Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
In a majority of patients with advanced SM, neoplastic MCs express the target receptor CD30.
The CD30-targeting drug brentuximab-vedotin blocks growth and survival in CD30+ neoplastic MCs which favors drug development in advanced SM.
Abstract
The Ki-1 antigen (CD30) is an established therapeutic target in patients with Hodgkin lymphoma and anaplastic large-cell lymphoma. We have recently shown that CD30 is expressed abundantly in the cytoplasm of neoplastic mast cells (MCs) in patients with advanced systemic mastocytosis (SM). In the current study, we asked whether CD30 is expressed on the surface of neoplastic MCs in advanced SM, and whether this surface structure may serve as therapeutic target in SM. As assessed by flow cytometry, CD30 was found to be expressed on the surface of neoplastic MCs in 3 of 25 patients (12%) with indolent SM, 4 of 7 patients (57%) with aggressive SM, and 4 of 7 patients (57%) with MC leukemia. The immature RAS-transformed human MC line MCPV-1.1 also expressed cell surface CD30, whereas the KIT-transformed MC line HMC-1.2 expressed no detectable CD30. The CD30-targeting antibody-conjugate brentuximab-vedotin inhibited proliferation in neoplastic MCs, with lower IC50 values obtained in CD30+ MCPV-1.1 cells (10 µg/mL) compared with CD30− HMC-1.2 cells (>50 µg/mL). In addition, brentuximab-vedotin suppressed the engraftment of MCPV-1.1 cells in NSG mice. Moreover, brentuximab-vedotin produced apoptosis in all CD30+ MC lines tested as well as in primary neoplastic MCs in patients with CD30+ SM, but did not induce apoptosis in neoplastic MCs in patients with CD30− SM. Furthermore, brentuximab-vedotin was found to downregulate anti-IgE–induced histamine release in CD30+ MCs. Finally, brentuximab-vedotin and the KIT D816V-targeting drug PKC412 produced synergistic growth-inhibitory effects in MCPV-1.1 cells. Together, CD30 is a promising new drug target for patients with CD30+ advanced SM.
Introduction
Systemic mastocytosis (SM) is a myeloid neoplasm defined by expansion and accumulation of neoplastic mast cells (MCs) in various organs.1-6 Based on clinical presentation and SM-related organ damage, indolent and aggressive variants of SM have been defined.6-10 Patients with indolent SM (ISM) usually suffer from mediator-related symptoms and/or from the cosmetic consequences of the disease. Otherwise, however, ISM patients have a normal or almost normal life expectancy without overt hematologic problems.1-4,11-14 In contrast, patients with advanced SM, including aggressive SM (ASM) and MC leukemia (MCL), have a dismal prognosis with short survival times.11-16 In these patients, the invasive growth of neoplastic MCs in the bone marrow (BM), liver, and other visceral organs leads to organ damage.11-16 Moreover, in advanced SM, neoplastic MCs are often resistant against various cytoreductive drugs.11-18 Therefore, these patients are candidates for experimental therapies. Indeed, several attempts have been made to develop more effective treatment approaches and to identify novel therapeutic targets in neoplastic MCs.17-20
In a vast majority of all patients with advanced SM, the transforming KIT mutation D816V is displayed by neoplastic cells.21-24 This mutation causes ligand-independent activation of KIT and is considered to contribute to malignant expansion of MCs in SM.2-6,25 Therefore, drugs interfering with the tyrosine kinase (TK) activity of KIT D816V have recently been used.17-20,26-32 These drugs include midostaurin (PKC412), nilotinib, and dasatinib.19,26-32 However, despite impressive effects in cell line models and a clinical trial using PKC412, these drugs may not be sufficient to induce long-lasting complete responses in ASM and MCL. More recently, we have shown that combinations of various KIT TK inhibitors (TKIs) exert synergistic growth-inhibitory effects on neoplastic MCs.19,27,32 However, in neoplastic MCs bearing KIT D816V, only a few drug combinations induced synergistic effects.32 Therefore, current research is seeking new targets and targeted drugs for ASM and MCL.
The Ki-1 antigen, also known as CD30, has long been recognized as a rather specific marker of Hodgkin disease and ALK+ anaplastic large-cell lymphomas.33,34 Other hematologic neoplasms are usually CD30−. However, recent data suggest that neoplastic MCs in advanced SM also express the Ki-1 antigen in their cytoplasm.35,36 Notably, whereas in ISM, most neoplastic MCs are CD30− cells, CD30 is expressed abundantly in the cytoplasm of MCs in patients with ASM and MCL.35,36 More recent data suggest that neoplastic MCs also express CD30 on their cell surface.37
In this study, we examined the expression of CD30 in various human MC lines and primary neoplastic MCs and asked whether CD30 may serve as a therapeutic target.
Materials and methods
Isolation and culture of primary cells
BM samples were obtained from 45 patients with SM (ISM, n = 25; SM with associated hematologic non-MC disease [SM-AHNMD], n = 6; ASM, n = 7; MCL, n = 7) and 6 controls (normal/reactive BM). BM mononuclear cells (MNCs) were isolated using Ficoll (supplemental Table 1, see supplemental Data available on the Blood Web site). All donors gave written informed consent. The study was approved by the ethics committee of the Medical University of Vienna. Human MC lines used in this study were HMC-1.1, HMC-1.2,19,38 MCPV-1.1, and MCPV-1.4.39 In addition, we used a canine mastocytoma cell line, C2.40 A detailed description of cell lines is provided in the supplemental Methods.
Multicolor flow cytometry
Heparinized BM cells (106 leukocytes per tube) of 51 donors were incubated with a phycoerythrin (PE)-labeled CD30 monoclonal antibody (mAb), allophycocyanin (APC)-labeled CD38 mAb, PE-Cy7–labeled CD117 mAb, APC-Cy7–labeled CD45 mAb, and Pacific Blue–labeled CD34 mAb (supplemental Table 2) at room temperature for 15 minutes. Then, erythrocytes were lysed in FACS lysing solution (BD Biosciences). Afterward, cells were washed and analyzed on a FACSCantoII (BD Biosciences) using FACSDiva (BD Biosciences) and FlowJo software (TreeStar). MCs were detected by their typical forward/side-scatter characteristics and their unique phenotype (CD45+/CD117++/CD34−). Flow cytometry results obtained with CD117++ MCs were expressed as ratio of median fluorescence intensities (MFIs) obtained with specific mAbs and MFIs obtained with isotype-matched control mAb (MFI test mAb:MFI control mAb). Results obtained from MFI calculations (ratio) were scored: MFI ratio, 10.01 to 100: ++; MFI ratio, 3.01 to 10: +; MFI ratio, 1.51 to 3: ±; MFI ratio, <1.5: –.
Immunohistochemistry and immunocytochemistry
Immunohistochemistry (IHC) was performed on paraffin-embedded BM sections obtained from 44 patients with SM using the indirect immunoperoxidase staining technique as reported.19 Immunocytochemistry (ICC) was performed as reported19 using cytospin preparations of HMC-1.1, HMC-1.2, MCPV-1.1, MCPV-1.4, and C2 cells as well as primary MCs obtained from 2 patients with MCL (#39, #43). Staining methods are described in detail in the supplemental Methods.
Quantitative PCR
Total RNA was isolated from MNCs of 29 patients with SM (ISM, n = 17; SM-AHNMD, n = 3; ASM, n = 5; MCL, n = 4) using the RNeasy MinElute Cleanup kit (Qiagen).41 The quantitative PCR (qPCR) technique is described in the supplemental Methods.
Measurement of sCD30 in patients’ sera
Serum levels of soluble CD30 (sCD30) were determined in 10 healthy controls and in 36 patients with mastocytosis, including 6 with cutaneous mastocytosis (CM), 25 with ISM, 3 with ASM, and 2 with MCL. Serum sCD30 levels were quantified using a commercial enzyme-linked immunosorbent assay (ELISA; eBioscience) following the manufacturer’s instructions. The detection limit of sCD30 in this assay was 6.4 ng/mL.
Evaluation of apoptosis and cell cycle progression in MC lines
For flow cytometric determination of apoptosis and viability, Annexin V/propidium iodide (PI) staining and active caspase-3 staining were performed as described.27,32 In brief, MCPV-1 cells, HMC-1 cells, and C2 cells were kept in control medium or brentuximab-vedotin (2.5-10 µg/mL) at 37°C for 96 hours. For Annexin V/PI staining, cells were incubated with Annexin V–fluorescein isothiocyanate (FITC) in binding buffer containing N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES; 10 mM, pH 7.4), NaCl (140 mM), and CaCl2 (2.5 mM). Thereafter, PI (1 µg/mL) was added and cells analyzed by flow cytometry. For active caspase-3 staining, cells were fixed in formaldehyde (2%) and permeabilized using methanol (100%) at −20°C for 30 minutes. Cells were analyzed on a FACSCalibur (BD Biosciences). For cell cycle studies, cells were incubated in control medium or in brentuximab-vedotin (2.5-10 µg/mL) for 96 hours. Then, cells were resuspended in 300 µL of permeabilization buffer (0.1% sodium acetate and 0.1% Triton X-100). Thereafter, 3 µL of RNAse (100 µg/mL) and 25 µL of PI were added. Cell cycle distribution was analyzed on a FACSCalibur as reported.27,32
Evaluation of apoptosis in primary neoplastic MCs
Primary BM cells obtained from 3 patients with CD30+ SM (ASM, n = 2; MCL, n = 1) and 3 with CD30− SM (ISM-AHNMD, n = 2; MCL, n = 1) were incubated in control medium or brentuximab-vedotin (2.5-10 µg/mL) at 37°C for 96 hours. Then, CD117++/CD34− MCs were examined by staining for Annexin V-FITC and 4′,6-diamidino-2-phenylindole (DAPI) or active caspase-3 as described.41 Apoptosis was expressed as percentage of Annexin V+ cells after gating for DAPI− cells (thereby excluding early apoptotic cells) and as a percentage of active caspase-3+ cells.
Evaluation of effects of brentuximab-vedotin on proliferation
HMC-1 cells, MCPV-1 cells, C2 cells (104 per well), and primary patient-derived BM-MNCs (106 per well) were seeded in 96-well plates (TPP) and incubated in increasing concentrations of brentuximab-vedotin (0.1-50 µg/mL) at 37°C for 96 hours. After incubation, 3H-thymidine (0.5 µCi per well) was added for 16 hours. Cells were then harvested and radioactivity determined in a β-counter (MicroBeta2; Perkin Elmer).19,27 To determine potential additive or synergistic drug effects, HMC-1.1, HMC-1.2, MCPV-1.1, MCPV-1.4, and C2 cells were exposed to brentuximab-vedotin and PKC412 as single agents or in combination at a fixed ratio of drug concentrations at 37°C for 96 hours.
Repopulation of MCs in NSG mice
Before being injected into NOD-SCID IL-2Rγnull (NSG) mice, CD30+ MCPV-1.1 cells were incubated in control medium or in brentuximab-vedotin (20-50 µg/mL) at 37°C for 1 hour. In a separate experiment, a more resistant subset of MCPV-1.1 cells were treated with control medium, brentuximab-vedotin (100 µg/mL), PKC412 (2 µM), or a combination of both drugs (same concentrations) at 37°C for 1 hour. Then, cells were checked for viability, resuspended in 0.15 mL of phosphate-buffered saline with 2% fetal calf serum, and injected into the tail vein of adult female NSG mice (3 × 106 per mouse, 5 mice per group) (The Jackson Laboratory). Prior to injection, mice were irradiated (2.4 Gy). After injection, mice were inspected daily and sacrificed as soon as symptoms occurred or after a maximum of 5 weeks. Then, BM cells were obtained from flushed femurs, tibias, and humeri, and MCPV-1.1 cells quantified by multicolor flow cytometry using mAbs against CD117 and CD45. Animal studies were approved by the ethics committee of the Medical University of Vienna and the University of Veterinary Medicine Vienna, and the national authority, according to §§26ff of the 2012 Animal Experiments Act. An animal experiment license was granted under BMWF-66.009/0296-II/3b/2011.
Histamine release experiments
Dextran-enriched blood basophils (BAs; 1 × 106/mL) obtained from healthy donors (n = 3) or patients with SM (CD30+, n = 3; CD30−, n = 3) were incubated in control medium or brentuximab-vedotin (0.1-10 µg/mL) at 37°C for 30 minutes. Then, cells were incubated in control histamine release (HR) buffer (HRB) or HRB containing anti-immunoglobulin E (IgE) antibody E-124.2.8 (1 µg/mL) at 37°C for 30 minutes. Then, cells were centrifuged at 4°C. Cell-free supernatants and cell suspensions were recovered and analyzed for histamine content by radioimmunoassay. HR was calculated as a percentage of released histamine compared with total (cellular + extracellular) histamine.
Evaluation of activation-linked cell surface antigens by flow cytometry
For examination of drug effects on expression of CD63 and CD203c on BAs and MCs, flow cytometry was performed. HMC-1.1, HMC-1.2, MCPV-1.1, and BAs from healthy donors (n = 3) were incubated in brentuximab-vedotin (0.1-10 µg/mL) or control medium for 30 minutes (37°C). Cell lines were then examined for expression of CD63 and CD203c using mAbs (supplemental Table 2) by flow cytometry. Drug-exposed primary BAs were further incubated with anti-IgE mAb E-124.2.8 (1 µg/mL) at 37°C for 15 minutes, washed, subjected to erythrocyte lysis, and analyzed by flow cytometry using mAbs against CD63 and CD203c. The anti-IgE–induced upregulation of CD63 and CD203c on primary BAs was calculated from mean fluorescence intensities (MFIs) obtained with stimulated (MFIstim) and unstimulated (MFIcontrol) cells and expressed as stimulation index (SI = MFIstim:MFIcontrol).
Statistical evaluation of data
Significances in differences in growth and apoptosis were determined by the Student t test for dependent samples. Results were considered significant when P < .05. Drug interactions (additive, synergistic, and antagonistic) were examined by calculating combination index (CI) values using CalcuSyn software (Biosoft).42 A CI value of 1 indicates an additive effect, whereas CI values below 1 indicate synergistic drug effects.
Results
Neoplastic MCs express cell surface CD30
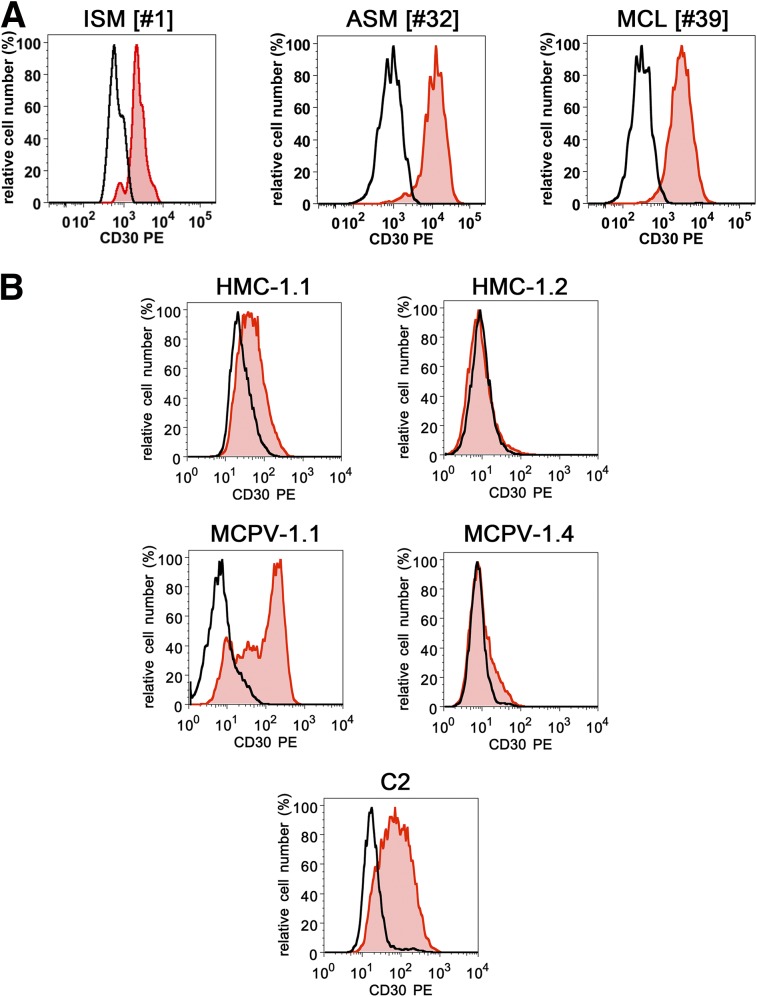
As assessed by flow cytometry, CD30 was expressed on neoplastic MCs in 3 of 25 patients (12%) with ISM, 4 of 7 (57%) with ASM, and 4 of 7 (57%) with MCL (Figure 1A). In most patients, CD30 expression in MCs was confirmed by IHC on BM sections (Table 1). Confirming previous studies,35 MCs were found to express cytoplasmic CD30 in most patients with advanced SM examined. However, in some of these patients, neoplastic MCs stained negative for cytoplasmic CD30 (Table 1). In most patients with ISM, neoplastic MCs expressed only low amounts or no cytoplasmic CD30. We also found a correlation between the type of SM and surface CD30 expression on MCs (Table 1). In particular, CD30 levels on MCs in patients with ASM and MCL were higher than that in ISM (median CD30 MFI: ASM/MCL, 4.24 vs ISM, 1.88, P < .05) (Table 1). However, in some patients with advanced SM (ASM/MCL) MCs stained negative for CD30. CD30 was also detected on the surface of MCPV-1.1 cells, an immature RAS-transformed human MC line, and in the canine mastocytoma cell line C2 (Figure 1B). The HMC-1.1 cell line expressed low levels of surface CD30, and HMC-1.2 cells stained negative for CD30 (Figure 1B). Expression of CD30 in MC lines and primary neoplastic MCs was also demonstrable by ICC and IHC (supplemental Figure 1). In most patients and all cell lines examined, expression of cytoplasmic CD30 was found to correlate with cell surface staining results (Table 1; supplemental Figure 1A).
Figure 1.
Expression of surface CD30 on neoplastic MCs. (A) BM cells of patients with ISM ([#1], left panel), ASM ([#32], middle panel), or MCL ([#39], right panel) were stained with antibodies against CD34, CD45, CD38, CD117, and CD30. After erythrocyte lysis, expression of CD30 on CD117++ (CD45+/CD34−) MCs was analyzed by flow cytometry on a FACSCantoII (BD Biosciences). The black open histograms show the isotype control and the red histograms represent CD30 expression on CD117++ MCs. Patients [#] refer to the numbers in Table 1. (B) HMC-1.1 cells, HMC-1.2 cells (top panel), MCPV-1.1 cells, MCPV-1.4 cells (middle panel), and C2 cells (bottom panel) were first incubated with Fc-blocking reagent and then with PE-labeled antibody Ber-H2 directed against CD30. After incubation for 15 minutes, expression of CD30 was analyzed by flow cytometry on a FACSCalibur (BD Biosciences). Black open histograms show the isotype-matched control antibody, and red histograms reactivity with the CD30 antibody.
Table 1.
Expression of CD30 on CD117++ MCs in patients with mastocytosis
| No. | Sex | Age, y | Diagnosis | Serum tryptase, ng/mL | CD30 surface expression on MCs (MFI ratio) | CD30 IHC |
|---|---|---|---|---|---|---|
| 1 | F | 68 | ISM | 42 | +(3.7) | ± |
| 2 | F | 63 | ISM | 32.7 | +(3.05) | ± |
| 3 | M | 50 | ISM | 19.7 | +(3.02) | ± |
| 4 | F | 63 | ISM | 77.8 | ±(1.94) | ± |
| 5 | M | 56 | ISM | 289 | ±(1.88) | + |
| 6 | F | 43 | ISM | 36.2 | ±(1.76) | ± |
| 7 | M | 55 | ISM | 124 | ±(2.03) | + |
| 8 | F | 53 | ISM | 61.2 | ±(2.44) | − |
| 9 | M | 24 | ISM | 53.7 | ±(2.08) | ± |
| 10 | M | 39 | ISM | 106 | ±(2.41) | ± |
| 11 | M | 47 | ISM | 82.5 | ±(2.00) | ± |
| 12 | M | 53 | ISM | 40.9 | ±(2.30) | ± |
| 13 | F | 35 | ISM | 34.1 | ±(1.57) | − |
| 14 | F | 57 | ISM | 11.2 | ±(1.58) | − |
| 15 | F | 57 | ISM | 55.1 | ±(2.23) | ± |
| 16 | M | 59 | ISM | 42.9 | ±(1.53) | − |
| 17 | M | 52 | ISM | 47.4 | ±(1.63) | ± |
| 18 | M | 64 | ISM | 33.5 | ±(2.08) | + |
| 19 | F | 36 | ISM | 19.5 | −(0.59) | − |
| 20 | F | 41 | ISM | 34.9 | −(1.12) | ± |
| 21 | F | 65 | ISM | 160 | −(1.33) | − |
| 22 | M | 45 | ISM | 31.1 | −(1.31) | ± |
| 23 | F | 47 | ISM | 28.8 | −(1.42) | ± |
| 24 | F | 38 | ISM | 16.8 | −(0.77) | ± |
| 25 | M | 29 | ISM | 31.1 | −(1.44) | − |
| 26 | M | 64 | ISM-AHNMD | 13.3 | ±(2.46) | − |
| 27 | M | 65 | ISM-AHNMD | 37.8 | −(0.98) | ± |
| 28 | F | 70 | ISM-AHNMD | 23 | −(0.83) | ± |
| 29 | M | 63 | ASM-AHNMD | 80 | +(8.93) | + |
| 30 | M | 70 | ASM-AHNMD | 48 | ±(1.64) | ± |
| 31 | F | 73 | ISM-AHNMD | 67.5 | +(5.8) | + |
| 32 | M | 75 | ASM | 789 | ++(12.93) | n.t. |
| 33 | M | 68 | ASM | 152 | +(3.94) | ± |
| 34 | F | 79 | ASM | 146 | +(3.50) | − |
| 35 | M | 66 | ASM | 103 | +(6.23) | ± |
| 36 | M | 71 | ASM | 113 | ±(1.98) | + |
| 37 | M | 74 | ASM | 152 | −(1.24) | − |
| 38 | F | 59 | ASM | 138 | −(1.43) | ± |
| 39 | F | 48 | MCL | 332 | +(9.79) | + |
| 40 | F | 62 | MCL | 547 | +(8.77) | ± |
| 41 | M | 75 | MCL | 431 | +(3.14) | + |
| 42 | M | 58 | MCL | 151 | +(3.04) | + |
| 43 | F | 54 | MCL | 904 | −(0.87) | − |
| 44 | F | 71 | MCL | 113 | −(1.4) | ± |
| 45 | M | 63 | MCL | 767 | −(1.05) | + |
| 46 | M | 44 | Normal BM | 35.5 | −(0.99) | − |
| 47 | M | 34 | Normal BM | 20.1 | −(1.31) | − |
| 48 | F | 32 | Normal BM | 14.8 | +(3.08)* | − |
| 49 | M | 53 | Normal BM | 18.1 | −(0.76) | − |
| 50 | F | 67 | Normal BM | 33.8 | −(1.1) | − |
| 51 | F | 51 | Normal BM | 21.1 | −(1.01) | − |
Score of antibody reactivity by flow cytometry: ++, MFI ratio 10-100; +, MFI ratio 3.01-9.99; ±, MFI ratio 1.51-3; −, MFI ratio <1.5.
Score of antibody reactivity by IHC: +, most cells (>50%) strongly reactive; ±, most cells (>50%) weakly reactive; −, <10% of cells reactive.
F, female; M, male; n.t., not tested.
In this patient, serum tryptase was slightly elevated and very few atypical MCs were found, but no KIT mutation was detected and no histologic evidence of SM could be substantiated.
Detection of increased serum levels of sCD30 in advanced SM
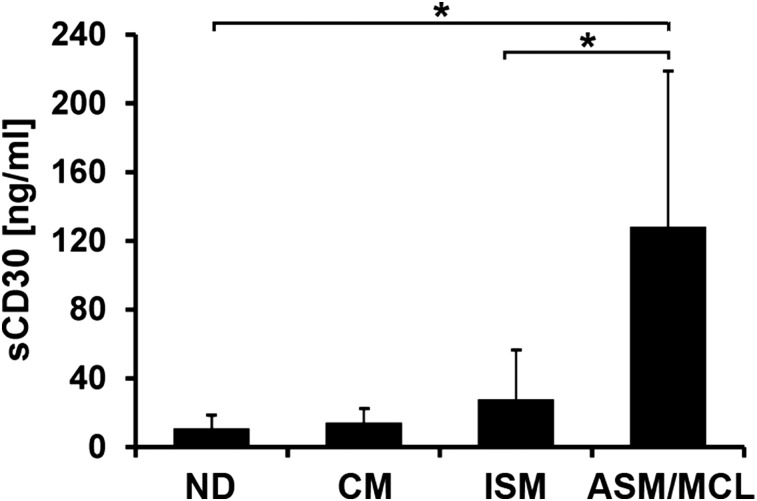
The levels of sCD30 in the sera of healthy controls (n = 10) ranged between 6.4 and 28.5 ng/mL (median, 8.1 ng/mL). In patients with mastocytosis, increased levels of sCD30 were detected. However, serum levels of sCD30 were found to vary among patients and to correlate with the variant of disease (Figure 2). In patients with CM (n = 6), sCD30 levels were only slightly elevated (median, 11.2 ng/mL; range, 7.3-31.0 ng/mL). Higher levels of sCD30 were detected in 25 patients with ISM (median, 21.0 ng/mL; range, 7.1-149.0 ng/mL) (Figure 2). The highest levels of sCD30 were measured in patients with ASM or MCL (n = 5), with a median of 129.0 ng/mL (Figure 2). However, no significant correlation between serum tryptase and sCD30 concentrations was found in our SM patients (not shown).
Figure 2.
Serum concentrations of sCD30 in patients with mastocytosis. Serum levels of sCD30 were determined in 10 normal (healthy) donors (ND), 6 patients with CM, 25 with ISM, 3 with ASM, and 2 with MCL. sCD30 levels were quantified using a commercial ELISA. The detection limit of this assay was found to be 6.4 ng/mL. Results represent sCD30 levels in each cohort (patients or controls) and represent the mean ± standard deviation (SD) of all donors in each group. *P < .05.
Expression of CD30 in neoplastic MCs is regulated by a MEK-dependent pathway
We next examined the regulation of expression of CD30 in neoplastic MCs. In a first step, we confirmed CD30 messenger RNA (mRNA) expression in CD30+ HMC-1.1 cells, MCPV-1.1 cells, and C2 cells as well as in primary MNCs of patients with MCL containing >90% MCs (supplemental Figure 2). In HMC-1.2 cells and MCPV-1.4 cells, CD30 transcripts were also detectable by qPCR, but the levels of CD30 mRNA expression were substantially lower compared with MCPV-1.1. These data suggest that surface levels of CD30 roughly correlate with mRNA expression levels. Next, we applied various signal transduction inhibitors. As visible in supplemental Figure 3, the mitogen-activated protein kinase kinase (MEK) inhibitors PD032509 and RDEA119 were found to downregulate expression of cell surface CD30 and CD30 mRNA levels in HMC-1.1 cells and MCPV-1.1 cells. The KIT-targeting TKI PKC412, the phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) blocker BEZ235, and the signal transducer and activator of transcription 5 (STAT5)-targeting drug piceatannol also decreased CD30 expression in HMC-1.1 and MCPV-1.1 cells, but the effects of these compounds were much weaker compared with the MEK inhibitors tested. The STAT5 inhibitor pimozide showed no effects on expression of CD30 in HMC-1.1 or MCPV-1.1 cells (not shown). These data suggest that expression of CD30 in neoplastic MCs is regulated by a MEK-dependent signaling pathway.
Brentuximab-vedotin inhibits proliferation of neoplastic MCs in vitro and the in vivo engraftment of MCPV-1.1 cells in NSG mice
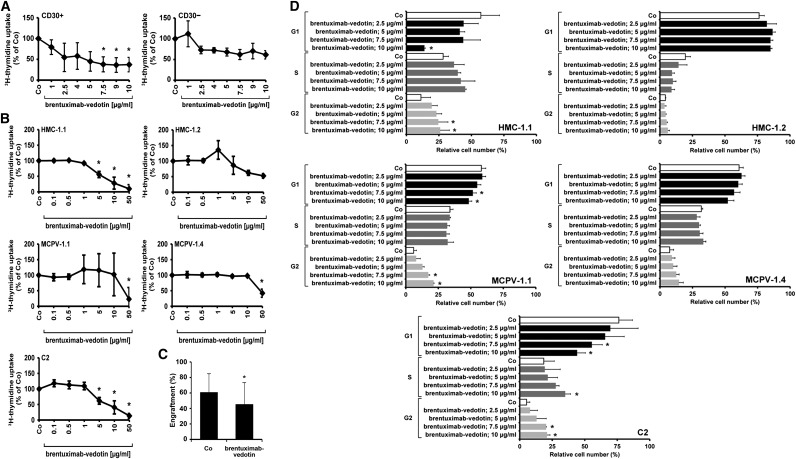
Brentuximab-vedotin inhibited the proliferation of primary neoplastic MCs in all CD30+ SM patients tested, whereas in SM patients in whom MCs stained negative for CD30, brentuximab-vedotin showed only weak effects or did not block the proliferation of neoplastic MCs (Figure 3A). Corresponding results were obtained in our cell line models: whereas brentuximab-vedotin produced strong and dose-dependent growth inhibition in the CD30+ MC lines MCPV-1.1, HMC-1.1, and C2, only weak effects of brentuximab-vedotin were seen in the CD30− cell lines MCPV-1.4 and HMC-1.2 (Figure 3B). We also examined the effect of brentuximab-vedotin on engraftment of MCPV-1.1 cells in NSG mice. In particular, preincubation of MCPV-1.1 cells with brentuximab-vedotin (20-50 µg/mL) resulted in reduced in vivo engraftment in NSG mice compared with control animals (Figure 3C).
Figure 3.
Effect of brentuximab-vedotin on proliferation and cell cycle distribution in neoplastic MCs. (A-B) Primary BM cells obtained from 3 patients with CD30+ SM (ASM, n = 1; MCL, n = 2; A, left panel), 3 patients with CD30− SM (ISM, n = 1; ISM-AHNMD, n = 1; MCL, n = 1; A, right panel), and cell lines (MCPV-1.1, MCPV-1.4, HMC-1, C2) (B) were incubated in control medium (Co) or in various concentrations of brentuximab-vedotin (0.1-50 µg/mL) at 37°C for 96 hours. After incubation, 3H-thymidine uptake was measured. Results represent the mean ± SD from 3 independent experiments. *P < .05. (C) MCPV-1.1 cells were incubated in control medium (Co) or in brentuximab-vedotin (20-50 µg/mL) at 37°C for 1 hour. After incubation, cells were washed and injected into the tail vein of NSG mice (3 × 106 cells/per mouse, 5 mice per group in 3 independent experiments). After 5 weeks, mice were sacrificed and MCPV-1.1 repopulation was measured by determining the percentage of CD45+/CD117+ cells in mouse BM samples by flow cytometry. Results represent mean ± SD from all mice in all experiments. *P < .05. (D) HMC-1.1 cells (top, left panel), HMC-1.2 cells (top, right panel), MCPV-1.1 cells (middle, left panel), MCPV-1.4 cells (middle, right panel), and C2 cells (bottom panel) were incubated in control medium or in various concentrations of brentuximab-vedotin (2.5-10 µg/mL) at 37°C for 96 hours. Then, cell cycle distribution was analyzed by flow cytometry. Results show relative cell numbers and represent the mean ± SD from 3 independent experiments. *P < .05.
Brentuximab-vedotin induces cell cycle arrest in CD30+ neoplastic MCs
Brentuximab-vedotin produced a G2/M cell cycle arrest in the CD30+ cell lines MCPV-1.1 and C2 (Figure 3D). At high concentrations, drug effects on cell cycle progression were also seen in HMC-1.1 cells (low CD30 expresser). By contrast, brentuximab-vedotin did not induce a cell cycle arrest in the CD30− cell lines HMC-1.2 and MCPV-1.4 (Figure 3D).
Brentuximab-vedotin induces apoptosis in CD30+ neoplastic MCs
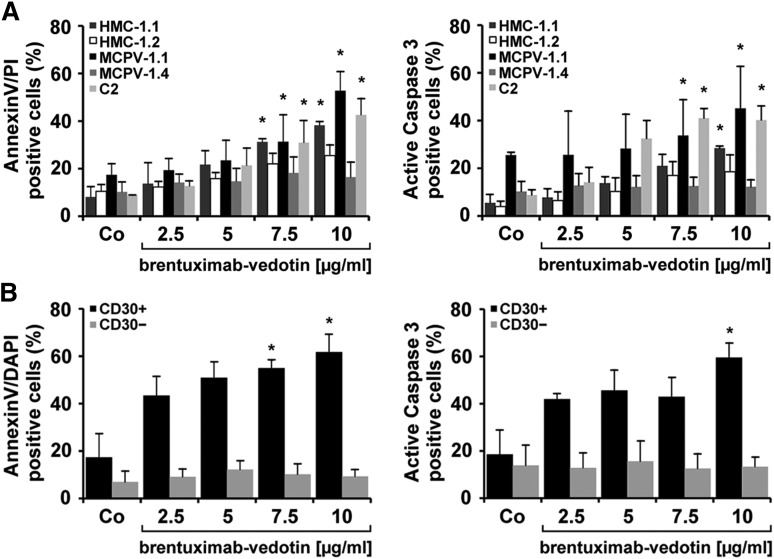
To investigate the mechanism of action of brentuximab-vedotin, we examined drug-exposed cells for signs of apoptosis by staining for Annexin V/PI and active caspase-3. In these experiments, brentuximab-vedotin produced dose-dependent apoptosis in MCPV-1.1 cells and C2 cells, and, less effectively, in HMC-1.1 cells (Figure 4A). By contrast, no significant increase in the number of apoptotic cells was seen in drug-exposed HMC-1.2 and MCPV-1.4 cells (Figure 4A). Next, we examined the effects of brentuximab-vedotin on primary CD30+ and CD30− neoplastic MCs. As shown in Figure 4B, brentuximab-vedotin induced dose-dependent apoptosis in CD30+ neoplastic MCs in all patients tested, whereas CD30− MCs did not respond to brentuximab-vedotin.
Figure 4.
Brentuximab-vedotin induces apoptosis in neoplastic MCs. (A) HMC-1 cells, MCPV-1.1 cells, MCPV-1.4 cells, and C2 cells were incubated in control medium or in medium containing brentuximab-vedotin (2.5-10 µg/mL) at 37°C for 96 hours. Cells were then stained for Annexin V and PI (left panel) or active caspase-3 (right panel) by flow cytometry. Apoptosis was evaluated in CD30+ MCPV-1.1 cells (black bars), CD30+ C2 cells (light gray bars), CD30+/− HMC-1.1 cells (gray bars), CD30− HMC-1.2 cells (open bars), and CD30− MCPV-1.4 cells (dark gray bars). Results are expressed as percentage of positive cells and represent the mean ± SD from 3 independent experiments. *P < .05. (B) Primary BM cells obtained from 3 patients with CD30+ SM (ASM, n = 2; MCL, n = 1) and 3 patients with CD30− SM (ISM-AHNMD, n = 2; MCL, n = 1) were incubated in control medium or brentuximab-vedotin (2.5-10 µg/mL) at 37°C for 96 hours. (Left panel) Cells were stained with a mAb against CD117 for MC detection, and for Annexin V. (Right panel) Cells were stained with a mAb against CD117 and a mAb against active caspase-3. Apoptosis was analyzed in CD30+ MCs (black bars) and in CD30− MCs (gray bars). Results are expressed as percentage of DAPI+/KIT+ cells (left panel) or as the percentage of KIT+ cells (right panel) and represent the mean ± SD from 3 independent experiments in each group of patients. *P < .05 compared with control.
Brentuximab-vedotin cooperates with PKC412 in inducing growth inhibition
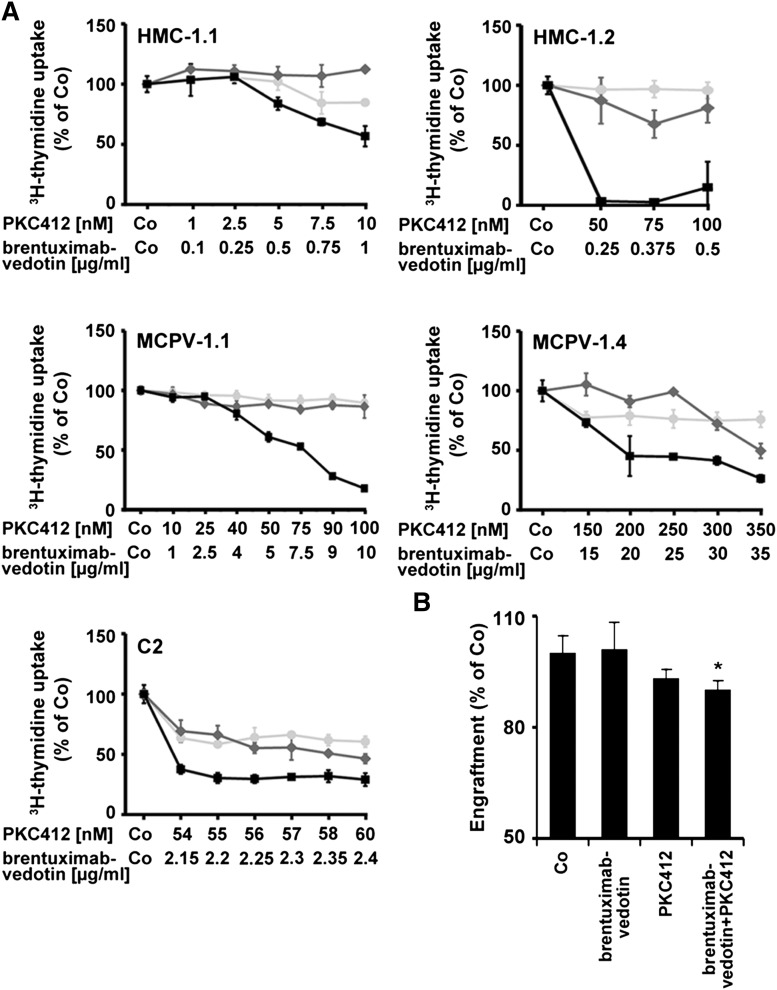
As visible in Figure 5A, brentuximab-vedotin and PKC412 were found to synergize in producing growth inhibition in all cell lines tested, including HMC-1.1, HMC-1.2, MCPV-1.1, and MCPV-1.4. In addition, we found that brentuximab-vedotin and PKC412 produce a cooperative effect on MCPV-1.1 engraftment in NSG mice. In particular, in 1 experiment, brentuximab-vedotin (100 µg/mL) alone did not suppress engraftment of more resistant MCPV-1.1 cells, but engraftment was suppressed effectively when cells were preincubated with the drug combination brentuximab-vedotin + PKC412 (Figure 5B).
Figure 5.
Effect of the drug combinations brentuximab-vedotin + PKC412 on proliferation of neoplastic MCs. (A) HMC-1.1 cells, HMC-1.2 cells, MCPV-1.1 cells, MCPV-1.4 cells, and C2 cells were incubated in control medium, brentuximab-vedotin (0.1-35 µg/mL, light gray symbols), PKC412 (1-350 nM, dark gray symbols), or a combination of both drugs at fixed ratio of drug concentration (black symbols) at 37°C for 96 hours. After incubation, 3H-thymidine uptake was measured. Results are expressed as percentage of control and show 1 typical experiment for each cell line. Almost identical results were obtained in at least 1 independent experiment in each cell line using the same drug concentrations and drug combination. (B) MCPV-1.1 cells were incubated in control medium (Co), brentuximab-vedotin (100 µg/mL), PKC412 (2 µM), or a combination of both drugs (same concentrations) at 37°C for 1 hour. Then, cells were washed and injected into the tail vein of NSG mice (3 × 106 cells per mouse; 4 mice per group). After 4 weeks, mice were sacrificed and MCPV-1.1 repopulation was measured by determining the percentage of CD45+/CD117+ cells in mouse BM samples by flow cytometry. Results represent engraftment as percentage of control. *P < .05.
Brentuximab-vedotin inhibits IgE-dependent HR in MCs and BAs
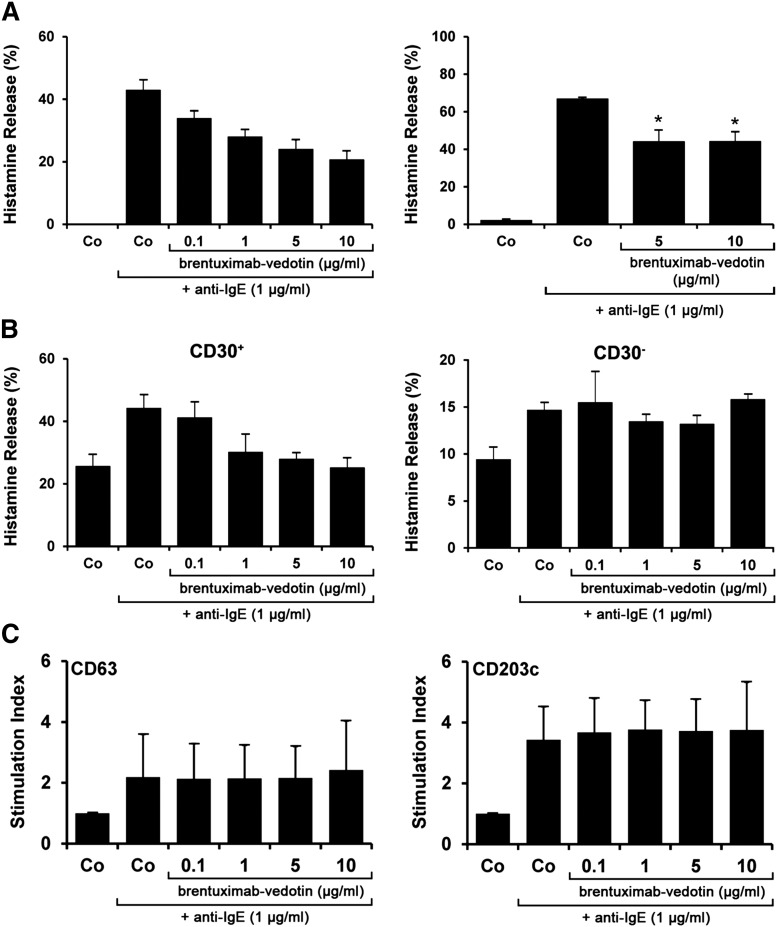
Patients with advanced SM not only suffer from the consequences of MC invasion in various organs but also from symptoms caused by mediators once these are released from neoplastic MCs after MC activation. Therefore, we were also interested to learn whether brentuximab-vedotin modulates histamine secretion in MCs. Over the dose range examined (0.1-10 µg/mL), brentuximab-vedotin did not induce HR from CD30+ MCs, CD30− MCs, or CD30− blood BAs (not shown). Rather, brentuximab-vedotin was found to inhibit anti-IgE-induced HR from normal BAs in a dose-dependent manner (Figure 6A). Furthermore, brentuximab-vedotin also suppressed anti-IgE-induced release of histamine in CD30+ MCs in patients with advanced SM, whereas no effects of brentuximab-vedotin on HR were seen in CD30− MCs (Figure 6B). Moreover, no substantial effects of brentuximab-vedotin on IgE-mediated upregulation of CD63 or CD203c on BAs were demonstrable (Figure 6C). Correspondingly, brentuximab-vedotin also failed to modulate the expression of CD63 and CD203c on HMC-1 cells and MCPV-1.1 cells (not shown).
Figure 6.
Effect of brentuximab-vedotin on IgE-dependent HR and activation in human BAs and MCs. (A-B) BAs obtained from healthy donors (n = 3) (A) and CD30+ or CD30− MCs from patients with mastocytosis (ASM, n = 2; MCL, n = 3; SM-AHNMD, n = 1) (B) were preincubated in control medium (Co) or in various concentrations of brentuximab-vedotin as indicated at 37°C for 30 minutes. Afterward, cells were exposed to anti-IgE (1 µg/mL) at 37°C for 30 minutes. After centrifugation, histamine concentrations were determined in supernatants and cell lysates. Histamine release is expressed as percentage of total histamine. Results represent the mean ± SD from 1 representative experiment (A; left panel) and represent the mean ± SD from 3 normal donors (A, right panel) and from 3 patients with mastocytosis in each panel (B). *P < .05. (C) BAs in whole-blood samples (n = 4) were preincubated in control medium (Co) or in medium containing various concentrations of brentuximab-vedotin (0.1-10 µg/mL) at 37°C for 30 minutes. Then, cells were exposed to anti-IgE antibody (1 µg/mL) for another 15 minutes (37°C). Thereafter, cells were stained with mAb directed against CD63 (left panel) or CD203c (right panel), and analyzed by multicolor flow cytometry as described in the “Material and methods.” BAs were defined as CD203c+ cells in all samples. Anti-IgE-induced upregulation of CD antigens was calculated from mean fluorescence intensities (MFIs) obtained with stimulated (MFIstim) and unstimulated (MFIcontrol) cells, and was expressed as SI (MFIstim: MFIcontrol). Results show SI values and represent the mean ± SD from 4 donors.
Discussion
In advanced SM, the malignant expansion and accumulation of neoplastic MCs in various organ systems leads to organ damage.1-6,12-16 For these patients, no effective therapy is available and the prognosis is poor. However, during the past few years, a number of potentially useful drug targets have been identified in neoplastic MCs.6,18,31,35-37,39 One of these potential targets appears to be the Ki-1 antigen, CD30. In the current study, we provide evidence that CD30 is expressed on the surface of MCs in advanced SM and that the CD30-targeting antibody-conjugate brentuximab-vedotin produces growth inhibition and apoptosis in neoplastic MCs. Moreover, our data show that brentuximab-vedotin and PKC412 exert synergistic growth-inhibitory effects on neoplastic MCs.
Recent data have shown that CD30 is expressed in the cytoplasm and on the surface of neoplastic MCs in SM.35-37,43-47 In an initial report, Sotlar et al described that CD30 is commonly and strongly expressed in the cytoplasm of neoplastic MCs in advanced SM, whereas in most patients with ISM, neoplastic MCs expressed only low amounts or did not exhibit cytoplasmic CD30.35 In the current study, we were able to confirm that CD30 is expressed in the cytoplasm of neoplastic MCs in advanced SM in most patients. In addition, our data show that in most patients with advanced SM, neoplastic MCs express cell surface CD30, which is important in the context of new treatment concepts using CD30-targeted therapy. Moreover, we found a rough correlation between surface expression of CD30 and a more advanced stage of the disease and also between expression of cytoplasmic CD30 and surface CD30 expression. However, these correlations were not significant, and in some patients with ASM or MCL, MCs did not exhibit CD30 on their cell surface. Moreover, we found that in a few patients with ISM, MCs express substantial amounts of CD30 on their cell surface. In addition, we were able to show that neoplastic MCs express substantial amounts of CD30 mRNA in our qPCR experiments, regardless of the variant of SM. Overall, these data are in line with the data published by Morgado et al37 and may have clinical implications. In fact, not all patients with advanced SM may benefit from CD30-targeting antibody treatment because in some of these patients, neoplastic MCs may not express surface CD30. In other words, our data are in favor of testing for CD30 surface expression on neoplastic MCs by flow cytometry before treatment with brentuximab-vedotin is considered.
Several different mechanisms may contribute to the variable expression of CD30 on neoplastic MCs in advanced SM. We therefore explored signaling pathways potentially contributing to CD30 expression. In these experiments, we were able to show that MEK-targeting drugs induce a significant decrease in expression of CD30 in neoplastic MCs. By contrast, inhibitors directed against KIT, PI3K, mTOR, or STAT5 showed only weak or no effects. These data suggest that expression and release of CD30 in neoplastic MCs is regulated by an RAS-MEK–dependent pathway. In this regard, it is noteworthy that RAS-activating mutations and other lesions potentially triggering the RAS-MEK pathway have been described in neoplastic MCs in patients with advanced SM.48,49
In a next step, we explored whether sCD30 is detectable in the serum of our patients with advanced SM. Indeed, we found increased levels of sCD30 in patients with SM compared with healthy controls. Increased sCD30 levels were found in all categories of SM, but not in patients with CM. Moreover, the median sCD30 level was higher in patients with advanced SM compared with ISM. Collectively, these data suggest that neoplastic MCs actively secrete CD30, especially in advanced SM. However, we were unable to show a correlation between sCD30 levels and serum tryptase levels in our patients.
The CD30-targeting antibody-drug conjugate brentuximab-vedotin reportedly inhibits the growth of CD30+ lymphoma cells.50-52 In the current study, we were able to show that brentuximab-vedotin suppresses the growth of primary neoplastic MCs as well as proliferation of CD30+ MC lines, including HMC-1.1 and MCPV-1.1 cells, and the CD30+ canine mastocytoma cell line C2. The concentrations of brentuximab-vedotin required to inhibit proliferation in primary neoplastic MCs and in the CD30+ cell lines correspond well with the drug concentrations that can be reached in patients treated with this drug.53 By contrast, the CD30− cell lines examined showed only a weak response to brentuximab-vedotin or did not respond to this drug. These data show that the antineoplastic effects of brentuximab-vedotin are largely dependent on surface expression of CD30. Corresponding data have been published for malignant lymphomas.54-56 Finally, we confirmed the growth-inhibitory effects of brentuximab-vedotin in an in vivo xenotransplantation assay using NSG mice and CD30+ MCPV-1.1 cells.
We next were interested in the molecular mechanism contributing to the antineoplastic actions of brentuximab-vedotin in MCs. In these experiments, we found that brentuximab-vedotin induces a G2/M cell cycle arrest as well as apoptosis in neoplastic MCs. Again, clear effects of brentuximab-vedotin were only seen in MCs expressing surface CD30, whereas in neoplastic MCs lacking surface CD30, no significant effects of brentuximab-vedotin were demonstrable. The apoptosis-inducing effects of brentuximab-vedotin on MCs were confirmed by staining for Annexin V/PI as well as staining for active caspase-3.
Most patients with ASM or MCL show clinically meaningful and sometimes even complete responses to midostaurin, also known as PKC412.30,57,58 However, responses are usually short-lived and often followed by a relapse.30,57,58 Therefore, research is currently seeking novel potent targeted drugs that can elicit synergistic growth-inhibitory effects when combined with PKC412. In the present study, we found that brentuximab-vedotin and PKC412 synergize with each other in inducing growth inhibition in CD30+ MCPV-1.1 cells. Based on these data it seems tempting to propose a clinical trial exploring antineoplastic effects of the drug combination PKC412 + brentuximab-vedotin in advanced SM.
Patients with SM often suffer from mediator-related symptoms. In these patients, MCs are not only increased in number but are also activated. In many cases, mediator release is triggered by IgE-dependent mechanisms, and the resulting symptoms represent a major clinical challenge.1-6 We were therefore interested to learn whether brentuximab-vedotin modulates histamine secretion from BAs or MCs. In initial safety-validation experiments, we were able to show that brentuximab-vedotin does not induce or promote HR from BAs or MCs, which is relevant clinically as it predicts that the drug will not induce anaphylactic reactions in vivo. Moreover, we found that brentuximab-vedotin counteracts IgE-dependent secretion of histamine in BAs and MCs. These data suggest that brentuximab-vedotin may exert beneficial effects on mediator-related symptoms in patients with SM. In this regard, it is noteworthy that clinical trials using brentuximab-vedotin in advanced SM have recently been initiated.
In summary, our data show that neoplastic MCs often express cell surface CD30 in advanced SM, and that the CD30-targeting antibody brentuximab-vedotin exerts strong antineoplastic effects on surface CD30+ MCs in these patients. In addition, our data show that brentuximab-vedotin synergizes with PKC412 in producing growth inhibition in neoplastic MCs. Whether these effects are clinically relevant and can be demonstrated in vivo in patients with ASM and MCL remains to be determined in clinical trials.
Acknowledgments
The authors thank Tina Bernthaler (University of Veterinary Medicine, Vienna, Austria) for excellent technical assistance.
This work was supported by the Austrian Science Fund (FWF): P 21173-B13, F 4611-B19, and F 4704-B20.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K. Blatt performed key laboratory experiments (proliferation and apoptosis assays, flow cytometry, and cell activation experiments) and wrote parts of the manuscript; S.C.-R. performed ICC and IHC staining; G.E. performed flow cytometry experiments; G.S. performed cell activation and histamine measurements; G.H. and M.M. contributed molecular studies and a vital cell line model (MCPV-1); M.S. and S.K. performed ELISA measurements; E.H., T.R., and M.W. provided mouse studies and a vital cell line model (C2); K. Bauer performed immunostainings of MCs; D.S. performed HR experiments; K.S., J.S., A.R., and H.-P.H. contributed patient material and immunostaining experiments; and P.V. contributed the study design and wrote the manuscript.
Conflict-of-interest disclosure: P.V., A.R., and H.-P.H. are consultants in a global Novartis trial. P.V. received a research grant from Novartis. The remaining authors declare no competing financial interests.
Sabine Cerny-Reiterer died before submission of this manuscript.
Correspondence: Peter Valent, Department of Internal Medicine I, Division of Hematology and Hemostaseology and Ludwig Boltzmann Cluster Oncology, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: peter.valent@meduniwien.ac.at.
References
- 1.Escribano L, Akin C, Castells M, Orfao A, Metcalfe DD. Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol. 2002;81(12):677–690. doi: 10.1007/s00277-002-0575-z. [DOI] [PubMed] [Google Scholar]
- 2.Valent P, Akin C, Sperr WR, et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol. 2003;122(5):695–717. doi: 10.1046/j.1365-2141.2003.04575.x. [DOI] [PubMed] [Google Scholar]
- 3.Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–432. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- 4.Valent P, Sperr WR, Schwartz LB, Horny HP. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114(1):3–11. doi: 10.1016/j.jaci.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112(4):946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3(4):497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 7.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25(7):603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 8.Valent P, Horny HP, Li CY, et al. Mastocytosis (mast cell disease). Pathology and genetics. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 1. Lyon, France: IARC Press; 2001:291-302. [Google Scholar]
- 9.Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435–453. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 10.Horny HP, Akin C, Metclafe DD, et al. Mastocytosis (mast cell disease). Pathology and genetics. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 2. Lyon, France: IARC Press; 2008:54-63. [Google Scholar]
- 11.Sperr WR, Escribano L, Jordan JH, et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001;25(7):529–536. doi: 10.1016/s0145-2126(01)00041-8. [DOI] [PubMed] [Google Scholar]
- 12.Valent P, Akin C, Sperr WR, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27(7):635–641. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 13.Lim KH, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727–5736. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 14.Pardanani A, Lim KH, Lasho TL, et al. Prognostically relevant breakdown of 123 patients with systemic mastocytosis associated with other myeloid malignancies. Blood. 2009;114(18):3769–3772. doi: 10.1182/blood-2009-05-220145. [DOI] [PubMed] [Google Scholar]
- 15.Travis WD, Li CY, Hoagland HC, Travis LB, Banks PM. Mast cell leukemia: report of a case and review of the literature. Mayo Clin Proc. 1986;61(12):957–966. doi: 10.1016/s0025-6196(12)62636-6. [DOI] [PubMed] [Google Scholar]
- 16.Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121(8):1285–1295. doi: 10.1182/blood-2012-07-442400. [DOI] [PubMed] [Google Scholar]
- 17.Tefferi A, Pardanani A. Clinical, genetic, and therapeutic insights into systemic mast cell disease. Curr Opin Hematol. 2004;11(1):58–64. doi: 10.1097/00062752-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Valent P, Ghannadan M, Akin C, et al. On the way to targeted therapy of mast cell neoplasms: identification of molecular targets in neoplastic mast cells and evaluation of arising treatment concepts. Eur J Clin Invest. 2004;34(suppl 2):41–52. doi: 10.1111/j.0960-135X.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 19.Gleixner KV, Mayerhofer M, Cerny-Reiterer S, et al. KIT-D816V-independent oncogenic signaling in neoplastic cells in systemic mastocytosis: role of Lyn and Btk activation and disruption by dasatinib and bosutinib. Blood. 2011;118(7):1885–1898. doi: 10.1182/blood-2010-06-289959. [DOI] [PubMed] [Google Scholar]
- 20.Valent P. Mastocytosis: a paradigmatic example of a rare disease with complex biology and pathology. Am J Cancer Res. 2013;3(2):159–172. [PMC free article] [PubMed] [Google Scholar]
- 21.Nagata H, Worobec AS, Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA. 1995;92(23):10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longley BJ, Tyrrell L, Lu SZ, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12(3):312–314. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 23.Fritsche-Polanz R, Jordan JH, Feix A, et al. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol. 2001;113(2):357–364. doi: 10.1046/j.1365-2141.2001.02783.x. [DOI] [PubMed] [Google Scholar]
- 24.Féger F, Ribadeau Dumas A, Leriche L, Valent P, Arock M. Kit and c-kit mutations in mastocytosis: a short overview with special reference to novel molecular and diagnostic concepts. Int Arch Allergy Immunol. 2002;127(2):110–114. doi: 10.1159/000048179. [DOI] [PubMed] [Google Scholar]
- 25.Furitsu T, Tsujimura T, Tono T, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993;92(4):1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Growney JD, Clark JJ, Adelsperger J, et al. Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412. Blood. 2005;106(2):721–724. doi: 10.1182/blood-2004-12-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleixner KV, Mayerhofer M, Aichberger KJ, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107(2):752–759. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 28.Schittenhelm MM, Shiraga S, Schroeder A, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66(1):473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 29.Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108(1):286–291. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- 30.Gotlib J, Berubé C, Growney JD, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106(8):2865–2870. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ustun C, DeRemer DL, Akin C. Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk Res. 2011;35(9):1143–1152. doi: 10.1016/j.leukres.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Gleixner KV, Mayerhofer M, Sonneck K, et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V-mutated oncogenic variant of KIT. Haematologica. 2007;92(11):1451–1459. doi: 10.3324/haematol.11339. [DOI] [PubMed] [Google Scholar]
- 33.Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848–858. [PubMed] [Google Scholar]
- 34.Chiarle R, Podda A, Prolla G, Gong J, Thorbecke GJ, Inghirami G. CD30 in normal and neoplastic cells. Clin Immunol. 1999;90(2):157–164. doi: 10.1006/clim.1998.4636. [DOI] [PubMed] [Google Scholar]
- 35.Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24(4):585–595. doi: 10.1038/modpathol.2010.224. [DOI] [PubMed] [Google Scholar]
- 36.Valent P, Sotlar K, Horny HP. Aberrant expression of CD30 in aggressive systemic mastocytosis and mast cell leukemia: a differential diagnosis to consider in aggressive hematopoietic CD30-positive neoplasms. Leuk Lymphoma. 2011;52(5):740–744. doi: 10.3109/10428194.2010.550072. [DOI] [PubMed] [Google Scholar]
- 37.Morgado JM, Perbellini O, Johnson RC, et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology. 2013;63(6):780–787. doi: 10.1111/his.12221. [DOI] [PubMed] [Google Scholar]
- 38.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12(4):345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 39.Hoermann G, Blatt K, Greiner G, et al. CD52 is a molecular target in advanced systemic mastocytosis. FASEB J. 2014;28(8):3540–3551. doi: 10.1096/fj.14-250894. [DOI] [PubMed] [Google Scholar]
- 40.DeVinney R, Gold WM. Establishment of two dog mastocytoma cell lines in continuous culture. Am J Respir Cell Mol Biol. 1990;3(5):413–420. doi: 10.1165/ajrcmb/3.5.413. [DOI] [PubMed] [Google Scholar]
- 41.Herrmann H, Kneidinger M, Cerny-Reiterer S, et al. The Hsp32 inhibitors SMA-ZnPP and PEG-ZnPP exert major growth-inhibitory effects on D34+/CD38+ and CD34+/CD38- AML progenitor cells. Curr Cancer Drug Targets. 2012;12(1):51–63. doi: 10.2174/156800912798888992. [DOI] [PubMed] [Google Scholar]
- 42.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 43.van Anrooij B, Kluin PM, Oude Elberink JN, Kluin-Nelemans JC. CD30 in systemic mastocytosis. Immunol Allergy Clin North Am. 2014;34(2):341–355. doi: 10.1016/j.iac.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Horny HP, Sotlar K, Valent P. Mastocytosis: immunophenotypical features of the transformed mast cells are unique among hematopoietic cells. Immunol Allergy Clin North Am. 2014;34(2):315–321. doi: 10.1016/j.iac.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arredondo AR, Jennings CD, Shier L, Sundram U, Gotlib J, George TI. CD30 expression in mastocytosis [abstract]. Lab Invest. 2011;91(1s):285A–286A. Abstract 1215. [Google Scholar]
- 46.Moonim MT, Kossier T, van Der Walt J, Wilkins B, Harrison CN, Radia DH. CD30/CD123 expression in systemic mastocytosis does not correlate with aggressive disease [abstract]. Blood. 2012;120(21) Abstract 1746. [Google Scholar]
- 47.Chiu A, Orazi A. Mastocytosis and related disorders. Semin Diagn Pathol. 2012;29(1):19–30. doi: 10.1053/j.semdp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Wilson TM, Maric I, Simakova O, et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011;96(3):459–463. doi: 10.3324/haematol.2010.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwaab J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122(14):2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 50.Newland AM, Li JX, Wasco LE, Aziz MT, Lowe DK. Brentuximab vedotin: a CD30-directed antibody-cytotoxic drug conjugate. Pharmacotherapy. 2013;33(1):93–104. doi: 10.1002/phar.1170. [DOI] [PubMed] [Google Scholar]
- 51.Bhatt S, Ashlock BM, Natkunam Y, et al. CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood. 2013;122(7):1233–1242. doi: 10.1182/blood-2013-01-481713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okeley NM, Miyamoto JB, Zhang X, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010;16(3):888–897. doi: 10.1158/1078-0432.CCR-09-2069. [DOI] [PubMed] [Google Scholar]
- 53.Ogura M, Tobinai K, Hatake K, et al. Phase I / II study of brentuximab vedotin in Japanese patients with relapsed or refractory CD30-positive Hodgkin’s lymphoma or systemic anaplastic large-cell lymphoma. Cancer Sci. 2014;105(7):840–846. doi: 10.1111/cas.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ansell SM. Brentuximab vedotin. Blood. 2014;124(22):3197–3200. doi: 10.1182/blood-2014-06-537514. [DOI] [PubMed] [Google Scholar]
- 55.Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125(9):1394–1402. doi: 10.1182/blood-2014-09-598763. [DOI] [PubMed] [Google Scholar]
- 56.Kumar A, Younes A. Role of CD30 targeting in malignant lymphoma. Curr Treat Options Oncol. 2014;15(2):210–225. doi: 10.1007/s11864-014-0275-7. [DOI] [PubMed] [Google Scholar]
- 57.Gotlib J, Kluin-Nelemans HC, George TI, et al. KIT inhibitor midostaurin in patients with advanced systemic mastocytosis: results of a planned interim analysis of the global CPKC412D2201 trial [abstract]. Blood. 2012;120(21) Abstract 799. [Google Scholar]
- 58.Gotlib J, Kluin-Nelemans HC, George TI, et al. Durable responses and improved quality of life with midostaurin (PKC412) in advanced systemic mastocytosis (SM): updated stage 1 results of the global D2201 trial [abstract]. Blood. 2013;122(21) Abstract 106. [Google Scholar]