Abstract
Mozambique's updated guideline for management of HIV-associated anemia prompts clinicians to consider opportunistic conditions, adverse drug reactions, and untreated immunosuppression in addition to iron deficiency, intestinal helminthes, and malaria. We prospectively evaluated this guideline in rural Zambézia Province. Likely cause(s) of anemia were determined through prespecified history, physical examination, and laboratory testing. Diagnoses were “etiologic” if laboratory confirmed (sputum microscopy, blood culture, Plasmodium falciparum malaria rapid test) or “syndromic” if not. To assess hemoglobin response, we used serial point-of-care measurements. We studied 324 ambulatory, anemic (hemoglobin <10 g/dl) HIV-infected adults. Study clinicians treated nearly all [315 (97.2%)] for suspected iron deficiency and/or helminthes; 56 (17.3%) had laboratory-confirmed malaria. Other assigned diagnoses included tuberculosis [30 (9.3%)], adverse drug reactions [26 (8.0%)], and bacteremia [13 (4.1%)]. Etiologic diagnosis was achieved in 79 (24.4%). Of 169 (52.2%) subjects who improved (hemoglobin increase of ≥1 g/dl without indications for hospitalization), only 65 (38.5%) received conventional management (iron supplementation, deworming, and/or antimalarials) alone. Thirty (9.3%) died and/or were hospitalized, and 125 (38.6%) were lost to follow-up. Multivariable linear and logistic regression models described better hemoglobin responses and/or outcomes in subjects with higher CD4+ T-lymphocyte counts, pre-enrollment antiretroviral therapy and/or co-trimoxazole prophylaxis, discontinuation of zidovudine for suspected adverse reaction, and smear-positive tuberculosis. Adverse outcomes were associated with fever, low body mass index, bacteremia, esophageal candidiasis, and low or missing CD4+ T cell counts. In this severely resource-limited setting, successful anemia management often required interventions other than conventional presumptive treatment, thus supporting Mozambique's guideline revision.
Introduction
Anemia, one of the “most intractable public health problems in Africa,” has historically been attributed to three principal causes: nutritional deficiencies, helminth infections, and malaria.1 Children and women of child-bearing age have comprised the two main target populations for standard anemia control initiatives. The advent of the human immunodeficiency virus (HIV) epidemic has now yielded a third population with a very high anemia burden, but standard international guidelines for first-level health workers in resource-constrained settings have not yet reflected the relevant differences in anemia etiology for HIV-infected vs. uninfected populations.
HIV-infected persons are vulnerable to the common causes of anemia: iron deficiency, intestinal parasites, and malaria.2–4 Malaria incidence and severity are greater in the presence of HIV, and the prognosis of HIV infection may be altered by concurrent helminth infection and/or deworming.5–8 Opportunistic and other complications of HIV infection further increase anemia risk. HIV viremia impairs hematopoiesis.9 Advanced HIV/AIDS disease is associated with lower hemoglobin (Hb) levels.10–12 HIV-associated immunosuppression increases the incidence of opportunistic conditions associated with anemia, including tuberculosis (TB), non-Hodgkin's lymphoma, oral candidiasis, chronic diarrhea, and Kaposi's sarcoma.9,13–17 HIV-associated anemia confers an increased risk of mortality.10,18–22 Though combination antiretroviral therapy (ART) generally improves anemia, specific antiretroviral drugs may exacerbate it, and persistent anemia after ART initiation is also associated with higher mortality. 19,23–26
In HIV-infected persons, HIV-associated causes of anemia may be more prevalent than the “usual” causes of anemia.12,27 In hospitalized Malawian adults with severe anemia, for example, mycobacterial infections and bacteremia were the predominant causes in HIV-infected patients, while iron deficiency and hookworm prevailed in HIV-uninfected people.28 Thus, in the presence of HIV, the differential diagnosis of anemia may be broader, and prior probabilities of possible causes may differ. In resource-limited settings characterized by large caseloads and scarce diagnostics, identification of the etiology of anemia in an HIV-infected individual may be difficult. Diagnosis is further complicated by the possibility of multiple concurrent conditions that simultaneously depress hemoglobin levels.
Mozambique is a low-income nation with a high prevalence of anemia in its HIV-infected population.29 The median Hb level was 9.9 g/dl in adults upon enrollment in HIV care in rural Zambézia Province, and Hb was <8.0 g/dl in 25% of TB/HIV patients at ART initiation.30,31 Malaria and intestinal helminthes are prevalent, and iron-deficiency anemia is thought to be common.32–34
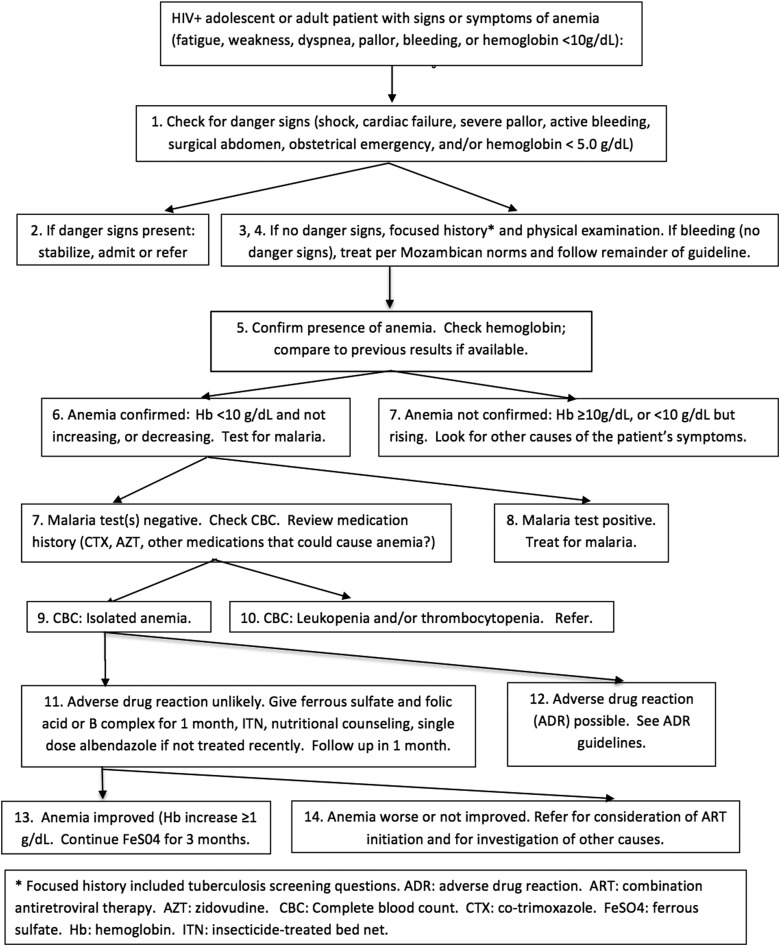
During Mozambique's initial response to the HIV epidemic, first-level health workers used a 2005 Mozambican guideline adapted from a standard World Health Organization guideline recommending that anemia (or pallor) be treated with presumptive iron supplementation, anthelminthics, and antimalarials.35 In 2009, the Mozambican Ministry of Health (MISAU) updated its anemia guideline to address several concerns: overprescription of antimalarials, underdetection of treatable HIV-associated causes of anemia, and possible increases in malaria and mortality risk resulting from overprescription of iron.36–39 The new anemia guideline (Fig. 1), designed for nonphysician clinicians providing HIV/AIDS care in resource-limited peripheral health centers, required hemoglobin and malaria testing and consideration of an expanded differential diagnosis that included TB, adverse drug reactions (ADRs), and HIV/AIDS Stage III anemia.11 Presumptive therapy with ferrous sulfate, folic acid, and anthelminthics was permitted. The anemia guideline focused only on initial management.
FIG. 1.
English translation (by the authors) of current Mozambican HIV/anemia guidelines.
In 2012, we conducted a prospective observational study (trial registration NCT 01681914, www.clinicaltrials.gov) in rural Zambézia Province (the estimated HIV prevalence based on 2009 survey data was 12.6% in adults 15–49 years old),40,41 with the goal of describing the performance of the new Mozambican anemia guideline when applied to the management of ambulatory, HIV-infected anemic adults in MISAU peripheral health facilities. We sought to determine the proportion of anemia cases that could be ascribed to specific cause(s) and the proportion improving with guideline-driven management, and to describe correlates of response to therapy.
Materials and Methods
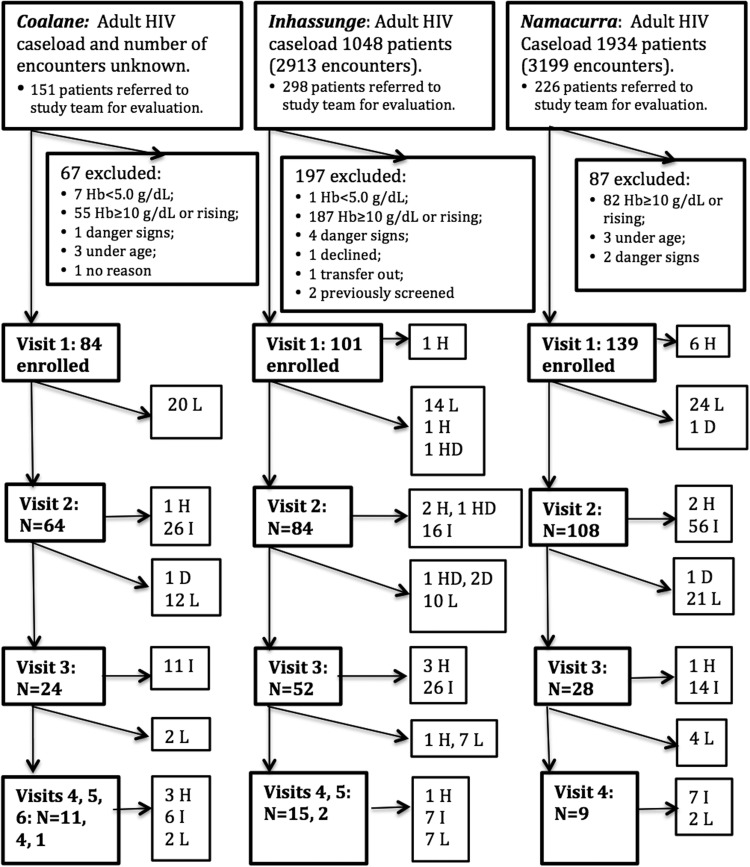
HIV-infected ambulatory patients ≥18 years of age presenting for scheduled (routine) or unscheduled care at three participating health centers, with current Hb <10 g/dl, were eligible (Fig. 2). All three health centers provided ongoing HIV/AIDS care, including ART. Random sampling was not logistically feasible. Clinic staff reviewed arriving patients' medical records in the outpatient HIV/AIDS clinic and in the prevention of mother-to-child transmission of HIV (PMTCT) clinic to identify those overdue for Hb testing. Records were reviewed approximately in the order in which patients arrived. Study laboratory technicians assisted site-based laboratories with Hb measurement, and study coordinators determined eligibility. Potential subjects were excluded if they presented with rising Hb, current ferrous sulfate treatment for anemia, inability to communicate in Portuguese or Echuabo (the major local language), or guideline-defined clinical “danger signs” (Hb <5 g/dl, shock, congestive heart failure, severe pallor, hemorrhage, acute abdomen, or obstetrical emergency) based on rapid preenrollment screening. Pregnant women and patients newly diagnosed with HIV were eligible. Study staff screened patients on weekdays, enrolling up to five patients per site per day. Recruitment began in April, during the peak malaria season, and ended in August, when malaria transmission still persisted but had declined from its seasonal peak.
FIG. 2.
Screening, eligibility, enrollment, and outcomes, by study site. Adult HIV caseload is the number of HIV-infected adults known to be in care at each site during the study period. Number of encounters is the estimated number of outpatient encounters for HIV-infected adults at each site during the period of recruitment. H, hospitalized; D, died; HD, died after hospitalization; L, lost to follow-up; I, improved. Diagonal lines: events between scheduled study visits.
Our general methods have been described previously in the context of a parallel study.42,43 Study coordinators recorded subjects' demographic and medical information. Study clinicians reviewed medical histories (including TB screening questions) and laboratory data, conducted physical examinations, and determined the most likely cause(s) of each subject's anemia. Diagnoses assigned by study clinicians were defined as “etiologic” if confirmed by laboratory methods (smear-positive pulmonary TB, culture-confirmed bacteremia, and rapid-test confirmed Plasmodium falciparum malaria) or “syndromic” if not laboratory confirmed. The syndromic diagnosis of adverse drug reaction was based on Mozambican national guidelines, derived in turn from World Health Organization standards.44,45 Study clinicians also addressed patients' other signs or symptoms, and managed ART and co-trimoxazole prophylaxis. The first follow-up Hb was usually measured 30 days after the enrollment visit and sooner if indicated. Community outreach workers visited subjects at home if they defaulted for scheduled visits or if blood cultures were positive, unless subjects specifically declined home visits.
Preplanned study endpoints were improvement (defined as a 1 g/dl increase in Hb either from enrollment or from Hb nadir), hospitalization, death, or loss to follow-up (LTFU). Subjects who were hospitalized with increasing Hb levels were classified as hospitalized. Hospitalization was defined as an endpoint because nonphysician clinicians were not authorized to provide in-patient care, and the anemia guideline was not meant for in-hospital use.
All subjects were tested for anemia and malaria at enrollment and retested at subsequent visits when indicated. Hemoglobin was measured using the HemoCue™ Hb 201 + analyzer (HemoCue Inc., Lake Forest, CA). We conducted quality control using blood specimens with known hemoglobin concentrations as measured by a Sysmex KX21N™ (Sysmex Corporation, Kobe, Japan) automated hematology analyzer because of global stockouts of HemoCue quality-control reagents. Eightcheck-3WP/X-Tra™ controls (Sysmex America, Lincolnshire, IL) were run daily to ensure the function of the Sysmex KX21N, except when Sysmex autoanalyzers were not functioning (for example, during reagent stockouts). Peripheral blood was tested for malaria antigenemia using the rapid ICT Malaria Plasmodium falciparum assay (ICT Diagnostics, Sydney, Australia). Positive tests were followed by light microscopy examination of thick and thin Giemsa-stained smears; three microscopists read each smear, although only the first reading was available at the time of the patient encounter.
For patients concurrently eligible for a parallel study of Mozambican fever guidelines,43 blood culture specimens (one set per subject) were collected at the first fever-study visit. We have described our methods for pathogen identification, antibiotic susceptibility testing, and quality control of blood cultures elsewhere.42
CD4+ T-lymphocyte (CD4) testing, complete blood counts (CBCs), and sputum microscopy for acid-fast bacilli (AFB) could also be ordered by study clinicians. These were not study procedures, although the study team provided quality assurance for CBCs and sputum AFB smears read by health center staff. Laboratory tests for iron and other micronutrient deficiencies, and for hemoglobinopathy, were not available.
Our target sample size was 324 subjects. We estimated that a cause of anemia would be identifiable in 60%. With 20% LTFU, if the true proportion of anemia cases with diagnosable/classifiable causes was 0.60, the 95% confidence interval (CI) would be 0.54, 0.66, and the lower limit of the CI would exclude 0.50.
We used proportions and medians to describe subject characteristics at enrollment. We evaluated correlates of Hb response with linear regression, and correlates of adverse outcomes with logistic regression models. To evaluate the first primary outcome (proportion of patients who were assigned an etiologic or syndromic diagnosis of anemia cause), we reviewed study instruments to identify diagnoses assigned by study clinicians. To evaluate the second primary outcome (proportion improving), we calculated the proportion of subjects whose Hb rose by ≥1 g/dl and who were discharged from the study. To characterize associations between subject and treatment characteristics and Hb evolution, we used bivariate and multivariable linear regression with Hb change from enrollment to first follow-up Hb measurement as the dependent variable to assess response to initial management. Because over 100 subjects remained in the study and often acquired additional diagnoses after the first follow-up Hb, we repeated these analyses using Hb change from enrollment (or nadir Hb) to final study Hb to assess response to overall management. Finally, we used bivariate and multivariable logistic regression to describe associations between subject or treatment characteristics and adverse outcomes.
The Mozambican National Bioethics Committee and Vanderbilt's Institutional Review Board approved the study protocol. Literate subjects gave written informed consent; illiterate subjects gave witnessed oral consent.
Results
We screened 675 ambulatory HIV-infected adults for enrollment (Fig. 2) and enrolled 324 (48.0%). Our subjects (Table 1) were predominantly female; the majority were on co-trimoxazole prophylaxis but were not receiving ART in spite of low CD4+ cell counts. Most who had already initiated ART [88/96 (91.7%)] were receiving zidovudine (ZDV)-based regimens.
Table 1.
Subject Characteristics at Study Entry, Overall and by Study Outcome
| Lost to follow-up, by hemoglobin trend at last study visit (n = 125; 38.6%) | ||||||
|---|---|---|---|---|---|---|
| Subject characteristics (n = 324) | All subjects (n = 324) N (%) or median (IQR) | Improved (n = 169; 52.2%) N (%) or median (IQR) | Hospitalized or died (n = 30; 9.3%) N (%) or median (IQR) | LTFU: No second Hb or no change in Hb (n = 70; 21.6%) N (%) or median (IQR) | LTFU: rising Hb (n = 37; 11.4%) N (%) or median (IQR) | LTFU: falling Hb (n = 18; 5.6%) N (%) or median (IQR) |
| Demographics | ||||||
| Study site | ||||||
| Coalane | 84 (25.9) | 43 (25.4) | 5 (16.7) | 27 (38.6) | 5 (13.5) | 4 (22.2) |
| Inhassunge | 101 (31.2) | 49 (29.0) | 14 (46.7) | 15 (21.4) | 16 (43.2) | 7 (38.9) |
| Namacurra | 139 (42.9) | 77 (45.6) | 11 (36.7) | 28 (40.0) | 16 (43.2) | 7 (38.9) |
| Gender, pregnancy | ||||||
| Male | 62 (19.1) | 39 (23.1) | 10 (33.3) | 8 (11.4) | 4 (10.8) | 1 (5.6) |
| Female, not pregnant | 193 (59.6) | 98 (58.0) | 19 (63.3) | 35 (50.0) | 28 (75.7) | 13 (72.2) |
| Pregnant | 69 (21.3) | 32 (18.9) | 1 (3.3) | 27 (38.6) | 5 (13.5) | 4 (22.2) |
| Age (years) | 29 (23, 36) | 29 (23, 37) | 31 (25, 36) | 25 (21, 34) | 28 (23, 38) | 30 (22, 40) |
| Illiterate | 148 (45.7) | 71 (42.1) | 13 (43.3) | 38 (54.3) | 16 (43.2) | 10 (55.6) |
| Did not accept home visits | 48 (14.8) | 20 (11.8) | 2 (6.7) | 18 (25.7) | 8 (21.6) | 0 |
| HIV/AIDS status | ||||||
| Most recent CD4 count (cells/μl) (73 missing) | 249 (112, 436) | 300 (150, 509) | 153 (86, 235) | 229 (85, 419) | 190 (121, 469) | 189 (70, 324) |
| Most recent CD4 count (cells/μl), categories | ||||||
| <200 | 106 (32.7) | 50 (29.6) | 15 (50) | 19 (27.4) | 16 (43.2) | 6 (33.3) |
| 200–349 | 51 (15.7) | 26 (15.4) | 7 (23.3) | 9 (12.9) | 5 (13.5) | 4 (22.2) |
| 350–499 | 43 (13.3) | 28 (16.6) | 1 (3.3) | 9 (12.9) | 4 (10.8) | 1 (5.6) |
| ≥500 | 51 (15.7) | 37 (21.9) | 1 (3.3) | 6 (8.6) | 6 (16.2) | 1 (5.6) |
| None available | 73 (22.5) | 28 (16.6) | 6 (20) | 27 (38.6) | 6 (16.2) | 6 (33.3) |
| On CTX | 237 (73.2) | 128 (75.7) | 18 (60) | 44 (62.9) | 32 (86.5) | 15 (83.3) |
| On ART | 96 (29.6) | 62 (36.7) | 8 (26.7) | 11 (15.7) | 11 (29.7) | 4 (22.2) |
| On ZDV (ART or PMTCT) | 114 (35.2) | 67 (39.6) | 8 (26.7) | 25 (35.7) | 10 (27.0) | 4 (22.2) |
| Malaria prevention | ||||||
| Used bed net on night before visit 1 (15 missing) | 155 (47.8) | 82 (50.6) | 13 (46.4) | 38 (58.5) | 16 (43.2) | 6 (35.3) |
| Indoor residual spraying (7 missing) | 100 (30.9) | 58 (34.3) | 7 (23.3) | 20 (28.6) | 9 (24.3) | 6 (33.3) |
| Clinical characteristics at enrollment (study visit one) | ||||||
| Temperature (axillary, °C) (4 missing) | 36.7 (36.2, 37.2) | 36.6 (36.2, 37.0) | 37.5 (37.0, 38.5) | 36.7 (36.2, 37.3) | 36.6 (36.1, 37.4) | 36.6 (36.2, 38.0) |
| Body mass index (kg/m2) (9 missing) | 19.0 (17.3, 21.2) | 19.2 (17.8, 21.1) | 17.3 (16.0, 18.3) | 19.8 (17.6, 22.0) | 19.5 (17.7, 21.5) | 17.0 (15.2, 21.1) |
| Hemoglobin (g/dl) at baseline | 8.8 (7.9, 9.6) | 8.8 (7.9, 9.4) | 8.2 (7.0, 9.0) | 8.7 (7.4, 9.6) | 9.5 (9.0, 9.8) | 9.1 (8.1, 9.7) |
| Key diagnoses and comorbidities at visit 1 (enrollment visit) | ||||||
| Smear-positive pulmonary TB | 2 (0.6) | 1 (0.6) | 0 | 1 (1.4) | 0 | 0 |
| Smear-negative pulmonary TB | 13 (4.0) | 8 (4.7) | 2 (6.7) | 3 (4.3) | 0 | 0 |
| TB suspect | 26 (8.0) | 10 (5.9) | 9 (30) | 3 (4.3) | 4 (10.8) | 0 |
| Bacteremiaa | 14 (4.3) | 4 (2.4) | 5 (16.7) | 2 (2.9) | 2 (5.4) | 1 (5.6) |
| Malaria (rapid test positive) | 50 (15.4) | 30 (17.8) | 3 (10) | 12 (17.1) | 3 (8.1) | 2 (11.1) |
| Malaria (slide-confirmed) (4 missing) | 39 (12.2) | 23 (13.6) | 2 (6.7) | 9 (12.9) | 3 (8.1) | 2 (11.1) |
| Oral candida | 12 (3.7) | 6 (3.6) | 4 (13.3) | 0 | 0 | 2 (11.1) |
| Esophageal candida | 4 (1.2) | 0 | 3 (10.0) | 0 | 0 | 1 (5.6) |
| Any bleeding | 4 (1.2) | 3 (1.8) | 0 | 1 (1.4) | 0 | 0 |
| Suspected Stage III anemia | 4 (1.2) | 2 (1.2) | 0 | 2 (2.9) | 0 | 0 |
| Suspected ADR, CTX | 5 (1.5) | 4 (2.4) | 0 | 0 | 1 (2.7) | 0 |
| Suspected ADR, ZDV | 9 (2.8) | 7 (4.1) | 1 (3.3) | 1 (1.4) | 0 | 0 |
Bacteremia could not be confirmed until 2 to 4 days after culture specimens were obtained.
ADR, adverse drug reaction; ART, combination antiretroviral therapy; CD4, CD4+ T-lymphocyte count; CTX, co-trimoxazole prophylaxis; Hb, hemoglobin (g/dl); IQR, interquartile range; LTFU, lost to follow-up; PMTCT, prevention of mother-to-child transmission of HIV, TB, tuberculosis; ZDV, zidovudine.
Table 2 describes diagnoses assigned by the study clinicians (our first primary outcome). Syndromic diagnoses predominated. Three hundred and fifteen subjects (97.2%) were assigned the syndromic diagnosis of iron deficiency (with or without presumed helminth infection). Only 79 (24.4%) subjects were assigned etiologic diagnoses (laboratory-confirmed malaria, tuberculosis, or bacteremia). Fifty-nine (18.2%) acquired the syndromic diagnoses of TB, malaria, ADR, stage III anemia, and/or bleeding (specifically contemplated in the current guideline). One hundred and seven (33.0%) never acquired diagnoses other than those emphasized in the previous anemia guideline: malaria, iron deficiency, and/or helminth infection.
Table 2.
Assigned Diagnoses and Clinical Management at Enrollment and Follow-up Visits
| Subjects not hospitalized at enrollment visit (n = 317)a | |||
|---|---|---|---|
| At enrollment visit: N (%) subjects not hospitalized at this visit | After enrollment but before final Hb measurement | After enrollment, any visit (includes subjects who had no follow-up hemoglobins) | |
| Key diagnoses and comorbidities | |||
| Iron deficiency (presumed; 1 missing) | 315 (99.4) | 0 | 0 |
| Intestinal helminth infection (presumed) | 293 (92.4) | 1 (0.3) | 2 (0.6) |
| Bleeding | 1 (0.3) | 1 (0.3) | 3 (0.9) |
| Suspected HIV/AIDS Stage III anemia, ART initiated | 4 (1.3) | 0 | 0 |
| Suspected ADR, ZDV discontinued | 9 (2.8) | 6 (1.9) | 6 (1.9) |
| Suspected ADR, CTX discontinued | 5 (1.6) | 12 (3.8) | 14 (4.4) |
| Malaria (rapid test positive) | 50 (15.8) | 2 (0.6) | 6 (1.9) |
| Malaria (slide-confirmed) | 38 (12.0) | 1 (0.3) | 4 (1.3) |
| Syndromically diagnosed malaria (rapid test negative) | 3 (0.9) | 0 | 1 (0.3) |
| Smear-positive pulmonary TB | 2 (0.6) | 1 (0.3) | 4 (1.3) |
| Smear-negative pulmonary TB | 14 (4.4) | 5 (1.6) | 10 (3.2) |
| TB suspect | 21 (6.7) | 3 (0.9) | 10 (3.2) |
| Bacteremia (confirmed)b | 12 (3.8) | 0 | 1 (0.3) |
| Oral candida | 9 (2.8) | 3 (0.9) | 7 (2.2) |
| Esophageal candida | 3 (0.9) | 0 | 2 (0.6) |
| Kaposi's sarcoma | 6 (1.9) | 1 (0.3) | 4 (1.3) |
| Management | |||
| Start ferrous sulfate/folic acid (1 missing) | 315 (99.4) | 0 | 0 |
| Start mebendazole or albendazole | 293 (92.4) | 1 (0.3) | 2 (0.6) |
| Start ART | 13 (4.1) | 10 (3.2) | 25 (7.9) |
| Start ZDV (ART or PMTCT regimen) | 15 (4.8) | 6 (1.9) | 17 (5.4) |
| Start CTX | 70 (22.2) | 1 (0.3) | 8 (2.5) |
| Stop ZDV | 6 (1.9) | 7 (2.2) | 8 (2.5) |
| Stop CTX | 5 (1.6) | 12 (3.8) | 14 (4.4) |
| Start antimalarial | 52 (16.5) | 2 (0.6) | 6 (1.9) |
| Start TB treatment | 4 (1.3) | 6 (1.9) | 12 (3.8) |
| Continue TB treatment | 12 (3.8) | N/A | N/A |
| Start antibiotics | 115 (36.4) | 9 (2.8) | 17 (5.4) |
| Start antifungals | 30 (9.5) | 1 (0.3) | 8 (2.5) |
| No recorded treatment other than ferrous sulfate, anthelmintics, and/or antimalarials | 120 (37.9) | 49 (15.5) | 106 (33.4) |
Seven additional subjects were hospitalized at the enrollment visit; the full lists of diagnoses acquired during these hospitalizations were unavailable and so these subjects have been excluded from the table.
Bacteremia could not be confirmed until 2 to 4 days after obtaining blood culture specimens. Two of the subjects hospitalized at enrollment were subsequently found to be bacteremic.
ADR, adverse drug reaction; ART, combination antiretroviral therapy; CTX, co-trimoxazole prophylaxis; Hb, hemoglobin; TB, tuberculosis; ZDV, zidovudine.
Slightly more than half of the subjects [169 (52.2%)] attained the study endpoint of “improvement” (the second primary outcome); 30 (9.3%) died or were hospitalized and 125 (38.6%) were LTFU. All adverse outcomes were associated with declining Hb, bacterial bloodstream infection [predominantly non-typhoid Salmonella (NTS)], low CD4 count in the absence of ART, suspected TB, febrile illness, and/or a recent diagnosis of HIV. Of 169 subjects who improved, 65 (38.5%) were managed only with conventional treatment (iron supplements, anthelminthics, and/or antimalarials) throughout the study, while the other patients required antibiotics, antifungals, TB treatment, or initiation or revision of ART. Fifty-two (41.6%) of those LTFU were known to be alive after the final study visit, based on chart review and active defaulter tracing.
Most subjects reached study endpoints between enrollment and the first follow-up Hb measurement. At enrollment, seven subjects (2.2%) were hospitalized; nearly all (99.4%) of the 317 not hospitalized were presumptively treated for iron deficiency, usually in combination with presumptive deworming. Antimalarials were prescribed for 49 subjects with positive malaria rapid tests (84.8% confirmed by microscopy). Fewer than half (120; 37.9%) were treated exclusively with conventional treatment at the first study visit.
Second Hb measurements were available for 240 subjects at a mean interval of 34 days after enrollment. The median initial Hb change was +1.0 g/dl (IQR 0.10, 1.95; range −5.3, +6.4). One hundred and twenty-four subjects (51.7%) achieved a ≥1.0 g/dl increase and therefore reached a study endpoint. Of the 116 subjects who did not achieve the target Hb increase and remained in the study, 51 (44.0%) had experienced Hb declines [median decline 0.90 g/dl (IQR −1.3, −0.3, range −5.8, −0.10)].
Table 3 describes linear associations between subject characteristics at enrollment and Hb evolution from enrollment to the second Hb measurement. In multivariable models, higher BMI was significantly and positively associated with Hb trend [+0.08 g/dl per 1 kg/m2 increase [0.02, 0.14)]. Covariates significantly but negatively associated with Hb trend were study sites [Coalane, coefficient: −0.62, (95% CI −1.15, −0.09) and Inhassunge, −0.98 (−1.45, −0.52)], higher baseline Hb [−0.38 per 1 g/dl increase (−0.57, −0.20)], esophageal candidiasis [−2.63 (−4.38, −0.87)], and ART initiation [predominantly zidovudine (ZDT) based] at enrollment [−1.43 (−2.50, −0.35)]. In bivariate models, higher temperature and the presence of undetected TB were also associated with Hb decline, and male sex with Hb increase, but these associations did not persist in multivariable analysis. In this and other models (see Table 5, discussed below), CD4-ART interactions could be assessed only for subjects with CD4 <350 cells/μl, owing to infrequent ART use in other subjects.
Table 3.
Association of Subject Characteristics at Study Entry and Hemoglobin Change at Second Hemoglobin Measurement: Results of Bivariate and Multivariable Linear Regression
| Subject characteristics | Bivariate coefficient for hemoglobin change from baseline to measurement 2 (g/dl), with 95% CI N = 240 | p | Multivariable coefficient for hemoglobin change from baseline to measurement 2 (g/dl), with 95% CI N = 232 | p |
|---|---|---|---|---|
| Demographics | ||||
| Study site | ||||
| Coalane | −0.50 (−1.04, 0.03) | 0.064 | −0.62 (−1.15, −0.09) | 0.022 |
| Inhassunge | −0.88 (−1.35, −0.40) | <0.001 | −0.98 (−1.45, −0.52) | <0.001 |
| Namacurra | Reference | Reference | Reference | |
| Gender, pregnancy status | ||||
| Male | 0.53 (0.00, 1.07) | 0.05 | — | |
| Female, not pregnant | Ref | Reference | — | |
| Pregnant | 0.39 (−0.18, 0.97) | 0.175 | — | |
| Age (years) | 0.005 (−0.02, 0.03) | 0.680 | ||
| Illiterate | −0.17 (−0.60, 0.26) | 0.437 | ||
| Did not accept home visits | 0.61 (−0.03, 1.25) | 0.061 | — | |
| HIV/AIDS status | ||||
| CD4 count adjusted for interaction with ART status at enrollmenta | ||||
| CD4 <350, no ART | −0.11 (−0.70, 0.48) | 0.719 | 0.10 (−0.46, 0.65) | 0.729 |
| CD4 <350, started ART | −1.51 (−2.62, −0.40) | 0.008 | −1.43 (−2.50, −0.36) | 0.009 |
| CD4 <350, on ART | −0.59 (−1.28, −0.10) | 0.095 | −0.40 (−1.04, 0.25) | 0.229 |
| CD4 >350 | Reference | Reference | ||
| No CD4 available | −0.38 (−0.25, +1.02) | 0.231 | 0.17 (−0.43, +0.77) | 0.586 |
| On zidovudine (ART or PMTCT) | 0.01 (−0.45, 0.43) | 0.964 | ||
| On co-trimoxazole prophylaxis | −0.05 (−0.56, 0.45) | 0.842 | ||
| Clinical characteristics at enrollment | ||||
| Temperature (axillary, °C) (4 missing) | −0.26 (−0.51, −0.02) | 0.038 | — | |
| Body mass index (kg/m2) (9 missing) | 0.08 (0.01, 0.15) | 0.017 | 0.08 (0.02, 0.14) | 0.010 |
| Hemoglobin (g/dl) at baseline | −0.25 (−0.44, −0.06) | 0.009 | −0.38 (−0.57, −0.20) | <0.001 |
| Smear-positive pulmonary TB | 1.22 (−2.08, 4.52) | 0.467 | ||
| Smear-negative pulmonary TB | −0.08 (−1.10, 0.94) | 0.876 | ||
| TB suspect | −0.35 (−1.20, 0.51) | 0.424 | ||
| Active TB, not diagnosed until after enrollment visit | −1.31 (−2.18, −0.45) | 0.003 | — | |
| Bacteremia (detected on blood culture drawn at enrollment visit) | 0.09 (−1.09, 1.28) | 0.878 | ||
| Malaria (rapid test positive) | −0.17 (−0.76, 0.41) | 0.557 | ||
| Malaria (slide-confirmed) | −0.11 (−0.77, 0.54) | 0.731 | ||
| Oral candida | 0.20 (−0.92, 1.32) | 0.726 | ||
| Esophageal candida | −3.06 (−4.93, −1.18) | 0.001 | −2.63 (−4.38, −0.87) | 0.003 |
| Suspected Stage III anemia | 0.57 (−1.77, 2.91) | 0.632 | ||
| Suspected ADR, CTX | 1.04 (−0.45, 2.52) | 0.170 | ||
| Suspected ADR, ZDV | 0.49 (−0.69, 1.68) | 0.413 | ||
| Management at visit 1 | ||||
| Start ART | −0.90 (−1.83, 0.04) | 0.060 | See above | |
| Start ZDV (for ART or PMTCT) | 0.38 (−0.73, 1.50) | 0.499 | ||
| Stop ZDV | 0.49 (−0.69, 1.68) | 0.413 | ||
| Start CTX | −0.20 (−0.74, 0.35) | 0.473 | ||
| Stop CTX | 1.04 (−0.48, 2.52) | 0.170 | ||
| Start antimalarial | −0.31 (−0.88, 0.26) | 0.280 | ||
| Start TB treatment | −0.22 (−2.14, 1.69) | 0.820 | ||
| Continue TB treatment | 0.12 (−1.00, 1.24) | 0.835 | ||
| Start antibiotics | −0.07 (−0.52, 0.37) | 0.743 | ||
| Start antifungals | 0.42 (−0.24, 1.08) | 0.211 | ||
| Start ferrous sulfateb | Undefined | |||
| Start anthelminthics | −0.60 (−1.48, 0.27) | 0.174 | ||
| No interventions aside from ferrous sulfate, anthelminthics, and/or antimalarials before hemoglobin 2 | 0.09 (−0.34, 0.52) | 0.685 | ||
| Constant | 3.28 (1.29, 5.26) | 0.001 | ||
Antiretroviral use was very infrequent in patients with CD4 ≥350 or missing.
100% ferrous sulfate prescription in subjects with nonmissing data.
ADR, adverse drug reaction; ART, combination antiretroviral therapy; CD4, CD4+ T-lymphocyte count, cells/μL; CI, confidence interval; CTX, co-trimoxazole prophylaxis; PMTCT, prevention of mother-to-child transmission of HIV; TB, tuberculosis; ZDV, zidovudine.
Table 5.
Association of Subject Characteristics at Study Entry with Adverse Outcomes (Hospitalization or Death): Results of Bivariate and Multivariable Linear Regression
| Subject characteristics (n = 324) | Bivariate OR for poor outcome (hospitalization or death), 95% CI | p | Multivariable OR for poor outcome (hospitalization or death), 95% CI | p |
|---|---|---|---|---|
| Demographics | ||||
| Study site | ||||
| Coalane | 0.81 (0.27, 2.49) | 0.719 | 0.15 (0.02, 0.99) | 0.049 |
| Inhassunge | 2.00 (0.84, 4.76) | 0.117 | 1.78 (0.52, 6.15) | 0.361 |
| Namacurra | Reference | Reference | ||
| Gender, pregnancy status | ||||
| Male | 1.32 (0.56, 3.10) | 0.520 | — | |
| Female, not pregnant | Reference | — | ||
| Pregnant | 0.16 (0.02, 1.25) | 0.081 | — | |
| Age (years) | 1.00 (0.96, 1.04) | 0.990 | ||
| Illiterate | 1.05 (0.48, 2.31) | 0.893 | ||
| Did not accept home visits | 0.53 (0.12, 2.41) | 0.412 | ||
| HIV/AIDS status | ||||
| Most recent CD4 count (cells/μl), categories with adjustment for 3 categories of ART at enrollment | ||||
| <350, no ART at enrollment | 8.77 (1.70, 45.36) | 0.010 | 8.94 (1.18, 67.49) | 0.034 |
| <350, start ART at enrollment | 5.38 (0.38, 75.38) | 0.212 | 3.29 (0.06, 181.82) | 0.561 |
| <350, already on ART at enrollment | 6.45 (1.09, 38.13) | 0.040 | 13.66 (1.31, 141.46) | 0.028 |
| > = 350 | Reference | Reference | ||
| No CD4 available | 6.92 (1.29, 37.17) | 0.024 | 12.39 (1.37, 111.82) | 0.025 |
| On co-trimoxazole prophylaxis at enrollment | 0.48 (0.21, 1.08) | 0.076 | 0.13 (0.03, 0.57) | 0.007 |
| On zidovudine (ART or prevention of mother-to-child transmission) at enrollment | 0.55 (0.23, 1.32) | 0.181 | ||
| Clinical characteristics at visit 1 | ||||
| Temperature (axillary, °C) (2 missing) | 2.91 (1.84, 4.62) | <0.001 | 2.21 (1.26, 3.87) | 0.005 |
| Body mass index (kg/m2) (7 missing) | 0.75 (0.64, 0.88) | <0.001 | 0.76 (0.61, 0.95) | 0.014 |
| Hemoglobin (g/dl) at baseline | 0.73 (0.53, 0.996) | 0.047 | — | |
| Key diagnoses and comorbidities at first or any study visit | ||||
| Lowest Hb recorded during study (Hb nadir) | 0.62 (0.47, 0.83) | 0.001 | 0.43 (0.25, 0.73) | 0.002 |
| Smear-positive pulmonary TB, first or any visita | Undefined | — | ||
| Smear-negative pulmonary TB, visit 1 | 6.81 (2.48, 18.69) | <0.001 | — | |
| TB suspect at visit 1 | 6.81 (2.48, 18.69) | <0.001 | — | |
| Active TB, not diagnosed until after enrollment visit (enrollment visit only) | 4.03 (1.22, 13.29) | 0.022 | — | — |
| Bacteremia (detected on blood culture drawn at this visit), visit 1 | 8.25 (2.07, 32.81) | 0.003 | 5.89 (0.85, 40.58) | 0.072 |
| Malaria (rapid test positive), visit 1 | 0.51 (0.15, 1.81) | 0.300 | ||
| Oral candida, any visit | 4.03 (1.22, 13.29) | 0.022 | — | |
| Esophageal candida (any visit)b | Undefined. | — | ||
| Any bleeding, any study visit | 5.96 (0.81, 44.09) | 0.080 | 110.21 (4.31, 2819.10) | 0.002 |
| Suspected Stage III Anemia, visit 1 or any visita | Undefined. | |||
| Suspected ADR CTX, any visit | 0.93 (0.20, 4.40) | 0.932 | ||
| Suspected ADR ZDV, any visit Hb | 2.74 (0.78, 9.53) | 0.114 | ||
| Management at any study visit | ||||
| Start ART, visit 1 | 0.69 (0.08, 5.76) | 0.735 | ||
| Start ZDV (ART or PMTCT), any visit | 0.31 (0.04, 2.40) | 0.262 | ||
| Stop ZDV, any study visit | 2.74 (0.78, 9.53) | 0.114 | ||
| Start CTX, any study visit | 0.89 (0.34, 2.34) | 0.816 | ||
| Stop CTX, any study visit | 0.93 (0.20, 4.40) | 0.932 | ||
| Start antimalarial, visit 1 | 0.71 (0.23, 2.19) | 0.555 | ||
| Start TB treatment, any study visit | 1.27 (0.26, 6.19) | 0.768 | ||
| Start antibiotics, any study visit | 1.97 (0.90, 4.31) | 0.089 | — | |
| Start antifungals, any study visit | 1.84 (0.71, 4.75) | 0.209 | ||
| Start iron supplements, first or any study visitc | Undefined. | |||
| Start anthelminthics, any study visit | 0.45 (0.13, 1.53) | 0.202 | ||
| No new interventions other than iron, anthelmintics, and/or antimalarial, all visits | 0.32 (0.17, 0.88) | 0.027 | — | |
Undefined: no adverse outcomes in this category.
Undefined: all subjects in this category had adverse outcomes.
Undefined: all subjects with known outcomes and non-missing data were started on iron at study visit 1.
ADR, adverse drug reaction; ART, combination antiretroviral therapy; CD4, CD4+ T-lymphocyte count, cells/μL; CI, confidence interval; CTX, co-trimoxazole prophylaxis; Hb, hemoglobin, g/dL; LTFU, lost to follow-up; OR, odds ratio; PMTCT, prevention of mother-to-child transmission of HIV; TB, tuberculosis; ZDV, zidovudine.
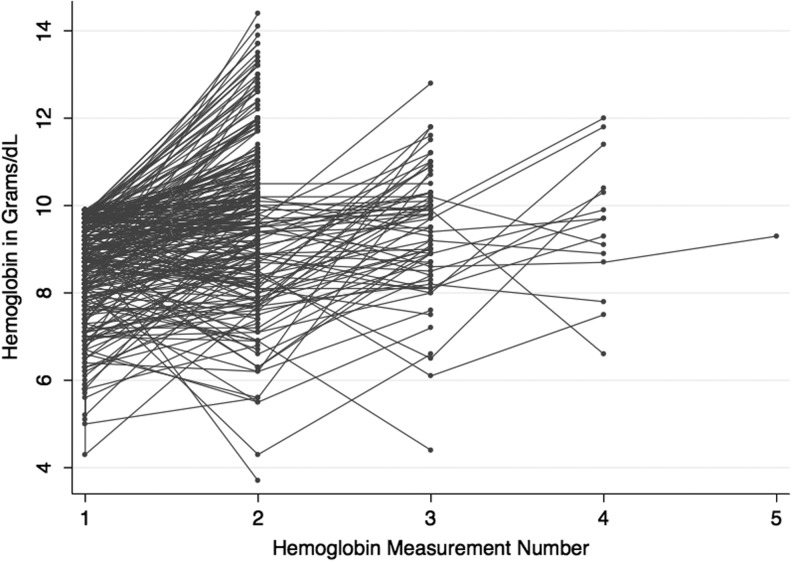
After the first follow-up Hb measurement, subjects who remained in the study had up to three subsequent Hb measurements before reaching endpoints. The evolution of anemia in individual subjects was heterogeneous throughout (Fig. 3). For example, one subject's Hb rose by 3.4 g/dl after iron supplementation and discontinuation of ZDV. Another's Hb fell by 2.3 g/dl after an episode of NTS bacteremia, then rose 5.4 g/dl after initiation of TB treatment, ART, and iron.
FIG. 3.
Hemoglobin Evolution in Study Subjects. Each line links the serial hemoglobin values for an individual study subject from study entry (hemoglobin measurement 1) to the final measurement before study endpoint (range: 1 to 5 measurements; x-axis). The y-axis describes measured hemoglobin values in grams per deciliter.
Anemia management after the first follow-up Hb differed from initial management. Initiation of TB treatment and ART regimen changes to address suspected ADR were more common after the first study visit, while new malaria treatment was less common (Table 2). Table 4 describes linear correlates of Hb response from enrollment or nadir (for those with hemoglobin improvement after decline) to final Hb. The multivariable model resembled that for initial Hb response, but detected three additional factors significantly and positively associated with Hb trend: continuation of ART from enrollment through study endpoint [coefficient 0.49 (0.11, 0.88)], discontinuation of ZDV for suspected ADR [1.08 (0.26, 1.89)], and diagnosis of smear-positive pulmonary TB [1.66 (0.13, 3.19)]. CD4 <350 was significantly associated with a negative Hb trend [−0.59 (−0.98, −0.20)].
Table 4.
Association of Subject and Treatment Characteristics at Any Study Visit with Hemoglobin Response from Hemoglobin Nadir to Last Hemoglobin Measurement: Results of Bivariate and Multivariable Linear Regression
| Subject characteristics (n = 240 subjects with at least 2 hemoglobin measurements)a | Bivariate coefficient, 95% CI | p | Multivariable coefficient, 95% CI | p |
|---|---|---|---|---|
| Demographics | ||||
| Study site | ||||
| Coalane | −0.42 (−0.92, 0.08) | 0.098 | −0.77 (−1.24, −0.31) | 0.001 |
| Inhassunge | −0.94 (−1.39, −0.49) | 0.000 | −1.06 (−1.46, −0.66) | <0.001 |
| Namacurra | Reference | Reference | ||
| Gender, pregnancy status | ||||
| Male | 0.41 (−0.09, 0.91) | 0.109 | ||
| Female, not pregnant | Reference | |||
| Pregnant | 0.32 (−0.22, 0.85) | 0.250 | ||
| Age (years) | 0.003 (−0.02, 0.02) | 0.741 | ||
| Illiterate | −0.01 (−0.42, 0.39) | 0.950 | ||
| Did not accept home visits | 0.50 (−0.09, 1.11) | 0.097 | — | |
| HIV/AIDS status | ||||
| Most recent CD4 count (cells/μl), categories | ||||
| <350 | −0.60 (−1.04,-0.16) | 0.008 | −0.59 (−0.98, −0.20) | 0.003 |
| ≥350 | Reference | Reference | ||
| None available | −0.04 (−0.62, 0.55) | 0.898 | −0.22 (−0.74, 0.29) | 0.393 |
| On ART at enrollment | 0.53 (0.11, 0.94) | 0.013 | — | |
| On CTX at enrollment | 0.11 (−0.38, 0.60) | 0.655 | ||
| On ZDV (ART or monotherapy for PMTCT) at enrollment | 0.45 (0.04, 0.86) | 0.032 | — | |
| Clinical characteristics at visit 1 | ||||
| Temperature (axillary, °C) (2 missing) | −0.27 (−0.50, −0.04) | 0.024 | — | |
| Body mass index (kg/m2) (7 missing) | 0.04 (−0.02, 0.10) | 0.237 | ||
| Hemoglobin (g/dl) at baseline | −0.33 (−0.50, −0.15) | <0.001 | −0.41 (−0.58, −0.24) | <0.001 |
| Key Diagnoses and comorbidities at any visit | ||||
| Smear-positive pulmonary TB | 1.92 (0.15, 3.70) | 0.034 | 1.66 (0.13, 3.19) | 0.034 |
| Smear-negative pulmonary TB | 0.38 (−0.42, 1.17) | 0.355 | ||
| Bacteremia (detected on blood culture drawn at this visit) | 0.04 (−0.96, 1.04) | 0.934 | ||
| Malaria (rapid test positive) | −0.28 (−0.80, 0.23) | 0.284 | ||
| Oral candida | −0.87 (−1.69, −0.06) | 0.036 | — | |
| Esophageal candida | −3.02 (−4.37, −1.68) | <0.001 | −2.39 (−3.60, −1.17) | <0.001 |
| Any bleeding | −1.84 (−3.22, −0.46) | 0.009 | −1.69 (−2.89, −0.50) | 0.006 |
| Suspected Stage III Anemia | 0.05 (−2.14, 2.25) | 0.964 | ||
| Management at any visit prior to endpoint visit | ||||
| Start antibiotics | −0.34 (−0.75, 0.07) | 0.101 | ||
| Start antimalarials | −0.43 (−0.95, 0.09) | 0.107 | ||
| Start antifungals | 0.19 (−0.42, 0.80) | 0.536 | ||
| Any TB treatment | 0.26 (−0.46, 0.98) | 0.474 | ||
| Start ART | −0.60 (−1.27, 0.08) | 0.083 | — | |
| Start PMTCT | 0.02 (−0.98, 1.02) | 0.967 | ||
| Start cotrimoxazole | −0.38 (−0.88, 0.12) | 0.133 | ||
| Interaction: ART/ZDV discontinuation | ||||
| On ART at study entry, no discontinuation of ZDV | 0.44 (−.004, 0.88) | 0.048 | 0.49 (0.11, 0.88) | 0.012 |
| On ART at study entry, ZDV discontinued | 1.09 (0.18, 2.01) | 0.019 | 1.08 (0.26, 1.90) | 0.010 |
| No ART | Reference | Reference | ||
| Interaction: CTX/CTX discontinuation | ||||
| On CTX at study entry, no discontinuation of CTX | 0.05 (−0.45, 0.54) | 0.858 | — | |
| On CTX at study entry, CTX discontinued | 1.01 (0.08, 1.94) | 0.033 | — | |
| No CTX | Reference | — | ||
| No management aside from iron supplements, anthelminthics, antimalarials | 0.32 (−0.10, 0.74) | 0.132 | ||
| Constant | 5.86 (4.29, 7.43) | <0.001 | ||
Subjects without second hemoglobin measurements either died, were hospitalized, or were lost to follow-up before the second measurement could be obtained.
ADR, adverse drug reaction; ART, combination antiretroviral therapy; CD4, CD4+ T-lymphocyte count; CI, confidence interval; CTX, co-trimoxazole prophylaxis; PMTCT, prevention of mother-to-child transmission; TB, tuberculosis; ZDV, zidovudine.
Logistic regression models (Table 5) assessing associations with the composite adverse outcome (hospitalization or death at any point in the study) echoed the linear regression findings, in that adverse outcomes were significantly associated with lower or missing CD4, lower BMI, and bleeding. They also described significant positive associations between adverse outcomes and increased axillary temperature on enrollment [OR 2.21 (1.26, 3.87 per 1°C elevation)] and significant negative associations with reported use of co-trimoxazole prophylaxis at enrollment [0.16 (0.04, 0.62)]. However, the odds ratios for association with adverse outcome were undefined for smear-positive pulmonary TB and suspected stage III anemia, prescription of ferrous sulfate, and esophageal candidiasis, because of small cell sizes.
Discussion
We recruited HIV-infected Mozambican adults with measured Hb <10 g/dl and no current ferrous sulfate supplementation as determined by chart review and subject interview. Because chart notes were usually quite brief, it is possible that some subjects had recently been given treatment doses of iron supplements, but it is not possible to quantify the effect of this potential misclassification based on available information.
Using a new Mozambican guideline, non-physician clinicians assigned etiologic or syndromic diagnoses of the presumed cause of anemia in all subjects not hospitalized upon enrollment. Although the syndromic diagnosis of iron deficiency (with or without helminth infection) was assigned almost universally, as it would have been using Mozambique's earlier guideline, fewer than one in five patients received antimalarials (seldom without confirmatory testing) and nearly one-third of subjects also received other HIV-associated diagnoses (e.g., TB, ADR, stage III anemia, oroesophageal candidiasis) that were not contemplated in the previous anemia guideline. This suggests that the new guideline did result in decreased overprescription of antimalarials and increased consideration of HIV-associated anemia, as hoped. However, barely half of subjects achieved the modest 1 g/dl Hb increase that defined “improvement.” Nearly 1 in 10 arrived at the composite adverse endpoint of hospitalization and/or death.
Subjects treated only with conventional anemia treatment (presumptive iron supplementation, deworming, and/or antimalarials) comprised fewer than half of those who improved. But improvement of a substantial minority with conventional treatment alone does suggest that the historic diagnoses of iron deficiency, intestinal helminthes, and malaria still contribute importantly to anemia burden in HIV-infected Mozambican adults, as anticipated, and must be retained in standard guidelines. It is possible that variability in treatment outcomes across study sites might be the result of differing malaria transmission intensities; malaria transmission was least intense in the site with the best outcomes.
Nearly all of those who failed to improve also received presumptive treatment with iron supplements and anthelminthics (with or without other interventions). These observed failures have several possible explanations. First, the subjects who failed to improve in spite of iron supplementation and deworming may not have had iron deficiency or helminth infection. Iron deficiency may be a less prominent contributor to anemia in HIV-infected patients. In Malawians with severe anemia, iron deficiency (assessed by complete blood count, serum ferritin, serum transferrin, and bone marrow aspirate) was present in only 16% of HIV-infected patients vs. 59% of HIV-uninfected patients.46 In pregnant HIV-infected Tanzanian women, although 48.3% were anemic, fewer than half of the anemic women were iron deficient (based on measurement of hemoglobin, serum ferritin, serum transferrin receptor, and C-reactive protein).4 Recent mass deworming campaigns may have decreased the historically high prevalence of helminth infections in Mozambique.
Second, our subjects may not have been adherent. In pregnant Pakistani women with severe anemia thought to be caused primarily by iron deficiency, better than 85% adherence was required to achieve a 65% response to therapy,47 but adherence to iron supplementation in pregnant Mozambican women has been reported as 67%.48 Lack of adherence could be caused by both patient factors and increasingly frequent pharmacy stockouts in the study environment.
Third, imprecision in point-of-care Hb testing may have failed to detect significant Hb increases. When used by trained laboratory personnel, the precision of the HemoCue is very good.49 The mean difference between our study HemoCue measurements and Sysmex analyzer readings was −0.29 g/dl (SD 0.03 g/dl). Thus, 95% of misclassification of Hb changes from enrollment to second measurement should lie in the range of 1.0 ± 0.12 g/dl. Nineteen (7.9%) of our subjects' responses at Hb measurement two lay within this range. But in our study subjects, variability in HemoCue performance would be more likely to result in overestimation than underestimation of improvement, because 14 (73.7%) of these 19 observed Hb changes were ≥1.0 g/dl, and could only have been failures misclassified as successes.
Fourth, the 1 g/dl Hb improvement threshold (attained at 1 month) may not have been ideal. We could not locate a published standard for Hb evolution with iron supplementation (with or without deworming and/or malaria treatment) in HIV-infected adults. In HIV-uninfected adults with uncomplicated iron deficiency anemia, expected Hb increases in response to oral iron have been variously stated as 0.04–0.05 g/dl/day, 2.0 g/dl every 3 weeks, 2.0–3.0 g/dl within 4 weeks, and 2.0 g/dl within 3 months.47,50–52 Because HIV-infected adults are unlikely to have “uncomplicated” iron deficiency, the higher targets may not be attainable in this rural Mozambican population. Also, normal day-to-day within-individual fluctuations in measured Hb have been found to have an SD of 0.82 g/dl.49 About half [129 (53.8%)] of subjects who had at least two Hb readings had Hb changes within the range of 1.0 ± 1.6 g/dl between enrollment and the second Hb. Thus, normal Hb fluctuations could have caused substantial nondifferential misclassification of individual responses to treatment. Though a higher threshold for Hb improvement would have alleviated concern about misclassification caused by normal fluctuations and/or the imprecision of HemoCue devices, it might have required a much longer period of observation to attain, and the longer wait could have caused avoidable delays in detection of other contributing causes of anemia.
Finally, subjects who did not respond to conventional therapy alone may have had other, undetected causes of anemia. Within the study, we believe that there was considerable underascertainment of many anemia-associated clinical entities: (1) advanced immunosuppression, because of slow CD4 turnaround times and the difficulty of clinical staging in the study environment;46 (2) adverse drug reactions, given low standard Hb thresholds for discontinuation of ZDV in the setting of ADR and frequent absence of baseline pre-ART Hb measurements; (3) TB, with long turnaround times for AFB smears, frequent stockouts of AFB reagents, and the scant availability of chest radiography; (4) other micronutrient deficiencies (e.g., vitamin A) that could not be evaluated in the study setting but were likely prevalent in this malnourished population; and/or (5) most Stage IV conditions, given the limited availability of diagnostics. Failure to diagnose and treat these possible causes of anemia may have led to anemia treatment failures.
Subjects who were treated with nonconventional anemia interventions, particularly those addressing HIV/AIDS itself, adverse drug reactions, and opportunistic infections, often improved. As anticipated within the Mozambican guidelines, ongoing ART, discontinuation of ZDV in the setting of suspected ADR, and treatment of smear-positive pulmonary TB were significantly associated with Hb improvement in our multivariable models. The Mozambican guideline did not anticipate the observed contribution of bacterial bloodstream infections to anemia burden, though this has been reported elsewhere and is associated with untreated AIDS.28,53 The observed association between active bleeding and hemoglobin decline is difficult to interpret because few subjects reported overt bleeding, and tests for occult bleeding were unavailable at study sites.
Anemia management in HIV-infected individuals should routinely consider ART initiation or regimen change and include TB evaluation. In Malawi, for example, 91% of HIV-infected patients with Hb ≤10 g/dl were reported to have CD4 <350 cells/μl and were eligible for ART.12 In South Africa, the prevalence of culture-confirmed TB was 26% and 40% in HIV-infected patients with moderate and severe anemia, respectively.27 Our study is limited by nonrandom selection, a paucity of local diagnostic capacity, and high dropout rates. Nonetheless, our findings support these recommendations to consider ART status and TB screening in anemic HIV-infected adults, and also support the Mozambican Ministry of Health's decision to update its previous guidelines for use in the context of HIV/AIDS care.
Further improvements to non-physician clinician management of anemic HIV-infected adults in Mozambique might include improved access to point-of-care CD4 and TB testing and to blood cultures; more specific instructions for management of Hb that is very low, falling, or slow to rise; the eventual introduction of laboratory tests that can accurately detect iron deficiency in the setting of HIV infection46; and the re-evaluation of thresholds for the definition of Hb “improvement” and for discontinuation of specific medications when ADR is suspected. More aggressive initiation of ART in eligible patients is critical. Given the high prevalence of anemia and the association between anemia and poor HIV/AIDS outcomes, we believe that further refinement of the approach to anemia management at first-level health centers is a high priority in resource-constrained settings.
Acknowledgments
Most importantly, we are grateful to the study subjects for their participation. We also thank the study teams, by working group: Jorge Fernandes Baulene, Maria Joaquina Arnaldo Chipendane, Carlos Domingos, Roque Pinto Azevedo Oliveira (site coordinators); António Fernando Ditone Macule, Atanásio Monteiro Fiscal, Marcelina Vicente Vatiua, Vidigal José Chaque, Francisco Basilio Malumbia, Elias Pedro Amade, Lorena Onofre Gonçalves (study clinicians); Gilberto Ali Ussene, Carlitos Goveia Pequenino (community outreach); Luis Morais, Rui Cipriano Colofite, Oscar Antonio, Argélio Armando Lubulino Onofre, Fernando Zita, Adelina Damas, Fernando Vilanculos (laboratory); Carmelinda Rocha, Menezes Domingos Madeira (data entry). In addition to the study team, many others generously provided support for the study design or implementation or Mozambican guideline development: Massada da Rocha, Moises Daniel Sitoi, Juma Azarate Jahar, Ligia Esther Bandera Elizastigui, Virginia Saldanha, the District Health Directorates of Inhassunge and Namacurra, the City Health Directorate of Quelimane, the Provincial Health Directorate of Zambézia Province, the clinical, nursing and counseling staff of the participating health centers, Rui Bastos, Rolanda Manuel, Alberto Baptista, Mohsin Sidat, Bill Wester, Lisa Nelson, Marcia Souza, Mark Micek, Paul Thottingal, I-TECH's curriculum development team (especially José Vallejo Torres, Pilar Martínez Martínez, Mónica Negrete, Florindo Martins Mudender, and María Ruano Camps), MISAU's Therapeutics Committee (Comité Terapéutico), Meredith Blevins, and Alfredo Vergara.
This research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement U2GPS000631 to Vanderbilt University. The REDCap (Research Electronic Data Capture; www.project-redcap.org/) on-line database was supported by grant UL1 TR000445 from NCATS/NIH. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position(s) of the Centers for Disease Control and Prevention or the National Institutes of Health.
Presented in part at the annual meeting of the American Society of Tropical Medicine and Hygiene in Washington, DC, November 13–16, 2013.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Crawley J: Reducing the burden of anemia in infants and young children in malaria-endemic countries of Africa: From evidence to action. Am J Trop Med Hyg 2004;71(Suppl 2):25–34 [PubMed] [Google Scholar]
- 2.Ivan E, Crowther NJ, Mutimura E, et al. : Helminthic infections rates and malaria in HIV-infected pregnant women on anti-retroviral therapy in Rwanda. PLoS Negl Trop Dis 2013;7(8):e2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fincham JE, Markus MB, and Adams VJ: Could control of soil-transmitted helminthic infection influence the HIV/AIDS pandemic. Acta Trop 2003;86:315–333 [DOI] [PubMed] [Google Scholar]
- 4.Kupka R, Msamanga GI, Mugusi F, et al. : Iron status is an important cause of anemia in HIV-infected Tanzanian women but is not related to accelerated HIV disease progression. J Nutr 2007;137(10):2317–2323 [DOI] [PubMed] [Google Scholar]
- 5.French N, Nakiyingi J, Lugada E, et al. : Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS 2001;15:899–906 [DOI] [PubMed] [Google Scholar]
- 6.Grimwade K, French N, Mbatha DD, et al. : HIV infection as a co-factor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS 2004;18:547–554 [DOI] [PubMed] [Google Scholar]
- 7.Elliott AM, Mawa PA, Joseph S, et al. : Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Trans R Soc Trop Med Hyg 2003;97:103–108 [DOI] [PubMed] [Google Scholar]
- 8.Walson JL, Otieno PA, Mbuchi M, et al. : Albendazole treatment of HIV-1 and helminth co-infection: A randomized, double-blind, placebo-controlled trial. AIDS 2008;22:1601–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redd AD, Avalos A, and Essex M: Infection of hematopoietic progenitor cells by HIV-1 subtype C, and its association with anemia in southern Africa. Blood 2007;110(9):3143–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Justice AC, Feinstein AR, and Wells CK: A new prognostic staging system for the acquired immunodeficiency syndrome. N Engl J Med 1989;320(21):1388–1393 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization: WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease. WHO Press, Geneva, 2006 [Google Scholar]
- 12.Page ID, McKew SJ, Kudzala AG, et al. : Screening HIV-infected adults in Malawi for anaemia: Impact on eligibility for antiretroviral therapy. Int J STD AIDS 2013;2:449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steketee RW, Wirima JJ, Bloland PB, et al. : Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg 1996;55(1 Suppl):42–49 [DOI] [PubMed] [Google Scholar]
- 14.van Geertruyden JP, Mulenga M, Chalwe V, et al. : Impact of HIV-1 infection on the hematological recovery after clinical malaria. J Acquir Immun Defic Syndr 2009;50(2):200–205 [DOI] [PubMed] [Google Scholar]
- 15.Kawai K, Villamor E, Mugusi FM, et al. : Predictors of change in nutritional and hemoglobin status among adults treated for tuberculosis in Tanzania. Int J Tuberc Lung Dis 2011;15(10):1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mounier N, Spina M, and Gisselbrecht C: Modern management of non-Hodgkin lymphoma in HIV-infected patients. Br J Haematol 2007;136(5):685–698 [DOI] [PubMed] [Google Scholar]
- 17.Makubi A, Okuma J, Spiegelman D, et al. : Burden and determinants of severe anemia among HIV-infected adults: Results from a large urban HIV program in Tanzania, East Africa. J Int Assoc Providers AIDS Care 2015;14(2):148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mocroft A, Kirk O, Barton SE, et al. : Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS 1999;13(8):943–950 [DOI] [PubMed] [Google Scholar]
- 19.Berhane K, Karim R, Cohen MH, et al. : Impact of highly active antiretroviral therapy on anemia and relationship between anemia and survival in a large cohort of HIV-infected women. Women's Interagency HIV Study. J Acquir Immun Defic Syndr 2004;37:1245–1252 [DOI] [PubMed] [Google Scholar]
- 20.Johannessen A, Naman E, Ngowi BJ, et al. : Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis 2008;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien ME, Kupka R, Msamanga GI, et al. : Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immun Defic Syndr 2005;40(2):219–225 [DOI] [PubMed] [Google Scholar]
- 22.Firnhaber C, Smeaton L, Saukila N, et al. : Comparisons of anemia, thrombocytopenia, and neutropenia at initiation of HIV antiretroviral therapy in Africa, Asia, and the Americas. Int J Infect Dis 2010;14(12):e1088–e1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Jaquet A, Bissagnene E, et al. : Short-term risk of anaemia following initiation of combination antiretroviral treatment in HIV-infected patients in countries in sub-Saharan Africa, Asia-Pacific, and central and South America. J Int AIDS Soc 2012;15(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy RA, Sunpath H, Kuritzkes DR, et al. : Antiretroviral therapy-associated toxicities in the resource-poor world: The challenge of a limited formulary. J Infect Dis 2007;196(Suppl 3):S449–456 [DOI] [PubMed] [Google Scholar]
- 25.Jaganath D, Walker AS, Ssali F, et al. : HIV-associated anemia after 96 weeks on therapy: Determinants across age ranges in Uganda and Zimbabwe. AIDS Res Hum Retroviruses 2014;30(6):523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giganti MJ, Limbada M, Mwango A, et al. : Six-month hemoglobin concentration and its association with subsequent mortality among adults on antiretroviral therapy in Lusaka, Zambia. J Acquir Immun Defic Syndr 2012;61:120–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerkhoff AD, Wood R, Vogt M, and Lawn SD: Predictive value of anemia for tuberculosis in HIV-infected patients in sub-Saharan Africa: An indication for routine microbiological investigation using new rapid assays. J Acquir Immune Defic Syndr 2014;66(1):33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis DK, Whitty CJM, Walsh AL, et al. : Treatable factors associated with severe anemia in adults admitted to medical wards in Blantyre, Malawi, an area of high HIV seroprevalence. Trans R Soc Trop Med Hyg 2005;99(8):561–567 [DOI] [PubMed] [Google Scholar]
- 29.Audet CM, Burlison J, Moon TD, et al. : Sociocultural and epidemiologic aspects of HIV/AIDS in Mozambique. BMC Int Health Hum Rights 2010;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon TD, Burlison JR, Blevins M, et al. : Enrolment and programmatic trends and predictors of antiretroviral therapy initiation from President's Emergency Plan for AIDS Relief (PEPFAR)-supported public HIV care and treatment sites in rural Mozambique. Int J STD AIDS 2011;22(11):621–627 [DOI] [PubMed] [Google Scholar]
- 31.Auld AF, Mbofana F, Shiraishi RW, et al. : Incidence and determinants of tuberculosis among adults initiating antiretroviral therapy–Mozambique, 2004–2008. PloS One 2013;8(1):e54665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.República de Moçambique. Ministério da Saúde. Direcção Nacional de Saúde Pública. Programa Nacional de Controlo da Malária. Inquérito Nacional sobre Indicadores de Malária em Moçambique (IIM-2007). Maputo: Ministério da Saúde; 2009 [Google Scholar]
- 33.Augusto G, Nala R, Casmo V, et al. : Geographic distribution and prevalence of schistosomiasis and soil-transmitted helminths among schoolchildren in Mozambique. Am J Trop Med Hyg 2009;81(5):799–803 [DOI] [PubMed] [Google Scholar]
- 34.Horjus P, Aguayo VM, Roley JA, et al. : School-based iron and folic acid supplementation for adolescent girls: Findings from Manica Province, Mozambique. Food Nutr Bull 2005;26(3):281–286 [DOI] [PubMed] [Google Scholar]
- 35.República de Moçambique. Ministério da Saúde: Guia de Avaliação Integrada do Adulto. Ministério da Saúde, Maputo, 2005 [Google Scholar]
- 36.Prentice AM, Verhoef H, and Cerami C: Iron fortification and malaria risk in children. JAMA 2013;310(9):914–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spottiswoode N, Fried M, Drakesmith H, and Duffy PE: Implications of malaria on iron deficiency control strategies. Adv Nutr 2012;3:570–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDermid JM, Jaye A, Schim van der Loeff MF, et al. : Elevated iron status strongly predicts mortality in West African adults with HIV infection. J Acquir Immun Defic Syndr 2007;46(4):498–507 [DOI] [PubMed] [Google Scholar]
- 39.McDermid JM, van der Loeff MF, Jaye A, et al. : Mortality in HIV infection is independently predicted by host iron status and SLC11A1 and HP genotypes, with new evidence of a gene-nutrient interaction. Am J Clin Nutr 2009;90(1):225–233 [DOI] [PubMed] [Google Scholar]
- 40.Ministério da Saúde. Instituto Nacional de Saúde: Inquérito Nacional de Prevalência, Riscos Comportamentais e Informação sobre o HIV e SIDA em Moçambique. Ministério da Saúde. Instituto Nacional de Saúde, Maputo, Mozambique, November2010 [Google Scholar]
- 41.Ministério de Saúde. Instituto Nacional de Saúde: Mozambique. National Survey on Prevalence, Behavioral Risks and Information about HIV and AIDS (2009 INSIDA). 2010; www.dhsprogram.com/pubs/pdf/SR179/SR179.pdf Accessed June10, 2015
- 42.Moon TD, Silva WP, Buene M, et al. : Bacteremia as a cause of fever in ambulatory, HIV-infected Mozambican adults: Results and policy implications from a prospective observational study. PloS One 2013;8(12):e83591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brentlinger PE, Silva WP, Buene M, et al. : Management of fever in ambulatory HIV-infected adults in resource-limited settings: Prospective observational evaluation of a new Mozambican guideline. J Acquir Immun Defic Syndr 2014;67(3):304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastos R, Manuel R, Osman N, et al. : Guia de Tratamento Antirretroviral e Infeções Oportunistas no Adulto, Adolescente e Grávida. Versão final. Ministério da Saúde, Maputo, Mozambique, 2009 [Google Scholar]
- 45.World Health Organization: Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: Towards universal access. Recommendations for a public health approach. World Health Organization, Geneva, 2006 [Google Scholar]
- 46.Lewis DK, Whitty CJM, Epino H, et al. : Interpreting tests for iron deficiency among adults in a high HIV prevalence African setting: Routine tests may lead to misdiagnosis. Trans R Soc Trop Med Hyg 2007;101:613–617 [DOI] [PubMed] [Google Scholar]
- 47.Christian P, Shahid F, Rizvi A, et al. : Treatment response to standard of care for severe anemia in pregnant women and effect of multivitamins and enhanced anthelminthics. Am J Clin Nutr 2009;89:853–861 [DOI] [PubMed] [Google Scholar]
- 48.Nwaru BI, Salome G, Abacassamo F, et al. : Adherence in a pragmatic randomized controlled trial on prophylactic iron supplementation during pregnancy in Maputo, Mozambique. Pub Health Nutr 2015;18(6):1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris SS, Ruel MT, Cohen RJ, et al. : Precision, accuracy, and reliability of hemoglobin assessment with use of capillary blood. Am J Clin Nutr 1999;69:1243–1248 [DOI] [PubMed] [Google Scholar]
- 50.Pasricha S-R, Flecknoe-Brown SC, Allen KJ, et al. : Diagnosis and management of iron deficiency anaemia: A clinical update. Med J Aust 2010;193(9):525–532 [DOI] [PubMed] [Google Scholar]
- 51.Ajmera AV, Shastri GS, Gajera MJ, and Judge TA: Suboptimal response to ferrous sulfate in iron-deficient patients taking omeprazole. Am J Ther 2012;19(3):185–189 [DOI] [PubMed] [Google Scholar]
- 52.Alleyne M, Horne MK, and Miller JL: Individualized treatment for iron-deficiency anemia in adults. Am J Med 2008;121(11):943–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crump JA, Ramadhani HO, Morrissey AB, et al. : Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis 2011;52(3):341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]