Summary
During 2013, cutaneous lesions developed in two men in the country of Georgia after they were exposed to ill cows. The men had never received vaccination against smallpox. Tests of lesion material with the use of a quantitative real-time polymerase-chain-reaction assay for non–variola virus orthopoxviruses were positive, and DNA sequence analysis implicated a novel orthopoxvirus species. During the ensuing epidemiologic investigation, no additional human cases were identified. However, serologic evidence of exposure to an orthopoxvirus was detected in cows in the patients’ herd and in captured rodents and shrews. A third case of human infection that occurred in 2010 was diagnosed retrospectively during testing of archived specimens that were originally submitted for tests to detect anthrax. Orthopoxvirus infection should be considered in persons in whom cutaneous lesions develop after contact with animals.
With the exception of the variola virus (the agent of smallpox), members of the orthopoxvirus genus are zoonotic.1,2 Antibodies that are generated after infection or vaccination with one orthopoxvirus species generally confer protection against other orthopoxvirus species.2–4 Thus, in the late 18th century, Benjamin Jesty and Edward Jenner successfully vaccinated patients against smallpox using less virulent orthopoxviruses that had been obtained from source patients with “cowpox.”2,5,6 Unlike patients with variola virus infection, patients with cowpox virus infection typically present with self-limited skin lesions. This infection is now most often acquired through contact with domestic animals such as cats.7 Humans are occasionally exposed to cowpox virus through contact with rodents, the natural reservoirs of the virus.2,7–10
In June 2013, the U.S. Centers for Disease Control and Prevention (CDC) and the National Center for Disease Control and Public Health of the country of Georgia tested specimens obtained from two cattle herders with suspected cowpox virus infection. Results of serologic testing and a general non–variola virus orthopoxvirus molecular assay suggested that both patients had orthopoxvirus infection, but follow-up molecular assays did not show that the virus belonged to a known orthopoxvirus species. Whole-genome sequencing followed by phylogenetic analysis led to the discovery of a novel orthopoxvirus species. We describe the clinical, phylogenetic, and epidemiologic features of this virus.
Case Reports
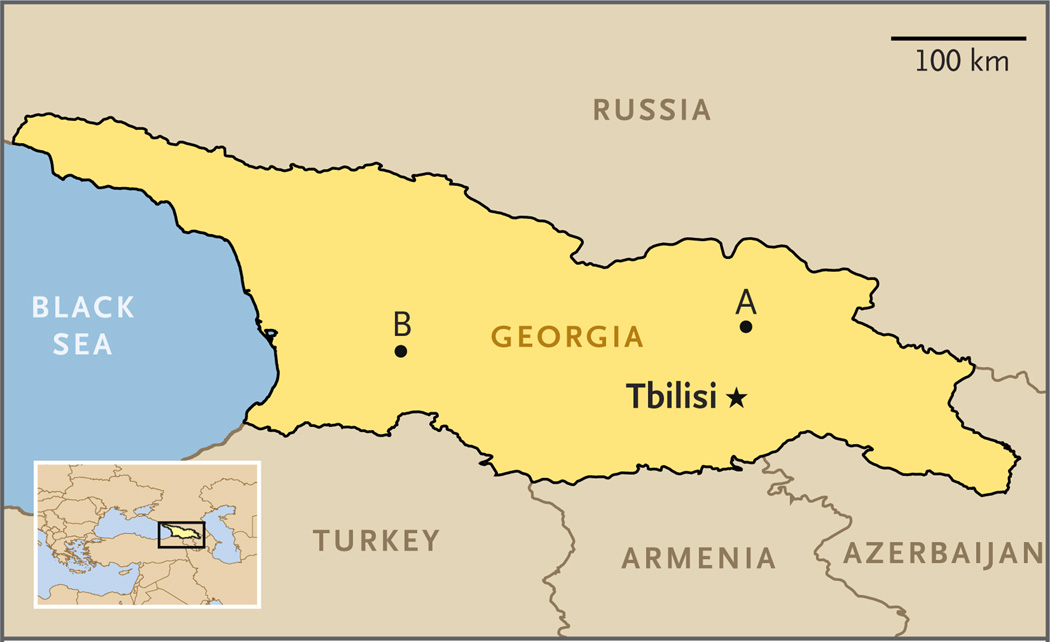
In June 2013, approximately 10 painful, pruritic lesions developed on both hands of a previously healthy 24-year-old man who had never received vaccination against smallpox. He lived in rural Georgia (Fig. 1), where he and three other men were responsible for a herd of 71 cows.
Figure 1. Location of Human Infections with the Novel Orthopoxvirus in Georgia.
The location marked A indicates where the two index patients began to have symptoms in 2013. The location marked B indicates where samples were obtained in 2010 from the patient in whom infection was diagnosed retrospectively.
Ten days before the onset of the patient’s illness, 10 cows had had lesions on their teats. All the cows fully recovered except for 1 cow in which there was contracture of a previously normal teat and decreased milk production (Fig. 2A).
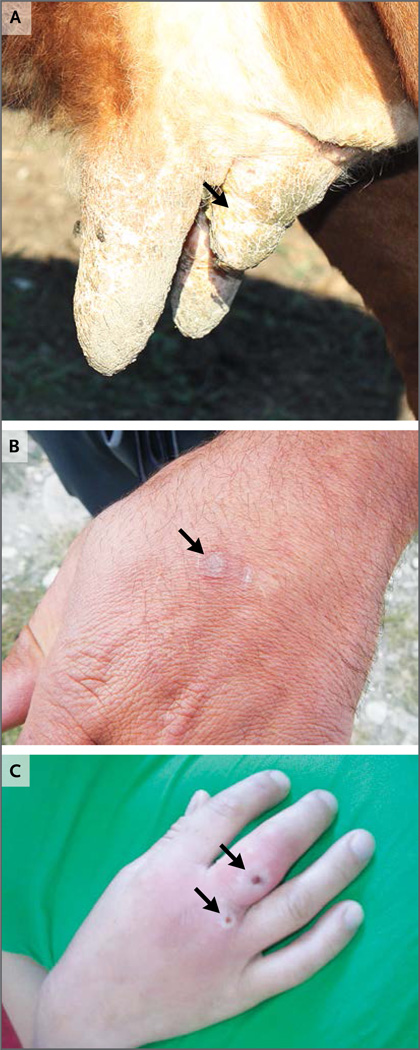
Figure 2. Clinical Manifestations and Sequelae of Infection with the Novel Orthopoxvirus.
Panel A shows a cow from the index herd tended to by the two index patients. The cow had lesions on its teats before the patients’ illnesses. After the cow’s illness, a previously normal teat became contracted (arrow) and had decreased milk production. Anti-orthopoxvirus IgG was detected in a serum sample obtained from this cow at an end-point titer of 1:400 or higher. Panel B shows a healed lesion (arrow) on the left hand of the first index patient approximately 3 months after the onset of illness. Panel C shows active lesions (arrows) on the right hand of a woman who received a retrospective diagnosis of infection with the novel virus. This photograph (courtesy of Maka Tsilosani) was taken in 2010 when the woman was ill.
Examination by a physician 2 weeks after the onset of the patient’s illness showed that the patient had a temperature of 39°C. Thick eschars were present, as was bilateral hand swelling and right axillary lymphadenopathy. The white-cell count was 6000 per cubic millimeter (35% neutrophils, 10% band forms, 50% lymphocytes, 2% eosinophils, and 3% monocytes). Cutaneous anthrax was not detected by means of laboratory testing.
Approximately 10 days after the first patient became ill, a lesion developed on each hand of a 36-year-old man who tended the same herd. This patient, who had an unremarkable medical history, reported that he had not received vaccination against smallpox. He had fever and chills on the same day that the lesions appeared; left axillary lymphadenopathy developed 5 days later. Given the patients’ history of exposure to ill cows and the presence of characteristic lesions, cowpox was suspected.
Methods
Diagnostic Testing of Patient Specimens
We used enzyme-linked immunosorbent assays (ELISAs) to detect anti-orthopoxvirus IgM and IgG in serum obtained from both index patients.11 Serum samples were considered to be positive for anti-orthopoxvirus IgG if there was a positive result on ELISA at a serum dilution of 1:160.
Material from cutaneous lesions was obtained with the use of clinical swabs. DNA was extracted from the swabs and screened with the use of quantitative real-time polymerase-chain-reaction (PCR) assays for non–variola virus orthopoxviruses.12 Additional quantitative PCR analyses for detection of specific orthopoxviruses (monkeypox, cowpox, and vaccinia viruses) were then performed to determine the species of the virus in the specimens that tested positive with the use of the screening assay.12–14 When a definitive determination of the species proved unsuccessful, primers targeting the highly conserved orthopoxvirus DNA-dependent RNA polymerase gene were used, and the PCR products were sequenced with the use of the Sanger method.15
Swab eluates were used to infect BSC-40 cells; DNA was extracted from cells that had an 80 to 100% cytopathic effect after one passage.12,13 Full genomic sequencing was performed at Otogenetics with the use of the Illumina HiSeq 2000 platform (Illumina). Sequence data were assembled into contigs with the use of CLC Genomics Workbench software (CLC Bio), and data were extracted from contigs on the basis of sequence similarity. Phylogenetic analysis was completed with the use of sequence data from nine genes located in the central conserved region of the orthopoxvirus genome.5
Epidemiologic Investigation
An investigation was initiated in the region where the two index patients lived to identify additional cases of infection in humans and animals and to determine the potential for human-to-human transmission. Persons who were considered to be at risk for infection, such as persons who worked with cattle (including cattle that belonged to herds other than the index herd) and persons who had come into physical contact with the index patients, were enrolled in the study in a convenience sample. Interviewees were asked whether they had had lesions that were suspicious for orthopoxvirus infection within the past year or whether they knew of other persons or animals with lesions.
Serum samples that were obtained from all consenting interviewees were tested for anti-orthopoxvirus IgM and IgG.11 We examined the heads and teats of the cows that were owned or tended by the interviewees, and we obtained serum samples from the cows when interviewees consented. The protocol underwent CDC human-subjects review and was determined not to be research involving human subjects; as such, approval by an institutional review board and written informed consent were not required.
Testing of Samples from Animals
To identify potential reservoirs for the virus, small mammals were captured in areas where the index herd had grazed during the week leading up to the initial illnesses in cows.16 The captured mammals underwent gross pathological examination, serum collection, and necropsy.17 DNA that was extracted from liver specimens was screened for non–variola virus orthopoxviruses by means of quantitative PCR.
Serum samples from cows and small mammals were tested for anti-orthopoxvirus IgG with the use of ELISA. ELISA plates were coated with the purified novel virus isolate, and serial dilutions of animal serum samples (up to 1:400) were added. Pierce Recombinant Protein A/G peroxidase conjugated at 1:5000 dilution was used as the secondary antibody.11
Retrospective Evaluation of Archived Clinical Specimens
Localized orthopoxvirus infections can resemble other cutaneous diseases such as anthrax. Thus, we obtained archived human clinical specimens that were originally submitted for diagnostic tests to detect anthrax but that had tested negative for anthrax.
We screened these specimens for non–variola virus orthopoxviruses with the use of quantitative PCR. Samples that tested positive underwent additional reactions to definitively determine the species of virus.
Results
Results of Diagnostic Tests of Patient Specimens
Anti-orthopoxvirus IgM and IgG were detected in serum samples obtained from both index patients approximately 3 weeks after the onset of illness. The IgG end-point titers were 1:640 and 1:2560.
Lesion specimens were obtained from both patients. These specimens were tested with the use of the non–variola virus orthopoxvirus screening quantitative PCR assay and yielded positive results (cycle-threshold values, 20.2 and 23.6). Subsequent assays to detect cowpox and vaccinia viruses were negative. Limited amplification occurred with the use of the monkeypox virus– specific assay (cycle-threshold values, 25.8 and 29.9). The amplified region of the DNA-dependent RNA polymerase gene shared 96% identity (490 of 513 nucleotides) with monkeypox virus Zaire 1979_005 (GenBank accession number, HM172544) and 98% identity (502 of 513 nucleotides) with cowpox virus UK2000_K2984 (GenBank accession number, HQ420900).15
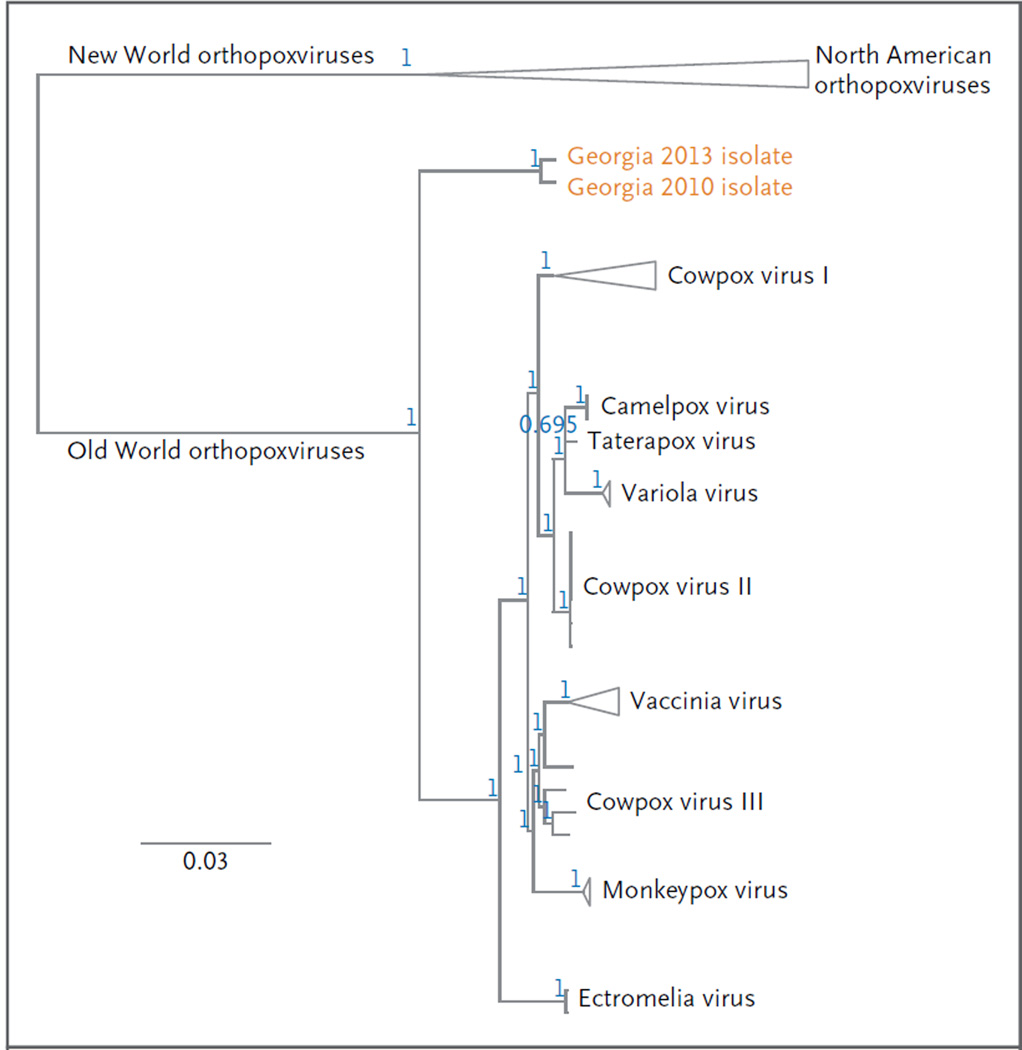
Viral DNA sequences from both patients were identical across nine conserved orthopoxvirus genes, with a total alignment length of 24,303 base pairs (GenBank accession numbers, KM046934–KM046942). Figure 3 shows the novel virus as a close relative to the remaining Old World orthopoxviruses.18, 19 Relationships within and between orthopoxvirus species were consistent with the current hypotheses regarding relationships among various orthopoxviruses, with high posterior probabilities supporting most nodes.
Figure 3. Phylogenetic Analysis of the Novel Orthopoxvirus.
The phylogenetic tree was constructed with the use of sequence data generated from the viruses that infected the patients in 2010 and 2013. The loci that were concatenated for phylogenetic analysis were vaccinia virus Copenhagen homologues A7L, A10L, A24R, D1R, D5R, E6R, E9L, H4L and J6R.5 The alignment was calculated with the use of Geneious software, version 6.1.2 (Biomatters); the Translation Align and ClustalW alignment options were implemented.18 The phylogenetic tree was inferred with the use of the MrBayes plug-in software, version 3.2.1, with a general time-reversible substitution model, inverse-gamma rate variation, chain length of 5 million generations, burn-in length of 1 million generations, subsampling every 1000 generations, and unconstrained branch lengths.19 The positions of the Georgian isolates were established by performing three successive phylogenetic analyses, from the subfamily level (Chordopoxvirinae) to the genus level (orthopoxvirus). Triangles indicate collapsed branches with a common origin. The position of the isolates from Georgia represents a deep divergence relative to other Eurasian isolates, suggesting a remote common ancestry with variola, monkeypox, vaccinia, and cowpox viruses (which probably diverged from one another more recently). The blue numbers at each node correspond to posterior probability values for each group. The scale bar corresponds to the number of substitutions per site.
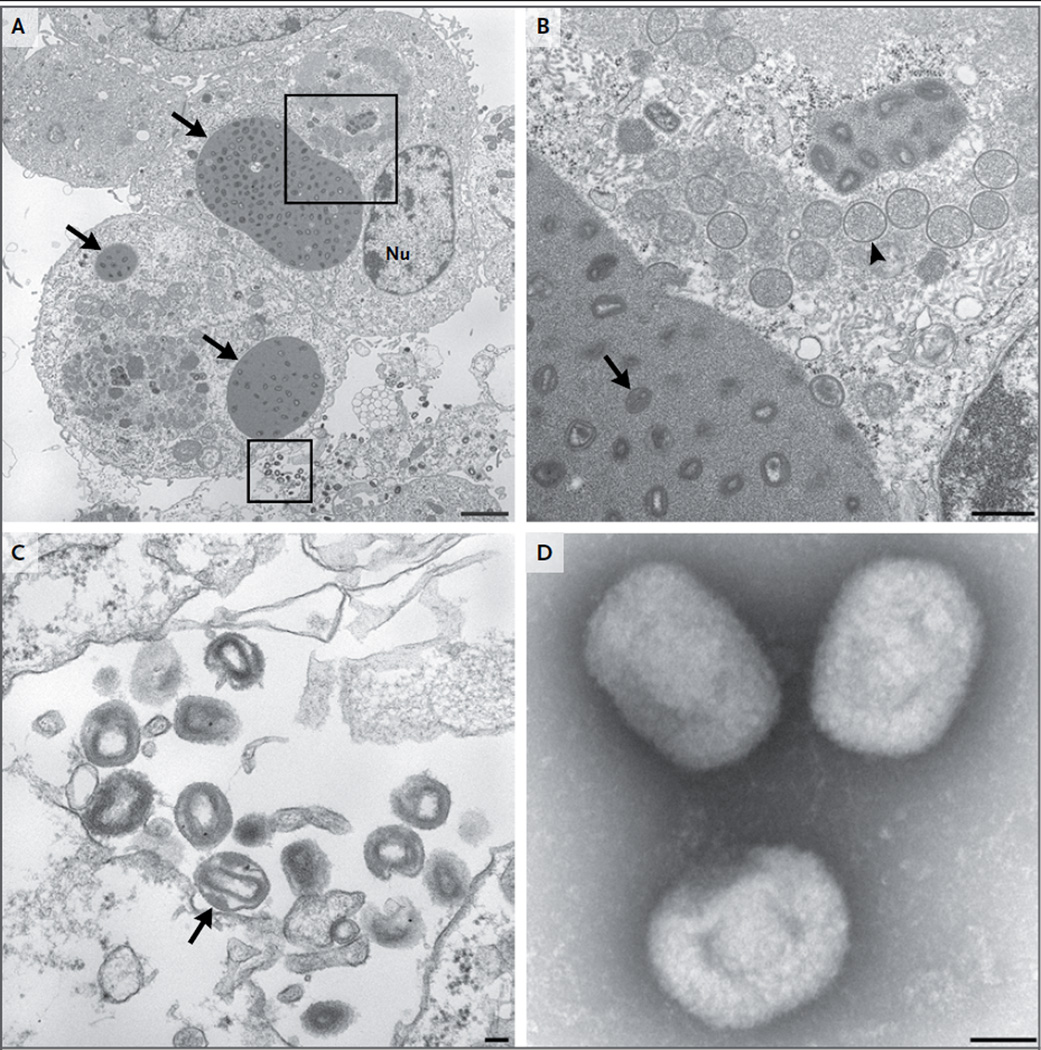
The virus was successfully cultured, and electron microscopy revealed viral particles that were characteristic of orthopoxviruses (Fig. 4).20, 21 This virus has been tentatively named Akhmeta virus in recognition of the region where it was identified.
Figure 4. The Novel Orthopoxvirus on Transmission Electron Microscopy.
Thin-section electron microscopy and negative-stain electron microscopy were performed, as previously described.20, 21 Panel A shows type A viral inclusion bodies (arrows) in infected cell-culture cells on thin-section electron microscopy. Nu denotes the cell nucleus. The scale bar represents 2 µm. Panel B shows the area in the upper box in Panel A at a higher magnification. Type A viral inclusion bodies, which are observed in infections caused by some orthopoxvirus species, are composed of proteinaceous material and mature virus particles (arrow). Type B viral inclusion bodies, also known as viral factories, are composed of spherical, immature virus particles (arrowhead). The scale bar represents 500 nm. Panel C shows a higher magnification of the extracellular mature virions shown in the area in the lower box in Panel A. A lateral body within a maturing virus particle (arrow) is shown. The scale bar represents 100 nm. Panel D shows brick-shaped virions, with short, whirled filaments on the surface on negativestain electron microscopy. The scale bar represents 100 nm.
Both patients had a full recovery except for scarring (Fig. 2B). Serologic testing 3 months after their original illnesses revealed the presence of anti-orthopoxvirus IgM and IgG (IgG endpoint titers in both patients, 1:2560).
Results of Epidemiologic Investigation
A total of 55 persons were interviewed during the epidemiologic investigation, but no additional cases of orthopoxvirus infection in humans were identified (Table 1). Serum samples were obtained from 37 interviewees. Although anti-orthopoxvirus IgM was not detected in any of the serum specimens, anti-orthopoxvirus IgG was detected in serum samples obtained from 22 persons (59%), 3 of whom reported no history of smallpox vaccination. Additional details are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Table 1.
Characteristics of At-Risk Persons Interviewed during the Epidemiologic Investigation of the Novel Orthopoxvirus Outbreak in Georgia.*
| Characteristic | Value |
|---|---|
| All persons interviewed | |
| Male sex — no./total no. (%) | 39/55 (71) |
| Reason for inclusion in investigation — no./total no. (%) | |
| Farmer, but did not own or herd domestic animals | 4/55 (7) |
| Owner or herder of domestic animals | 43/55 (78) |
| Veterinarian | 3/55 (5) |
| Household contact of an index patient | 5/55 (9) |
| Persons for whom age and history of smallpox vaccination were known | |
| Age — yr | |
| Median | 51 |
| Range | 14–88 |
| Born before 1981 — no./total no. (%) | 38/47 (81) |
| Smallpox vaccination status | |
| Reported vaccination — no./total no. (%) | 29/47 (62) |
| Born before 1981 — no./total no. (%) | 29/29 (100) |
| No reported vaccination — no./total no. (%) | 18/47 (38) |
| Born before 1981 — no./total no. (%) | 9/18 (50) |
| Persons from whom serum samples were obtained | |
| Anti-orthopoxvirus IgM detected — no./total no. | 0/37 |
| Anti-orthopoxvirus IgG detected — no./total no. (%) | 22/37 (59) |
| No reported smallpox vaccination — no./total no. (%) | 3/22 (14) |
| Born before 1981 — no./total no. (%) | 21/22 (95) |
Routine smallpox vaccination was discontinued in Georgia before 1981. Percentages may not sum to 100 because of rounding.
No lesions that were indicative of active infection were identified in cows. Serum samples were obtained from 11 cows in the index herd, 4 of which were reported to have had teat lesions in June 2013. Anti-orthopoxvirus IgG was detected in serum samples at end-point titers of 1:400 or higher in 9 cows (including the 4 cows that were reported to have been ill previously) and at endpoint titers of 1:200 in 2 cows.
Serum samples were obtained from 24 cows in other herds in which illness had not been reported. Anti-orthopoxvirus IgG was detected in serum obtained from only 1 of these cows (endpoint titer, 1:200).
Results of Tests of Samples from Small Mammals
Three shrews and 31 rodents were captured (Table S1 in the Supplementary Appendix). None of the animals had gross or molecular evidence of orthopoxvirus infection. However, anti-orthopoxvirus IgG was detected in serum samples obtained from all 3 shrews and 9 rodents.
Results of Retrospective Evaluation of Archived Clinical Specimens
We tested 154 archived human specimens that had been obtained during the period from 2009 through 2014. One specimen from the year 2010 was positive for non–variola virus orthopoxviruses. This isolate shared 99.4% sequence identity (257 nucleotide differences) with the isolates from the two index patients across nine conserved genes (GenBank accession numbers, KM053243–KM053251), and together these isolates form a unique clade (Fig. 3).
This specimen was obtained from a woman who owned cattle and who was born after the discontinuation of routine smallpox vaccination in Georgia. She had lesions on her hand (Fig. 2C), fever, and lymphadenopathy during her illness. She lived more than 250 km away from the original two index patients.
Discussion
The two index patients described here became ill after occupational exposure to ill cows. Although cowpox was initially suspected, subsequent molecular analyses confirmed that the infection was caused by a novel orthopoxvirus species. This unique virus is the deepest branching lineage of all known Old World orthopoxviruses. Our findings raise important questions about the control of orthopoxviruses through public health efforts in the post–smallpox vaccination era, and they underscore the need to improve clinical awareness of poxviruses.
Misdiagnoses of orthopoxvirus infections are likely to occur if clinical criteria alone are applied without laboratory testing.4 For example, humans who have orthopoxvirus infections after contact with sick cows might receive a “cowpox” diagnosis, but this practice has given rise to a taxonomically inaccurate organization of distinct orthopoxvirus species into a single group known as “cowpox virus.”5,22 Whether the clinical heterogeneity that is sometimes observed in patients with a cowpox diagnosis is due to the fact that some infections are caused by an orthopoxvirus that is not a cowpox virus has yet to be determined.7 We cannot draw conclusions on the full spectrum of disease caused by this novel virus. However, it is notable that the first patient, who did not have risk factors for severe infection with either the cowpox virus or vaccinia virus, had a burden of lesions in excess of that commonly seen with other zoonotic orthopoxviruses (with the exception of monkeypox virus).7,14,23–25
Although we found no evidence of human-to-human transmission of this virus, the index patients’ history of exposure to ill cows and the serologic data from cows suggest that it is zoonotic. Cows are not routinely vaccinated against orthopoxviruses, and detection of anti-orthopoxvirus IgG in cows is therefore indicative of exposure to a naturally circulating orthopoxvirus. Unfortunately, our serologic assays do not distinguish among various species of orthopoxviruses, and the seropositive cows could have been exposed to an orthopoxvirus other than this novel virus.
As is the case for several other zoonotic orthopoxviruses, we suspect that cows are an incidental host and that the natural reservoirs are small mammals.8–10,26,27 Our detection of anti-orthopoxvirus IgG in rodents and shrews provides support for this hypothesis and is consistent with previous findings in Georgia.16,28 Data that would better characterize the ecologic characteristics of this virus are lacking. Such data, which might shed light on interventions to protect public health, could also help to identify the effects of this virus on agriculture.
Vaccination against smallpox provides cross-protection against an array of orthopoxviruses, but more than half the current world population was born after the global discontinuation of routine vaccination against smallpox in 1980.2,29,30 This waning population immunity against orthopoxviruses could facilitate the emergence of non–variola virus orthopoxviruses such as monkeypox virus.3,4,22,25,30,31 It is possible that vaccination against smallpox confers protection against this virus as well, since both of the index patients had a humoral response with antibodies known to cross-react against other members of this genus. Furthermore, the three cases described here all occurred in persons without a known history of smallpox vaccination.
Our findings highlight the need to strengthen global surveillance for orthopoxvirus infections in both humans and animals. The majority of modern cowpox virus infections have been reported in Europe; however, as shown by the cases described here, similar orthopoxvirus infections occur across a broader geographic range and are probably the “tip of the iceberg.”32 It is fortuitous that the two index patients came to the attention of the National Center for Disease Control and Public Health and the CDC. These cases speak to the importance of training physicians and veterinarians to identify poxvirus infections.25 Our retrospective identification of a third human case in 2010 provides support for our concern that this virus had caused unrecognized human infections across Georgia for years.
Future surveillance will benefit from the use of a syndromic approach, rather than an exclusively pathogen-specific approach, for nonhealing cutaneous lesions. As surveillance improves, so will our understanding of the genetic diversity of this genus, which is essential for the development of reliable diagnostic tests. The ability to rapidly identify an orthopoxvirus is critical for global health security because this genus includes potential agents of bioterrorism. Thus, it is notable that this novel virus cross-reacted with currently available rapid diagnostic tests for monkeypox virus, which in turn led to delays in definitively identifying the etiologic agent.3,25,31
Finally, our analysis suggests that this virus has remote common ancestry with variola, monkeypox, vaccinia, and cowpox viruses (all of which probably diverged from each other more recently). The unique phylogenetic position of this virus may provide insights about factors that determine the virulence of orthopoxviruses.22
Supplementary Material
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC.
We thank the patients and other persons who agreed to be interviewed during this investigation. The following persons provided essential assistance during the field investigation and subsequent laboratory testing of samples: Tamuna Bolkvadze, Whitni Davidson, Jeffrey Doty, Christopher Duggar, Amiran Gamkrelidze, M.D., Paata Imnadze, M.D., Lali Madzgarashvili, Matthew Mauldin, Ph.D., Yoshinori Nakazawa, Ph.D., Irakli Sikharulidze, M.D., Misha Sokhadze, D.V.M., Shota Tsanava, M.D., Giorgi Turabelidze, Olga Urazova, and Kimberly Wilkins. We also thank Yoshinori Nakazawa for assistance with figures.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Dhar AD, Werchniak AE, Li Y, et al. Tanapox infection in a college student. N Engl J Med. 2004;350:361–366. doi: 10.1056/NEJMoa031467. [DOI] [PubMed] [Google Scholar]
- 2.Pahlitzsch R, Hammarin AL, Widell A. A case of facial cellulitis and necrotizing lymphadenitis due to cowpox virus infection. Clin Infect Dis. 2006;43:737–742. doi: 10.1086/506937. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds MG, Damon IK. Outbreaks of human monkeypox after cessation of smallpox vaccination. Trends Microbiol. 2012;20:80–87. doi: 10.1016/j.tim.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Shchelkunov SN. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 2013;9(12):e1003756. doi: 10.1371/journal.ppat.1003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll DS, Emerson GL, Li Y, et al. Chasing Jenner’s vaccine: revisiting cowpox virus classification. PLoS One. 2011;6(8):e23086. doi: 10.1371/journal.pone.0023086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross CP, Sepkowitz KA. The myth of the medical breakthrough: smallpox, vaccination, and Jenner reconsidered. Int J Infect Dis. 1998;3:54–60. doi: 10.1016/s1201-9712(98)90096-0. [DOI] [PubMed] [Google Scholar]
- 7.Baxby D, Bennett M, Getty B. Human cowpox 1969–93: a review based on 54 cases. Br J Dermatol. 1994;131:598–607. doi: 10.1111/j.1365-2133.1994.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 8.Bennett M, Crouch AJ, Begon M, et al. Cowpox in British voles and mice. J Comp Pathol. 1997;116:35–44. doi: 10.1016/s0021-9975(97)80041-2. [DOI] [PubMed] [Google Scholar]
- 9.Baxby D. Is cowpox misnamed? A review of 10 human cases. Br Med J. 1977;1:1379–1381. doi: 10.1136/bmj.1.6073.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chantrey J, Meyer H, Baxby D, et al. Cowpox: reservoir hosts and geographic range. Epidemiol Infect. 1999;122:455–460. doi: 10.1017/s0950268899002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karem KL, Reynolds M, Braden Z, et al. Characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–872. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Zhao H, Wilkins K, Hughes C, Damon IK. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169:223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCollum AM, Austin C, Nawrocki J, et al. Investigation of the first laboratory-acquired human cowpox virus infection in the United States. J Infect Dis. 2012;206:63–68. doi: 10.1093/infdis/jis302. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Meyer H, Zhao H, Damon IK. GC content-based pan-pox universal PCR assays for poxvirus detection. J Clin Microbiol. 2010;48:268–276. doi: 10.1128/JCM.01697-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsanava SA, Sakvarelidze LA, Shelukhina EM. Serologic survey of wild rodents in Georgia for antibodies to orthopoxviruses. Acta Virol. 1989;33:91. [PubMed] [Google Scholar]
- 17.Reynolds MG, Carroll DS, Olson VA, et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: evidence for multi-species involvement in the absence of widespread human disease. Am J Trop Med Hyg. 2010;82:746–754. doi: 10.4269/ajtmh.2010.09-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearse M, Moir R, Wilson A, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 20.Goldsmith CS, Whistler T, Rollin PE, et al. Elucidation of Nipah virus morphogenesis and replication using ultrastructural and molecular approaches. Virus Res. 2003;92:89–98. doi: 10.1016/s0168-1702(02)00323-4. [DOI] [PubMed] [Google Scholar]
- 21.Emerson GL, Nordhausen R, Garner MM, et al. Novel poxvirus in big brown bats, northwestern United States. Emerg Infect Dis. 2013;19:1002–1004. doi: 10.3201/eid1906.121713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabrowski PW, Radonić A, Kurth A, Nitsche A. Genome-wide comparison of cowpox viruses reveals a new clade related to Variola virus. PLoS One. 2013;8(12):e79953. doi: 10.1371/journal.pone.0079953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damle AS, Gaikwad AA, Patwardhan NS, Duthade MM, Sheikh NS, Deshmukh DG. Outbreak of human buffalopox infection. J Glob Infect Dis. 2011;3:187–188. doi: 10.4103/0974-777X.81698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campe H, Zimmermann P, Glos K, et al. Cowpox virus transmission from pet rats to humans, Germany. Emerg Infect Dis. 2009;15:777–780. doi: 10.3201/eid1505.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frey SE, Belshe RB. Poxvirus zoonoses — putting pocks into context. N Engl J Med. 2004;350:324–327. doi: 10.1056/NEJMp038208. [DOI] [PubMed] [Google Scholar]
- 26.da Fonseca FG, Trindade GS, Silva RL, Bonjardim CA, Ferreira PC, Kroon EG. Characterization of a vaccinia-like virus isolated in a Brazilian forest. J Gen Virol. 2002;83:223–228. doi: 10.1099/0022-1317-83-1-223. [DOI] [PubMed] [Google Scholar]
- 27.Tryland M, Sandvik T, Mehl R, Bennett M, Traavik T, Olsvik O. Serosurvey for orthopoxviruses in rodents and shrews from Norway. J Wildl Dis. 1998;34:240–250. doi: 10.7589/0090-3558-34.2.240. [DOI] [PubMed] [Google Scholar]
- 28.Tsanava ShA, Marennikova SS, Sakvarelidze LA, Shelukhina EM, Ianova NN. Isolation of cowpox virus from the red-tailed jird. Vopr Virusol. 1989;34:95–97. (In Russian.) [PubMed] [Google Scholar]
- 29.Berthet N, Nakouné E, Whist E, et al. Maculopapular lesions in the Central African Republic. Lancet. 2011;378:1354. doi: 10.1016/S0140-6736(11)61142-2. [DOI] [PubMed] [Google Scholar]
- 30.Mombouli JV, Ostroff SM. The remaining smallpox stocks: the healthiest outcome. Lancet. 2012;379:10–12. doi: 10.1016/S0140-6736(11)60694-6. [DOI] [PubMed] [Google Scholar]
- 31.Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinnunen PM, Henttonen H, Hoffmann B, et al. Orthopox virus infections in Eurasian wild rodents. Vector Borne Zoonotic Dis. 2011;11:1133–1140. doi: 10.1089/vbz.2010.0170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.