Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
The major subpopulation of platelets involved in thrombus development form via regulated necrosis involving cyclophilin D.
Necrotic platelets may be targeted independent of platelet activation.
Abstract
A subpopulation of platelets fulfills a procoagulant role in hemostasis and thrombosis by enabling the thrombin burst required for fibrin formation and clot stability at the site of vascular injury. Excess procoagulant activity is linked with pathological thrombosis. The identity of the procoagulant platelet has been elusive. The cell death marker 4-[N-(S-glutathionylacetyl)amino]phenylarsonous acid (GSAO) rapidly enters a subpopulation of agonist-stimulated platelets via an organic anion-transporting polypeptide and is retained in the cytosol through covalent reaction with protein dithiols. Labeling with GSAO, together with exposure of P-selectin, distinguishes necrotic from apoptotic platelets and correlates with procoagulant potential. GSAO+ platelets form in occluding murine thrombi after ferric chloride injury and are attenuated with megakaryocyte-directed deletion of the cyclophilin D gene. These platelets form a procoagulant surface, supporting fibrin formation, and reduction in GSAO+ platelets is associated with reduction in platelet thrombus size and fibrin formation. Analysis of platelets from human subjects receiving aspirin therapy indicates that these procoagulant platelets form despite aspirin therapy, but are attenuated by inhibition of the necrosis pathway. These findings indicate that the major subpopulation of platelets involved in fibrin formation are formed via regulated necrosis involving cyclophilin D, and that they may be targeted independent of platelet activation.
Introduction
Activated platelets have a dual role in hemostasis and thrombosis. They aggregate to form the platelet plug and also provide the surface for the assembly of the coagulation factors. There is increasing evidence that these roles are performed by separate subpopulations of platelets within the thrombus.1 In particular, a subset of activated platelets, which have been termed “procoagulant platelets,” have distinct properties, including the ability to support thrombin generation.2 Procoagulant platelets are able to assemble the soluble coagulation factors on the membrane surface into the tenase (FIXa/FVIIIa/FX) and prothrombinase (FXa/FVa/FII) complexes to generate the thrombin burst required for fibrin formation and thrombus stability. It is theorized that these platelets form the link between the unstable activated platelet aggregate and the coagulation system-mediated stabilization of the thrombus; however, the exact nature of this population remains to be defined. Procoagulant platelets are characterized by a sustained rise in intracellular calcium and phosphatidylserine exposure on the outer membrane. Several reports indicate that there is imperfect correlation between phosphatidylserine exposure and presence of coagulation factors on the platelet surface. Thus, although phosphatidylserine is necessary for procoagulant activity, phosphatidylserine exposure alone is insufficient to define the procoagulant platelet.3-5 Moreover, even after activation with strong agonists, only some platelets will expose phosphatidylserine, and only a subset of these are procoagulant.
Excess procoagulant activity can tip the balance from physiological hemostasis to pathological thrombosis, which means that platelet procoagulant activity is a profoundly important concept. Elevated levels of “coated” platelets, defined as platelets with high levels of retained procoagulant proteins, have been demonstrated in patients with ischemic stroke and recurrent ischemic stroke, whereas low levels correlate with hemorrhagic complications of stroke.6 Identifying and understanding this subset is particularly important. It has been differentially proposed that the procoagulant platelet is formed via activation of apoptosis pathways,7 or necrosis pathways through cyclophilin D-dependent mitochondrial permeability transition pore formation.8 Most studies concur that combination thrombin and collagen stimulation is required for maximal generation of the procoagulant phenotype.5,9 The study of the physiological and pathological role of the procoagulant platelet in vivo, however, has been thwarted by the lack of a suitable marker.10-12 Use of annexin V or lactadherin to study phosphatidylserine-positive platelets is problematic, as only a subset of platelets elaborating phosphatidylserine are procoagulant, and the labeling probes are inhibitory to the process being studied in the doses required for in vivo imaging.13,14
A key feature of procoagulant platelets is that their morphological features, as seen under phase and electron microscopy, resemble features of “necrosis” in mammalian nucleated cells. For example, there is rearrangement of the membrane phospholipid symmetry with externalization of phosphatidylserine, microvesiculation, ballooning of the cell with cytoskeletal disruption, and loss of membrane integrity. Traditional markers of loss of membrane integrity that bind to nuclear material such as propidium iodide or Sytox dyes cannot be used in the anucleate platelet. Thus, we investigated the use of a cell death imaging agent that complexes with cytoplasmic ligands, 4-[N-(S-glutathionylacetyl)amino] phenylarsonous acid (GSAO),15-17 to determine whether we could identify the procoagulant platelet in vitro and in vivo. Using GSAO, we show that necrotic platelets are generated within a few seconds of agonist stimulation in vitro and are procoagulant. They are also generated in vivo during formation of occlusive murine thrombi and provide a procoagulant surface. In addition, analysis of platelets from human subjects receiving aspirin indicates that procoagulant necrotic platelets form despite aspirin therapy, but are attenuated by inhibition of the cyclophilin D-dependent necrosis pathway.
Methods
GSAO labeling of platelets
Whole blood was collected from healthy volunteers into 1:9 volume of 4% citrate and then dispensed into 20% volume of citrate-dextrose solution (Sigma-Aldrich). Platelet-rich plasma was isolated after brake-free centrifugation for 20 min at 260g and left to rest for 10 minutes at room temperature on a slow rocker. Platelets were pelleted at 930g for 10 minutes, platelet-poor plasma was removed, and the pellet was gently resuspended into phosphate-buffered saline containing 20% volume acid citrate dextrose and 1.5 µM prostaglandin E1 (Sigma-Aldrich). Platelets were washed once and resuspended into modified N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)/Tyrodes buffer (10 mM HEPES at pH 7.5 buffer containing 0.14 M NaCl, 1 mM MgCl2, and 5 mM glucose). Research was carried out according to the principles of the Declaration of Helsinki and approved by the institution’s ethics review board.
Washed human platelets were resuspended at 5 × 107/mL in modified HEPES/Tyrodes buffer supplemented with 2.5 mM CaCl2. Platelets were left unstimulated or stimulated with ionomycin (10 µM; Sigma-Aldrich), α-thrombin (0.1 U/mL; Sigma-Aldrich), collagen (0-10 µg/mL; Optigen Scientific), collagen-related peptide (0-10 µg/mL; Sigma-Aldrich), or thrombin (0.1 U/mL) plus collagen (5 µg/mL) for 10 min at 37°C. On some occasions, platelets were incubated with the cyclophilin D inhibitor cyclosporine A (2 µM; Sigma-Aldrich) for 15 minutes before agonist stimulation. On other occasions, platelets were incubated with the pancaspase inhibitor ZVAD FMK (200 μM; Promega) for 1 h before stimulation with ABT-737 (30 μM) for 2 h at 37°C. Platelets were washed once after agonist stimulation, incubated with phycoerythrin-labeled anti-CD62P monoclonal antibody (5% vol/vol; BD Biosciences) and GSAO-AF647 or control GSCA-AF647 (1 µM) for 30 min at room temperature, washed once, and analyzed immediately by flow cytometry on a BD Fortessa LSR. On some occasions, thrombin/collagen-stimulated platelets were incubated with 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS; 0-200 µM; Sigma-Aldrich) for 10 min at 37°C before labeling. GSAO and GSCA were produced and conjugated to Alexa Fluor 647, as described previously.15,16,18 On some occasions, washed platelets were also labeled with annexin V-Pacific Blue (5% vol/vol; Life Technologies).
Methods for measurement of platelet mitochondrial membrane potential, platelet surface FXa, platelet procoagulant potential, imaging and analysis of murine thrombi, aspirin study participants, and protocol and statistical analysis are found in the supplemental Methods, available on the Blood Web site.
Results
Rationale for the platelet necrosis marker
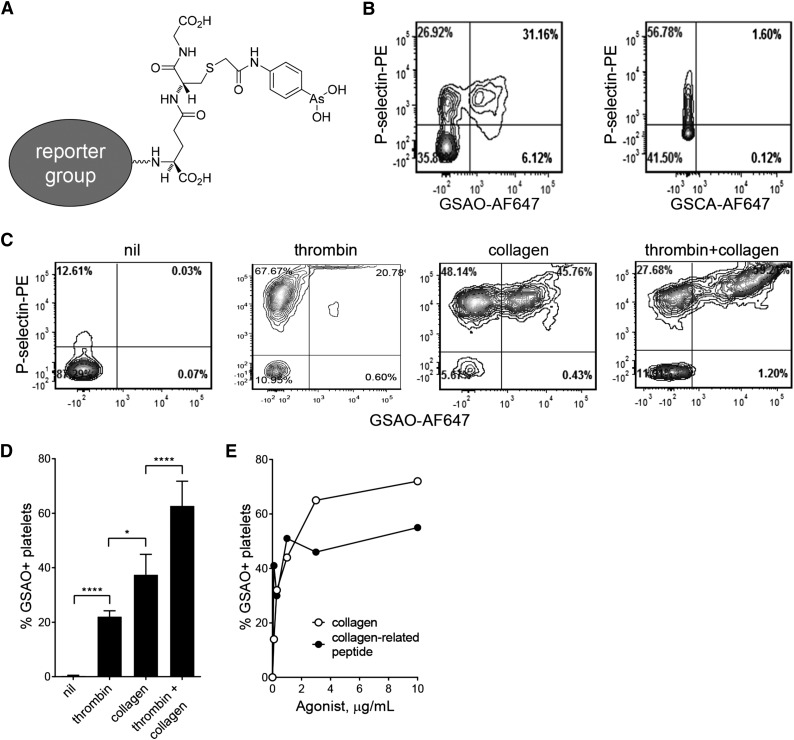
GSAO is a tripeptide trivalent arsenical that has recently been characterized as an imaging agent for necrotic and late apoptotic nucleated cells15-17 (Figure 1A). When tagged with a reporter compound at the γ-glutamyl residue of the glutathione moiety, the compound is unable to cross the plasma membrane of viable cells. However, when plasma membrane permeability changes in necrosis or late apoptosis, GSAO enters the cell and is retained in the cytoplasm by covalent bonding to proteins containing closely spaced cysteine thiols.15 The intracellular protein targets for trivalent arsenicals vastly outnumber the extracellular targets. For instance, the intensity of GSAO labeling of dying/dead nucleated cells is at least 1000-fold higher than labeling of viable cells.15 We reasoned that GSAO might also selectively label the anucleate necrotic/apoptotic platelet.
Figure 1.
GSAO labels a subpopulation of activated platelets. (A) Structure of GSAO and site of conjugation of AF647, Oregon Green, or biotin. (B) Washed human platelets were stimulated with calcium ionophore (1 µM) and activation and plasma membrane compromise measured by flow cytometry. Activated platelets were detected from surface elaboration of P-selectin and plasma membrane compromise by labeling with GSAO-AF647 or negative control GSCA-AF647. (C) Washed human platelets were left unstimulated or stimulated with thrombin (0.1 U/mL), collagen (5 µg/mL), or thrombin (0.1 U/mL) and collagen (5 µg/mL), and exposure of P-selectin and labeling with GSAO-AF647 were measured by flow cytometry. (D) Platelet stimulation with both thrombin and collagen results in significantly more labeling with GSAO-AF647 than with either agonist alone. (E) Washed human platelets were stimulated with collagen or collagen-related peptide, and labeling with GSAO-AF647 was measured by flow cytometry.
GSAO marking of dying/dead nucleated cells is compatible with a number of different reporter groups, including hydrophilic fluorophores, a biotin label, or radioisotopes.15-17 We employed conjugates of GSAO with AF647, AF546, or Oregon Green in this study. A GSAO control compound, GSCA [4-(N-[(S-glutathionyl) acetyl]amino)benzoic acid], contains an inert carboxylic acid group in place of the chemically reactive As(III) in GSAO. GSCA has the same biodistribution as GSAO but does not react with proteins, so washes out of cells.
GSAO labels a subpopulation of activated platelets
Calcium ionophore treatment of platelets leads to a sustained rise in intracellular calcium and surface elaboration of phosphatidylserine, which are features of both the procoagulant and necrotic phenotype.19 Washed human platelets were treated with calcium ionophore and labeled with GSAO-AF647 and an antibody against the α-granule marker, P-selectin. GSAO-AF647 was consistently retained in a subpopulation of platelets that coexpress P-selectin (Figure 1B). Resting platelets not exposed to ionophore did not label with GSAO-AF647. The control compound, GSCA-AF647, showed no labeling of ionophore-treated platelets.
Dual stimulation with thrombin and collagen is known to be superior to either agonist alone in the generation of the procoagulant platelet.7,8,20 To determine whether GSAO labeling reflects this agonist profile, washed human platelets were stimulated with thrombin, collagen, or both thrombin and collagen. Thrombin generated minimal GSAO+ platelets, collagen stimulation resulted in a moderate proportion, and simultaneous stimulation with thrombin and collagen resulted in a marked increase in GSAO+ platelets (Figure 1C-D). To determine whether signaling through the collagen receptor, GPVI, was sufficient to generate the GSAO+ phenotype, we compared the maximal proportion of GSAO+ platelets generated by collagen compared with the GPVI-specific activator, collagen-related peptide. The majority of the collagen effect was recapitulated with the peptide (Figure 1E).
GSAO rapidly enters the activated platelet subpopulation via an organic anion-transporting polypeptide
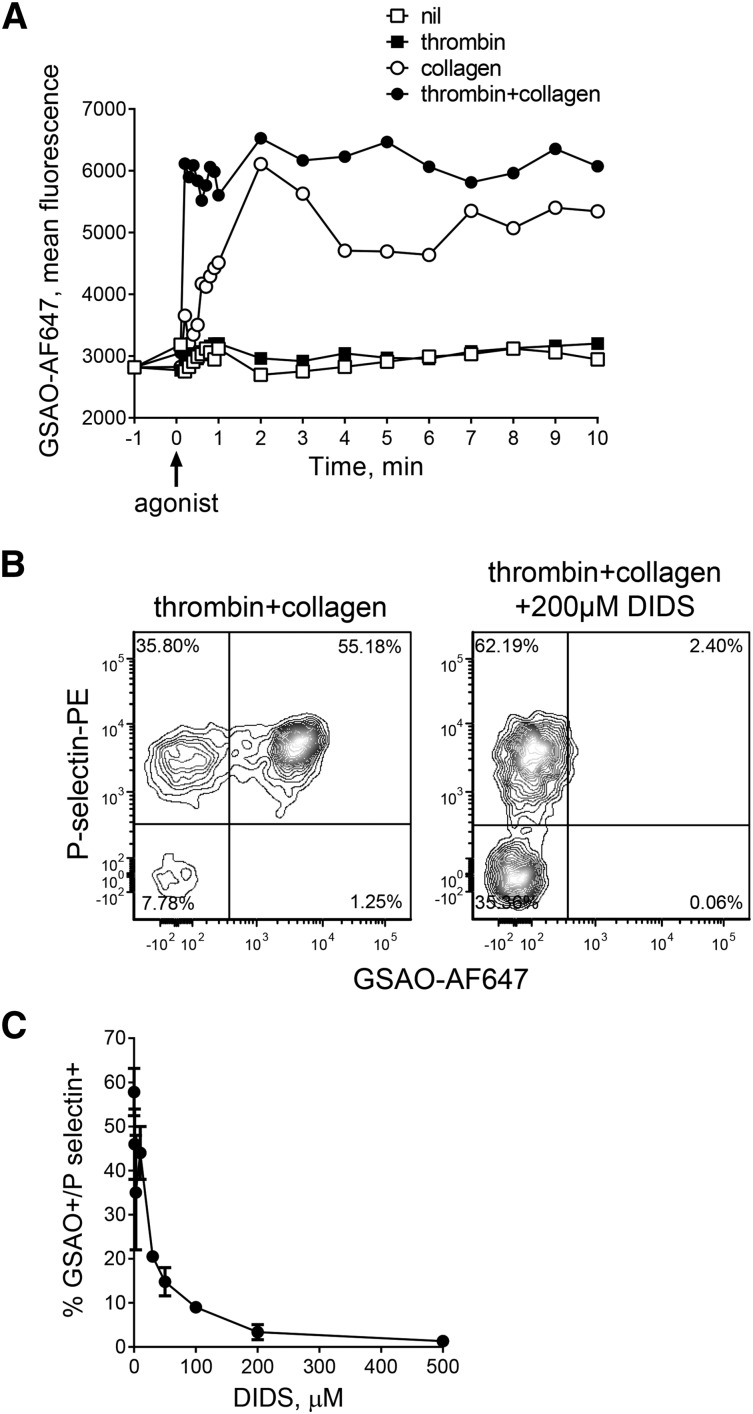
The rate of GSAO+ platelet generation after agonist exposure was measured using time-lapse flow cytometry. Results are reported for GSAO+ events based on lead in time before agonist exposure threshold. Resting platelets showed no increase in GSAO labeling over the course of 10 minutes. Platelets exposed to thrombin demonstrate little GSAO labeling compared with unstimulated platelets. Collagen alone resulted in a gradual increase in GSAO labeling over the course of 60 seconds, whereas combination thrombin and collagen stimulation resulted in a rapid initial rise in GSAO labeling that peaked in 12 seconds and was sustained during the 10 minutes of the experiment (Figure 2A).
Figure 2.
GSAO rapidly enters the activated platelet subpopulation via an organic anion-transporting polypeptide. (A) The kinetics of labeling of stimulated platelets with GSAO-AF647 was measured by time-lapse flow cytometry. Flow plots are representative of n ≥ 3 separate experiments, and bars and data points are from n ≥ 3 separate experiments. *P < .05; ****P < .0001. (B-C) Washed human platelets were stimulated with thrombin (0.1 U/mL) and collagen (5 µg/mL), incubated with 0 to 200 µM DIDS for 10 minutes, and then exposure of P-selectin and labeling with GSAO-AF647 were measured by flow cytometry. DIDS inhibited labeling of P-selectin+ activated platelets at a half-maximal concentration of ∼30 µM. The data points and errors are the mean ± range of 1 to 5 experiments.
The organic anion-transporting polypeptide (OATP) family has been implicated in transport across the plasma membrane of organic anions in the same class as GSAO.21,22 Nine members of the OATP family have been reported in humans,23 and platelets express OATP2B1.24 DIDS is an inhibitor of OATP class B transporters,25 so we tested its effect on GSAO labeling of agonist-stimulated platelets. DIDS inhibited GSAO labeling of P-selectin+-activated platelets (Figure 2B) at a half-maximal concentration of ∼30 µM (Figure 2C). GSAO is retained in the platelet cytoplasm through covalent reaction with closely spaced protein cysteine thiols15 (data not shown).
Labeling with GSAO and exposure of P-selectin distinguishes necrotic from apoptotic platelets
Bcl-XL-dependent apoptosis is involved in regulation of platelet life span, although the role of platelet apoptosis in thrombosis is controversial. To determine whether GSAO can differentiate agonist-induced necrotic vs apoptotic platelets, platelet apoptosis was triggered using the BH3 mimetic ABT-737. GSAO labeled P-selectin-negative apoptotic platelets (Figure 3A-B). The GSAO+ population also labeled with a polycaspase marker and annexin V, both indicators of apoptotic cells. The pancaspase inhibitor, ZVADFMK, reduced ABT-737-induced GSAO+/P-selectin− platelets but had no effect on GSAO labeling of thrombin and collagen-stimulated platelets (Figure 3C), confirming that the agonist-induced platelets are not undergoing apoptotic cell death. These results indicate that apoptotic platelets are GSAO+/P-selectin−, whereas necrotic platelets are GSAO+/P-selectin+ (supplemental Table 1). Washed platelets from healthy donors were untreated or stimulated with thrombin, collagen, or thrombin and collagen and examined for apoptosis (GSAO+/P-selectin−) and necrosis (GSAO+/P-selectin+) (Figure 3D). These agonists trigger platelet necrosis, but not apoptosis.
Figure 3.
Labeling with GSAO and exposure of P-selectin distinguishes necrotic from apoptotic platelets. (A) Washed human platelets were incubated with 30 µM of the BH3 mimetic ABT-737 for 2 hours, and exposure of P-selectin and labeling with GSAO-AF647, control GSCA-AF647, polycaspase-FAM, or annexin V-PB were measured by flow cytometry. (B) Washed human platelets were incubated with increasing concentrations of ABT-737 for 2 hours, and labeling with GSAO-AF647 and exposure of P-selection were measured by flow cytometry. The percentage of GSAO+ platelets are graphed with respect to P-selectin exposure. (C) Washed human platelets were preincubated with 200 µM ZVADFMK or vehicle control for 15 minutes and then incubated with 30 µM ABT-737 for 2 hours (part F), or stimulated with thrombin (0.1 U/mL) and collagen (5 µg/mL) for 10 minutes (part G). Labeling with GSAO-AF647 and exposure of P-selection were measured by flow cytometry. (D) Washed human platelets from healthy donors (n = 5-11) were untreated or stimulated with thrombin (0.1 U/mL), collagen (5 µg/mL), or thrombin (0.1 U/mL) and collagen (5 µg/mL) for 10 min. Labeling with GSAO-AF647 and exposure of P-selection was measured by flow cytometry, and the results were expressed as GSAO+ platelets that are P-selectin− or P-selection+. The error bars are mean ± standard deviation.
GSAO marks platelets undergoing cyclophilin D-dependent necrosis
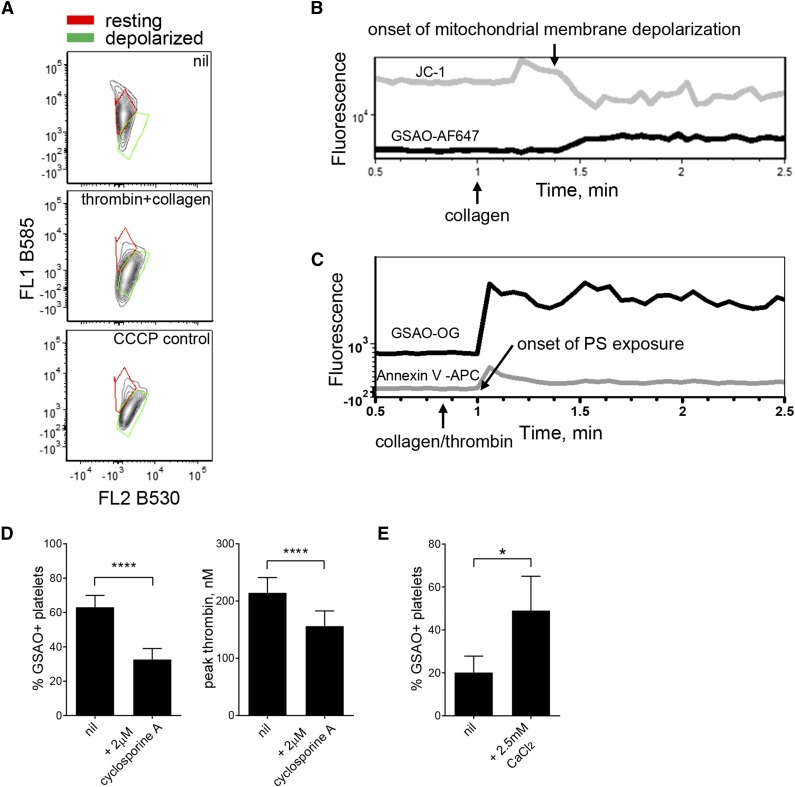
The cyclophilin D-dependent regulated necrosis pathway is characterized by agonist stimulation, raised intracellular calcium, formation of the mitochondrial transition pore, and loss of mitochondrial membrane potential. Dual agonist stimulation of platelets results in loss of mitochondrial transmembrane potential (Figure 4A). The onset of mitochondrial depolarization (Figure 4B) and phosphatidylserine exposure (Figure 4C) are coincident with GSAO labeling. Direct mitochondrial membrane depolarization using m-chlorophenylhydrazone does not result in GSAO labeling, indicating that GSAO entry into necrotic platelets is not triggered by direct mitochondrial perturbation (supplemental Figure 1).
Figure 4.
GSAO marks platelets undergoing cyclophilin D-dependent necrosis. (A) Washed human platelets were untreated or stimulated with thrombin (0.1 U/mL) and collagen (5 µg/mL) or the mitochondrial membrane disrupter m-chlorophenylhydrazone (4 μM). Mitochondrial transmembrane potential was measured using the cationic dye, JC-1. The ratio of distribution of JC-1 between the mitochondria (red fluorescence) and cytosol (green fluorescence) reflects mitochondrial transmembrane potential. Results are representative of n ≥ 3 separate experiments. (B-C) The correlation between loss of platelet mitochondrial transmembrane potential (JC-1 loss of B585 fluorescence) or phosphatidylserine exposure (annexin V labeling) and labeling with GSAO-AF647 after collagen (5 µg/mL) or thrombin (0.1 U/mL) and collagen (5 µg/mL) stimulation was measured by time-lapse flow cytometry. (D) Washed human platelets were untreated or preincubated with the cyclophilin D inhibitor, cyclosporine A (CysA, 2 μM), for 15 minutes before stimulation with thrombin (0.1 U/mL) and collagen (5 µg/mL). Labeling with GSAO-AF647 was measured by flow cytometry and procoagulant potential assessed using the Calibrated Automated Thrombogram (n = 3-6; ****P < .0001). (E) Washed human platelets were preincubated with CaCl2 for 15 minutes and then untreated or stimulated with thrombin (0.1 U/mL) and collagen (5 µg/mL). Labeling with GSAO-AF647 was measured by flow cytometry (n = 8; *P < .05).
Cyclophilin D is involved in formation of the mitochondrial permeability transition pore, and inhibition of this protein blunts pore formation and necrosis.26 Inhibiting cyclophilin D with cyclosporine A reduced GSAO labeling and procoagulant potential of agonist-stimulated platelets (Figure 4D). Increases in cytosolic calcium levels are an important event in cell necrosis, leading to a chain of molecular events resulting in bioenergetics failure.27 As anticipated, addition of calcium promoted GSAO+ agonist-stimulated platelets (Figure 5F).
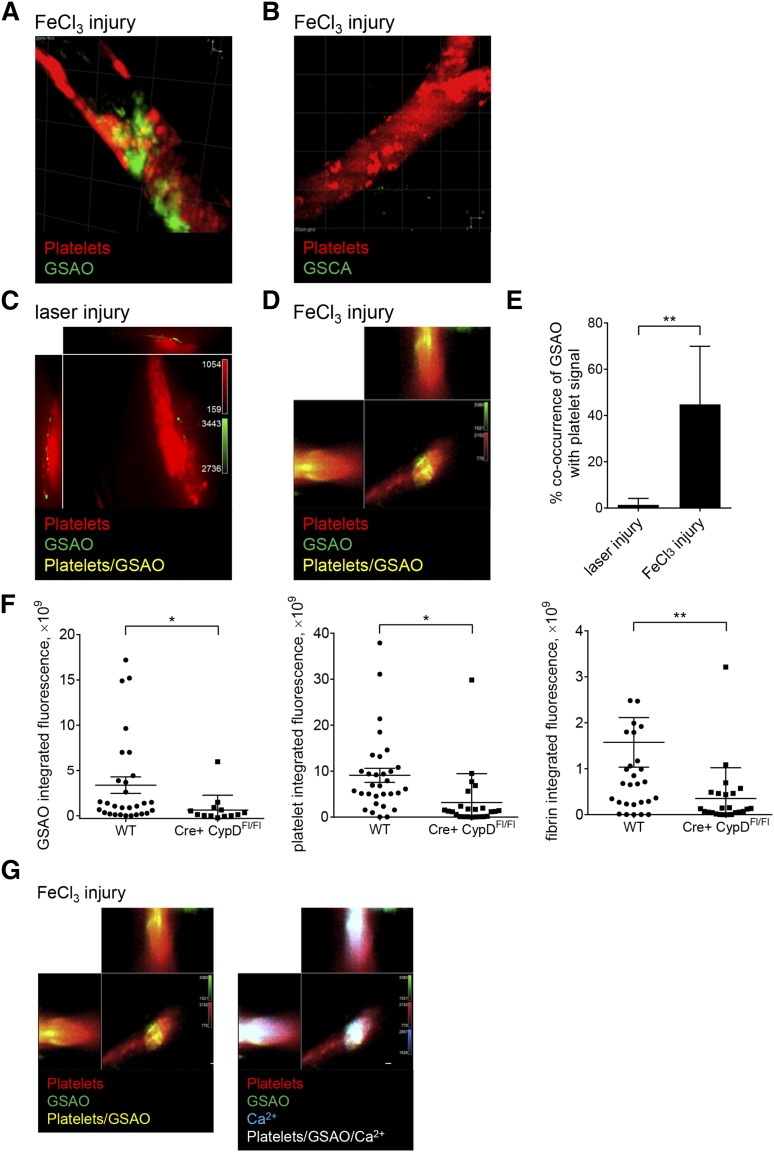
Figure 5.
GSAO+ platelets form in occluding murine thrombi and are attenuated with megakaryocyte-directed deletion of the cyclophilin D gene. (A-B) Dylight 649-conjugated antiplatelet CD42b antibody and GSAO-Oregon Green or control compound GSCA-Oregon green were injected into the murine circulation and thrombus initiated in the cremaster muscle arterioles by FeCl3 injury. Thrombi were captured by confocal intravital video microscopy, and images are displayed as 3-dimensional reconstructions (maximum intensity projection mode). Occlusive thrombi incorporate GSAO-Oregon green, but not the control compound. (C-E) Dylight 649-conjugated antiplatelet CD42b and GSAO-Oregon Green were injected into the murine circulation and thrombus initiated in the cremaster muscle arterioles by either laser (C) or FeCl3 (D) injury. Thrombi were captured by confocal intravital video microscopy and images displayed as cross-sectional orthogonal views for single-plane colocalization. There was minimal GSAO signal in the nonocclusive thrombus initiated by laser injury, but extensive signal in the occlusive thrombus initiated by FeCl3 injury (C; n = 6-8 in 6-8 different mice; Manders correlation coefficient, **P < .01). (F) Dylight 649-conjugated anti-platelet CD42b antibody, GSAO-AF546, and AF488-conjugated anti-fibrin antibody were injected into the murine circulation of wild-type (WT) or platelet-specific cyclophilin D-deficient (PF4Cre+ CypDFl/Fl) mice and thrombus initiated in the cremaster muscle arterioles by FeCl3 injury. Thrombi were captured by confocal intravital video microscopy and images analyzed for integrated GSAO, platelet, and fibrin fluorescence (WT n = 4, 29 thrombi; PF4Cre+ CypDFl/Fl n = 3, 15 thrombi; *P < .05; **P < .001). (G) Dylight 649-conjugated anti-platelet CD42b antibody, GSAO-Oregon Green, and calcium-sensing dye rhodamine 2 were injected into the murine circulation and thrombus initiated in the cremaster muscle arterioles by FeCl3 injury. Thrombus was visualized 10 minutes after injury. Images are represented as cross-sectional orthogonal views of single confocal plane displaying platelets and GSAO (left) with rhodamine signal (right). Persistence of high calcium signal is demonstrated in the GSAO+ platelets consistent with necrotic cell death.
GSAO+ platelets form in occluding murine thrombi and are attenuated with megakaryocyte-directed deletion of the cyclophilin D gene
It is important that GSAO not interfere with thrombus formation for it to be used as an in vivo marker of procoagulant necrotic platelets. GSAO-AF647 or unconjugated GSAO does not trigger platelet apoptosis or necrosis (supplemental Figure 2). GSAO-Oregon Green (1 µM) also had no effect on in vitro platelet aggregation in response to collagen, thrombin, adenosine 5′-diphosphate, or epinephrine, and there was no effect on coagulation in a 1-step clotting assay (data not shown). GSAO-AF750 (0.1 µg/g mouse) had no effect on platelet accumulation or fibrin formation in the murine laser injury model of thrombosis (data not shown).
Two methods of initiation of thrombosis in the murine cremaster arteriolar circulation were compared and contrasted to explore the functional relevance of GSAO+ platelets in vivo: the endothelial stimulation laser injury model that is primarily dependent on thrombin for thrombus formation and not dependent on extravascular collagen exposure,28,29 and the FeCl3 chemical injury model.30 Dylight-conjugated antiplatelet CD42b antibody, GSAO-Oregon Green, GSAO-AF546, GSAO-AF647, or appropriately labeled control compound GSCA were introduced to the murine circulation, and arterioles were injured and thrombi imaged within 10 to 30 minutes. Thrombus formation was imaged in real time by 3 laser confocal intravital microscopy. Two-dimensional orthogonal sections and 3-dimensional high resolution images were reconstructed. GSAO-AF750 is detectable in the murine circulation up to 3 hours after intravenous injection and persists within necrotic lesions for at least 6 hours,17 so thrombi are imaged well before GSAO is cleared from the circulation. A 3000-Da dextran tracer has been shown to permeate the shell and core of laser-induced platelet thrombi.31,32 GSAO-fluorophore has a molecular mass of ∼1500 Da and is expected to readily diffuse within thrombi.
GSAO-Oregon Green labeled a subset of platelets within thrombi induced by FeCl3 injury (Figure 5A), whereas control GSCA-Oregon Green was not retained (Figure 5B). There were minimal GSAO+ platelets in the nonoccluding thrombi induced by laser injury (Figure 5C). Approximately 1% of the platelet signal in the laser injury model co-occurred with the GSAO signal compared with 44% in the occlusive platelet aggregates of the FeCl3 injury model (range, 11%-80%) (Figure 5D-E). There were reduced GSAO+ platelets in thrombi initiated by FeCl3 injury in cyclophilin D-deficient mice, which associated with reduced platelets and fibrin (Figure 5F; supplemental Figure 3). GSAO+ platelets were also characterized by high intracellular calcium levels, which is consistent with necrosis (Figure 5G).
GSAO marks functionally procoagulant platelets
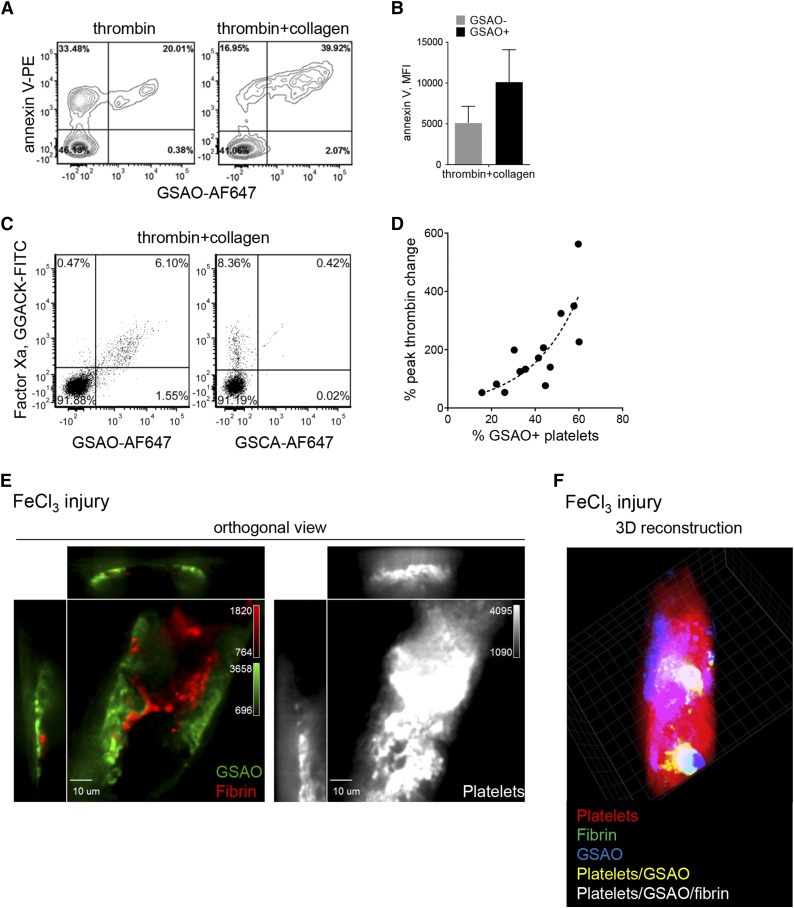
The relationship between the activated and procoagulant phenotype of platelets remains controversial.33,34 Procoagulant platelets are characterized by the presence of phosphatidylserine on the platelet surface, surface binding of plasma-derived activated clotting factors, ability to generate thrombin, and promotion of fibrin formation. Regardless of the agonist stimulus, 100% of GSAO+ platelets were positive for α-granule P-selectin, which implies that α-granule release is a prerequisite for GSAO labeling after agonist stimulation (Figure 1). Effectively all GSAO+ platelets bound annexin V, indicating externalization of phosphatidylserine (Figure 6A-B), which is a hallmark of necrosis.35 Of note, a subpopulation of GSAO− platelets also bound annexin V, so platelet necrosis is not required for exposure of phosphatidylserine.
Figure 6.
GSAO marks functionally procoagulant platelets. (A-B) Washed human platelets were stimulated with thrombin (0.1 U/mL) or thrombin (0.1 U/mL) and collagen (5 µg/mL), and phosphatidylserine externalization was assessed by annexin V binding. The mean fluorescence intensity of annexin V binding to GSAO− and GSAO+ platelets is shown in B. Flow plots and bars are representative of n ≥ 3 separate experiments. (C) Platelet-rich plasma was recalcified and stimulated with thrombin (1 U/mL) and collagen (5 µg/mL) with fibrin polymerization inhibition. FXa on the platelet surface was detected using the small molecule inhibitor, Glu-Gly-Arg chloromethyl ketone-fluorescein isothiocyanate. Of the FXa+ platelets, 93% were co-labeled with GSAO. (D) Washed human platelets were stimulated with thrombin (0.1 U/mL) or thrombin (0.1 U/mL) and collagen (5 µg/mL), and their procoagulant potential was assessed using the Calibrated Automated Thrombogram. Correlation between peak thrombin time and the percentage of GSAO+ platelets in the preparation (n = 18). The dotted line is the nonlinear least squares fit of the data to a single exponential (r2 = 0.77; P < .001). (E) Dylight 649-conjugated anti-platelet CD42b antibody, GSAO-AF546, and AF488-conjugated anti-fibrin antibody were injected into the murine circulation and thrombus initiated in the cremaster muscle arterioles by FeCl3 injury. Images are represented as cross-sectional orthogonal views of single confocal plane separately displaying GSAO and fibrin signal (left) and platelet signal (right). Fibrin signal preferentially localized with the GSAO+ platelets (representative image, n = 9 images in 3 independent mice). (F) 3D reconstruction of an occlusive thrombus.
The synthetic covalent inhibitor of factor Xa (FXa), fluorescein isothiocyanate-labeled Glu-Gly-Arg chloromethyl ketone, was used to detect this activated coagulation factor on the platelet surface. There was a high correlation (χ2 = 15 793; P < .000) between GSAO labeling and FXa on the agonist-activated platelet surface (Figure 6C). The sensitivity and specificity for detection of FXa on the platelet surface by GSAO labeling are 99% and 91%, respectively. To assess the functional procoagulant potential of the GSAO+ agonist-stimulated platelets, peak thrombin values were measured using the global coagulation assay, Calibrated Automated Thrombogram. There was an exponential correlation between the percentage change in peak thrombin and percentage change in GSAO+ platelets (Figure 6D). These results demonstrate that GSAO+ necrotic platelets are functionally procoagulant.
To determine whether GSAO+ platelets support coagulation in vivo, we looked for localization of GSAO+ platelets with fibrin within thrombi induced by FeCl3 injury in murine cremaster arterioles. Fibrin is the end product of activation of the coagulation factors. Fibrin strands are shown adjacent to GSAO+ platelets in the 2-dimensional cross-sectional sections (Figure 6E). The triple localization of platelets, GSAO, and fibrin is demonstrated by the white in the 3-dimensional reconstruction of an occlusive thrombus (Figure 6F; supplemental Methods). These findings demonstrate that GSAO identifies procoagulant platelets in vitro and in vivo. Together, these results demonstrate that the GSAO+ platelets generated during thrombus formation form via the cyclophilin D-dependent necrosis pathway and are procoagulant.
Aspirin ingestion sensitizes platelets to cyclophilin D inhibition in human subjects
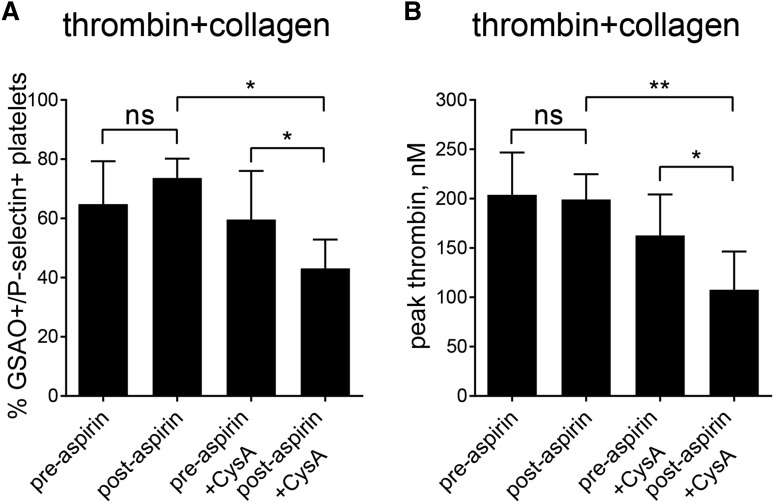
The standard pharmacological means for prevention of cardiovascular events is by inhibiting platelet cyclooxygenase-1 with aspirin, which suppresses platelet activation. To explore the possibility of targeting platelet activation (cyclooxygenase-1) and necrosis (cyclophilin D) pathways in combination, we compared the effect of ex vivo cyclosporine A treatment on thrombin and collagen activation of platelets from healthy volunteers before and after 7 days of aspirin ingestion. Aspirin ingestion resulted in no change in the GSAO+/P-selectin+ necrotic (Figure 7A) or procoagulant (Figure 7B) potential of activated platelets. However, cyclosporine A treatment of preaspirin platelets resulted in significant decrease in GSAO+/P-selectin+ necrotic platelets and corresponding procoagulant function, and this effect was significantly more pronounced in the postaspirin platelets. These results indicate that targeting of platelet activation by aspirin alone does not affect formation of procoagulant platelets, but aspirin treatment sensitizes platelets to cyclophilin D blockade, thereby increasing the degree to which thrombin production can be inhibited.
Figure 7.
Aspirin ingestion sensitizes platelets to cyclophilin D inhibition in human subjects. Platelets from healthy volunteers were examined before and after 7 days of aspirin ingestion. Washed platelets were activated with thrombin (0.1 U/mL) and collagen (5 µg/mL) and GSAO+/P-selectin+ necrotic platelets measured by flow cytometry (A) and procoagulant potential assessed using the Calibrated Automated Thrombogram (B). Aspirin ingestion resulted in no change in the GSAO+/P-selectin+ necrotic or procoagulant potential of activated platelets. CysA treatment of preaspirin platelets resulted in a decrease in GSAO+/P-selectin+ necrotic platelets (n = 5;* P < .05) and procoagulant function (n = 5; **P < .01), and this effect was more pronounced in the postaspirin platelets (n = 5; *P < .05).
Discussion
The concept of the “procoagulant” platelet is central to our current model of hemostasis. It localizes coagulation activation and fibrin formation to the activated platelet clot. However, although there is agreement with regard to the functional importance of the procoagulant platelet phenotype, there remains much debate about its identity. There have been a number of candidates proposed by different groups.
The “coated” population of platelets originally described by Dale are characterized by surface-bound serotonin-derived α-granule proteins including factor Va, which naturally led to speculation that coated platelets were the proteins that support coagulation.36 However, investigation in flow-based ex vivo models indicated that the coated platelet population did not fully correlate with phosphatidylserine-positive platelets, nor were they spatially associated with fibrin strands.37 Platelets with a sustained intracellular calcium signal have been labeled “SCIP” platelets and are known to generate fibrin, but again, measuring SCIP platelets during thrombus formation is difficult. Schoenwaelder and colleagues identified that “apoptotic” platelets support thrombin generation in vitro7; however, inhibition of the Bcl-XL proapoptotic pathway using BH3 mimetics resulted in reduced thrombus formation in murine studies.38 Increasingly, it has been proposed that the procoagulant platelet is undergoing a form of necrosis.2,12 Jobe and colleagues identified a cyclophilin D-dependent, mitochondrial permeability transition pore-dependent platelet subpopulation with distinct biochemical features that could be considered “necrotic.” These platelets appear to support prothrombinase and are another good candidate for the procoagulant platelet.8 The functional importance of the cyclophilin D-dependent pathway in thrombus formation has been strengthened by the recent finding that platelets from Pfif−/− mice that lack cyclophilin D show reduction in collagen-dependent thrombus formation.9 The way these different populations may relate to one another or may overlap remains unclear. Another persistent difficulty in this field is identifying whether these platelet populations, largely characterized in vitro, have distinct roles in vivo. High-resolution optical and fluorescence microscopy in murine models of thrombosis has produced insights. Structurally different platelet subpopulations have been identified in different thrombosis models.37 GSAO has allowed resolution of some of these questions.
GSAO, in conjunction with the α-granule marker, P-selectin, can identify a population of platelets with features of cyclophilin D-dependent necrosis that have a functionally procoagulant phenotype. The GSAO+/P-selectin+ population has properties of the coated platelet, including phosphatidylserine exposure and loss of mitochondrial membrane potential.33,39 The GSAO+ population is likely to contain “coated” platelets; however, this “coat” may be formed secondary to phosphatidylserine exposure, rather than representing a separate physiologically discrete population.1 The lack of complete overlap between annexin V positivity and procoagulant platelets is well described4,19,20 and is reflected in our findings, which show that only a subset of the annexin V+ platelets are GSAO+. GSAO+ platelets also show sustained high-level intracellular calcium, which is characteristic of SCIP platelets. In addition, GSAO+ platelets undergo cyclophilin D-dependent opening of the mitochondrial permeability transition pore, which are the platelets described by Jobe and colleagues.8,9,40 The time course studies indicate that the appearance of the necrotic platelet occurs within a few seconds after agonist stimulation. Indeed, a transition from the activated platelet to the necrotic platelet was not apparent. This suggests that platelets going down the cyclophilin D-dependent necrotic pathway are committed early after stimulation and are less likely to be merely the end stage of prolonged exposure to strong agonists. Furthermore, the observation that entry of GSAO-AF647 appears to be via a specific anion channel suggests that the formation of this platelet subset is via a regulated process.
The formation of GSAO+ platelets within the developing thrombus has been visualized in real time in murine arterioles. The GSAO+ platelets are spatially associated with sites of fibrin formation in the platelet aggregate, and attenuation of GSAO+ platelets in megakaryocyte-specific CypD−/−-deficient mice is associated with decreased fibrin formation. Together, these results imply they are providing a procoagulant surface during thrombus formation. Furthermore, not all agonists initiating formation of the platelet aggregate result in platelet necrosis. The FeCl3 injury model resulting in an occlusive thrombus was associated with GSAO+ platelets, whereas very few GSAO+ platelets were generated in the laser injury nonocclusive thrombus model. There is minimal FXa and FVa in the laser-induced platelet thrombus,41 which supports our observation of a minimal GSAO+ procoagulant surface and helps explain why fibrin generation is independent of platelet thrombus formation in this model.42 The role of the mitochondrial-depolarized platelet in thrombus formation is controversial. Abaeva and colleagues argued that these platelets form a fibrin(ogen) cap on the surface of the thrombus to limit growth.34 Liu and colleagues demonstrated that platelets dependent on cyclophilin D-mediated mitochondrial depolarization have impaired aggregation potential as a result of secondary inactivation of the αIIbβ3 integrin and found increased thrombus size in cyclophilin D-deficient platelets after rose Bengal injury to murine mesenteric arterioles.40 In contrast, we observed a marked decrease in GSAO+ platelets, thrombus size, and fibrin formation in cyclophilin D-deficient platelets after FeCl3 injury to murine cremaster muscle arterioles. These findings suggest the cyclophilin D-dependent procoagulant platelet may alter the balance between aggregation and thrombus stabilization, depending on the thrombus stimulus.
The necrotic platelet has implications for potential therapeutic intervention in thrombotic diseases. Our ex vivo analysis of platelets from human subjects receiving aspirin therapy indicates that procoagulant necrotic platelets form despite aspirin therapy, but their numbers are reduced by inhibition of the mitochondrial necrosis pathway. This finding indicates that formation of the procoagulant platelet is not directly dependent on platelet activation pathways, and that this functional population can be targeted separately. Furthermore, aspirin appeared to enhance the effect of necrosis inhibition on thrombin generation. This suggests that future therapies directed against procoagulant platelets could be combined with antiplatelet agents. Notably, the propensity for formation of “coated” platelets after exogenous GPVI agonist and thrombin stimulation has been observed to decrease after platelet inhibition using aspirin or the adenosine 5′-diphosphate receptor antagonist, clopidogrel.43,44 The assay used for “coated” platelets measures the capture of fibrinogen on the platelet surface, which is likely to reflect all activated αIIbβ3 platelets, rather than the procoagulant platelet subset. This provides further evidence of the imperfect overlap between “coated” and “procoagulant” platelets.
Blocking the procoagulant platelet is attractive in thrombosis, as it would not affect the platelet aggregate required for hemostasis, and unlike systemic anticoagulant therapy, the effect would be localized to those lesions that are more likely to be pathological. For example, patients who have persistent ischemic events when receiving antiplatelet therapy may benefit.45 In addition, testing peripheral blood for propensity to generate GSAO+ necrotic platelets may be a useful biomarker for prediction of recurrent ischemic events, particularly for patients already receiving aspirin therapy.
Acknowledgments
We thank Robert Lindeman and Benjamin Kile for many helpful discussions and Gabrielle Pennings for technical support. Microscopy was performed at the Biomedical Imaging Facility and flow cytometry at the Biological Resources Imaging Laboratory at the University of New South Wales.
The studies were supported by grants from the National Health and Medical Research Council of Australia, Kanematsu Memorial Research Fund, and South Eastern Area Laboratory Services Haematology Research Fund.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.M.H., L.A., E.G., L.P., and H.C. designed the research and analyzed data; P.J.H. conceived the study and wrote the manuscript; and V.M.Y.C. conceived the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philip Hogg, Chris O’Brien Lifehouse, Level 6, 119-143 Missenden Rd, Camperdown, NSW 2050, Australia; e-mail: philip.hogg@ctc.usyd.edu.au.
References
- 1.Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: different populations, different functions. J Thromb Haemost. 2013;11(1):2–16. doi: 10.1111/jth.12045. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP. Arterial thrombosis--insidious, unpredictable and deadly. Nat Med. 2011;17(11):1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 3.Butenas S, van’t Veer C, Mann KG. “Normal” thrombin generation. Blood. 1999;94(7):2169–2178. [PubMed] [Google Scholar]
- 4.Kempton CL, Hoffman M, Roberts HR, Monroe DM. Platelet heterogeneity: variation in coagulation complexes on platelet subpopulations. Arterioscler Thromb Vasc Biol. 2005;25(4):861–866. doi: 10.1161/01.ATV.0000155987.26583.9b. [DOI] [PubMed] [Google Scholar]
- 5.London FS, Marcinkiewicz M, Walsh PN. A subpopulation of platelets responds to thrombin- or SFLLRN-stimulation with binding sites for factor IXa. J Biol Chem. 2004;279(19):19854–19859. doi: 10.1074/jbc.M310624200. [DOI] [PubMed] [Google Scholar]
- 6.Prodan CI, Stoner JA, Cowan LD, Dale GL. Higher coated-platelet levels are associated with stroke recurrence following nonlacunar brain infarction. J Cereb Blood Flow Metab. 2013;33(2):287–292. doi: 10.1038/jcbfm.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoenwaelder SM, Yuan Y, Josefsson EC, et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 2009;114(3):663–666. doi: 10.1182/blood-2009-01-200345. [DOI] [PubMed] [Google Scholar]
- 8.Jobe SM, Wilson KM, Leo L, et al. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood. 2008;111(3):1257–1265. doi: 10.1182/blood-2007-05-092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattheij NJ, Gilio K, van Kruchten R, et al. Dual mechanism of integrin αIIbβ3 closure in procoagulant platelets. J Biol Chem. 2013;288(19):13325–13336. doi: 10.1074/jbc.M112.428359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchard BA, Paradis AK, Brummel-Ziedins KE. Measurement of procoagulant platelet subpopulations in whole blood: development of an assay for population-based studies. Thromb Res. 2011;127(1):62–64. doi: 10.1016/j.thromres.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gyulkhandanyan AV, Mutlu A, Freedman J, Leytin V. Markers of platelet apoptosis: methodology and applications. J Thromb Thrombolysis. 2012;33(4):397–411. doi: 10.1007/s11239-012-0688-8. [DOI] [PubMed] [Google Scholar]
- 12.Jackson SP, Schoenwaelder SM. Procoagulant platelets: are they necrotic? Blood. 2010;116(12):2011–2018. doi: 10.1182/blood-2010-01-261669. [DOI] [PubMed] [Google Scholar]
- 13.Kuijpers MJ, Munnix IC, Cosemans JM, et al. Key role of platelet procoagulant activity in tissue factor-and collagen-dependent thrombus formation in arterioles and venules in vivo differential sensitivity to thrombin inhibition. Microcirculation. 2008;15(4):269–282. doi: 10.1080/10739680701653517. [DOI] [PubMed] [Google Scholar]
- 14.Shi J, Pipe SW, Rasmussen JT, Heegaard CW, Gilbert GE. Lactadherin blocks thrombosis and hemostasis in vivo: correlation with platelet phosphatidylserine exposure. J Thromb Haemost. 2008;6(7):1167–1174. doi: 10.1111/j.1538-7836.2008.03010.x. [DOI] [PubMed] [Google Scholar]
- 15.Park D, Don AS, Massamiri T, et al. Noninvasive imaging of cell death using an Hsp90 ligand. J Am Chem Soc. 2011;133(9):2832–2835. doi: 10.1021/ja110226y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park D, Xie BW, Van Beek ER, et al. Optical imaging of treatment-related tumor cell death using a heat shock protein-90 alkylator. Mol Pharm. 2013;10(10):3882–3891. doi: 10.1021/mp4003464. [DOI] [PubMed] [Google Scholar]
- 17.Xie BW, Park D, Van Beek ER, et al. Optical imaging of cell death in traumatic brain injury using a heat shock protein-90 alkylator. Cell Death Dis. 2013;4:e473. doi: 10.1038/cddis.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Don AS, Kisker O, Dilda P, et al. A peptide trivalent arsenical inhibits tumor angiogenesis by perturbing mitochondrial function in angiogenic endothelial cells. Cancer Cell. 2003;3(5):497–509. doi: 10.1016/s1535-6108(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 19.Bevers EM, Comfurius P, van Rijn JL, Hemker HC, Zwaal RF. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur J Biochem. 1982;122(2):429–436. doi: 10.1111/j.1432-1033.1982.tb05898.x. [DOI] [PubMed] [Google Scholar]
- 20.Fager AM, Wood JP, Bouchard BA, Feng P, Tracy PB. Properties of procoagulant platelets: defining and characterizing the subpopulation binding a functional prothrombinase. Arterioscler Thromb Vasc Biol. 2010;30(12):2400–2407. doi: 10.1161/ATVBAHA.110.216531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dilda PJ, Decollogne S, Weerakoon L, et al. Optimization of the antitumor efficacy of a synthetic mitochondrial toxin by increasing the residence time in the cytosol. J Med Chem. 2009;52(20):6209–6216. doi: 10.1021/jm9008339. [DOI] [PubMed] [Google Scholar]
- 22.Dilda PJ, Ramsay EE, Corti A, Pompella A, Hogg PJ. Metabolism of the tumor angiogenesis inhibitor 4-(N-(S-Glutathionylacetyl)amino)phenylarsonous acid. J Biol Chem. 2008;283(51):35428–35434. doi: 10.1074/jbc.M804470200. [DOI] [PubMed] [Google Scholar]
- 23.Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609(1):1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- 24.Niessen J, Jedlitschky G, Grube M, et al. Human platelets express organic anion-transporting peptide 2B1, an uptake transporter for atorvastatin. Drug Metab Dispos. 2009;37(5):1129–1137. doi: 10.1124/dmd.108.024570. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306(2):703–708. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa T, Shimizu S, Watanabe T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434(7033):652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 27.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 28.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8(10):1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 29.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117(4):953–960. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo. Blood. 2006;107(10):3902–3906. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voronov RS, Stalker TJ, Brass LF, Diamond SL. Simulation of intrathrombus fluid and solute transport using in vivo clot structures with single platelet resolution. Ann Biomed Eng. 2013;41(6):1297–1307. doi: 10.1007/s10439-013-0764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dale GL, Friese P, Batar P, et al. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature. 2002;415(6868):175–179. doi: 10.1038/415175a. [DOI] [PubMed] [Google Scholar]
- 34.Abaeva AA, Canault M, Kotova YN, et al. Procoagulant platelets form an α-granule protein-covered “cap” on their surface that promotes their attachment to aggregates. J Biol Chem. 2013;288(41):29621–29632. doi: 10.1074/jbc.M113.474163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bratosin D, Mitrofan L, Palii C, Estaquier J, Montreuil J. Novel fluorescence assay using calcein-AM for the determination of human erythrocyte viability and aging. Cytometry A. 2005;66(1):78–84. doi: 10.1002/cyto.a.20152. [DOI] [PubMed] [Google Scholar]
- 36.Dale GL. Coated-platelets: an emerging component of the procoagulant response. J Thromb Haemost. 2005;3(10):2185–2192. doi: 10.1111/j.1538-7836.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- 37.Munnix IC, Kuijpers MJ, Auger J, et al. Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation: regulation by transient integrin activation. Arterioscler Thromb Vasc Biol. 2007;27(11):2484–2490. doi: 10.1161/ATVBAHA.107.151100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenwaelder SM, Jarman KE, Gardiner EE, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118(6):1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- 39.Remenyi G, Szasz R, Friese P, Dale GL. Role of mitochondrial permeability transition pore in coated-platelet formation. Arterioscler Thromb Vasc Biol. 2005;25(2):467–471. doi: 10.1161/01.ATV.0000152726.49229.bf. [DOI] [PubMed] [Google Scholar]
- 40.Liu F, Gamez G, Myers DR, Clemmons W, Lam WA, Jobe SM. Mitochondrially mediated integrin αIIbβ3 protein inactivation limits thrombus growth. J Biol Chem. 2013;288(42):30672–30681. doi: 10.1074/jbc.M113.472688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanciu L, Krishnaswamy S, Camire RM. New insights into the spatiotemporal localization of prothrombinase in vivo. Blood. 2014;124(11):1705–1714. doi: 10.1182/blood-2014-03-565010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandendries ER, Hamilton JR, Coughlin SR, Furie B, Furie BC. Par4 is required for platelet thrombus propagation but not fibrin generation in a mouse model of thrombosis. Proc Natl Acad Sci USA. 2007;104(1):288–292. doi: 10.1073/pnas.0610188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prodan CI, Joseph PM, Vincent AS, Dale GL. Coated-platelet levels are influenced by smoking, aspirin, and selective serotonin reuptake inhibitors. J Thromb Haemost. 2007;5(10):2149–2151. doi: 10.1111/j.1538-7836.2007.02691.x. [DOI] [PubMed] [Google Scholar]
- 44.Norgard NB, Saya S, Hann CL, Hennebry TA, Schechter E, Dale GL. Clopidogrel attenuates coated-platelet production in patients undergoing elective coronary catheterization. J Cardiovasc Pharmacol. 2008;52(6):536–539. doi: 10.1097/FJC.0b013e3181907390. [DOI] [PubMed] [Google Scholar]
- 45.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]