Abstract
INTRODUCTION
High-sensitivity cardiac troponin assays have potent prognostic value in stable cardiovascular disease cohorts. Our objective was to assess the prognostic utility of a novel cardiac troponin I (cTnI) high-sensitivity assay, independently and in combination with amino-terminal pro–B-type natriuretic peptide (NT-proBNP), for the future development of heart failure and mortality in the general community.
METHODS
A well-characterized community-based cohort of 2042 participants underwent clinical assessment and echocardiographic evaluation. Baseline measurements of cTnI with a high-sensitivity assay and NT-proBNP were obtained in 1843 individuals. Participants were followed for new-onset heart failure and mortality with median (25th, 75th percentile) follow-up of 10.7 (7.9, 11.6) and 12.1 (10.4, 13.0) years, respectively.
RESULTS
When measured with a high-sensitivity assay, cTnI greater than the sex-specific 80th percentile was independently predictive of heart failure [hazard ratio 2.56 (95% confidence interval 1.88 – 3.50), P < 0.001] and mortality [1.91(1.49 – 2.46), P < 0.001] beyond conventional risk factors in this community-based cohort, with significant increases in the net reclassification improvement for heart failure. The prognostic utility of cTnI measured with a high-sensitivity assay goes beyond NT-proBNP, yet our data suggest that these 2 assays are complementary and most beneficial when evaluated together in identifying at-risk individuals in the community.
CONCLUSIONS
Our findings lay the foundation for prospective studies aimed at identification of individuals at high risk by use of a multimarker approach, followed by aggressive prevention strategies to prevent subsequent heart failure.
High-sensitivity cardiac troponin assays manifest potent prognostic value in stable cardiovascular disease cohorts such as those with heart failure (HF)5 and can be deployed to predict an increased propensity to the development of HF in the general population (1, 2–5). This predictive value is based in part on the fact these high-sensitivity assays measure values of cardiac troponin in nearly all patients, in contrast to conventional assays (6). Higher values of high-sensitivity assay cardiac troponin in putatively healthy individuals seem to represent subtle cardiovascular comorbidities and pathophysiologic pathways or processes that increase the risk for subsequent development of HF (7). We have previously reported the distribution and determinants of cardiac troponin I (cTnI) assessed by a novel high-sensitivity assay in a well-characterized community-based cohort (7). The objective of the current study was to assess the prognostic utility of this novel high-sensitivity assay for cTnI to predict the development of HF and/or mortality in this cohort. We also evaluated the extent to which amino-terminal pro–B-type natriuretic peptide (NT-proBNP), a well-established prognostic marker of HF and mortality in the general community, augments the prognostic abilities of cTnI as measured with a high-sensitivity assay.
Methods
The Mayo Foundation and Olmsted Medical Center Institutional Review Boards approved this study.
STUDY POPULATION
With the resources of the Rochester Epidemiology Project, a random sample of 2042 Olmsted County, Minnesota, residents age≥45 was identified from 1997 to 2000. The design and selection criteria of this community-based cohort study as well as the characteristics of the Olmsted County population have been previously described (8–11). Of the 2042 total participants, 39 (1.9%) were excluded due to symptomatic HF [stage C and D HF by American College of Cardiology/American Heart Association (ACC/AHA) guidelines (12)]. Of the remaining 2003 individuals, a comprehensive history, physical examination, echocardiogram, and samples for measurement of cTnI by high-sensitivity assay and NT-proBNP were obtained in 1843 (93%), and only these individuals are included in these analyses.
MEDICAL RECORD REVIEW AND OUTCOME MEASURES
All Olmsted County, Minnesota, healthcare providers have maintained a unified medical record, which is indexed by the Rochester Epidemiology Project. Each participant underwent a focused physical examination that included measurement of blood pressure, height, and weight. Trained nurse abstractors reviewed each participant’s medical record, and each subject completed medication questionnaires. Body mass index (BMI), myocardial infarction, and coronary artery disease (CAD) were defined by use of established criteria as previously described (10). Diabetes was defined as a fasting glucose>126 mg/dL (7.0 mmol/L) or a diagnosis in the medical record. Criteria for hypertension included at least 1 of the following: 1) systolic blood pressure>140 mmHg, 2) diastolic blood pressure >90 mmHg, and 3) diagnosis in the medical record with concomitant antihypertensive medical therapy. Hyperlipidemia was defined as total cholesterol>200 mg/dL (>5.2 mmol/L) or a diagnosis of hyperlipidemia in the medical record. Smoking status was defined as never, prior, or active. Glomerular filtration rate was estimated by use of the Modification of Diet in Renal Disease (13) formula. Participants were followed from enrollment (August 1997 to September 2000) until last known follow-up through March 2012, at which time they were censored. This provided a median (25th, 75th percentile) follow-up of 12.1 (10.4, 13.0) years for mortality. Participants were also followed for the incidence of HF. HF was defined as International Classification of Diseases, Revision 9 codes 402 or 428 as previously reported (14, 15). The median (25th, 75th percentile) follow-up for HF was 10.7 (7.9, 11.6) years.
DOPPLER ECHOCARDIOGRAPHY
In Olmsted County, all echocardiograms are performed and interpreted in the Mayo Clinic Echocardiographic Laboratory. M-mode, 2-dimensional (2D), Doppler, and Doppler tissue imaging were performed according to guidelines of the American Society of Echocardiography (16). Digital echocardiographic data containing a minimum of 3 consecutive beats (5 in atrial fibrillation) were acquired and transferred to a server for storage and archiving (ProSolv Echo Management System, Problem Solving Concepts). In each participant, ejection fraction was measured by M-mode by use of the modified Quinones formula, quantitative 2D (biplane Simpson), and semiquantitative 2D (visual estimate), as described previously (16, 17). We performed 2D and color Doppler imaging to screen for valvular disease, defined as greater than mild stenosis or regurgitation of 1 or more valves as defined by the American Society of Echocardiography (18, 19). Pulsed-wave Doppler examination of mitral (before and with Valsalva maneuver) and pulmonary venous inflow, as well as Doppler tissue imaging of the mitral annulus, was performed in each subject. Diastolic function was categorized as normal, mildly impaired (defined as impaired relaxation without evidence of increase filling pressures), moderately impaired (defined as impaired relaxation associated with moderate elevation of filling pressures or pseudonormal filling), and severely impaired (defined as advanced reduction in compliance) (10, 20). Diastolic dysfunction was defined as moderate or severe dysfunction. Left ventricular mass was calculated according to the Devereux formula (21) and indexed to body surface area. The presence of left ventricular hypertrophy was defined on the basis of left ventricular mass index >130 g/m2 for men and >100 g/m2 for women (22). The presence of left atrial (LA) enlargement was defined as LA volume index >33 mL/m2 in men and >30 mL/m2 in women (23).
BIOMARKER ASSESSMENT
Blood was collected on the same day as the echocardiogram in heparin (for high-sensitivity cTnI assay) and EDTA (for NT-proBNP assay) Vacutainer tubes, placed on ice, centrifuged within 2 h, separated into multiple aliquots, and placed in a freezer at - 80 °C. We used a new, never-thawed aliquot for each assay. We measured cTnI data with a prototype high-sensitivity assay on the Dimension Vista® 1500 System (Siemens Healthcare Diagnostics) as previously described (7). The limit of detection for cTnI with this high-sensitivity assay was 0.8 ng/L. Detectable cTnI concentrations (≥0.8 ng/L) were present in 1716 of the 1843 (93%) participants, with a median (25th, 75th percentile) value of 3 (2, 5) ng/L and a mean (SD) value of 7 (20) ng/L. Functional sensitivity for the assay at a 10% CV was 3.0 ng/L. Total imprecision (CV) was 8.5% at 4.4 ng/L and 4.6% at 11.8 ng/L (24). In a well-characterized healthy cohort (n = 565) from the current study population, the 95th and 99th percentile (95% CI) values were 6 (4–13) and 33 (22–155) ng/L for females and 17 (9–36) and 55 (32–124) ng/L for males, respectively (7). We measured plasma NT-proBNP with the Elecsys proBNP electrochemiluminescence immunoassay on the Elecsys 2010 (Roche Diagnostics) as previously described (11, 25, 26). The lower limit of detection is 5 pg/mL, with interassay and intraassay variability of 3.1% at 2021 pg/mL and 2.5% at 388 pg/mL, respectively.
STATISTICAL ANALYSIS
Continuous variables were expressed as a mean (SD) and categorical variables as n (%). Due to the skewed distributions of the high-sensitivity assay cTnI and NTproBNP results, analyses were performed after log transformation of these variables, and statistical analysis that confirmed the variables then met criteria for use with normalized statistical approaches. We estimated survival and event-free rates using the Kaplan–Meier method. The association of outcomes with clinical and echocardiographic variables and high-sensitivity assay cTnI concentrations were assessed by use of Cox proportional hazards regression. Hazard ratios (HRs) and 95% CIs were calculated (a) from unadjusted analyses, (b) after adjustment for age and sex, and (c) from a multivariable model (multivariable model 1) with conventional cardiovascular risk factors including age, sex, BMI, systolic blood pressure, creatinine (log), smoking status, and presence of hypertension, diabetes mellitus, and CAD. A final model (multivariable model 2) included both conventional cardiovascular risk factors and echocardiographic abnormalities (ejection fraction <50%, diastolic dysfunction, valvular dysfunction, left ventricular hypertrophy, LA enlargement, and wall motion abnormalities). HRs for continuous high-sensitivity assay cTnI are presented as per 1SD increment after logarithmic transformation. We also analyzed high-sensitivity assay cTnI and NT-proBNP results as dichotomous variables defined as being above the sex-specific 80th percentile. We chose the 80th percentile because it allows for selection of a high-risk cohort, has implications for an interventional trial, and is consistent with partition values in the biomarker literature (14, 27). We also provide data on other cutoff values in Supplemental Table 1, which accompanies the online version of this article at http://www.clinchem.org/content/vol60/issue9. Survival c-statistics were calculated by use of timeto- event data, to evaluate the incremental improvement in discrimination with high-sensitivity assay cTnI for mortality and HF. High-sensitivity assay cTnI was added to 2 multivariable models: first to multivariable model 1, which included traditional cardiovascular risk factors, and second to multivariable model 1 with the addition of NT-proBNP. Differences between survival c-statistics were calculated on the basis of 1000 bootstrap samples, and P values and CIs for these differences were also calculated. Integrated discrimination improvement (IDI) and net reclassification improvement (NRI) for the addition of the logarithm of high-sensitivity assay cTnI to multivariable model 1 were calculated according to the methods of Pencina et al. (28). All IDI and NRI calculations were done within the Cox proportional hazards framework, which extends these measures appropriately for time-to-event data. Because NRI calculations are highly sensitive to chosen cut points, and because there are no prespecified cut points for long-term follow-up in individuals with these specific outcomes, the category-free NRI (NRI >0) was used. The NRI was divided into “event NRI” and “nonevent NRI” so that they could be individually evaluated. The event NRI is the amount of correct reclassification that occurred among participants who had events, whereas the nonevent NRI is the amount of correct reclassification among participants who did not have events.
Results
STUDY POPULATION
Characteristics of the total study population (n =1843) are shown in Table 1. The mean age was 62 years, and 52% of participants were female. Median (25th, 75th percentile) high-sensitivity assay cTnI and NTproBNP values were 3 (2, 5) and 69 (28, 149) ng/L, respectively.
Table 1.
Baseline characteristics of the total population.a
| Characteristic | Study population |
|---|---|
| n | 1843 |
| Age, years | 62 (53, 71) |
| Women | 52 |
| BMI, kg/m2 | 28 (25, 31) |
| Systolic blood pressure, mmHg | 131 (116, 146) |
| Diastolic blood pressure, mmHg | 73 (67, 80) |
| Hypertension | 28 |
| Serum creatinine, mg/dL | 0.8 (0.7, 1.0) |
| Glomerular filtration rate, mL/min | 81 (71, 89) |
| Total cholesterol, mg/dL | 201 (179, 225) |
| HDL cholesterol, mg/dL | 43 (36, 55) |
| NT-proBNP, ng/L | 69 (28, 149) |
| High-sensitivity cTnI, ng/L | 3 (2, 5) |
| Diabetes | 7 |
| Coronary artery disease | 12 |
| Ejection fraction | 65 (60, 68) |
| Ejection fraction <50% | 4 |
| Diastolic dysfunction | 5 |
| Left atrial volume index, mL/m2 | 44 (36, 55) |
| Left ventricular mass index, g/m2 | 94 (82, 109) |
Data are median (25th, 75th percentile) or %. For conversion of traditional units to SI units, multiply by 0.0259 for total cholesterol (mmol/L) and 88.4 for serum creatinine (μmol/L).
PROGNOSTIC VALUE OF HIGH-SENSITIVITY cTnI
High-sensitivity assay cTnI was significantly higher in individuals who developed HF compared with those who did not (5.8 vs 2.6 ng/L, P < 0.0001) and in individuals who died compared with those who did not (5.8 vs 2.5 ng/L, P < 0.0001).
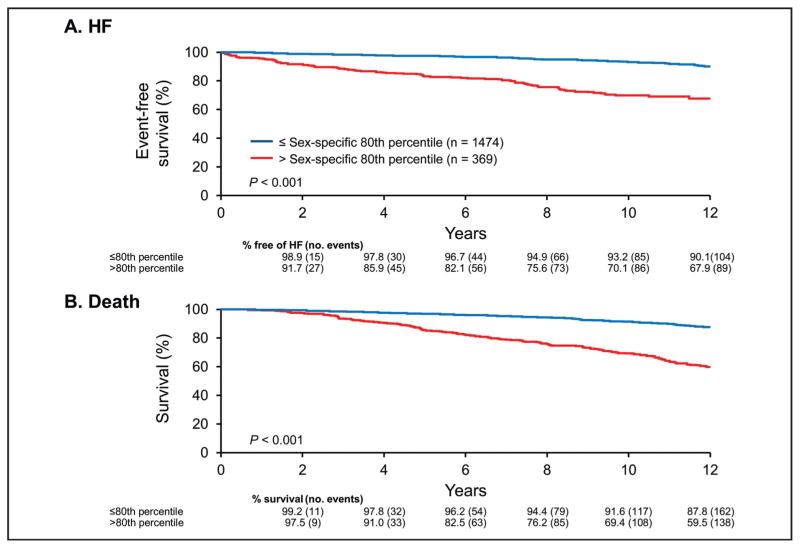
HF events (Fig. 1A) and mortality (Fig. 1B) significantly increased among participants with a high-sensitivity assay cTnI concentration above the sex-specific 80th percentile (males, cTnI > 7.8 ng/L; females, cTnI > 4.9 ng/L) compared with participants with concentrations less than the 80th percentile. HRs for high-sensitivity assay cTnI as both a continuous (1SD increment) and a categorical (above the sex-specific 80th percentile) variable are shown in Table 2. In unadjusted models, high-sensitivity assay cTnI concentrations were significantly associated with HF and mortality. High-sensitivity assay cTnI remained predictive for the development of HF and/or mortality in models adjusted for age, sex, and conventional cardiovascular risk factors including hypertension, diabetes mellitus, BMI, smoking status, and creatinine. Importantly, even after adjustment for conventional risk factors and cardiac structure and functional abnormalities assessed by echocardiography, high-sensitivity assay cTnI retained its independent prognostic utility for the prediction of HF when analyzed as a continuous variable.
Fig. 1. Kaplan–Meier curves for the unadjusted cumulative incidence in the sample population (n = 1843) for HF (A) and mortality (B) according to high-sensitivity cTnI values above (red line) and below (blue line) the sex-specific 80th percentile.
Follow-up results are truncated after 12 years.
Table 2.
HF and mortality according to baseline high-sensitivity cTnI.a
| Outcome | HR per 1SD increment in log high-sensitivity cTnI values (95% CI) | P | HR for high-sensitivity cTnI values >80th percentile (95% CI) | P |
|---|---|---|---|---|
| HF | ||||
| Unadjusted | 1.91 (1.70–2.15) | <0.0001 | 4.59 (3.48–6.07) | <0.0001 |
| Age and sex | 1.70 (1.47–1.96) | <0.0001 | 3.02 (2.25–4.06) | <0.0001 |
| Model 1 | 1.62 (1.38–1.90) | <0.0001 | 2.56 (1.88–3.50) | <0.0001 |
| Model 2 | 1.33 (1.04–1.69) | 0.023 | 1.43 (0.92–2.28) | 0.114 |
| Model 3 | 1.42 (1.20–1.68) | <0.0001 | 2.04 (1.46–2.83) | <0.0001 |
| Mortality | ||||
| Unadjusted | 1.79 (1.64–1.96) | <0.0001 | 4.17 (3.35–5.19) | <0.0001 |
| Age and sex | 1.43 (1.26–1.62) | <0.0001 | 2.12 (1.67–2.68) | <0.0001 |
| Model 1 | 1.36 (1.18–1.56) | <0.0001 | 1.91 (1.49–2.46) | <0.0001 |
| Model 2 | 1.12 (0.90–1.39) | 0.330 | 1.53 (1.07–2.17) | 0.019 |
| Model 3 | 1.15 (0.98–1.33) | 0.075 | 1.41 (1.08–1.85) | 0.012 |
Model 1 was adjusted for age, sex, systolic blood pressure, creatinine (log), BMI, smoking status, and presence of hypertension, diabetes, and coronary artery disease. Model 2 was adjusted for all variables in Model 1 plus ejection fraction <50%, moderate/severe diastolic dysfunction, left ventricular hypertrophy, left atrial enlargement, and presence of wall motion abnormalities. Model 3 was adjusted for all variables in Model 1 plus NT-proBNP.
For predicting HF, the addition of high-sensitivity assay cTnI to conventional cardiovascular risk factors improved discrimination, with an increase in the area under the curve (AUC) (c-statistic) from 0.774 to 0.793 (P = 0.02) and a significant IDI (IDI 0.014, P < 0.001, relative IDI 65.7%) and NRI [NRI 45% (P < 0.001), correct reclassification of events (13%) and nonevents (32%)]. For predicting mortality, the addition of high-sensitivity assay cTnI to conventional cardiovascular risk factors improved discrimination, with an increase in the AUC (c-statistic) from 0.813 to 0.816 (P = 0.19) and a significant IDI (IDI 0.008, P = 0.001, relative IDI 8.8%) and NRI [NRI 25% (P < 0.001), correct reclassification of events (2%) and nonevents (23%)].
ADDITIVE PROGNOSTIC UTILITY OF HIGH-SENSITIVITY ASSAY cTnI BEYOND NT-proBNP
We assessed the additive prognostic utility of high-sensitivity assay cTnI to NT-proBNP, a highly prognostic biomarker for HF and mortality (Table 2). When high-sensitivity assay cTnI was added to the model of conventional risk factors and NT-proBNP, there was significant additive prognostic ability of the model for HF and mortality. For predicting HF, the addition of high-sensitivity assay cTnI to conventional cardiovascular risk factors and NT-proBNP improved discrimination, with an increase in the AUC (c-statistic) from 0.796 to 0.809 (P=0.06) and a significant IDI (IDI 0.008, P=0.01, relative IDI 17.9%) and NRI [NRI 33% (P < 0.001), correct reclassification of events (10%) and nonevents (23%)]. For predicting mortality, the addition of high sensitivity assay cTnI to conventional cardiovascular risk factors and NT-proBNP did not significantly improve discrimination, with no increase in the AUC (c-statistic) (0.828 to 0.828; P=0.92) and lack of a significant IDI (IDI 0.002, P = 0.08, relative IDI 1.7%) or NRI [NRI 6% (P = 0.38), incorrect reclassification of mortality events (−4%) and correct classification of nonevents (10%)].
COMBINED PROGNOSTIC UTILITY OF HIGH-SENSITIVITY ASSAY cTnI AND NT-proBNP
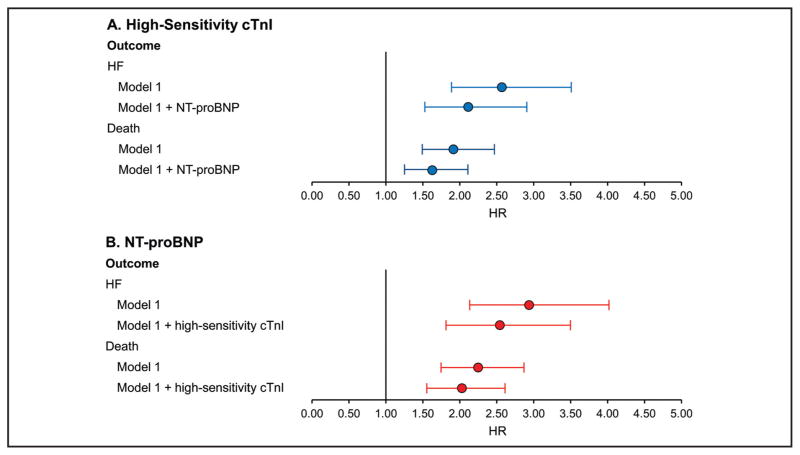
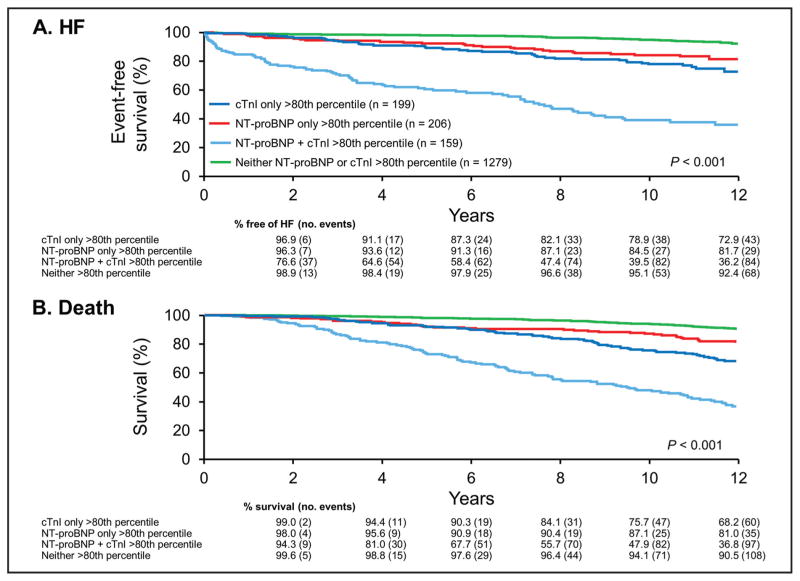
Adjusted models (Fig. 2) suggested that both high-sensitivity assay cTnI and NT-proBNP were independently prognostic for HF and mortality. Neither the addition of NT-proBNP to models of high-sensitivity assay cTnI or addition of high-sensitivity assay cTnI to models of NT-proBNP significantly reduced the prognostic value of the other. Indeed, the incidence of both HF and mortality among participants with highsensitivity assay cTnI and NT-proBNP concentrations above the sex-specific 80th percentile was significantly higher than in individuals with only high-sensitivity assay cTnI concentrations or NT-proBNP concentrations above the sex-specific 80th percentile (HF and mortality are shown in Fig. 3).
Fig. 2. HRs for high-sensitivity cTnI (A) and NT-proBNP (B) for HF and mortality. Multivariable model 1 included adjustment for age, sex, BMI, systolic blood pressure, creatinine (log), smoking status, and presence of hypertension, diabetes, and coronary artery disease.
(A), NT-proBNP is added to multivariable model 1; (B), high-sensitivity cTnI is added to multivariable model 1. Data are presented as HRs with 95% CIs.
Fig. 3. Kaplan–Meier curves for the unadjusted cumulative incidence in the sample population (n = 1843) for HF (A) and mortality (B) according to high-sensitivity cTnI and NT-proBNP above and below the sex-specific 80th percentile.
Light blue is both high-sensitivity cTnI and NT-proBNP above the 80th percentile (n = 159, 9%). Dark blue is high-sensitivity cTnI only above the 80th percentile (n = 199, 11%). Red is NT-proBNP only above the 80th percentile (n = 206, 11%). Green is neither high-sensitivity cTnI or NT-proBNP above the 80th percentile (n = 1279, 69%).
Discussion
The current findings demonstrate that circulating cTnI assessed by a high-sensitivity assay was prognostic beyond conventional risk factors for HF and mortality in a community-based population without known HF at baseline. High-sensitivity assay cTnI identified high-risk participants years before the onset of HF or mortality. These results are novel for this high-sensitivity assay for cTnI in the general community and confirm similar observations with cTnT high-sensitivity assay (1, 29) and other cTnI high-sensitivity assays (3, 4). Importantly, the prognostic utility of high-sensitivity assay cTnI for HF goes beyond the prognostic value of cardiac structural abnormalities that can be assessed by in-depth echocardiography, including even subtle abnormalities such as those due to diastolic dysfunction. Finally, the prognostic utility of high-sensitivity assay cTnI goes beyond that of NT-proBNP for predicting HF and mortality, and our data suggest that these 2 assays are complementary in identification of at-risk individuals in the general community. We have previously reported detectable concentrations of cTnI as measured by a high-sensitivity assay in the majority (93%) of individuals in the general community (7). Although occult structural or CAD may account for some of the reasons for higher troponin values, even among individuals without structural heart disease, detectable cTnI was present in 88%. The current report demonstrates that in the general community free of HF, higher circulating concentrations of high-sensitivity assay cTnI are associated with increased risk of HF and mortality. This higher risk is likely secondary to occult cardiovascular comorbidities only detectable by the biomarker. Such comorbidities may induce secondary neurohumoral activation due to mechanical stretch and associated cellular stress, with subsequent release of cardiac troponin into the circulation. Hence the use of the high-sensitivity assay for cardiac troponin allows for the detection of increased risk before the onset of clinically detectable risk factors. Identification of high-risk cohorts is paramount for trials aimed at the prevention of HF. Predicting risk in asymptomatic ambulatory populations allows therapeutic efforts to be focused on high-risk individuals vs dilution over a low- or average-risk population. Indeed, the recent Screening to Prevent Heart Failure (STOP-HF) trial suggests that BNP-based screening and guided therapy for HF have favorable outcomes compared with the standard of care (30). Such prevention trials are essential to decrease the burden of HF, because even asymptomatic disease is associated with high morbidity, and there have been no new HF therapeutic agents in more than a decade. These and other recently published data suggest that the combination of biomarkers, particularly high-sensitivity assay cTnI and NT-proBNP, can provide further stratification of cardiovascular risk and allow for even more targeted prevention efforts in the community (31, 32). Although some clinicians may view these data as important, there is a need for additional studies to define the appropriate therapeutic interventions before implementing these combined biomarker measurement strategies clinically. This is not only what we advocate, but also is consistent with the recommendations of the AHA position paper in this area (33). There are now sufficiently robust data that a clinical trial attempting to prevent HF development could be developed on the basis of these biomarkers. The Atherosclerosis Risk in Communities (ARIC) group recently demonstrated that a simplified model including NT-proBNP, what some would argue is a less-sensitive cardiac troponin high-sensitivity assay (34), age, sex, and race was similar in prognosis ability to a more complex model that used extensive clinical and historical assessment (29). This concept considerably lowers the burden of using such complex and laborious models to identify patients for therapeutic trials and potentially, once robust strategies are defined, in clinical practices as well. Analysis of data from the current study and event rates in Olmsted County, Minnesota, demonstrate a combined mortality/HF event-free rate at 5 years of 61%. By use of a 20% relative rate reduction in the intervention group (event-free rate of 69%), 550 research subjects would be needed per group (biomarker-guided and standard of care). Overall, our data suggest that cTnI assessed by a high-sensitivity assay is an independent marker for mortality in the general community. However, the improvement in the c-statistic for mortality is small and, when assessed in addition to NT-proBNP, is not significant. Nonetheless, our data (NRI, Fig. 2, and Fig. 3) support the independent and additive prognostic value of high-sensitivity assay cTnI and NT-proBNP for HF and mortality. Although previous studies reported the prognostic ability of high-sensitivity assay cTnI in the general community and diseased cohorts (4, 5, 31, 35, 36), our study adds strength to their findings by the in-depth characterization of the current cohort, including assessment of diastolic dysfunction. Nonetheless, there are several limitations to the current study, as it was limited to individuals who were primarily white and who were 45 years and older. Caution should be observed with extrapolation of our findings to other age and ethnic groups. In conclusion, we report that cTnI assessed by high-sensitivity assay is prognostic beyond traditional cardiovascular risk factors for HF and mortality in the general HF-free community. Further, the prognostic utility of high-sensitivity assay cTnI goes beyond that of NT-proBNP, and our data suggest that these 2 assays are complementary in identification of at-risk individuals in the general community. Our findings lay the foundation for prospective studies aimed at identification of high-risk individuals by use of a multimarker approach followed by aggressive prevention efforts to reduce HF-associated morbidity and mortality.
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
- Employment or Leadership: None declared.
- Consultant or Advisory Role: M.M. Redfield, Novartis; A.S. Jaffe, Beckman-Coulter, Radiometer, Roche, Abbott, Alere, Critical Diagnostics, Ortho Diagnostics, Trinity, ET Healthcare, and Amgen.
- Stock Ownership: None declared.
- Honoraria: M.M. Redfield, HFSA Educational Session; A.S. Jaffe, theheart.org.
- Research Funding: NIH-funded (HL36634) research assessing natriuretic peptides as therapeutics in heart failure and hypertension.
- Expert Testimony: None declared.
- Patents: None declared.
- Other Remuneration: M.M. Redfield, royalties from Anexon.
- Role of Sponsor: No sponsor was declared.
References
- 1.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apple FS, Steffen LM, Pearce LA, Murakami MM, Luepker RV. Increased cardiac troponin I as measured by a high-sensitivity assay is associated with high odds of cardiovascular death: the Minnesota Heart Survey. Clin Chem. 2012;58:930–5. doi: 10.1373/clinchem.2011.179176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeller T, Tunstall-Pedoe H, Saarela O, Ojeda F, Schnabel RB, Tuovinen T, et al. High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: the Morgam Biomarker Project Scottish Cohort. Eur Heart J. 2014;35:271–81. doi: 10.1093/eurheartj/eht406. [DOI] [PubMed] [Google Scholar]
- 5.Leistner DM, Klotsche J, Pieper L, Stalla GK, Lehnert H, Silber S, et al. Circulating troponin as measured by a sensitive assay for cardiovascular risk assessment in primary prevention. Clin Chem. 2012;58:200–8. doi: 10.1373/clinchem.2011.174292. [DOI] [PubMed] [Google Scholar]
- 6.Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem. 2009;55:1303–6. doi: 10.1373/clinchem.2009.128363. [DOI] [PubMed] [Google Scholar]
- 7.McKie PM, Heublein DM, Scott CG, Gantzer ML, Mehta RA, Rodeheffer RJ, et al. Defining highsensitivity cardiac troponin concentrations in the community. Clin Chem. 2013;59:1099–107. doi: 10.1373/clinchem.2012.198614. [DOI] [PubMed] [Google Scholar]
- 8.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–9. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen SJ, Mahoney DW, Redfield MM, Bailey KR, Burnett JC, Jr, Rodeheffer RJ. Participation bias in a population-based echocardiography study. Ann Epidemiol. 2004;14:579–84. doi: 10.1016/j.annepidem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 11.Costello-Boerrigter LC, Boerrigter G, Redfield MM, Rodeheffer RJ, Urban LH, Mahoney DW, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–53. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 14.McKie PM, Cataliotti A, Lahr BD, Martin FL, Redfield MM, Bailey KR, et al. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage a/b heart failure subjects. J Am Coll Cardiol. 2010;55:2140–7. doi: 10.1016/j.jacc.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macheret F, Boerrigter G, McKie P, Costello-Boerrigter L, Lahr B, Heublein D, et al. Pro-B-type natriuretic peptide(1–108) circulates in the general community: plasma determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2011;57:1386–95. doi: 10.1016/j.jacc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Jr, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–53. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s rosetta stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 21.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 22.Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987;59:956–60. doi: 10.1016/0002-9149(87)91133-7. [DOI] [PubMed] [Google Scholar]
- 23.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036–43. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 24.Christenson R, Gantzer M, Duh S, Cervelli D, deFilippi C. Analytical and clinical validation of a next-generation “high-sensitivity” cardiac troponin I assay on the Dimension Vista® system (Abstract) Clin Chem. 2010;56(6 Suppl):A132. [Google Scholar]
- 25.Collinson PO, Barnes SC, Gaze DC, Galasko G, Lahiri A, Senior R. Analytical performance of the N terminal pro B type natriuretic peptide (NTproBNP) assay on the Elecsys 1010 and 2010 analysers. Eur J Heart Fail. 2004;6:365–8. doi: 10.1016/j.ejheart.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 26.McKie PM, Rodeheffer RJ, Cataliotti A, Martin FL, Urban LH, Mahoney DW, et al. Amino-terminal proB-type natriuretic peptide and B-type natriuretic peptide: biomarkers for mortality in a large community-based cohort free of heart failure. Hypertension. 2006;47:874–80. doi: 10.1161/01.HYP.0000216794.24161.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 29.Nambi V, Liu X, Chambless LE, de Lemos JA, Virani SS, Agarwal S, et al. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk: the Atherosclerosis Risk in Communities Study. Clin Chem. 2013;59:1802–10. doi: 10.1373/clinchem.2013.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, et al. Natriuretic peptide based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310:66–74. doi: 10.1001/jama.2013.7588. [DOI] [PubMed] [Google Scholar]
- 31.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58:54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 35.Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–9. doi: 10.1016/j.jacc.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 36.Xue Y, Clopton P, Peacock WF, Maisel AS. Serial changes in high-sensitive troponin I predict outcome in patients with decompensated heart failure. Eur J Heart Fail. 2011;13:37–42. doi: 10.1093/eurjhf/hfq210. [DOI] [PubMed] [Google Scholar]