Abstract
Significance: Nitric oxide (NO) is a critical signaling molecule marked by complex chemistry and varied biological responses depending on the context of the redox environment. In the setting of inflammation, NO can not only contribute to tissue injury and be causative of oxidative damage but can also signal as an adaptive molecule to limit inflammatory signaling in multiple cell types and tissues. Recent Advances: An advance in our understanding of NO biology was the recognition of the nitrate-nitrite-NO axis, whereby nitrate (predominantly from dietary sources) could be converted to nitrite and nitrite could be reduced to NO. Critical Issues: Intriguingly, the recognition of multiple enzymes that serve as nitrite reductases in the setting of hypoxia or ischemia established the concept of nitrite as a circulating endocrine reservoir of NO, with the selective release of NO at sites that were primed for this reaction. This review highlights the anti-inflammatory roles of nitrite in numerous clinical conditions, including ischemia/reperfusion, transplant, cardiac arrest, and vascular injury, and in gastrointestinal inflammation. Future Directions: These preclinical and clinical investigations set up further clinical trials and studies that elucidate the endogenous role this pathway plays in protection against inflammatory signaling. Antioxid. Redox Signal. 23, 328–339.
The Nitrate–Nitrite–Nitric Oxide Cycle
Arginine metabolism by nitric oxide synthases (NOSs), predominantly endothelial NOS (eNOS), accounts for a significant portion of endogenous NO production as well as much of the circulating levels of nitrite (NO2−) in the body. However, dietary intake of nitrates and nitrites also contributes to an estimated 30–50% of the circulating pool of these anions. Nitrates are found endogenously in many foods, particularly green leafy vegetables (74). Ingested nitrate is absorbed in the stomach and intestines and subsequently secreted in concentrated levels in the salivary glands (75), forming the so-called enterosalivary circulation of nitrate. This concentrated nitrate is reduced to nitrite via facultative anaerobic bacteria in the oral cavity (33). Nitrite is then swallowed as part of saliva and reabsorbed by the gut. The expected rise in systemic nitrite after ingestion of nitrate is not seen if saliva is expelled instead of swallowed (75). Furthermore, antimicrobial mouthwash diminishes levels of these commensal bacteria and blunts the increase in circulating nitrite following dietary nitrate intake. Thus, confirming the importance of bacteria in reducing nitrate to nitrite (13, 42).
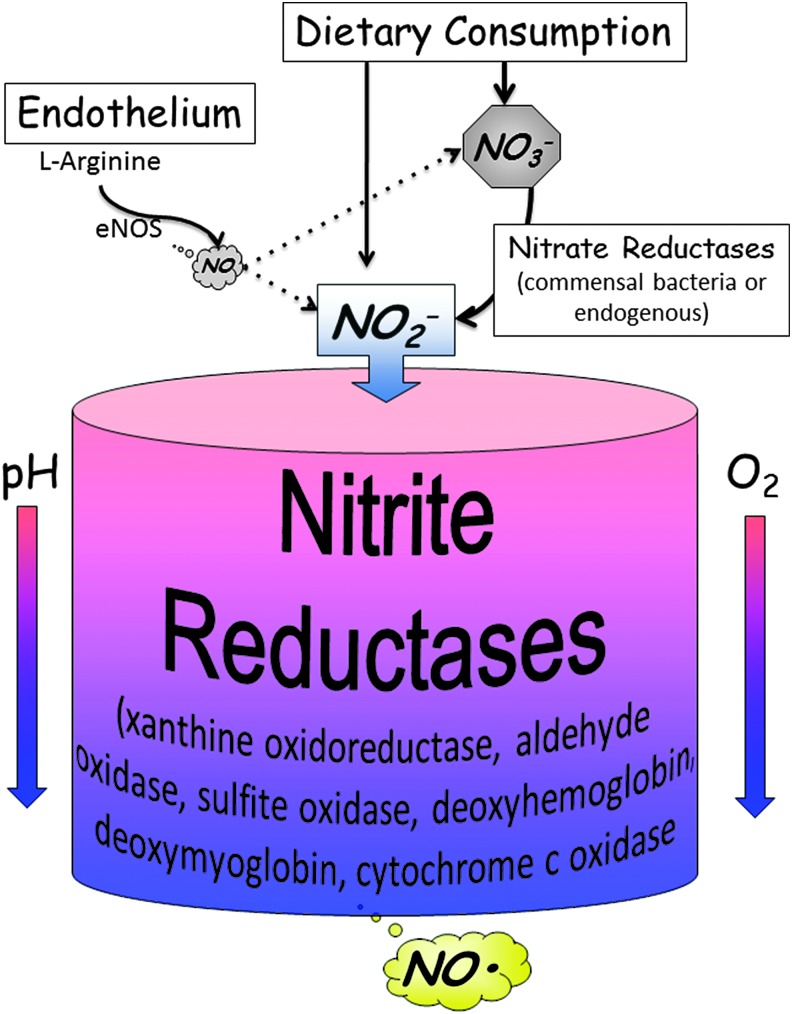
Circulating nitrite was once thought to be a by-product formed from the breakdown of NO, and that nitrite was a relative end metabolite that did not serve any biological function. Not only was the conversion of dietary nitrate to nitrite discovered but the recognition of nitrite as a molecule that can be converted back to biologically active NO was also elucidated over the past decade. NO from NOS enzymes functions as an autocrine and/or paracrine messenger and has a very short half-life. The findings that dietary nitrates/nitrites or the conversion of NO to nitrite that can then lead to the circulation of nitrite throughout the body with subsequent conversion back to NO suggest a mechanism whereby nitrite can serve as a circulating endocrine reservoir of NO (Fig. 1).
FIG. 1.
The nitrate-nitrite-NO axis. Dietary consumption of nitrates or nitrites leads to increased levels of each of these anions in the circulation. Nitrate is secreted in saliva at high concentrations and is converted to nitrite by commensal bacteria within the glands. Some nitrite is converted to NO within the low acidic environment of the stomach. The rest that is absorbed, then circulates, and can be converted to nitrite in tissues or blood by enzymes that possess nitrite reductase activity. The nitrite reductase activity of most of these enzymes is favored under conditions of hypoxia or low pH. NO, nitric oxide.
It should be noted that the focus on inorganic nitrate and nitrite molecules in this review is in contrast to the already widely used organic nitrate compounds such as isosorbide mononitrate and nitroglycerin. The differences in pharmacology and bioactivation of organic nitrate compounds are reviewed elsewhere (84).
The reduction of nitrite to NO occurs predominantly in the setting of hypoxemia or acidosis (111). NOS enzymes require oxygen as a substrate. Thus, these arginine-NOS-independent mechanisms for NO generation perhaps serve as redundant pathways of NO production specifically in cells or tissues that are relatively hypoxic. Indeed, circulating nitrite has been shown to produce vasodilation through NO signaling in a model of human exercise in which NOS was blocked (39), suggesting that this pathway functions as a viable alternative for NO production.
Nitrite reductases
There are multiple described pathways for the reduction of nitrite to NO, including xanthine oxidoreductase (XOR), aldehyde oxidase (AO), and a variety of heme-associated proteins, including hemoglobin, myoglobin, and cytochromes, more extensively reviewed elsewhere (25, 60, 85).
XOR and AO are molybdopterin-containing proteins with iron–sulfur centers and an FAD-binding site (22, 69). Nitrite is reduced to NO by molybdenum. Molybdenum is subsequently reduced through one of two pathways depending on oxygen tension and NADH (69). The first pathway involves reduction of molybdenum by xanthine or 2,3-dihydroxybenzaldehyde. The FAD site is open and thus the reduction of nitrite competes with that of oxygen (generating superoxide and hydrogen peroxide), ultimately favoring this pathway in hypoxic conditions.
The second pathway has NADH bound to the FAD site, blocking the competitive reduction of oxygen and rendering this independent of oxygen conditions (69). A similar mechanism is present for the reduction of nitrite by AO (68). Recent work has identified a new molybdopterin-containing enzyme: mitochondrial amidoxime-reducing component. This process produces nitrite at similar rates as XOR; however, it appears to generate a more sustained level of NO. This may represent a new important pathway in hypoxic NOS-independent nitric oxide production (100).
Tissue levels of XOR are variable between organs and influenced by multiple factors. Hypoxia, acidemia, and a variety of inflammatory cytokines can induce XOR expression in tissues, endothelium, and red blood cells (23, 44, 59). During inflammatory/hypoxic conditions, XOR is sequestered by negatively charged glycosaminoglycans found on vascular endothelial cell surfaces (50). The importance of both XOR and AO in a variety of disease states has been investigated through the use of specific inhibitors. As discussed later, these mechanisms may be most important in extreme ischemic insults clinically seen with obstruction of major vessels or during organ transplantation.
A variety of heme proteins, including hemoglobin, myoglobin, and cytochromes, have also been implicated in nitrite reduction (60). Similar to the molybdopterin-containing enzymes, the heme group reduces nitrite in conditions of hypoxia or acidemia. Hemoglobin functions as a nitrite reductase through a reaction involving deoxyhemoglobin with iron in a reduced state, nitrite, and a proton, resulting in NO, methemoglobin, and hydroxide (32, 52). This process is dependent on both the allosteric state of hemoglobin and oxygen saturation, with the highest rate of reaction occurring with allosteric relaxed state hemoglobin near an oxygen saturation of 60% (51, 52).
While this may implicate the erythrocyte as a potential source of NO, it must be considered in the context of the known rapid NO scavenging by hemoglobin. Oxygenated hemoglobin reacts rapidly with NO to form nitrate and methemoglobin (35). The estimated half-life of NO in whole blood is 1.8 ms (71). This limits the ability of NO generated in the erythrocyte to effectively diffuse out of the erythrocyte and act on the vasculature. Various hypotheses exist to circumvent the immediate scavenging by hemoglobin, including the generation of more stable intermediates such as S-nitrosylated hemoglobin (55) or iron–nitrosyl hemoglobin species (93), which may ultimately allow for NO release outside of the erythrocyte. Further work has suggested that perhaps NO release from the erythrocyte itself is not necessary to enact a vascular response and that nitrite reduction results in the release of ATP from the erythrocyte that mediates vasodilation (24).
Like oxyhemoglobin, oxymyoglobin is a potent NO scavenger, whereas deoxymyoglobin reduces nitrite to NO in a similar manner as deoxyhemoglobin, but up to 30 times faster (67). This leads to increased local NO in muscular tissue such as cardiomyocytes and vascular smooth muscles. NO generated in this manner is thought to regulate the muscular respiratory status through impacting the mitochondria, discussed later, thus serving as an important homeostatic regulator in periods of muscle hypoxia (46, 90).
Cytochrome c also contains heme and under certain conditions it too may function as a nitrite reductase. Exposure of cytochrome c to hypochlorite induces oxidation of the enzyme that affords it the ability to reduce nitrite (28). This may relate specifically to instances of mitochondrial injury coupled with neutrophil activation. Further studies have found that the ability of cytochrome c to reduce nitrite is dependent upon the pentacoordinate form of heme, which is also induced by anionic phospholipids (7).
Effects of nitrites on the cell and signaling
The effects of nitrate/nitrite and NO are diverse. Perhaps the most direct and best described pathway involves the binding of NO to soluble guanylate cyclase (sGC) leading to the generation of cGMP, activation of cGMP-dependent kinase pathways, and an ultimate myriad of effects, including vascular relaxation, changes in erythrocyte deformability, platelet aggregation, and leukocyte–endothelial interactions (14, 37, 62, 66, 80).
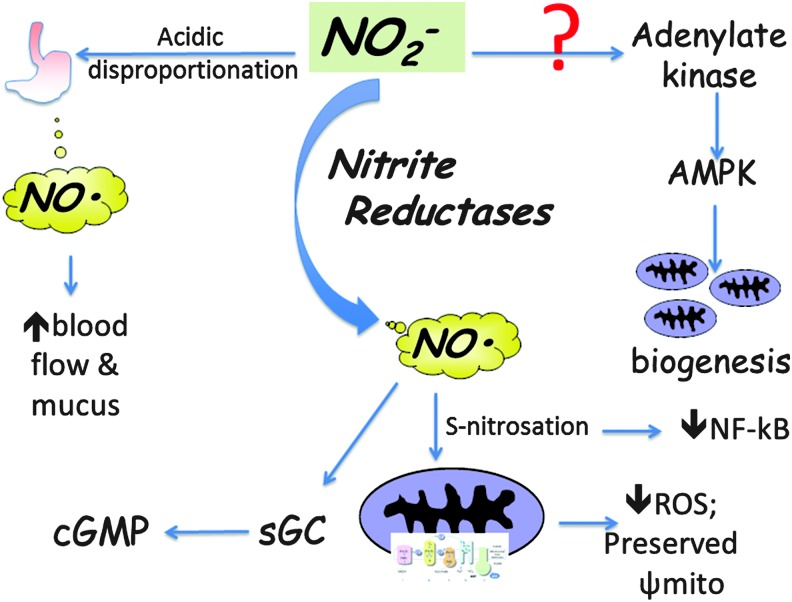
The mitochondria appear to be central to many of the effects of nitrite/NO in mediating cellular protection (Fig. 2). In normoxic conditions, nitrite treatment of cardiomyocytes stimulated mitochondrial fusion through protein kinase A-dependent phosphorylation of Drp1 and downstream activation of AMPK (86). Nitrite itself has been shown to directly increase AMPK, resulting in mitochondrial biogenesis in an NO and cGMP-independent manner (78). In healthy volunteers, dietary supplementation of nitrate (reduced to nitrite as described above) reduced expression of ATP/ADP, ultimately limiting proton flux and improving oxidative phosphorylation efficiency (65). This and the above findings may prime the host to be more resistant to ischemic periods.
FIG. 2.
Nitrite and anti-inflammatory signaling. Multiple studies have highlighted NO-dependent anti-inflammatory effects of nitrite. These effects have been mediated via activation of sGC or S-nitrosation of proteins such as mitochondrial complex I. Other downstream pathways that may not depend directly on reduction to NO have been suggested, including activation of adenylate kinase-AMPK signaling and modulating NF-κB. NF-κB, nuclear factor-kappa B; sGC, soluble guanylate cyclase.
With the aforementioned pathways of nitrite reduction to NO in settings of hypoxemia, it is not surprising that there has been much investigation into the role of nitrite in ischemia/reperfusion (IR) injury. The hallmark of IR injury involves the depletion of high-energy stores, resulting in mitochondrial dysfunction with subsequent reactive oxygen species (ROS) generation upon reperfusion inducing injury. Clinically, this injury manifests in various scenarios such as reperfusion after myocardial infarction and graft reperfusion in transplant to be expanded upon later.
During the ischemic insult, nitrite mediates cytoprotection through changes in mitochondrial function. Nitrite is capable of the inhibiting activity of complex I of the electron transport chain via S-nitrosation, effectively decreasing the generation of mitochondrial ROS and preventing changes in mitochondrial permeability and release of cytochrome c and potential deleterious effects of reperfusion (20, 70, 97).
Furthermore, NO has the capability to modify cysteine residues in proteins, resulting in S-nitrosylation that influences protein structure and ultimately function (41). Inhaled NO results in elevated S-nitrosothiols such as S-nitrocysteine (15). S-nitrocysteine is specifically transported into cells via the L-type amino acid transporter (LAT) system (110). These compounds can influence cellular signaling in a manner independent of merely serving as NO intermediaries (17, 110). Rather, once transported, intracellularly S-nitrocysteine can undergo transnitrosation reactions with other thiol groups producing other S-nitrosated proteins (17). As extensively reviewed elsewhere, S-nitrosylation can influence a host of transcriptions factors, including nuclear factor-kappa B (NF-κB), nuclear factor erythroid 2-related factor 2 (Nrf2), and p53 (95).
A specific example is S-nitrosoglutathione (GSNO), the S-nitrosylated form of glutathione (GSH). This may occur through various mechanisms at both high and low oxygen tensions (16). GSNO, like other nitrosylated products, has multiple potential targets in the cell. Overall, GSNO appears to support anti-inflammatory properties through modulation of NF-κB, tumor necrosis factor-α (TNFα), and monocyte chemoattractant protein-1 (MCP-1), among others (30). Nitrite itself, independent of NO, has also been shown to be able to directly nitrosylate a variety of important signaling pathways, including heme oxygenase-1, heat shock protein 70, and cytochrome P450 activity, at physiologic nitrite levels (19). This may represent an important NO-independent manner of nitrite/nitrate/NO-mediated cellular response.
Conversely, there is evidence of detrimental nitrosylation and NO species. For example, in the presence of oxidants such as superoxide radicals and hydrogen peroxide, NO can be oxidized to peroxynitrite anion and nitrogen dioxide (89). This can lead to the generation of nitrotyrosine, another post-translational protein modification that has been implicated in various diseases, including atheroma generation (99).
Overall, nitrite and NO have a myriad of potential targets through a variety of mechanisms with a complex interplay from the specific redox environment. There is unlikely to be one single pathway to explain the potential beneficial or detrimental effects.
Anti-inflammatory Role of Nitrite
The past decade has seen an increase in the number of publications demonstrating biological function of nitrite and the involvement of the nitrate-nitrite reductase-NO axis in a number of different biological processes. The remainder of this review highlights findings that illustrate nitrite and nitrite signaling to be critical in regulating inflammation in multiple and varied pathophysiological settings (Table 1). The stability of nitrate and nitrite compared with NO and the ability to deliver systemically and perhaps induce a local release of NO at a site where the biology favors the reduction to NO have created a renaissance of interest in NO-based therapies for disease.
Table 1.
Inflammatory Conditions in Which Nitrite Has Been Shown to Protect Against Tissue Injury in Preclinical Models
| Ischemia/reperfusion |
| Cardiac IR (6, 18, 34, 46, 56, 91, 96, 98) |
| Hepatic IR (32, 70) |
| Cardiac arrest (31) |
| Transplantation/rejection (83, 102, 109) |
| Vascular injury |
| Balloon injury/neointimal hyperplasia (1) |
| Pulmonary arterial hypertension (36) |
| Toxemia/shock |
| Endotoxemia/lipopolysaccharide (27) |
| Tumor necrosis factor-α |
| Gastrointestinal injury |
| IBD/stress injury/NSAID injury (9, 54, 77, 106) |
| Necrotizing enterocolitis (108) |
IR, ischemia/reperfusion; NSAID, nonsteroidal anti-inflammatory drug.
Ischemia and reperfusion injury
It was first shown in rat heart that nitrite infusion could reduce infarct size, an effect that was negated by the administration of a nitric oxide scavenger (107). Nitrite administration protected equally against ischemia in eNOS knockout mice, further confirming that the protective effects were conferred in an NO-dependent, but NOS-independent, manner (34). Additional studies have also shown a benefit of oral supplementation of nitrate in reducing infarct size (18), reinforcing the clinical significance of the reduction of dietary nitrate to nitrite as previously described. The exact pathway for this nitrite-mediated protection is debatable. One in vivo study in rats showed dependence on XOR as inhibition with allopurinol mitigated protective effects of nitrite infusion (6).
Alternatively, another study investigating NO generation in heart homogenates found a dependence on deoxygenated myoglobin and no effect from XOR on NO production (96). One may assume that differences in preparation may account for the differences between XOR dependence in one model and myoglobin in the other. However, work looking at myoglobin knockout mice again illustrates the dependence of myoglobin in the cardiac muscle as knockout mice produced half as much NO upon exposure to hypoxia and nitrite compared with controls (46). Furthermore, S-nitrosothiols as described earlier have been shown to be cardioprotective in reperfusion injury. Pretreatment of rat hearts with S-nitrosocysteine improved postischemic contractile dysfunction and necrosis. This protection was independent of NO release and was blocked by inhibiting the LAT system specific to S-nitrosocysteine (48).
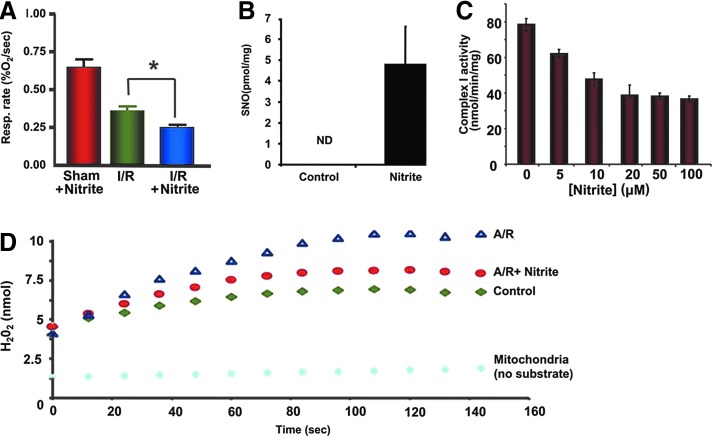
Similar findings are demonstrated in liver models of IR, with nitrite-derived protection via XOR that is lost in the setting of NO scavengers (73). The liver contains nearly 10 times more XOR and AO at baseline than cardiac tissue (67). Thus, these pathways may be more important in the liver where levels of myoglobin are lower compared with cardiac tissue. In parallel to cardiac models, dietary nitrite has also been found to be cytoprotective in liver IR (Fig. 3) (88).
FIG. 3.
Nitrite results in nitrosation, inhibition of mitochondrial complex I activity, and decreased oxidative damage in ischemia/reperfusion. (A) Quantification of copper (I)/cysteine-based chemiluminescence traces of mitochondria isolated from rats treated with 480 nmol of saline or nitrite 24 h earlier. (B) Respiratory rates of mitochondria isolated from mice 5 h after they were subjected to hepatic IR in vivo. (C) Complex I activity of isolated mitochondria decreased with increasing concentrations of nitrite. (D) Nitrite decreased oxidative damage in mitochondria. Representative traces of ROS production measured by amplex red in mitochondria without substrate (light blue), in the presence of glutamate/malate and ADP before anoxia/reoxygenation (green), and after anoxia/reoxygenation in the presence (red) and absence (blue) of 20 μM nitrite. Adopted from Shiva et al. (97). IR, ischemia/reperfusion; ROS, reactive oxygen species. *p<0.01.
Nitrite has also been implicated as a potential endocrine mediator of the so-called remote ischemic preconditioning response. This refers to the finding that ischemia and reperfusion in one region of the body result in protection from a second ischemic insult in another region. This was first demonstrated in the myocardium where IR of the circumflex artery resulted in diminished infarct size in subsequent ischemia in the left anterior descending coronary artery distribution (87).
There are several proposed mediators for this response, likely acting in concert, reviewed elsewhere (38). Nitrite has been implicated as one of the potential important mediators of this protective response. Recent work has demonstrated that nitrite generated in the remotely ischemic tissue, through shear stress-activated eNOS, is capable of mediating myocardial protection in subsequent myocardial ischemia by generation of NO and through mitochondrial regulation as discussed earlier. These beneficial effects are lost in myoglobin knockout mice, again implicating myoglobin as an important nitrite reductase in cardiac tissue (91).
Additionally, in a more global IR model of cardiac arrest, nitrite has been shown to protect against cardiac and brain injuries. In these models, global ischemia and cardiopulmonary resuscitation were associated with depletion of nitrite from the blood and tissue. This presumed metabolism of nitrite in this model suggests that nitrite and nitrite reductases may be part of an important endogenous response. A single low dose of intravenous nitrite compared with blinded saline placebo given at cardiopulmonary resuscitation initiation improved cardiac function, survival, and neurological outcomes (31).
In a stroke model in rats with temporary occlusion of the middle cerebral artery, administration of sodium nitrate upon reperfusion was found to be neuroprotective, with these beneficial effects negated by the administration of a direct NO scavenger (58). However, in perhaps a more clinically relevant model of stroke, the coadministration of nitrite at the time of reperfusion with recombinant tissue plasminogen activator did not result in appreciable differences between histology, clinical characteristics, or magnetic resonance imaging characteristics between groups at 48 h (94). Different dosing strategies (bolus vs. infusion), the incorporation of tissue plasminogen, the use of older rats, and assessing later time points, may account for the seemingly contrasting results between the two previous studies.
Stemming from these investigations, a phase 2, randomized, double-blind placebo-controlled trial investigating the role of intravenous sodium nitrite in acute ST-elevated myocardial infarction (STEMI) was recently completed. Patients received either 70 micromoles of sodium nitrite intravenously or placebo 5 min before percutaneous coronary intervention (PCI). In this study, there was no difference in the primary outcome of infarct size or secondary outcomes of elevated cardiac enzymes, left ventricular end-diastolic volume, end-systolic volume, or ejection fraction with nitrite infusion (98).
A subsequent phase two trial was performed that investigated whether local delivery with intracoronary injection of nitrite during primary PCI might improve infarct size in STEMI (56). Although intracoronary nitrite infusion did not alter infarct size, there was a trend to an improved myocardial salvage index and a significant reduction in the composite outcome measurement termed major adverse cardiac events (MACE). In a subgroup of patients with low angiographic flow rates (thrombolysis in myocardial infarction [TIMI] flow ≤1), nitrite reduced infarct size and MACE and improved the myocardial salvage index. This may indicate that the benefit of nitrite therapy may only be realized in patients with severely decreased flow (TIMI ≤1), resulting in an ischemic environment favoring nitrite reduction as described previously. Of note there were no major clinical side effects from treatment, including equivalence to controls in drop in systolic blood pressure and methemoglobin levels. These authors concluded that a phase III clinical trial assessing intracoronary nitrite administration as an adjunct to PCI in STEMI patients would be warranted.
Organ transplantation
Tissue injury and inflammation in organ transplantation are multifactorial. There is a combination of organ injury that occurs as part of the cold IR process as well as the immunological sequelae of graft and host interactions. Syngeneic transplant models presumably induce less of the latter immunologic injury, allowing for study of the effects of IR.
In a syngeneic model of rat lung transplant, grafts and recipients were treated with nitrite. Graft function was improved in rats receiving organs stored in nitrite solution and in recipients pretreated with intravenous nitrite before reperfusion of the transplanted lung. The effects of nitrite were dependent on NO and the activation of guanylate cyclase as both NO scavenger and guanylate cyclase inhibitor reversed the protective effects. Furthermore, IR injury was worse in animals receiving allopurinol. This suggests a role for XOR as the critical nitrite reductase in this setting (102). A follow-up study has shown dose-dependent improvement in graft function with pretreatment via nebulized nitrite in the same model of rat lung transplant, representing an alternative method of administering nitrites (83).
In an allogeneic model of heart transplantation, the influence of dietary nitrates/nitrites and supplemental sodium nitrite was investigated. Mice were randomized to receive a diet low in nitrates and nitrites (NOx-low) or standard chow, as well as water supplemented with or without nitrite. Serum nitrite/nitrate levels were significantly higher in animals given nitrite water for 30 days and lower in the animals fed with NOx-low. Supplementation of drinking water with nitrite enhanced heart graft survival to a median of >120 days compared with 49.5 days in animals fed a standard diet. In contrast, NOx dietary restriction resulted in significant early rejection of allografts (30.5 days). Markers of inflammation (mRNA levels for interferon gamma [IFN-γ] or TNFα) were significantly less with nitrite supplementation (109).
Stemming from these experiments, inhaled nitric oxide has been investigated in randomized clinical trials in patients undergoing liver transplantation (63, 64). Inhaled NO during operative transplantation was shown to have anti-inflammatory effects and trended toward improved graft function months after the transplant. Inhaled NO has long been known to decrease pulmonary vascular resistance, improving pulmonary hypertension, through direct vasodilation of pulmonary vasculature (11, 36). Inhaled NO has been shown to exhibit systemic effects, particularly when eNOS is inhibited, with increased nitrosyl hemoglobin and nitrite levels potentially acting as intermediates for systemic NO delivery (82). Systemic effects through potential intermediates were also hypothesized in these transplant studies as blood levels of nitrate, nitrite, and nitrosyl hemoglobin were increased following inhaled NO therapy. The authors hypothesized that the delivery of NO to the graft may be via oxidation of NO to nitrite in the lungs, release into the systemic circulation, followed by reduction back to NO in the tissue, although this has not been tested directly.
An alternative hypothesis stems around the release of arginase upon liver reperfusion, resulting in depletion of arginine and a decrease in NO. This may ultimately lead to pulmonary vascular injury and vasoconstriction, which in turn influences hepatic blood flow and function. Exogenously inhaled NO would provide a source of NO during this period of arginine depletion and thus limit pulmonary injury and protect hepatic graft function. Nonetheless, these findings are intriguing, yet far from conclusive, that inhaled NO and nitrite are beneficial in human liver transplant. There is an ongoing phase II clinical trial investigating sodium nitrite administration in lung transplantation (clinicaltrials.gov NCT01715883).
Vascular injury and inflammation
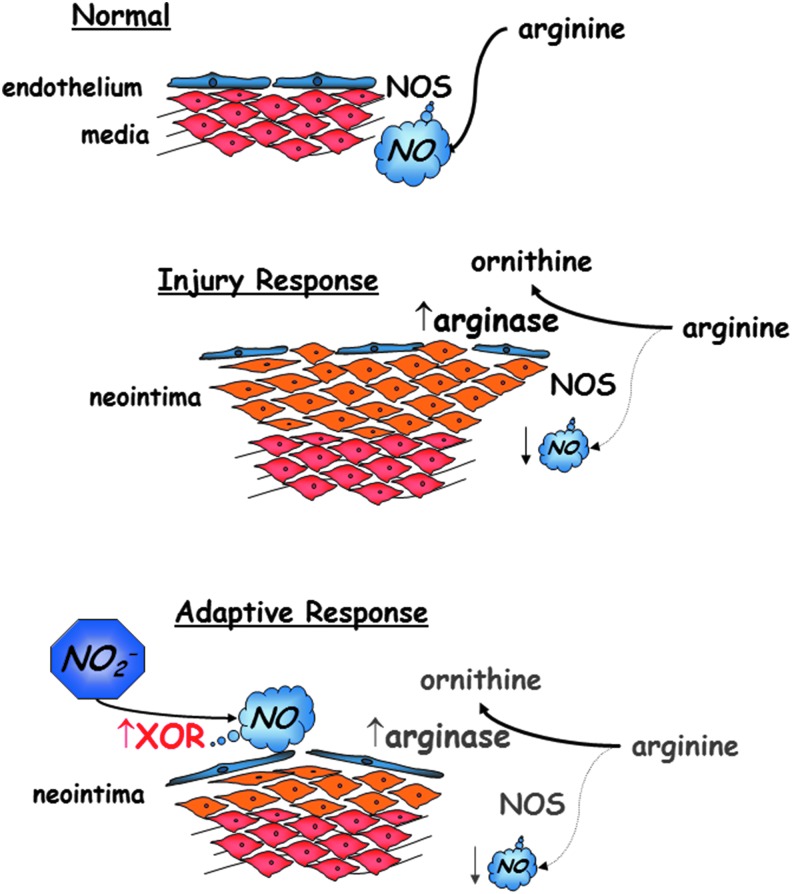
There is a large body of work investigating the influence of nitrite on vascular biology, which is not reviewed here. However, several studies highlight a role for nitrite on vascular inflammatory processes in both the systemic and pulmonary vasculature. Nitrates and nitrites have been investigated in a rat carotid artery balloon injury model. Balloon injury results in local inflammation and the formation of neointimal hyperplasia marked by smooth muscle cell proliferation and hypertrophy of the intima of the arterial wall. Alef et al. demonstrated that this arterial injury is marked by an increase in arginase-1 levels within the vascular wall, which leads to a dysregulation of the arginine-NOS-NO axis and perhaps shifts signaling to non-NOS-dependent NO production. To support this, rats fed the NOx-low diet demonstrated worse arterial injury compared with rats fed standard chow.
Furthermore, injury also increased XOR levels and activity in the arterial wall, and a diet supplemented with tungsten that inhibits the activity of XOR (as well as other molybdoenzymes) also intensified arterial injury. Sodium nitrite delivered intraperitoneally, orally, or as an inhaled nebulized treatment protected against the formation of intimal hyperplasia (Fig. 4) (1).
FIG. 4.
Nitrite compensates for a dysregulated arginine-NOS-NO axis in the setting of vascular injury and inflammation. Following angioplasty injury, vessels express increase in levels of arginase-1 with increased activity and decreased activity of NOS enzymes. However, increased expression and activity of XOR can lead to increased NO production from the reduction of nitrite. NOS, nitric oxide synthase; XOR, xanthine oxidoreductase.
In a subsequent study, nitrite was demonstrated to increase mitochondrial biogenesis within the vascular wall (78). The in vitro component of these investigations found that the influence of nitrite on smooth muscle cell mitochondrial biogenesis in the setting of hypoxia was independent of sGC, suggesting an NO-independent mechanism. Furthermore, these studies suggested a mechanism that was dependent on adenylate kinase activation with downstream activation of AMP kinase phosphorylation.
Toxemia/shock
Severe sepsis represents a major healthcare burden. In the United States, the annual case incidence for severe sepsis, defined as documented infection and acute organ failure, is greater than 750,000, with an associated mortality rate of nearly 30% (3). The underlying pathophysiology is heterogeneous and involves a complex host response and inter-relationship between pro- and anti-inflammatory signaling that results in tissue damage, coagulation cascade abnormalities, microvascular damage, tissue hypoxia, and eventually organ injury and failure (4).
Murine models of endotoxemia with systemic administration of lipopolysaccharide (LPS) result in depressed vasopressor response. This blunted response was reversed by blockade of NOS by L-NMMA, and subsequently depressed with administration of l-arginine (57). Acute lung injury is a common finding in sepsis. Mice deficient in inducible NOS were found to be resistant to acute lung injury stemming from LPS injection (61). In mouse models of toxemia with either LPS or TNFα administration, nitrite was found to be protective against the development of hypothermia and mortality. This protection was independent of NOS signaling, and interestingly, nitrite in this model did not have an effect on mitochondrial complex I activity (27). These data suggested less mitochondrial oxidant injury as demonstrated by preserved function of the mitochondrial enzyme, aconitase, in those mice treated with nitrite (27). The effects of nitrite in these sepsis models were seemingly NO dependent and through sGC as the protective effects of nitrite were lost in mice deficient in sGC (21, 27). The importance of sGC in sepsis models was further confirmed with the ability to reactivate sGC with BAY 58-2667, an independent sGC activator with potentially more direct effects further downstream from NO signaling (104).
These data along with multiple other studies led to the hypothesis that NO was a key mediator in the development of septic shock and that it may serve as a therapeutic target (29). This ultimately led to a large phase III clinical trial randomizing patients in septic shock to receive an intravenous NOS inhibitor. The trial was discontinued early due to increased mortality in patients receiving the study drug. Interestingly, the disparity was from a higher proportion of cardiovascular deaths despite a lower incidence of deaths from multisystem organ failure (72). There were many flaws with this study, yet these data coupled with mounting evidence from experimental sepsis models showing potential benefit of NO donors [reviewed in Cauwels and Brouckaert (26)] have flipped the view of NO signaling in sepsis from detrimental to perhaps beneficial.
In septic patients, the administration of nitroglycerin was shown to provide a significant increase in microvascular flow, despite a drop in mean arterial pressure (101). The decrease in mean arterial pressures with NO administration is a potential deleterious gross hemodynamic effect. Utilizing nitrite as a source of NO that is only converted in tissues endogenously primed for reduction of nitrite could avoid nonspecific effects as well as the systemic vasodilation. However, in a follow-up study, in which a strict resuscitation protocol was followed, there was no demonstrable difference in microcirculatory perfusion with equivalent dosing of nitroglycerin (12).
Recently, a trial utilizing inhaled nitric oxide as an alternative method of delivery was tested in sepsis. Again, after establishing adequate resuscitation with fluid and vasopressors, patients were randomized to inhaled 40 ppm NO for 6 h. While plasma nitrite levels increased in the treatment group, there was no difference in microcirculatory perfusion or in secondary outcomes of organ dysfunction (103). These studies suggest that administration of NO or nitroglycerin affords no benefit once other goals of sepsis treatment (adequate perfusion through fluid and vasopressor support) have been met. However, there may still be a role in looking at earlier time points. As suggested by one animal study, administration of inhaled nitric oxide at the time of septic insult (by cecal ligation and puncture) blunted sepsis-induced acute lung injury (92). Further studies are warranted to tease out the therapeutic potential of nitrate/nitrite/NO in sepsis.
Gastrointestinal inflammation
Gastric ulcers induced by the use of nonsteroidal anti-inflammatory drugs (NSAIDs) continue to be problematic in the clinical setting (45). Under normal conditions, cyclooxygenase (COX) enzymes in the gastric mucosa produce prostaglandins that maintain adequate blood flow to the mucosa and stimulate production of protective mucus (2). By inhibiting COX, NSAIDs predispose the mucosa to injury by endogenous and exogenous insults. NO has been shown to play a protective role in the gastric mucosa (106). As described earlier, the enterosalivary circulation of nitrate and nitrite provides nitrite in relatively high levels to the stomach mucosa. In this acidic environment, equilibrium exists between protonated nitrite, nitrosyl cation, and dinitrogen trioxide. Dinitrogen trioxide is itself a strong nitrosating species able to enter tissue and release NO (43). The nitrate-nitrite-NO pathway is believed to play an important role in gastric defense against pathogens (10) and stress injury (77) by stimulating blood flow and mucus production (9).
In the stomach, the generation of mucus is controlled by genes from the mucin family, MUC6 and MUC5AC (47). The gastric mucus layer consists of two layers, one loosely adherent and other firmly attached to the mucosa (5). Dietary nitrate induces MUC6 mRNA, which plays an important role in the inner firmly attached mucus layer (81). The importance of this mucosal layer and its maintenance by nitrite have also been extended to the colon, where a murine model for inflammatory bowel disease revealed that nitrite supplementation was both protective and therapeutic by preserving the mucosal layer and goblet cell abundance (54).
In the small bowel, the pathophysiology of NSAID damage is less understood. It appears that the injury is caused by inhibition of prostaglandin and uncoupling of mitochondrial oxidative phosphorylation that results in leukocyte activation and chemotaxis with subsequent inflammation and ulceration (76). Mechanistically, NSAIDs cause damage to the microvascular endothelium leading to upregulation of P-selectin and intercellular adhesion molecule-1 (ICAM-1) and recruitment of neutrophils, which play an important part as an effector cell in the injury caused by NSAIDs (8, 79, 105). Accumulation of neutrophils has been greatly reduced by treatment with nitrate evidenced by reduced levels of myeloperoxidase and downregulation of P-selectin (53). Additional effects of nitrite on other endothelial adhesion molecules cannot be excluded, and effects on levels of ICAM-1 were also observed (53).
A recent study has linked the nitrate-nitrite-NO signaling pathway to the innate immune system and the development of necrotizing enterocolitis (NEC), a disease of premature infants characterized by intestinal necrosis. In an experimental model of NEC, it was found that deficiency in eNOS increased toll-like receptor 4 (TLR-4) signaling and worsened disease severity. This mucosal injury was associated with impaired intestinal perfusion and endothelial damage. Breast feeding has long been recognized as protective in NEC, perhaps through the higher levels of nitrates/nitrites compared with formula (108).
Furthermore, nitrite supplementation preserved the intestinal microcirculation and reduced the severity of NEC. Interestingly, colostrum has higher levels of nitrite versus breast milk, which contains relatively higher nitrates (49). From a teleological standpoint, this may be based upon the requirement of colonization of the neonate with nitrite-reducing bacteria and gain of function of the related enterosalivary pathway before nitrate can be effectively processed. Of course, there are other components in breast milk thought to be protective in preventing NEC, including lactoferrin, various growth factors, and nondigestible carbohydrates (40). It is unlikely that the nitrate axis alone is responsible for modulating disease development, but the above studies are intriguing.
Conclusion
Over the past several decades, nitrite and nitrate have progressed from by-products of nitric oxide metabolism to a potentially important NOS-independent signaling. Most studies reveal NO/cGMP-dependent signaling; however, several studies suggest signaling independent of these pathways (Fig. 2). Overall, the mechanisms, involved intermediates, and targets are varied and dependent upon local and systemic factors. Future clinical studies will need to be analyzed carefully to detect beneficial clinical scenarios (for example, subgroup analysis in PCI revealing improvement with low TIMI flow). There is still more to be learned before the compelling benchtop data translate into clinically useful interventions.
Abbreviations Used
- AO
aldehyde oxidase
- COX
cyclooxygenase
- eNOS
endothelial NOS
- GSH
glutathione
- GSNO
S-nitrosoglutathione
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon gamma
- IR
ischemia/reperfusion injury
- LAT
L-type amino acid transporter
- LPS
lipopolysaccharide
- MACE
major adverse cardiac events
- MCP-1
monocyte chemoattractant protein-1
- NEC
necrotizing enterocolitis
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide
- NOS
NO synthase
- Nrf2
nuclear factor erythroid 2-related factor 2
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PCI
percutaneous coronary intervention
- ROS
reactive oxygen species
- sGC
soluble guanylate cyclase
- STEMI
ST-elevated myocardial infarction
- TIMI
thrombolysis in myocardial infarction
- TLR-4
toll-like receptor 4
- TNFα
tumor necrosis factor-α
- XOR
xanthine oxidoreductase
References
- 1.Alef MJ, Vallabhaneni R, Carchman E, Morris SM, Shiva S, Wang Y, Kelley EE, Tarpey MM, Gladwin MT, Tzeng E, and Zuckerbraun BS. Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. J Clin Invest 121: 1646–1656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen A, Flemström G, Garner A, and Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev 73: 823–857, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, and Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Angus DC. and van der Poll T. Severe sepsis and septic shock. N Engl J Med 369: 840–851, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Atuma C, Strugala V, Allen A, and Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280: G922–G929, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Baker JE, Su J, Fu X, Hsu A, Gross GJ, Tweddell JS, and Hogg N. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and KATP channels. J Mol Cell Cardiol 43: 437–444, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu S, Azarova NA, Font MD, King SB, Hogg N, Gladwin MT, Shiva S, and Kim-Shapiro DB. Nitrite reductase activity of cytochrome c. J Biol Chem 283: 32590–32597, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck PL, Xavier R, Lu N, Nanda NN, Dinauer M, Podolsky DK, and Seed B. Mechanisms of NSAID-induced gastrointestinal injury defined using mutant mice. Gastroenterology 119: 699–705, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Björne H, Petersson J, Phillipson M, Weitzberg E, Holm L, and Lundberg JO. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. J Clin Invest 113: 106–114, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Björne H, Weitzberg E, and Lundberg JO. Intragastric generation of antimicrobial nitrogen oxides from saliva—physiological and therapeutic considerations. Free Radic Biol Med 41: 1404–1412, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Bloch KD, Ichinose F, Roberts JD, and Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc Res 75: 339–348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boerma EC, Koopmans M, Konijn A, Kaiferova K, Bakker AJ, van Roon EN, Buter H, Bruins N, Egbers PH, Gerritsen RT, Koetsier PM, Kingma WP, Kuiper MA, and Ince C. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med 38: 93–100, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Bondonno CP, Liu AH, Croft KD, Considine MJ, Puddey IB, Woodman RJ, and Hodgson JM. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am J Hypertens 28: 572–575, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Bor-Kucukatay M, Wenby RB, Meiselman HJ, and Baskurt OK. Effects of nitric oxide on red blood cell deformability. Am J Physiol Heart Circ Physiol 284: H1577–H1584, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Brahmajothi MV, Mason SN, Whorton AR, McMahon TJ, and Auten RL. Transport rather than diffusion-dependent route for nitric oxide gas activity in alveolar epithelium. Free Radic Biol Med 49: 294–300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broniowska KA, Diers AR, and Hogg N. S-nitrosoglutathione. Biochim Biophys Acta 1830: 3173–3181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broniowska KA, Zhang Y, and Hogg N. Requirement of transmembrane transport for S-nitrosocysteine-dependent modification of intracellular thiols. J Biol Chem 281: 33835–33841, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, and Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A 104: 19144–19149, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, and Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 1: 290–297, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, and Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394: 627–634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buys ES, Cauwels A, Raher MJ, Passeri JJ, Hobai I, Cawley SM, Rauwerdink KM, Thibault H, Sips PY, Thoonen R, Scherrer-Crosbie M, Ichinose F, Brouckaert P, and Bloch KD. sGC(alpha)1(beta)1 attenuates cardiac dysfunction and mortality in murine inflammatory shock models. Am J Physiol Heart Circ Physiol 297: H654–H663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calzi ML, Raviolo C, Ghibaudi E, de Gioia L, Salmona M, Cazzaniga G, Kurosaki M, Terao M, and Garattini E. Purification, cDNA cloning, and tissue distribution of bovine liver aldehyde oxidase. J Biol Chem 270: 31037–31045, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Cantu-Medellin N. and Kelley EE. Xanthine oxidoreductase-catalyzed reduction of nitrite to nitric oxide: insights regarding where, when and how. Nitric Oxide 34: 19–26, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Z, Bell JB, Mohanty JG, Nagababu E, and Rifkind JM. Nitrite enhances RBC hypoxic ATP synthesis and the release of ATP into the vasculature: a new mechanism for nitrite-induced vasodilation. Am J Physiol Heart Circ Physiol 297: H1494–H1503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castiglione N, Rinaldo S, Giardina G, Stelitano V, and Cutruzzolà F. Nitrite and nitrite reductases: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 17: 684–716, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Cauwels A. and Brouckaert P. Nitrite regulation of shock. Cardiovasc Res 89: 553–559, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Cauwels A, Buys ES, Thoonen R, Geary L, Delanghe J, Shiva S, and Brouckaert P. Nitrite protects against morbidity and mortality associated with TNF- or LPS-induced shock in a soluble guanylate cyclase-dependent manner. J Exp Med 206: 2915–2924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YR, Chen CL, Liu X, Li H, Zweier JL, and Mason RP. Involvement of protein radical, protein aggregation, and effects on NO metabolism in the hypochlorite-mediated oxidation of mitochondrial cytochrome c. Free Radic Biol Med 37: 1591–1603, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Cobb JP. and Danner RL. Nitric oxide and septic shock. J Am Med Assoc 275: 1192–1196, 1996 [PubMed] [Google Scholar]
- 30.Corti A, Franzini M, Scataglini I, and Pompella A. Mechanisms and targets of the modulatory action of S-nitrosoglutathione (GSNO) on inflammatory cytokines expression. Arch Biochem Biophys 562: 80–91, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, and Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation 120: 897–905, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, and Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem 256: 12393–12398, 1981 [PubMed] [Google Scholar]
- 33.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, and Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med 1: 546–551, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Duranski MR, Greer JJM, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, and Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest 115: 1232–1240, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, and Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry 35: 6976–6983, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Frostell C, Fratacci MD, Wain JC, Jones R, and Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 83: 2038–2047, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Gaboury J, Woodman RC, Granger DN, Reinhardt P, and Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol 265: H862–H867, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Gill R, Kuriakose R, Gertz ZM, Salloum FN, Xi L, and Kukreja RC. Remote ischemic preconditioning for myocardial protection: update on mechanisms and clinical relevance. Mol Cell Biochem 402: 41–49, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, and Cannon RO. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A 97: 11482–11487, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Good M, Sodhi CP, and David J. Evidence-based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev Clin Immunol 10: 875–884, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gould N, Doulias PT, Tenopoulou M, Raju K, and Ischiropoulos H. Regulation of protein function and signaling by reversible cysteine s-nitrosylation. J Biol Chem 288: 26473–26479, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Govoni M, Jansson EA, Weitzberg E, and Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19: 333–337, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Habermeyer M, Roth A, Guth S, Diel P, Engel K-H, Epe B, Fürst P, Heinz V, Humpf H-U, Joost H-G, Knorr D, de Kok T, Kulling S, Lampen A, Marko D, Rechkemmer G, Rietjens I, Stadler RH, Vieths S, Vogel R, Steinberg P, and Eisenbrand G. Nitrate and nitrite in the diet: how to assess their benefit and risk for human health. Mol Nutr Food Res 59: 106–128, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 33: 774–797, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Hawkey CJ. and Langman MJS. Non-steroidal anti-inflammatory drugs: overall risks and management. Complementary roles for COX-2 inhibitors and proton pump inhibitors. Gut 52: 600–608, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff H-J, Goedecke A, Schrader J, Gladwin MT, Kelm M, and Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A 105: 10256–10261, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, and Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci 49: 1598–1606, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Hogg N, Broniowska KA, Novalija J, Kettenhofen NJ, and Novalija E. Role of S-nitrosothiol transport in the cardioprotective effects of S-nitrosocysteine in rat hearts. Free Radic Biol Med 43: 1086–1094, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Hord NG, Ghannam JS, Garg HK, Berens PD, and Bryan NS. Nitrate and nitrite content of human, formula, bovine, and soy milks: implications for dietary nitrite and nitrate recommendations. Breastfeed Med 6: 393–399, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, and Freeman BA. Binding of xanthine oxidase to vascular endothelium: kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem 274: 4985–4994, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Huang KT, Keszler A, Patel N, Patell RP, Gladwin MT, Kim-Shapiro DB, and Hogg N. The reaction between nitrite and deoxyhemoglobin: Reassessment of reaction kinetics and stoichiometry. J Biol Chem 280: 31126–31131, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, and Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest 115: 2099–2107, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jädert C, Petersson J, Massena S, Ahl D, Grapensparr L, Holm L, Lundberg JO, and Phillipson M. Decreased leukocyte recruitment by inorganic nitrate and nitrite in microvascular inflammation and NSAID-induced intestinal injury. Free Radic Biol Med 52: 683–692, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Jädert C, Phillipson M, Holm L, Lundberg JO, and Borniquel S. Preventive and therapeutic effects of nitrite supplementation in experimental inflammatory bowel disease. Redox Biol 2: 73–81, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia L, Bonaventura C, Bonaventura J, and Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380: 221–226, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Jones DA, Pellaton C, Velmurugan S, Andiapen M, Antoniou S, van Eijl S, Webb AJ, Westwood M, Parmar M, Mathur A, and Ahluwalia A. Randomized phase 2 trial of intra-coronary nitrite during acute myocardial infarction. Circ Res 116, 437–447, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Julou-Schaeffer G, Gray GA, Fleming I, Schott C, Parratt JR, and Stoclet JC. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol 259: H1038–H1043, 1990 [DOI] [PubMed] [Google Scholar]
- 58.Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, and Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke 37: 2744–2750, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Kelley EE, Hock T, Khoo NKH, Richardson GR, Johnson KK, Powell PC, Giles GI, Agarwal A, Lancaster JR, and Tarpey MM. Moderate hypoxia induces xanthine oxidoreductase activity in arterial endothelial cells. Free Radic Biol Med 40: 952–959, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Kim-Shapiro DB. and Gladwin MT. Mechanisms of nitrite bioactivation. Nitric Oxide 38: 58–68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristof AS, Goldberg P, Laubach V, and Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med 158: 1883–1889, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Kubes P, Suzuki M, and Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A 88: 4651–4655, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lang JD, Smith AB, Brandon A, Bradley KM, Liu Y, Li W, Crowe DR, Jhala NC, Cross RC, Frenette L, Martay K, Vater YL, Vitin AA, Dembo GA, DuBay DA, Bynon JS, Szychowski JM, Reyes JD, Halldorson JB, Rayhill SC, Dick AA, Bakthavatsalam R, Brandenberger J, Broeckel-Elrod JA, Sissons-Ross L, Jordan T, Chen LY, Siriussawakul A, Eckhoff DE, and Patel RP. A randomized clinical trial testing the anti-inflammatory effects of preemptive inhaled nitric oxide in human liver transplantation. PLoS One 9: e86053, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lang JD, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, and Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest 117: 2583–2591, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, and Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 13: 149–159, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, Huang PL, and Scalia R. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am J Physiol 276: H1943–H1950, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Li H, Cui H, Kundu TK, Alzawahra W, and Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: Critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem 283: 17855–17863, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li H, Kundu TK, and Zweier JL. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J Biol Chem 284: 33850–33858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, Samouilov A, Liu X, and Zweier JL. Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation. J Biol Chem 279: 16939–16946, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Liu B, Tewari AK, Zhang L, Green-Church KB, Zweier JL, Chen YR, and He G. Proteomic analysis of protein tyrosine nitration after ischemia reperfusion injury: mitochondria as the major target. Biochim Biophys Acta 1794: 476–485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X, Miller MJS, Joshi MS, Sadowska-Krowicka H, Clark DA, and Lancaster JR. Diffusion-limited reaction of nitric oxide with erythrocytes vs free hemoglobin. FASEB J 12: A82–A82, 1998 [DOI] [PubMed] [Google Scholar]
- 72.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, and Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 32: 21–30, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Lü P, Liu F, Yao Z, Wang CY, Chen DD, Tian Y, Zhang JH, and Wu YH. Nitrite-derived nitric oxide by xanthine oxidoreductase protects the liver against ischemia-reperfusion injury. Hepatobiliary Pancreat Dis Int 4: 350–355, 2005 [PubMed] [Google Scholar]
- 74.Lundberg JO. Cardiovascular prevention by dietary nitrate and nitrite. Am J Physiol Heart Circ Physiol 296: H1221–H1223, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Lundberg JO. and Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 37: 395–400, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, and Hyodo I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr 48: 107–111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyoshi M, Kasahara E, Park A-M, Hiramoto K, Minamiyama Y, Takemura S, Sato EF, and Inoue M. Dietary nitrate inhibits stress-induced gastric mucosal injury in the rat. Free Radic Res 37: 85–90, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Mo L, Wang Y, Geary L, Corey C, Alef MJ, Beer-Stolz D, Zuckerbraun BS, and Shiva S. Nitrite activates AMP kinase to stimulate mitochondrial biogenesis independent of soluble guanylate cyclase. Free Radic Biol Med 53: 1440–1450, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morise Z, Granger DN, Fuseler JW, Anderson DC, and Grisham MB. Indomethacin induced gastropathy in CD18, intercellular adhesion molecule 1, or P-selectin deficient mice. Gut 45: 523–528, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murad F. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med 355: 2003–2011, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Nordman H, Davies JR, Lindell G, de Bolós C, Real F, and Carlstedt I. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem J 364: 191–200, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O Cannon R III, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, and Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest 108: 279–287, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okamoto T, Tang X, Janocha A, Farver CF, Gladwin MT, and McCurry KR. Nebulized nitrite protects rat lung grafts from ischemia reperfusion injury. J Thorac Cardiovasc Surg 145: 1108–1116, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Omar SA, Artime E, and Webb AJ. A comparison of organic and inorganic nitrates/nitrites. Nitric Oxide 26: 229–240, 2012 [DOI] [PubMed] [Google Scholar]
- 85.Omar SA, and Webb AJ. Nitrite reduction and cardiovascular protection. J Mol Cell Cardiol 73: 57–69, 2014 [DOI] [PubMed] [Google Scholar]
- 86.Pride CK, Mo L, Quesnelle K, Dagda RK, Murillo D, Geary L, Corey C, Portella R, Zharikov S, St Croix C, Maniar S, Chu CT, Khoo NK, and Shiva S. Nitrite activates protein kinase A in normoxia to mediate mitochondrial fusion and tolerance to ischaemia/reperfusion. Cardiovasc Res 101: 57–68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Przyklenk K, Bauer B, Ovize M, Kloner RA, and Whittaker P. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87: 893–899, 1993 [DOI] [PubMed] [Google Scholar]
- 88.Raat NJH, Noguchi AC, Liu VB, Raghavachari N, Liu D, Xu X, Shiva S, Munson PJ, and Gladwin MT. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radic Biol Med 47: 510–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A 101: 4003–4008, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rassaf T, Flögel U, Drexhage C, Hendgen-Cotta U, Kelm M, and Schrader J. Nitrite reductase function of deoxymyoglobin: Oxygen sensor and regulator of cardiac energetics and function. Circ Res 100: 1749–1754, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, and Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res 114: 1601–1610, 2014 [DOI] [PubMed] [Google Scholar]
- 92.Razavi HM, Werhun R, Scott JA, Weicker S, Wang LF, McCormack DG, and Mehta S. Effects of inhaled nitric oxide in a mouse model of sepsis-induced acute lung injury. Crit Care Med 30: 868–873, 2002 [DOI] [PubMed] [Google Scholar]
- 93.Salgado MT, Nagababu E, and Rifkind JM. Quantification of intermediates formed during the reduction of nitrite by deoxyhemoglobin. J Biol Chem 284: 12710–12718, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schatlo B, Henning EC, Pluta RM, Latour LL, Golpayegani N, Merrill MJ, Lewin N, Chen Y, and Oldfield EH. Nitrite does not provide additional protection to thrombolysis in a rat model of stroke with delayed reperfusion. J Cereb Blood Flow Metab 28: 482–489, 2008 [DOI] [PubMed] [Google Scholar]
- 95.Sha Y. and Marshall HE. S-nitrosylation in the regulation of gene transcription. Biochim Biophys Acta 1820: 701–711, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, and Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res 100: 654–661, 2007 [DOI] [PubMed] [Google Scholar]
- 97.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, and Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204: 2089–2102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siddiqi N, Neil C, Bruce M, MacLennan G, Cotton S, Papadopoulou S, Feelisch M, Bunce N, Lim PO, Hildick-Smith D, Horowitz J, Madhani M, Boon N, Dawson D, Kaski JC, and Frenneaux M. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI). Eur Heart J 35: 1255–1262, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Souza JM, Peluffo G, and Radi R. Protein tyrosine nitration-Functional alteration or just a biomarker? Free Radic Biol Med 45: 357–366, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Sparacino-Watkins CE, Tejero J, Sun B, Gauthier MC, Thomas J, Ragireddy V, Merchan BA, Wang J, Azarov I, Basu P, and Gladwin MT. Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. J Biol Chem 289: 10345–10358, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, and Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 360: 1395–1396, 2002 [DOI] [PubMed] [Google Scholar]
- 102.Sugimoto R, Okamoto T, Nakao A, Zhan J, Wang Y, Kohmoto J, Tokita D, Farver CF, Tarpey MM, Billiar TR, Gladwin MT, and McCurry KR. Nitrite reduces acute lung injury and improves survival in a rat lung transplantation model. Am J Transplant 12: 2938–2948, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Trzeciak S, Glaspey LJ, Dellinger RP, Durflinger P, Anderson K, Dezfulian C, Roberts BW, Chansky ME, Parrillo JE, and Hollenberg SM. Randomized controlled trial of inhaled nitric oxide for the treatment of microcirculatory dysfunction in patients with sepsis. Crit Care Med 42: 2482–2492, 2014 [DOI] [PubMed] [Google Scholar]
- 104.Vandendriessche B, Rogge E, Goossens V, Vandenabeele P, Stasch JP, Brouckaert P, and Cauwels A. The soluble guanylate cyclase activator BAY 58–2667 protects against morbidity and mortality in endotoxic shock by recoupling organ systems. PLoS One 8: e72155, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wallace JL, McKnight W, Miyasaka M, Tamatani T, Paulson J, Anderson DC, Granger DN, and Kubes P. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am J Physiol 265: G993–G998, 1993 [DOI] [PubMed] [Google Scholar]
- 106.Wallace JL. and Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology 119: 512–520, 2000 [DOI] [PubMed] [Google Scholar]
- 107.Webb A, Bond R, McLean P, Uppal R, Benjamin N, and Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A 101: 13683–13688, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, Neal MD, Jia H, Lin J, Ma C, Branca MF, Prindle T, Richardson WM, Ozolek J, Billiar TR, Binion DG, Gladwin MT, and Hackam DJ. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci U S A 110: 9451–9456, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhan J, Nakao A, Sugimoto R, Dhupar R, Wang Y, Wang Z, Billiar TR, and McCurry KR. Orally administered nitrite attenuates cardiac allograft rejection in rats. Surgery 146: 155–165, 2009 [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y. and Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci U S A 101: 7891–7896, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zweier JL, Wang P, Samouilov A, and Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1: 804–809, 1995 [DOI] [PubMed] [Google Scholar]