a reversed phase C18 column as described on a Water Acquity UPLC system interfaced with an API 5000 mass spectrometer. The assay is linear over the range of 50–1,500 fmol TFV-DP/sample. TFV-DP and FTC-TP concentrations in lysed PBMC matrix were assayed with a validated LC-MS/MS method as described previously.22 The dynamic range was 2.5–2,000 fmol/sample for TFV-DP and 0.1–200 pmol/sample for FTC-TP. Five million PBMCs were typically assayed per PBMC sample resulting in an LLOQ of 0.5 fmol/106 cells for TFV-DP and 0.02 pmol/106 cells for FTC-TP.
Pharmacokinetic and statistical analysis
Adverse event frequency across study regimens was assessed using a chi-square test. Concentrations at each time point were summarized using descriptive statistics. Dose proportionality was assessed across study regimens using dose-adjusted predose concentrations (Cτ) for each day of observation through day 35: the Kruskal–Wallis test was used to test for equality of these dose-adjusted concentrations. [Note: Because of several outliers and small sample size within each dosing arm (9 to 13 participants), the nonparametric Kruskal–Wallis test was used.] For observation times with statistically significant differences, pairwise comparisons between study regimens were then tested using Wilcoxon rank sum tests with post hoc corrections (Bonferroni). Time to steady state was assessed by nonparametric ANOVA within a dose cohort among all observation times. Statistically significant differences were further tested for differences between consecutive sampling times using paired Wilcoxon rank sum tests with post hoc corrections (Bonferroni). To assess dose accumulation, the concentration prior to the second dose was compared (paired Wilcoxon rank sum test) to each of the subsequent steady-state predose concentrations for each individual. Total cell to CD4+ cell TFV-DP and FTC-TP ratios were tested for statistical significance using the one sample Wilcoxon signed rank test. Bivariate parameter correlations between drug analyte-matrix pairs were tested using the Spearman rank correlation test.
The terminal half-life for TFV-DP was estimated based on the PBMC sample collected 24 h after the final dose. This steady-state terminal half-life estimate was compared to an estimate based on superposition of 28 daily single doses based on the single dose TFV-DP concentration–time profile reported in Louissaint, et al. and Chen, et al., as reported subsequent to the design of HPTN 066, both of which indicate TFV-DP peaks occurring over a 3- to 4-day period prior to beginning its terminal decay.20,25 Intersubject variability was calculated from the percent coefficient of variation (%CV) for all subjects within a given sample day and reported as median (range) from among all steady-state sample times (D7 through D35) CV%s. Intrasubject variation was calculated from the CV% for each subject across all steady-state sample days (D7 through D35) and reported as median (range) from among all subjects CV%s. For all comparison tests, p-values ≤0.05 were considered statistically significant.
Receiver operating characteristics curves were explored to select concentration thresholds for each dose frequency and each matrix-analyte pair. All steady-state concentrations (day 7 to day 35) were pooled in this analysis. Statistical significance was evaluated using area under the curve and 95% confidence interval. Threshold concentrations optimized for greater than 90% sensitivity and for greater than 90% specificity were selected based on the ROC analysis. Generalized estimating equations (GEE) were also used for this analysis given the repeated measures in multiple individuals (SAS version 13.2, SAS Institute, Cary, NC).
Results
Demographics, disposition, adverse events
Forty-nine participants were enrolled for which adverse event data are reported (safety cohort). Baseline demographics were comparable between the four treatment regimens (Table 1). There were no differences in baseline values between the tissue-fluid and blood only cohort. Forty-five research participants were evaluable for PK assessments (PK cohort). Of the four participants not evaluable for PK assessments, all refused further participation at some point during the study for a variety of reasons (refused to continue after randomization to daily DOT, incarceration, spouse demands, family member health demands on time). The PK cohort was composed of the originally planned 32 participants plus 13 additional participants. Ten were replacement participants necessitated by compromised PBMC samples in the original cohort since PBMC TFV-DP and FTC-TP concentration informed critical study endpoints. Three additional individuals were initially determined not to be evaluable by protocol, but were later included in the PK analysis based on reevaluation of inclusion criteria in light of study data that differed greatly from a priori expectations.
Table 1.
Demographic Characteristics
| Arm 1 | Arm 2 | Arm 4 | Arm 7 | |
|---|---|---|---|---|
| Dose frequency | 1 tab 1×/wk | 1 tab 2×/wk | 2 tabs 2×/wk | 1 tab daily |
| Number of participants | 9 | 12 | 13 | 15 |
| Age, median years (IQR) | 30 (25–36) | 25 (22–35) | 32 (27–39) | 31 (24–37) |
| Gender, n (%) | ||||
| Male | 4 (44) | 6 (50) | 5 (38) | 5 (33) |
| Female | 5 (56) | 6 (50) | 8 (62) | 10 (67) |
| Race, n (%) | ||||
| Asian | 1 (11) | 0 (0) | 0 (0) | 0 (0) |
| Black | 3 (33) | 5 (42) | 7 (54) | 8 (53) |
| White | 4 (44) | 5 (42) | 6 (46) | 6 (40) |
| Other | 1 (11) | 2 (17) | 0 (0) | 1 (7) |
Briefly, because TFV-DP half-life in healthy subjects was far below the value in HIV-infected patients (see below) (1) time to steady-state concentration occurred far sooner (day 7), thus making day 28 and day 35 less essential, and (2) time to undetectable TFV-DP concentrations after cessation of dosing occurred far sooner than expected. These observations rendered analysis of TFV-DP concentrations at day 49 far less important. Accordingly, the study team agreed to include three individuals. All research clinic doses given were observed by study personnel.
All dosing regimens were well tolerated. A total of 58 adverse events (AEs) occurred in 32 (65%) of the 49 participants and were all mild or moderate in severity; 55% of these AEs were attributed to study drugs. There were no serious AEs. Regardless of attribution, the most frequent AEs were nausea and/or vomiting (10%), musculoskeletal pain (9%), headache (7%), fatigue (7%), hypophosphatemia (9%), anemia (5%), lower gastrointestinal disturbance (5%), and skin disorders (5%), with all other AEs occurring in less than 5% of research participants. There was no association between dosing regimen and AE frequency (p > 0.05).
Tenofovir serum and PBMC pharmacokinetics
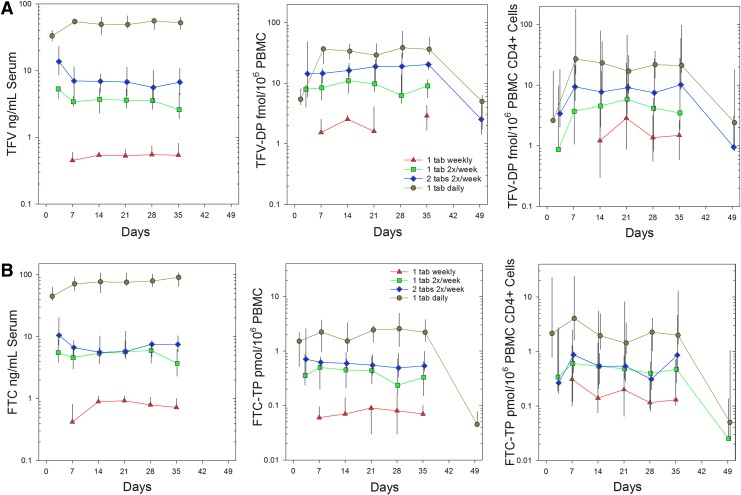
Concentration–time profiles for TFV, PBMC TFV-DP, and PBMC CD4+ TFV-DP in the four treatment regimens (Fig. 3A) all indicate a rise to, and achievement of, plateau concentrations by the first week on drug (day 7); plateau prodrug or metabolite concentrations were maintained for 5 weeks (day 35); following the last dose, concentrations fell toward and often below the lower limits of assay quantification. TFV in serum and TFV-DP in PBMCs reached steady state within 7 days in the daily cohort. In less frequent dosing cohorts, differences between predose concentrations for the second dose and the first weekly observation was significant only for serum TFV and not TFV-DP. TFV-DP in blood CD4+ cells was not different between any sample times within any dose frequency arm. Among TFV analytes, the steady-state trough concentration (Css) was greater than the single dose trough concentration (Cτ,1) only for serum TFV and PBMC TFV-DP in the daily dosing cohort [Cτ,ss/Cτ,1 median (IQR) among individual medians, 1.6 (1.5, 1.6) and 4.8 (4.5, 5.2), respectively] and for PBMC CD4+ cells in both the four/week arm [2.4 (2.1, 2.7)] and daily arm [8.9 (7.9, 9.2)]; these observations are indicative of drug accumulation.
FIG. 3.
(A) Tenofovir analyte predose median (IQR) concentration vs. time relationships for all dosing regimens: one tablet weekly, red triangle; one tablet, two times per week, green square; two tablets twice weekly, blue diamond; one tablet daily, gold circle. (B) Emtricitabine moiety predose median (IQR) concentration vs. time relationships for all dosing regimens: one tablet weekly, red triangle; one tablet, two times per week, green square; two tablets twice weekly, blue diamond; one tablet daily, gold circle.
The typical serum TFV Cτ values across 5 weeks (median of each of five weekly medians) indicate a disproportionate increase when transitioning from four doses weekly to daily dosing (Table 2A). In contrast, typical Cτ PBMC TFV-DP increased proportionally with increasing dose frequency from weekly to daily dosing. PBMC CD4+ cell subsets at steady state were dose proportional four of five study weeks (only Arm 1 was not dose proportional with the other arms in that week). Based on statistical analyses of dose-adjusted Cτ, serum TFV was not dose proportional in any week throughout the study, which is as expected for trough concentrations (p < 0.05). PBMC TDV-DP was dose proportional for all, but one, sampling time (day 14) across treatment regimens. PBMC CD4+ cells were dose proportional at all times.
Table 2A.
Steady-State Tenofovir Pharmacokinetic Summary by Analyte and Location
| Matrix | Analyte | TFV LLOQa | Arm 1 | Arm 2 | Arm 4 | Arm 7 |
|---|---|---|---|---|---|---|
| Dose frequency | 1 tab 1×/wk | 1 tab 2×/wk | 2 tabs 2×/wk | 1 tab daily | ||
| Serumb | TFV | 0.31 ng/ml | 0.5 (0.5–0.6) | 3.6 (2.6–3.7) | 5.9 (4.6–6.4) | 52.2 (49.0–55.6) |
| PBMC totalb | TFV-DP | 0.5 fmol/106 cells | 1.6 (BLQc–2.9) | 9.1 (6.3–11.0) | 18.8 (14.5–20.4) | 36.3 (29.0–38.5) |
| PBMC CD4+ onlyb | TFV-DP | 0.5 fmol/106 cells | 1.7 (BLQ–18.7) | 4.0 (BLQ–91.2) | 14.6 (7.8–100.6) | 24.1 (0.4–117.8) |
| Colon homogenated | TFV | 0.01 ng/mge | 0.02 (0.01–0.10) | 0.23 (0.05–3.28) | 1.58 (0.21–4.75) | 5.42 (0.21–20.54) |
| Colon homogenated | TFV-DP | 5 fmol/mge | 16 (BLQ–21) | 27 (13–762) | 186 (58–412) | 206 (0–595) |
| Colon total cellsd | TFV-DP | 0.5 fmol/106 cells | 37 (2–122) | 40 (0.6–84.7) | 64 (BLQ–277) | 160 (18–340) |
| Vagina homogenated | TFV | 0.02 ng/mge | BLQ, BLQ | BLQ, BLQ, BLQ | BLQ, BLQ | 0.02, 0.18 |
| Vagina homogenated | TFV-DP | 5 fmol/mge | BLQ, BLQ | BLQ, BLQ | BLQ, BLQ | BLQ, 41 |
| Vagina total cellsd | TFV-DP | 0.5 fmol/106 cells | BLQ, 1038 | BLQ, BLQ | BLQ, 5.7 | BLQ, BLQ |
| Rectal fluidd | TFV | 1.25 ng/sponge | 3.7, 6.8 | BLQ | 25.8 | 1617 |
| Cervicovaginal fluidd | TFV | 5 ng/ml | 126 | BLQ, 127 | 18, 77 | 625, 6290 |
| Semend | TFV | 25 ng/ml | BLQ, BLQ | BLQ,BLQ | 34, 98 | 409, 877 |
LLOQ, lower limit of assay quantitation.
Median (range) of individual research participant medians at steady state (day 7–day 35).
BLQ, below limit of assay quantitation. (Note: sample mass varies greatly, especially for tissue and tissue cells, with substantial impact on true assay sensitivity.)
Median (range) of day 35 concentrations; when three or fewer are available, all values are shown without parentheses.
For tissue homogenates, values are reported as “per mg” based on conversion from “per sample” divided by sample weight. LLOQ per mg is based on median of sample weight and LLOQ per sample calculated for each sample.
TFV, tenofovir; TFV-DP, tenofovir diphosphate.
Two weeks after the final dose, day 49, serum TFV concentrations were below the LLOQ (BLQ) in all arms, except for two of eight (25%) in the four/week arm and four of 10 (40%) in the daily arm [median (IQR), BLQ (BLQ, 0.36)]. For PBMC TFV-DP, the median (IQR) was above LLOQ in seven of eight of the four/week participants [median (IQR) 2.54 (1.43, 4.48)] and eight of 10 daily arm participants [4.98 (1.75, 6.34)]. TFV-DP in the two and one dose per week cohorts was two of 10 and one of eight, respectively. The TFV-DP concentration in PBMC was greater than in the CD4+ subsets with a median (IQR) PBMC/CD4+ ratio of 1.25 (0.4, 2.5) (p < 0.001).
Between day 7 and day 35, median interindividual coefficients of variation ranged from 33% to 82%, 50% to 121%, and 46% to 71% for serum TFV, PBMC TFV-DP, and CD4+ TFV-DP Cτ, respectively (Table 3). Interindividual variability in TFV and TFV-DP Cτ increased with less frequent dosing. Compared to interindividual variability, intraindividual variability had a lower range of 17% to 34% in the daily dosing and double-dose twice weekly (4×/week) treatment regimens for serum and PBMC. Intrasubject and intersubject drug concentration variabilities were similar for CD4+ cells (13–65%).
Table 3.
Inter- and Intraindividual Variability
| Plasma TFV | Plasma FTC | PBMC TFV-DP | PBMC FTC-TP | CD4+ TFV-DP | CD4+ FTC-TP | |
|---|---|---|---|---|---|---|
| Intersubject | ||||||
| 1 tab 1×/wk | 82 (77–92) | 86 (82–105) | 121 (85–171) | 70 (50–88) | 53 (46–92) | 75 (52–91) |
| 1 tab 2×/wk | 41 (36–70) | 49 (34–65) | 61 (47–164) | 78 (68–193) | 46 (26–52) | 64 (52–87) |
| 2 tabs 2×/wk | 60 (42–63) | 48 (32–99) | 49 (32–59) | 54 (44–67) | 51 (29–63) | 53 (26–58) |
| 1 tab daily | 33 (30–49) | 33 (28–54) | 50 (33–66) | 63 (54–81) | 71 (24–87) | 62 (25–86) |
| Intrasubject | ||||||
| 2 tabs 2×/wk | 24 (10–78) | 18 (7–70) | 23 (18–79) | 25 (10–74) | 16 (1–63) | 13 (5–66) |
| 1 tab daily | 17 (6–56) | 14 (8–53) | 34 (14–77) | 32 (21–79) | 55 (11–95) | 65 (10–96) |
Median (range) of CV% of steady state (day 7–day 35) values.
Intersubject variability calculated from CV% for all subjects within a given sample day; median (range) is from among D7, D14, D21, D28, D35 CV%s.
Intrasubject variability calculated from CV% for each subject across all his or her steady-state samples days (D7–D35); median (range) is from among all subjects CV%s. Only higher dose frequency is included due to predominance of BLQ values.
Emtricitabine serum and PBMC pharmacokinetics
Similar to TFV moieties, concentration–time profiles for serum FTC, PBMC FTC-TP, and PBMC CD4+ FTC-TP in the four treatment regimens all achieved plateau concentrations by the first week (day 7), maintained this concentration for the rest of the dosing period (through day 35), then decayed near to or below assay quantification limits by day 49 (Fig. 3B). FTC in serum and FTC-TP in PBMCs reached steady state within 7 days of dosing (Table 2). Fractional accumulation of serum FTC at steady state seen in the daily dosing arm, 1.6 (1.5, 1.6), was similar in magnitude to serum TFV; daily dosing PBMC FTC-TP accumulation at steady state, 1.8 (1.7, 1.8) was less than half the accumulation seen for PBMC TFV-DP. There was no evidence of PBMC CD4+ cell accumulation in any regimen.
Table 2B.
Steady State Emtricitabine Pharmacokinetic Summary by Analyte and Location
| Matrix | Analyte | FTC LLOQa | Arm 1 | Arm 2 | Arm 4 | Arm 7 |
|---|---|---|---|---|---|---|
| Dose frequency | 1 tab 1×/wk | 1 tab 2×/wk | 2 tabs 2×/wk | 1 tab daily | ||
| Serumb | FTC | 0.31 ng/ml | 0.8 (0.4–0.9) | 5.4 (3.7–6.0) | 6.7 (5.4–7.6) | 70.9 (67.7–81.9) |
| PBMC totalb | FTC-TP | 0.02 pmol/106 cells | 0.1 (0.1–0.1) | 0.4 (0.2–0.5) | 0.6 (0.5–0.6) | 2.2 (1.5–2.6) |
| PBMC CD4+ onlyb | FTC-TP | 0.02 pmol/106 cells | 0.1 (0.1–1.7) | 0.5 (0.1–6.5) | 1.0 (0.3–6.9) | 3.2 (0.1–12.3) |
| Colon homogenatec | FTC | 0.04 ng/mgd | 0.13 (BLQe–0.21) | 0.49 (0.20–2.08) | 1.22 (0.36–5.74) | 20.5 (1.87–49.32) |
| Colon total cellsc | FTC-TP | 0.5 fmol/106 cells | BLQ (BLQ–BLQ) | BLQ (BLQ–1.2) | BLQ (BLQ–6.6) | BLQ (BLQ–0.14) |
| Vagina homogenatec | FTC | 0.08 ng/mg | BLQ, BLQ | BLQ, BLQ, 0.38 | BLQ, 0.14 | 3.02, 3.31 |
| Vagina total cellsc | FTC-TP | 0.5 fmol/106 cells | BLQ, BLQ | BLQ, BLQ | BLQ, BLQ | BLQ, BLQ |
| Rectal fluidc | FTC | 5 ng/sponge | BLQ,BLQ | BLQ | BLQ | 20.8 |
| Cervicovaginal fluidc | FTC | 20 ng/ml | 124 | BLQ, 182 | 143, 145 | 2330, 7420 |
| Semenb | FTC | 5 ng/ml | BLQ, 7.4 | 21, 23 | 13, 27 | 907, 992 |
LLOQ, lower limit of assay quantitation.
Median (range) of individual research participant medians at steady state (day 7–day 35).
Median (range) of day 35 concentrations; when three or fewer values are available, all values are shown without parentheses.
For tissue homogenates, values are reported as “per mg” based on conversion from “per sample” divided by sample weight. LLOQ per mg is based on median of sample weight and LLOQ per sample calculated for each sample.
BLQ, below limit of assay quantitation. (Note: sample mass varies greatly, especially for tissue and tissue cells, with substantial impact of true assay sensitivity.)
FTC, emtricitabine; FTC-TP, emtricitabine triphosphate.
All FTC analyte Cτ values across 5 weeks failed to demonstrate dose proportionality (all p < 0.05) with the daily dosing arm being disproportionately larger than the less frequent dosing cohorts (Table 2B). At week 49, the only detectable analyte was FTC-TP in the daily dosing cohort with seven of seven participant samples above the LLOQ, median (IQR) 2.2 (1.5–2.6) pmol/106 cells. The FTC-TP concentration in PBMCs was slightly more than half of the concentration in CD4+ subsets with a median (IQR) PBMC/CD4+ ratio of 0.58 (0.18, 0.96) (p < 0.001).
Interindividual variability was similar to TFV moieties across all treatment regimens with a range of 33–86% for serum FTC, 54–70% for PBMC FTC-TP, and 53–75% for CD4+ FTC-TP and increased with decreasing dosing frequency. Intraindividual variability was 14–32% in the daily dosing and double-dose twice weekly regimens for serum and PBMCs. It increased to 65% in the daily dosing CD4+ samples.
Tissue-fluid sampling cohort
Where quantifiable, steady-state day 35 concentrations of all analytes in nearly all matrices of the tissue-fluid sampling cohort increased with increasing weekly cumulative dose (Table 2). Many concentrations were not quantifiable, especially in infrequent dosing arms. All CD4+ cell subset data were below the limits of quantification. Except for the vaginal homogenate TFV and FTC concentrations with daily dosing, nearly all vaginal samples were below quantitative limits. Accordingly, dose proportionality was not tested. Of all matrices, colon tissue and tissue cells for TFV and TFV-DP and colon tissue homogenate for FTC-TP were the only matrix–analyte combination consistently detectable for most subjects in all treatment regimens. Concentrations of TFV analytes, where quantifiable, were higher in colon tissue and cells than in vaginal tissue and cells (except for a single TFV-DP vaginal tissue cell value in the weekly cohort). FTC was also greater in colon compared to vaginal tissue homogenates. By day 49, concentrations were consistently detectable (roughly half of participants) only in the four or seven dose per week regimens and only for TFV and TFV-DP in colon tissue homogenate and cells, semen, and CVF. At day 49, FTC was detected in half or fewer of participants in colon tissue homogenates in ≥2×/week groups and only in the daily group in semen.
Intercompartment correlations
Serum TFV and FTC correlated with a high degree of statistical significance (p < 0.001) and relatively high correlation coefficients [all r > 0.5 except PBMC CD4+ cells (r > 0.24)] with all drug analytes assessed in all sites (where values were quantifiable). Beyond serum, concentrations were best correlated with the concentrations in an adjacent anatomic compartment (e.g., serum with PBMC or total cells with CD4+ cells) or parent and active drug in a given compartment, for example, TFV with TFV-DP in colon tissue homogenate (r = 0.87, p < 0.001) and PBMC and colon TFV-DP (r = 0.62, p < 0.001) and FTC-TP (r = 0.832, p < 0.001). The notably absent correlations were between vaginal tissue TFV, TFV-DP, and FTC and any other drug-matrix sample type where no correlations outside vaginal tissue were statistically significant, though these were correlated with each other (r = 0.55, p = 0.03). Vaginal tissue FTC-TP was not assessed. There were no statistically significant correlations between creatinine or creatinine clearance and serum TFV or FTC Cτ concentrations. There were no statistically significant gender differences in any blood or colon tissue or fluid parameters.
ROC and adherence threshold analysis
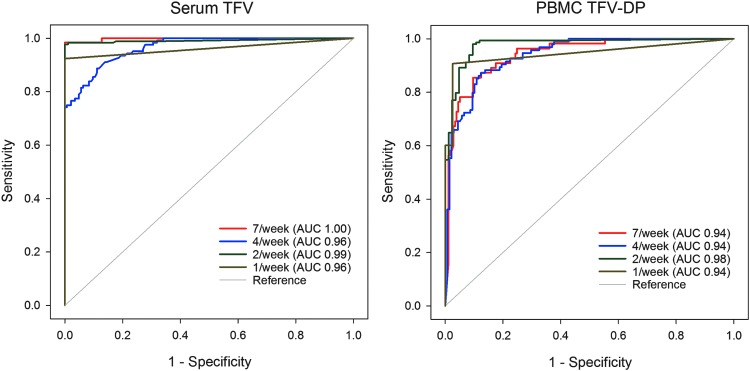
ROC analysis of all pooled steady-state observations indicates an excellent ability to discriminate between adherence levels based on ROC curves (Fig. 4) and a high AUC for all analytes (TFV, TFV-DP, FTC, FTC-TP) and matrices (plasma, PBMCs, PBMC CD4+ cells). AUC values ranged from 0.93 to 1.00 for all analytes in plasma and PBMCs. PBMC CD4+ cells were a little lower, from 0.81 to 0.94. Threshold concentrations indicating a minimum number of doses per week or greater were selected to optimize for sensitivity greater than 90% and specificity greater than 90% (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid). For example, selecting thresholds to be inclusive, by optimizing for greater than or equal to 90% sensitivity, plasma TFV concentrations of at least 35.5, 4.2, 2.5, and 0.5 ng/ml or PBMC TFV-DP concentrations of 16.8, 9.9, 5.2, and 1.6 fmol/106 cells indicate adherence consistent with at least seven, four, two, and one dose in the prior week, respectively. Strictly controlling for repeated measures in a given individual with a GEE analysis yielded highly concordant results with those reported above, which pooled 5 weeks of observations from among all subjects.
FIG. 4.
Receiver operating characteristic (ROC) curves for plasma tenofovir (TFV) (left) and PBMC TFV-DP (right). The legend for each figure indicates the dose frequency ranging from one to seven doses per week and the associated area under the curve (AUC) for the ROC curve.
Discussion
We describe the concentrations of serum TFV and FTC and PBMC TFV-DP and FTC-TP in four dosing regimens using directly observed TDF/FTC dosing to establish 100% adherence benchmarks for dose taking ranging from weekly to daily. The finding of dose proportionality for TFV-DP allows for the simple proportional extrapolation of TFV-DP concentration to estimate adherence (e.g., 50% adherence would yield 50% the expected 100% benchmark). While the precision of such estimates is limited on an individual basis due to the large interindividual variability compared to serum concentrations, the relatively smaller intraindividual variability allows improved precision in adherence estimates if baseline PK data, after an observed dose, are available.12,23,26 In a clinical study sample, absence of detectable PBMC TFV-DP suggests no doses taken within the prior week, similar to plasma or serum. This was an unexpectedly short period of “look back” due to a shorter TFV-DP half-life than expected.
For TFV, FTC, and FTC-TP, the lack of dose proportionality for predose concentrations was anticipated based on a shorter drug half-life relative to dosing interval compared to TFV-DP in the less than daily dosing regimens. This does not preclude the use of these moieties for adherence assessment, but it indicates a greater sensitivity to timing of doses on drug concentration, which complicates their use for quantitative adherence assessment. Furthermore, due to the shorter half-life of TFV and FTC in serum, drug concentration measurements have the capacity to reflect only recent (several days) dosing compared to 1 week of dosing for PBMC TFV-DP. From other reports, precise adherence pattern estimates within an individual using plasma TFV are not possible for even the prior 3 days.12
Despite these limitations, on a population basis, plasma TFV predicts PrEP efficacy, given the close relationship between plasma concentration, adherence, and efficacy. More specifically, applying daily dosing steady-state serum TFV concentration benchmarks from HPTN 066 to PrEP clinical trials, we find median concentrations (1) consistent with plasma concentrations reported in the Partners in Prevention, CDC TDF2, and Bangkok PWID PrEP studies (high levels of HIV protection), (2) higher than in iPrEx (moderate protection), and (3) far higher than in FEM-PrEP and VOICE (no protection).1–6 Furthermore, the combination of serum TFV and PBMC TFV-DP data, given their different half-lives and time to steady state (see below), makes it possible to identify a white-coat effect—dosing only in the day(s) prior to concentration assessment—by the combination serum or plasma TFV concentration indicating dosing within the prior day (>49 ng/ml) and PBMC TFV-DP indicating less than daily dosing (<29 fmol/106 cells). White coat effect is important to exclude in interpreting therapeutic drug monitoring data in clinical trials.27
Threshold concentrations associated with a discrete number of doses in the prior week serve as adherence benchmarks when using pharmacologic measures to quantify recent adherence in a study participant. Using an ROC assessment, these pharmacologic measures are quite powerful in their ability to discriminate among adherence levels. In addition to describing the typical values (point estimates) associated with each weekly dosing frequency, we used an ROC approach to provide benchmarks that are either more inclusive (sensitivity optimized) or more stringent (specificity optimized), depending on the use of the thresholds. Because our sampling occurred at concentration troughs (predose) based on precise dosing regimens and because there are many ways to take four, two, or one pill in any prior week, the benchmarks using shorter half-life plasma TFV, FTC, and FTC-TP concentrations are more of an average from among a range of possibilities. The long half-life of TFV-DP in PBMCs and CD4+ cells is far more resistant to this effect.
Adherence benchmark values from this study may not extrapolate to other studies where different sample processing and/or analytical procedures may be used. TFV-DP values in this study are similar to values from several studies in healthy volunteers,20,25,28 but are lower than values from several other studies in both healthy volunteers29,30 and HIV-infected patients.17,31,32 Additional investigation is required to understand the reason for the differences in TFV-DP among these studies. Until the differences are explained, benchmarks will need to remain specific to the laboratory performing the PBMC processing and PK sample analysis in the benchmark study (e.g., HPTN 066, STRAND, and Cell-PrEP) and the randomized controlled trial in which adherence estimates are desired.
A major challenge of using drug concentrations as biomarkers for adherence is the underlying interindividual and intraindividual PK variability that also influences drug concentrations. To isolate PK-related variability, we eliminated variability due to adherence through directly observed therapy and characterized the intraindividual variability (CV%) of serum and PBMC TFV-DP and FTC-TP as 17% to 34% and 14% to 32%, respectively. These values will inform future interpretation of drug concentration values and help determine accurate sample size estimates for studies that use drug concentration monitoring as an adherence measure.
The 7 days required to reach steady-state provides a cautionary note when initiating periodic PrEP dosing regimens given the delay in achieving protective concentrations. Based on iPrEx protective concentrations (EC90), single daily oral doses do not achieve these levels for 5 to 7 days; double doses daily would be expected to achieve the iPrEx targets in less time given the dose proportionality observed. Consistent with our findings, the Cell-PrEP study reported that 89% of a population would achieve iPrEx protective concentrations after seven daily TDF/FTC doses.30 These time to protection estimates, however, are applicable only to MSM/TGW since dose frequency associated with high levels of protection in women appear to be higher than in MSM. Furthermore, the TFV-DP half-life in vaginal tissue cells is several times longer than in colon tissue cells and would, therefore, take longer to achieve protective levels even if protective tissue concentrations were similar.20
The 7 day time to steady state of TFV-DP and the 5-fold accumulation of TFV-DP at steady state are sooner and lower, respectively, than the expected 3–4 week time to steady state and 9-fold accumulation we anticipated based on previous reports (which we used to design this study) in HIV-infected individuals of an approximately 150 h terminal half-life.15–17 Our findings are consistent with a 48–53 h TFV-DP half-life that we reported in two prior studies. The first is a single oral TDF dose study in healthy women using an accelerator mass spectrometry analytical method and three terminal concentrations over 2 weeks. The second is a multiple dose oral TDF study using a similar TFV-DP UPLC-MS/MS analytical method as in HPTN 066 and a population PK model-based estimation of TFV-DP half-life.20,26 Of note, the PBMC TFV-DP terminal half-life could not be estimated directly in HPTN 066 using terminal half-life due to the inability to use the penultimate day 35 data given the sustained 3–4 day postdose PBMC TFV-DP plateau noted in several recent reports.20,25,28 The plateau was not known at the time HPTN 066 was designed and would bias the half-life estimates upward.
The Cell-PrEP study reported that >90% of the TFV-DP steady-state concentration was achieved after 12 daily doses and 97% of FTC-TP steady-state was achieved after seven daily doses.30 These values are internally consistent with their reported 84 h half-life. The slightly longer time to TFV-DP steady state in Cell-PrEP—12 daily doses vs. seven daily doses in HPTN 066—may be related to the different study design used. Specifically, HPTN 066 used paired comparisons of discrete sequential weekly observations to identify the first week in which concentrations do not rise, therefore, 12 doses is not a possible outcome. Cell-PrEP modeled time as a continuous variable and did not limit the analysis to discrete week-long observation periods. Furthermore, given the PK estimate variability reported in both HPTN 066 and Cell-PrEP, we do not believe the numeric differences in time to steady state are truly different.
We do not have a good explanation for the wide range in absolute TFV-DP values (ranging from 36 to 160 fmol/106 cells) or differences in half-life estimates (ranging from 48 to 164 h) among studies.15–17,20,26 Some of this may be due to variation in study design or pharmacokinetic modeling methods as noted above. We do not have any suitable biological reasons to explain this apparent difference. Some have suggested that the state of immune activation—higher in some HIV+ patients compared to healthy uninfected research participants—may account for the difference. However, in vitro studies of phytohemagglutinin ± interlukin-2-stimulated PBMCs (surrogates for increased immune activation in HIV+ patients) show that TFV-DP is actually lower, not higher, and consistent with a shorter half-life, when compared to resting cells (surrogates for healthy volunteers).33,34 Furthermore, the longest and shortest PBMC TFV-DP half-life studies are in healthy volunteers.20,35
In our tissue-fluid sampling cohort, steady-state TFV-DP concentrations at day 35 were higher in colonic tissue homogenate than in vaginal tissue homogenate—a pattern described in prior single dose studies, which sampled tissue at 24 h.20,36 Cell-specific TFV-DP differences are less clear due to our many BQL results, which cannot necessarily be interpreted as lower in concentration since fewer biopsies were available for analysis from vaginal compared to colon tissue. However, in a previous report, the colonic greater than vaginal tissue homogenate differences were not seen in total cells extracted from tissue.20 There were no differences in concentration between men and women for any drug moiety or location.
We did not sample at time points earlier than 1 week or more frequently than weekly, so we could not evaluate whether steady state had been achieved at an even earlier time point (likely for FTC-TP). However, another study reported a longer, not shorter, time to TFV-DP steady state.30 Because our primary outcomes were assessed using carefully timed trough concentrations, our estimates are biased downward for both concentration expectations for a given dose frequency and variability compared to a clinical trial context employing random sampling times. We did not perform frequent sampling of drug moieties to establish individual PK parameters for these drug moieties. The combination of individual PK parameters prior to or within a clinical study would significantly improve the precision of adherence estimates. The study is also limited by scarce data in tissue and fluid samples, especially vaginal, which limits precision and precludes evaluating tissue and fluid drug concentrations as adherence benchmarks for these matrices.
This report highlights 100% adherence serum and PBMC drug concentration benchmarks for weekly to daily dose frequency of TDF/FTC, which is dose proportional only for TFV-DP. A 1-week lead-in period of TDF/FTC dosing is necessary to achieve steady-state concentrations of TFV-DP and FTC-TP in PBMCs. Interindividual variability and intraindividual variability greatly differ depending on the drug analyte being assessed. Threshold concentrations for plasma and PBMC analytes differentiate very well among various numbers of doses taken in the prior week as surrogate for levels of adherence. These results provide useful data for the design and interpretation of future PrEP studies.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the contributions of the research participants. The following individuals were indispensable in the planning and conduct of the study: University of North Carolina—Trent Stephens, Nicholas Shaheen, MD MPH, Ryan Madanick MD, Evan Dellon MD MPH, staff of the UNC CTU (UM1AI069423), CTSA nursing staff, and UNC CFAR CPAC laboratory and immunology personnel (P30AI50410); The Johns Hopkins University—Linda Lee, MD, Kathleen Truelove, Teresa Parsons, and the staff of the Hopkins CTU (UM1AI069465), Hopkins Institute for Clinical and Translational Research (UL1TR001079), the Drug Development Unit, the Clinical Pharmacology Analytical Laboratory, the Hopkins CFAR Laboratory Core (P30AI094189); the staff of the University of Colorado CAVP. HPTN 066 laboratory grant support was provided by the National Institute of Allergy and Infectious Disease (NIAID) UM1AI068613.
Author Disclosure Statement
C. Hendrix has received grant support from Gilead Sciences in the past, managed by the Johns Hopkins University.
References
- 1.Baeten JM, Donnell D, Ndase P, et al. : Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. : Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choopanya K, Martin M, Suntharasamai P, et al. : Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013;381:2083–2090 [DOI] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. : Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367:423–434 [DOI] [PubMed] [Google Scholar]
- 5.Marrazzo JM, Ramjee G, Richardson B, et al. : Pre-exposure prophylaxis for HIV in women: Daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003). New Engl J Med 2015;372:509–51825651245 [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, et al. : Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012;367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrix CW: Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design. Cell 2013;155:515–518 [DOI] [PubMed] [Google Scholar]
- 8.Donnell D, Baeten JM, Bumpus NN, et al. : HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014;66:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karim SS, Kashuba AD, Werner L, and Karim QA: Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: Implications for HIV prevention in women. Lancet 2011;378:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agot K, Taylor D, Corneli AL, et al. : Accuracy of self-report and pill-count measures of adherence in the FEM-PrEP clinical trial: Implications for future HIV-prevention trials. AIDS Behav 2015;19:743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Straten A, Montgomery ET, Hartmann M, and Minnis A: Methodological lessons from clinical trials and the future of microbicide research. Curr HIV/AIDS Rep 2013;10:89–102 [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedula A, Fossler MJ, and Hendrix CW: Estimation of tenofovir's population pharmacokinetic parameters without reliable dosing histories and application to tracing dosing history using simulation strategies. J Clin Pharmacol 2014;54:150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barditch-Crovo P, Deeks SG, Collier A, et al. : Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother 2001;45:2733–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gish RG, Leung NW, Wright TL, et al. : Dose range study of pharmacokinetics, safety, and preliminary antiviral activity of emtricitabine in adults with hepatitis B virus infection. Antimicrob Agents Chemother 2002;46:1734–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pruvost A, Negredo E, Benech H, et al. : Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 2005;49:1907–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pruvost A, Negredo E, Theodoro F, et al. : Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): Investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob Agents Chemother 2009;53:1937–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins T, Veikley W, St Claire RL 3rd, et al. : Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J Acquir Immune Defic Syndr 2005;39:406–411 [DOI] [PubMed] [Google Scholar]
- 18.Adams JL, Patterson KB, Prince HM, et al. : Single and multiple dose pharmacokinetics of dolutegravir in the genital tract of HIV negative women. Antivir Ther 2013;18:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz JL, Rountree W, Kashuba AD, et al. : A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One 2011;6:e25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louissaint NA, Cao YJ, Skipper PL, et al. : Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 2013;29:1443–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller MJ, Madan RP, Torres NM, et al. : A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One 2011;6:e16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bushman LR, Kiser JJ, Rower JE, et al. : Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal 2011;56:390–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrix CW, Chen BA, Guddera V, et al. : MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One 2013;8:e55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiFrancesco R, Taylor CR, Rosenkranz SL, et al. : Adding value to antiretroviral proficiency testing. Bioanalysis 2014;6:2721–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Flexner C, Liberman RG, et al. : Biphasic elimination of tenofovir diphosphate and nonlinear pharmacokinetics of zidovudine triphosphate in a microdosing study. J Acquir Immune Defic Syndr 2012;61:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns RN, Hendrix CW, Fossler MJ, and Chaturvedula A: Population pharmacokinetics of tenofovir and tenofovir-diphosphate in healthy women. J Clin Pharmacol 2015;55:629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podsadecki TJ, Vrijens BC, Tousset EP, et al. : “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials 2008;9:238–246 [DOI] [PubMed] [Google Scholar]
- 28.Jackson A, Moyle G, Watson V, et al. : Tenofovir, emtricitabine intracellular and plasma, and efavirenz plasma concentration decay following drug intake cessation: Implications for HIV treatment and prevention. J Acquir Immune Defic Syndr 2013;62:275–281 [DOI] [PubMed] [Google Scholar]
- 29.Anderson PL, Glidden DV, Liu A, et al. : Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012;4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seifert SM, Glidden DV, Meditz AL, et al. : Dose response for starting and stopping HIV preexposure prophylaxis for men who have sex with men. Clin Infect Dis 2015;60:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams JL, Sykes C, Menezes P, et al. : Tenofovir diphosphate and emtricitabine triphosphate concentrations in blood cells compared with isolated peripheral blood mononuclear cells: A new measure of antiretroviral adherence? J Acquir Immune Defic Syndr 2013;62:260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiser JJ, Aquilante CL, Anderson PL, et al. : Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr 2008;47:298–303 [DOI] [PubMed] [Google Scholar]
- 33.Robbins BL, Wilcox CK, Fridland A, and Rodman J: Metabolism of tenofovir and didanosine in quiescent or stimulated human peripheral blood mononuclear cells. Pharmacotherapy 2003;23(6):695–701 [DOI] [PubMed] [Google Scholar]
- 34.Robbins BL, Srinivas RV, Kim C, et al. : Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother 1998;42(3):612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson A, Moyle G, Watson V, et al. : Tenofovir, emtricitabine intracellular and plasma, and efavirenz plasma concentration decay following drug intake cessation: Implications for HIV treatment and prevention. J Acquir Immune Defic Syndr 2013;62(3):275–281 [DOI] [PubMed] [Google Scholar]
- 36.Patterson KB, Prince HA, Kraft E, et al. : Penetration of tenofovir and emtricitabine in mucosal tissues: Implications for prevention of HIV-1 transmission. Sci Transl Med 2011;3:112re114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.