Abstract
Background
Postoperative stroke remains one of the most devastating complications after cardiac surgery. Variations in stroke rates and ability to rescue from mortality after stroke between surgical centers are not understood. This study evaluated patient risk and institutional factors associated with likelihood of postoperative stroke as well as hospital variation in risk-adjusted stroke and rates of failure to rescue (FTR) after stroke after cardiac surgery.
Methods
Patient records from The Society of Thoracic Surgeons' multiinstitutional certified database for cardiac operations (2001 to 2011) were analyzed. The relative contribution of patient- and hospital-related factors to the likelihood of postoperative stroke was assessed by univariate and multivariate analyses. Variations in risk-adjusted stroke and rates of FTR after stroke were compared, and impact of stroke on hospital resource utilization and costs were evaluated.
Results
A total of 57,387 patients was included. Postoperative stroke rate was 1.5%, with significant variation across hospitals (range, 0.8% to 2%, p < 0.001). Stroke patients (versus no stroke patients) presented with more comorbid disease and higher risk profiles (The Society of Thoracic Surgeons predicted risk of mortality, 3% versus 1%, p < 0.001). Mortality was expectedly higher after stroke compared with no stroke (18% versus 2%, p < 0.001). Postoperative stroke was associated with nearly double the total cost of hospitalization. After risk adjustment, individual hospitals demonstrated a strong association with likelihood for stroke (p < 0.001). Furthermore, high-performing hospitals with low stroke rates performed fewer aortic valve operations, more coronary artery bypass graft operations, and accrued longer intensive care unit lengths of stay. Significant hospital variations were observed for risk-adjusted stroke and rates of FTR after stroke (both p<0.001).
Conclusions
Institutional variation, more so than individual patient risk factors, is highly associated with postoperative stroke and FTR rates after stroke after cardiac surgery. Postoperative stroke remains significantly associated with mortality and morbidity. Institutional practice patterns may confer a disproportionate influence on postoperative stroke independent of case mix. Understanding differences between high and low performing centers is essential to improving outcomes, costs, and hospital quality.
Postoperative stroke remains one of the most devastating complications after cardiac surgery. The overall incidence of stroke related to cardiac surgery remains rather ill defined, with reported ranges of 0.4% to 14% [1–6], depending on patient populations, cardiac procedures, approach to diagnosis, and variations in imaging modalities, as well as varying clinical definitions for this postoperative event. Moreover, the timing of most perioperative strokes varies as well, with approximately 30% to 40% occurring intraoperatively, and the large majority of strokes occur within the first 1 to 2 days after surgery [3, 7–9]. The primary etiologic events responsible for intraoperative stroke include cerebral hypoperfusion and embolism (atheroembolism, thromboembolism, air embolism), whereas cardiogenic embolism appears to be the most common culprit for stroke in the postoperative setting [3, 7, 8, 10, 11].
Failure to rescue (FTR) is defined as mortality after major complication. Recent evidence supports reduction in FTR as a mechanism to reduce postoperative mortality after surgery [12]. Indeed, the Agency of Health Care Research and Quality (AHRQ) has recently identified FTR as one of 20 patient safety indicators for patients in the United States [13]. Among postoperative cardiac surgery complications, FTR after stroke is attractive to evaluate because response after postoperative stroke represents a potentially modifiable scenario that may associated with variabilities in disability and death. Whereas prior reports have analyzed FTR in various surgical populations [14–18], reports evaluating FTR after cardiac surgery remain limited [18, 19]. Moreover, the identification of risk factors for postoperative stroke has the potential to greatly impact FTR status in the future.
The purpose of this study was threefold: (1) to characterize variations in stroke rates after cardiac surgery among Virginia Cardiac Surgery Quality Initiative (VCSQI) centers; (2) to identify patient-, operation-, and hospital-related factors associated with the likelihood of postoperative stroke; and (3) to characterize hospital variability in risk-adjusted postoperative stroke and FTR after stroke rates. We hypothesized that significant variations in hospital stroke and FTR after stroke rates exist after cardiac surgery within a multiinstitutional cohort of patients.
Material and Methods
Hospital Institutional Review Board at each participating VCSQI center exempted this analysis from formal review owing to the secondary analysis of the deidentified VCSQI data registry and absence of patient identifiers, and also because the VCSQI data are principally collected for quality analysis and not exclusively for research.
Patients and Data Acquisition
Deidentified VCSQI patient records were evaluated for the period January 1, 2001, through December 31, 2011 (n = 57,387). All patient records represented patients who had undergone cardiac surgery operations at VCSQI centers who had The Society of Thoracic Surgeons (STS) predicted risk of mortality (PROM) scores calculated. Patient records were stratified according to postoperative permanent stroke status as defined within the STS adult cardiac database. Subsequently, FTR after stroke status was established based on mortality status after stroke. Included cardiac operations represent standard surgical approaches. Patient preoperative risk was assessed by calculated STS predicted risk indices: STS PROM and STS predicted risk of mortality and morbidity (PROMM), as these risk indexes were more completely captured in the abstracted VCSQI data than other indexes, including STS predicted risk of stroke. Total costs of hospitalization were estimated using uniform methodology among all VCSQI centers, as described elsewhere [20].
Measured Outcomes
The primary outcomes of interest included (1) risk-adjusted associations between postoperative stroke and patient-, operation-, and hospital-related risk factors; and (2) risk-adjusted postoperative stroke and FTR after stroke (mortality after stroke) rates between hospitals. Secondary outcomes included unadjusted differences in the associations between stroke and patient risk profiles, operation-related factors, and hospital resource utilization. Traditional STS definitions were applied for all analyzed variables [21].
Statistical Analysis
Study outcomes and study design were established a priori before data collection. Categoric variables are expressed as group percentages, and continuous variables are expressed as either mean ± SD or median (25th, 75th percentile). Either Pearson's χ2 or the Fisher's exact test of association was used to compare categorical variables. Either analysis of variance or the Mann-Whitney U test was used to compare continuous data. Standard statistical significance was determined by a p value less than 0.05.
Hierarchical, multiple logistic regression was used to estimate adjusted associations between various preoperative and operative risk factors and the likelihood of postoperative stroke. Postoperative events (eg, postoperative atrial fibrillation) were not adjusted for as modeled factors. All model covariates were selected based on tests of significance (p < 0.05) on univariate analyses. The model was adjusted for the influence of each entered risk factor. Clustering at the hospital level was considered in the hierarchical structure of the statistical model to adjust for the influence of correlated events within hospitals. The relative strength of association between modeled factors and the likelihood of postoperative stroke was determined by each factors likelihood ratio (Wald χ2 test statistic) within the regression model. Model discrimination was assessed by the area under the receiver-operating characteristics curve, and the Hosmer-Lemeshow test was used to verify model calibration. Risk-adjusted postoperative stroke and FTR after stroke rates were determined for each hospital, calculated based on the relationship between expected (or predicted) and observed event rates determined by regression modeling, as described elsewhere [22]. Risk-adjusted event rates, adjusted for baseline STS predicted risk, type of operation, operative year, and annual hospital volume, were compared across hospitals. Predictive Analytics SoftWare (PASW) with complex sampling module software, version 21.0.0 (IBM Corporation, Somers, NY), was used for all data manipulation and statistical analyses.
Results
Impact of Postoperative Stroke and Distribution of Patient Characteristics and Operative Features
The overall stroke event rate was 1.5% (n = 845) among all patients undergoing cardiac operations during the study, with significant variation across hospitals (range, 0.8% to 2.0%; Fig 1). The overall mortality rate among stroke patients was 18% and significantly higher compared with patients without a complication of stroke (2%, p < 0.001). Postoperative stroke was associated with nearly double the total cost of hospitalization ($46,395 versus $25,254, p < 0.001). Table 1 displays unadjusted comparisons of patient risk factors stratified by stroke status. Stroke patients were on average older, more commonly female, and presented with a higher burden of preoperative comorbid disease, including peripheral arterial disease, heart failure, and diabetes mellitus. As a result, stroke patients had higher calculated median STS PROM (3.5% versus 1.5%) and PROMM (18.2% versus 11.0%) compared with patients without stroke (both p < 0.001). Postoperative stroke was less common after elective operations and performance of isolated CABG operations, whereas stroke was more common after urgent or emergent operations as well as isolated valve and combined valve and coronary artery bypass graft (CABG) procedures (all p < 0.05).
Fig 1. Distribution of unadjusted postoperative stroke rates across VCSQI hospitals.

Table 1. Patient Characteristics and Profiles for All Cardiac Arrest Patients Stratified by Postoperative Stroke Status.
| Characteristics | Stroke (n = 845) | No Stroke (n = 56,542) | p Value |
|---|---|---|---|
| Patient age, years | 69 ± 10 | 65 ± 11 | <0.001 |
| Male | 62 | 71 | <0.001 |
| Hypertension | 86 | 79 | <0.001 |
| Diabetes mellitus | 47 | 37 | <0.001 |
| Cerebrovascular disease | 30 | 14 | <0.001 |
| Chronic lung disease (severe) | 3.6 | 2.5 | 0.08 |
| Peripheral arterial disease | 22 | 14 | <0.001 |
| Heart failure | 32 | 17 | <0.001 |
| Ejection fraction, % | 50 (37, 60) | 55 (45, 60) | <0.001 |
| Previous CABG | 20 | 16 | <0.001 |
| Previous valve | 6 | 5 | <0.001 |
| Elective operation | 39 | 47 | <0.001 |
| STS PROMM, % | 18.2 (9.6, 25.6) | 10.9 (7.4, 17.8) | <0.001 |
| STS PROM, % | 3.5 (1.5, 6.5) | 1.5 (0.7, 3.1) | <0.001 |
| Operation | <0.001 | ||
| AV replacement | 8.2 | 7.7 | |
| AV replacement + CABG | 10.4 | 6.6 | |
| CABG only | 72.4 | 80.4 | |
| MV repair | 0.9 | 1.1 | |
| MV repair + CABG | 1.4 | 0.8 | |
| MV replacement + CABG | 2.7 | 1.3 | |
| MV replacement only | 3.9 | 2.1 |
Categoric variables are expressed as group percentages, and continuous variables as mean ± SD or median (25th, 75th percentile).
CABG = coronary artery bypass graft surgery; MV = mitral valve; PROM = predicted risk of mortality; PROMM = predicted risk of mortality and morbidity; STS = The Society of Thoracic Surgeons.
Risk-Adjusted Associations Between Stroke and Patient-, Hospital-, and Operation-Related Factors
To account for baseline differences in patient risk profiles, risk-adjusted associations between the likelihood for postoperative stroke and various patient-, hospital-, and operation-related factors were estimated (Table 2). After adjusting for these confounding influences, individual hospital demonstrated the strongest relative association (Wald χ2 likelihood ratio 71.43) with the likelihood for postoperative stroke. Other significant factors associated with postoperative stroke included patient age, operating surgeon, nonelective operative status, procedure type, and preoperative cerebrovascular disease, peripheral vascular disease, hypertension, heart failure, and renal failure. The statistical performance of the logistic regression model achieved adequate discrimination with an area under the curve of 0.78. The calibration of the model was adequate, with Hosmer-Lemeshow p less than 0.95.
Table 2. Risk-Adjusted Associations Among Patient, Operation, and Hospital-Related Risk Factors for Outcome of Postoperative Stroke.
| Risk Factor | Likelihood Ratio (Wald) | AOR | p Value |
|---|---|---|---|
| Hospital | 71.43 | … | <0.001 |
| Surgeon | 61.20 | … | 0.01 |
| Age | 60.76 | 1.04 | <0.001 |
| Nonelective status | 60.35 | 4.20 | <0.001 |
| Cerebrovascular disease | 33.71 | 1.88 | <0.001 |
| Renal failure | 14.31 | 1.70 | <0.001 |
| Heart failure | 13.00 | 1.50 | <0.001 |
| Male | 9.68 | 0.74 | <0.001 |
| Hypertension | 9.33 | 1.51 | <0.001 |
| Procedure type | 7.44 | 0.02 | |
| CABG only (reference) | … | … | |
| AV replacement | 1.28 | 0.18 | |
| AV replacement + CABG | 1.53 | 0.04 | |
| MV repair | 0.87 | 0.11 | |
| MV repair + CABG | 1.40 | 0.86 | |
| MV replacement + CABG | 2.10 | 0.03 | |
| MV replacement only | 1.73 | 0.04 | |
| Peripheral vascular disease | 5.46 | 1.31 | 0.02 |
| Year | 0.81 | … | 0.73 |
Model performance: area under receiver-operating characteristics curve = 0.78, Hosmer-Lemeshow p = 0.95, Nagelkerke pseudo R2 = 0.25.
AOR = adjusted odds ratio; AV = aortic valve; CABG = coronary artery bypass graft surgery; MV = mitral valve.
Interhospital Variability in Risk-Adjusted Event Rates
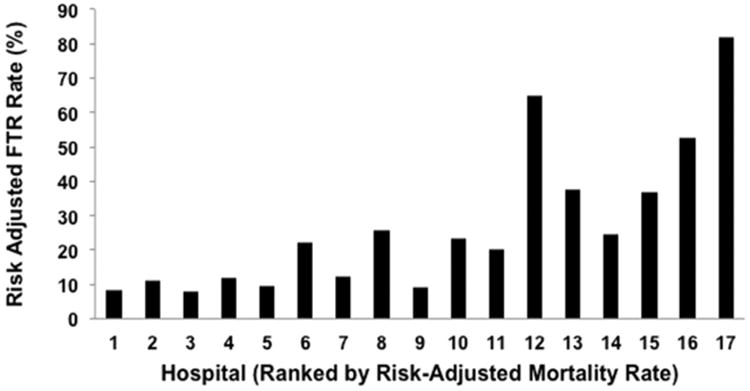
After estimating that individual hospital demonstrated the strongest association with the risk-adjusted likelihood for postoperative stroke compared with other modeled factors, we compared risk-adjusted postoperative stroke and FTR after stroke rates among hospitals. As a result, significant variations were demonstrated in overall risk-adjusted postoperative stroke rate (range, 0.12% to 5.7%, p < 0.001; Fig 2) and FTR after stroke rate (range, 7.7% to 82.0%, p < 0.001; Fig 3) across hospitals. Interestingly, when hospitals were ranked by risk-adjusted stroke event rates, highest performing centers (ie, hospitals with lowest risk-adjusted stroke rates) demonstrated a trend toward the performance of fewer aortic valve operations (5% to 6% versus 7% to 17%), more CABG operations (83% to 87% versus 65% to 83%) and accrued longer intensive care unit lengths of stay (48 versus 32 to 43 hours, all p < 0.001) compared with lower performing centers (ie, hospitals with highest risk-adjusted stroke rates).
Fig 2. Comparisons of risk-adjusted postoperative stroke rates across hospitals (ranked according to risk-adjusted mortality rates, p < 0.001).
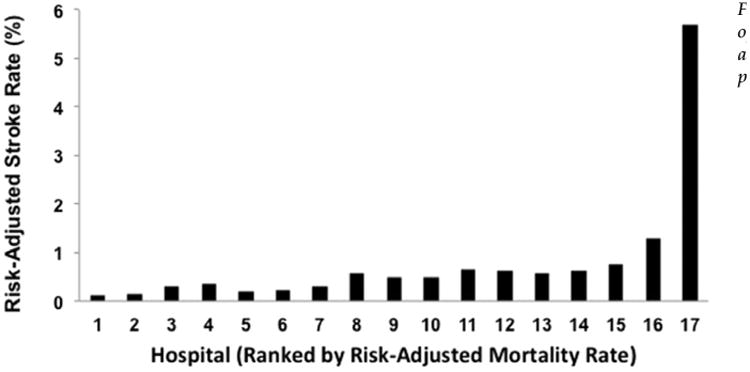
Fig 3. Comparisons of risk-adjusted failure to rescue (FTR) after postoperative stroke rates across hospitals (ranked according to risk-adjusted mortality rates, p < 0.001).
Comment
The present study reports the impact of postoperative stroke after performance of cardiac surgery in the Commonwealth of Virginia with particular attention to hospital variations in risk-adjusted event rates. Through the evaluation of more than 57,000 patient records from an 11-year study, the data also demonstrate the relative strength of association that exist between various patient-, operation-, and hospital-related factors and the likelihood of postoperative stroke. The results suggest that the influence of individual hospital is more strongly associated with postoperative stroke than patient preoperative risk profile or surgeon. Significant variations in hospital risk-adjusted stroke rate and FTR after stroke rate also suggest that the identification of institutional differences in preventive measures and responses after stroke may play a primary role in improving overall patient outcomes. The reported results, therefore, have significant clinical implications related to patient safety and quality efforts designed to address this postoperative event.
The first aim of this study was to characterize the variation in postoperative stroke rate across all 17 participating VCSQI centers. As demonstrated in Figure 1, significant variation in overall unadjusted stroke rates was apparent, with incidence of stroke ranging from 0.8% to 2.0%. These results are consistent with previously reported overall stroke rates after cardiac surgery in other retrospective and national registry reports. In a recent analysis of isolated CABG outcomes reported in the STS adult cardiac surgery database, stroke rates varied from 1.6% (1999) to 1.2% (2009) [23]. Similarly, the STS noted stroke rate after mitral valve procedures has been reported to be 1.1% for mitral repair and 1.6% after mitral replacement [24]. Another report describing the late incidence and development of stroke after aortic and mitral valve operations reported stroke rates ranging as high as 1.3% to 1.4% per year for aortic valve replacements and 1.3% to 2.3% for mitral replacement [25]. In the present study, significant variations in risk-adjusted stroke event rates were also demonstrated (range, 0.12% to 5.7%). Importantly, the reported risk-adjusted event rates for postoperative stroke were less than 1% for all but two centers, which had risk-adjusted stroke rates of 1.3% and 5.7%. Variations in reported stroke rate are often related to a multitude of different factors, including differences in diagnostic modalities used to identify postoperative strokes and varying clinical definitions of this complication. Unfortunately, at the time of study, the granularity of the VCSQI data registry was not sufficient to examine possible factors and processes of care related to the occurrence of postoperative stroke such as single versus partial aortic cross-clamp techniques, degree of aortic and valvular calcification, timing of postoperative antiplatelet therapy, and duration of or approach to the treatment of atrial fibrillation. However, the clinical definition of permanent postoperative stroke, consistent with that captured by the STS adult cardiac database, was utilized and uniform across VCSQI centers.
The second aim of this study was to identify patient-, operation-, and hospital-related factors associated with the likelihood of postoperative stroke. The reported results demonstrated significant associations between several patient and operative risk factors and the likelihood of postoperative stroke, including increasing patient age, being female, and presence of culprit comorbid disease states (hypertension, cerebrovascular disease, peripheral vascular disease, heart failure, and renal failure). In addition, the type and status (elective versus nonelective) of a cardiac operation was also identified as a potential risk factor for stroke. These results are in agreement with those of other series [1–6, 8, 25, 26]. Perhaps most interesting, however, was the estimated relationship between individual hospital and the likelihood of postoperative stroke. Even after adjusting for differences in comorbid disease, case mix, individual surgeon, and the clustering of correlated events occurring within each hospital, these data suggest that institutional practice may have the most significant influence on the risk of postoperative stroke.
The third objective of this study was to further characterize the variability that may exist in postoperative stroke and FTR after stroke rates taking into consideration baseline patient and operative risk. Specifically, FTR status after stoke was investigated as we believed it was reasonable to consider that hospital variations existed in the ability of different hospitals to respond and “rescue” patients from mortality after sustaining a postoperative stroke and that such data would establish a foundation on which to base future investigation of best practice patterns to reduce the occurrence and impact of stroke in the perioperative setting. The present results are complementary to accumulated surgical data devoted to the examination of FTR as it relates to interhospital variations in mortality.
Several surgical series have demonstrated associations between postoperative complication rates and mortality, including cardiac surgical populations [14–18]. One particular report of Medicare patients undergoing several different subspecialty operations, including CABG and aortic and mitral valve operations, demonstrated differences in overall FTR rates (6.8% versus 16.7%) between lowest and highest performing hospitals [14]. Similarly, Reddy and colleagues [18] reported 45,904 adult cardiac surgery patients in Michigan, demonstrating that low mortality hospitals appear to have superior overall FTR abilities compared with high mortality hospitals based on the evaluation of composite morbidity [18]. The results of our study add to this increasing body of evidence related to hospital differences in cardiac surgery-specific complications. Unlike other series, which have evaluated FTR in terms of mortality after composite complications, unique in this analysis is the focus on the specific relationship that exists between postoperative stroke and mortality as a more specific FTR event. These results suggest that it may be possible to identify processes of care within high performing hospitals (ie, those with low risk-adjusted mortality, stroke, and FTR after stroke event rates) to reduce the clinical impact of postoperative stroke in lower performing hospitals in the future. Further analyses by the VCSQI are needed to better define these processes within participating centers.
A few additional noteworthy observations were revealed in this analysis. An interesting trend was observed for longer total intensive care unit length of stay as well as the performance of lower risk operations among hospitals with low risk-adjusted stroke rates compared with hospitals with higher risk-adjusted stroke rates. Although the exact circumstances surrounding individual patient encounters cannot be further described in this analysis, it is possible that such trends may partially reflect differences in postoperative management philosophy and practice protocols related to early or expedited patient discharge from the intensive care unit. Furthermore, despite differences in hospital case mix between hospitals, incremental differences in risk-adjusted stroke event rates were apparent between hospitals. These findings are critical as previous studies suggest that postoperative complication rates after cardiac surgery are largely determined by patient-related characteristics and risk factors [12].
The results of this study have significant clinical and healthcare policy-related implications. The demonstration of significant differences in risk-adjusted stroke and FTR after stroke rates among 17 different cardiac surgical centers suggests that differences in the approach to pre-operative, intraoperative, and postoperative prevention and management of stroke have the potential to greatly improve patient outcomes in other hospitals. In addition, FTR as a metric (after composite complications) has recently been endorsed by the National Quality Forum as a performance measure for which hospitals are subject to evaluation [12–15, 27, 28]. Therefore, cardiac surgeon leadership in hospital-specific efforts designed to facilitate early recognition and response to postoperative neurologic complications is essential to improve patient outcomes and quality measures.
The present study has limitations. First, the secondary analysis of the deidentified VCSQI data registry limited the ability to scrutinize certain patient encounter data. As a result, more specific details related to the circumstances surrounding perioperative stroke events, including perioperative period, location (operating room, intensive care unit, or ward), underlying etiology (ischemic or embolic), extent of cerebral damage, success of stroke response protocols, aortic cross-clamp and aortic annular debridement strategies, and diagnostic imaging modalities used to identify postoperative strokes remain unknown. As the VCSQI works to further investigate individual hospital differences in ability to rescue after stroke, we anticipate the prospective collection of these data points for future analyses. Second, the retrospective study design renders it subject to inherent selection bias. Third, reported results describe estimated associations and do not describe direct cause and effect relationships between analyzed factors. All analyses were limited to short-term, operative outcomes and do not provide insight into the long-term outcomes, including neurologic sequelae or quality of life after stroke. Surgical subgroup analyses, including on-pump versus off-pump CABG, were not performed. Alternatively, we chose to adjust for the impact of case mix among hospitals by including operation type as a factor in our risk-adjustment models. Examination of specific surgical populations may prove beneficial for future analyses. Finally, a small potential for unrecognized miscoding of data in any data registry is possible.
In conclusion, the presented data demonstrates that significant intercenter variation exists in unadjusted and risk-adjusted postoperative stroke rates after cardiac surgery. Postoperative stroke remains a significant source of mortality and morbidity. Institutional factors appear to confer a strong influence on postoperative stroke independent of case mix and comorbid disease. Correlations between risk-adjusted stroke and FTR after stroke rates appear to exist for Virginia cardiac surgical centers. Understanding differences between high- and low-performing centers is essential to improving patient outcomes, costs, and hospital quality.
Acknowledgments
The authors wish to thank George J. Stukenborg, PhD, for his statistical guidance. Virginia Cardiac Surgery Quality Initiative investigators: Drs LaPar, Crosby, Kern, Kron, and Ailawadi, University of Virginia, Charlottesville; Dr Speir, Inova Heart and Vascular Institute, Falls Church; Dr Rich, Mid Atlantic Cardiothoracic Surgeons, Norfolk; and Dr Quader, Virginia Commonwealth University, Richmond, Virginia.
Footnotes
Presented at the Sixty-first Annual Meeting of the Southern Thoracic Surgical Association, Tucson, AZ, Nov 5-8, 2014.
References
- 1.Anyanwu AC, Filsoufi F, Salzberg SP, et al. Epidemiology of stroke after cardiac surgery in the current era. J Thorac Cardiovasc Surg. 2007;134:1121–7. doi: 10.1016/j.jtcvs.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Gardner TJ, Horneffer PJ, Manolio TA, et al. Stroke following coronary artery bypass grafting: a ten-year study. Ann Thorac Surg. 1985;40:574–81. doi: 10.1016/s0003-4975(10)60352-9. [DOI] [PubMed] [Google Scholar]
- 3.Hogue CW, Murphy SF, Schechtman KB, et al. Risk factors for early or delayed stroke after cardiac surgery. Circulation. 1999;100:642–7. doi: 10.1161/01.cir.100.6.642. [DOI] [PubMed] [Google Scholar]
- 4.Puskas JD, Winston AD, Wright CE, et al. Stroke after coronary artery operation: incidence, correlates, outcome, and cost. Ann Thorac Surg. 2000;69:1053–6. doi: 10.1016/s0003-4975(99)01569-6. [DOI] [PubMed] [Google Scholar]
- 5.Roach GW, Kanchuger M, Mangano CM, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335:1857–63. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 6.Stamou SC, Hill PC, Dangas G, et al. Stroke after coronary artery bypass: incidence, predictors, and clinical outcome. Stroke. 2001;32:1508–13. doi: 10.1161/01.str.32.7.1508. [DOI] [PubMed] [Google Scholar]
- 7.Likosky DS, Marrin CA, Caplan LR, et al. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Stroke. 2003;34:2830–4. doi: 10.1161/01.STR.0000098650.12386.B3. [DOI] [PubMed] [Google Scholar]
- 8.McKhann GM, Grega MA, Borowicz LM, et al. Stroke and encephalopathy after cardiac surgery: an update. Stroke. 2006;37:562–71. doi: 10.1161/01.STR.0000199032.78782.6c. [DOI] [PubMed] [Google Scholar]
- 9.Tarakji KG, Sabik JF, Bhudia SK, et al. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA. 2011;305:381–90. doi: 10.1001/jama.2011.37. [DOI] [PubMed] [Google Scholar]
- 10.Barbut D, Grassineau D, Lis E, et al. Posterior distribution of infarcts in strokes related to cardiac operations. Ann Thorac Surg. 1998;65:1656–9. doi: 10.1016/s0003-4975(98)00272-0. [DOI] [PubMed] [Google Scholar]
- 11.Lahtinen J, Biancari F, Salmela E, et al. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. Ann Thorac Surg. 2004;77:1241–4. doi: 10.1016/j.athoracsur.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 12.Silber JH, Williams SV, Krakauer H, et al. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30:615–29. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality (AHRQ) quality indicators. Patient safety indicators: technical specifications. [Accessed October 1, 2014]; Available at: http://www.qualityindicators.ahrq.gov.
- 14.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in Medicare patients. Ann Surg. 2009;250:1029–34. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 15.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–75. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 16.Glance LG, Dick AW, Meredith JW, et al. Variation in hospital complication rates and failure-to-rescue for trauma patients. Ann Surg. 2011;253:811–6. doi: 10.1097/SLA.0b013e318211d872. [DOI] [PubMed] [Google Scholar]
- 17.Pasquali SK, He X, Jacobs JP, et al. Evaluation of failure to rescue as a quality metric in pediatric heart surgery: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2012;94:573–80. doi: 10.1016/j.athoracsur.2012.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy HG, Shih T, Englesbe MJ, et al. Analyzing “failure to rescue”: is this an opportunity for outcome improvement in cardiac surgery? Ann Thorac Surg. 2013;95:1976–81. doi: 10.1016/j.athoracsur.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaPar DJ, Ghanta RK, Kern JA, et al. Hospital variation in mortality from cardiac arrest after cardiac surgery: an opportunity for improvement? Ann Thorac Surg. 2014;98:534–40. doi: 10.1016/j.athoracsur.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaPar DJ, Crosby IK, Ailawadi G, et al. Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. J Thorac Cardiovasc Surg. 2013;145:796–804. doi: 10.1016/j.jtcvs.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 21.The Society of Thoracic Surgeons. STS adult cardiac surgery database data specifications (version 2.81) [Accessed October 1, 2014]; Available at: http://www.sts.org/sites/default/files/documents/Stsadultcvdataspecificationsv2_81.pdf.
- 22.Hannan EL, Wu C, DeLong ER, et al. Predicting risk-adjusted mortality for CABG surgery: logistic versus hierarchical logistic models. Med Care. 2005;43:726–35. doi: 10.1097/01.mlr.0000167802.27044.44. [DOI] [PubMed] [Google Scholar]
- 23.ElBardissi AW, Aranki SF, Sheng S, et al. Trends in isolated coronary artery bypass grafting: an analysis of The Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143:273–81. doi: 10.1016/j.jtcvs.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Gammie JS, Sheng S, Griffith BP, et al. Trends in mitral valve surgery in the United States: results from The Society of Thoracic Surgeons adult cardiac surgery database. Ann Thorac Surg. 2009;87:1431–9. doi: 10.1016/j.athoracsur.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 25.Ruel M, Masters RG, Rubens FD, et al. Late incidence and determinants of stroke after aortic and mitral valve replacement. Ann Thorac Surg. 2004;78:77–84. doi: 10.1016/j.athoracsur.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 26.Charlesworth DC, Likosky DS, Marrin CA, et al. Development and validation of a prediction model for strokes after coronary artery bypass grafting. Ann Thorac Surg. 2003;76:436–43. doi: 10.1016/s0003-4975(03)00528-9. [DOI] [PubMed] [Google Scholar]
- 27.Silber JH, Rosenbaum PR, Schwartz JS, et al. Evaluation of the complication rate as a measure of quality of care in coronary artery bypass graft surgery. JAMA. 1995;274:317–23. [PubMed] [Google Scholar]
- 28.National Quality Forum. Measuring performance. [Accessed October 1, 2014]; Available at: http://www.qualityforum.org/measures_list.


